Adenosine Receptors Profile in Fibromuscular Dysplasia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Inclusion Criteria for Patients
- Patients aged between 18 to 80 years.
- Patients with multifocal FMD of a renal or cervical artery with typical aspect “ string of beads “ appearance.
- Patients willing to participate in the study.
2.3. Inclusion Criteria for the Control Group
- Subjects aged between 18 to 80 years.
- Subjects without history of personal or familial fibromuscular dysplasia.
- Subjects without story of cardiovascular disease or inflammatory process.
- Subjects free from medical treatment.
- Subjects willing to participate in the study.
2.4. Exclusion Criteria
- Patients less than 18 or more than 80 years of age.
- Renal failure with GFR < 30 mL/min.
- Pregnant women.
- Patients with symptomatic atheromatous cardiovascular disease.
- Patient with inflammatory or chronic disease.
- Patient with a history of cancer or active cancer.
- Patient refusing to participate in the study.
2.5. Adenosine Blood Level (ABL) Measurement
2.5.1. Blood Samples Collection
2.5.2. Blood Samples Extraction
2.5.3. Adenosine Concentration
2.6. Expression of Adenosine Receptors in PBMC
Western Blots
3. Statistical Analysis
4. Results
4.1. Patients
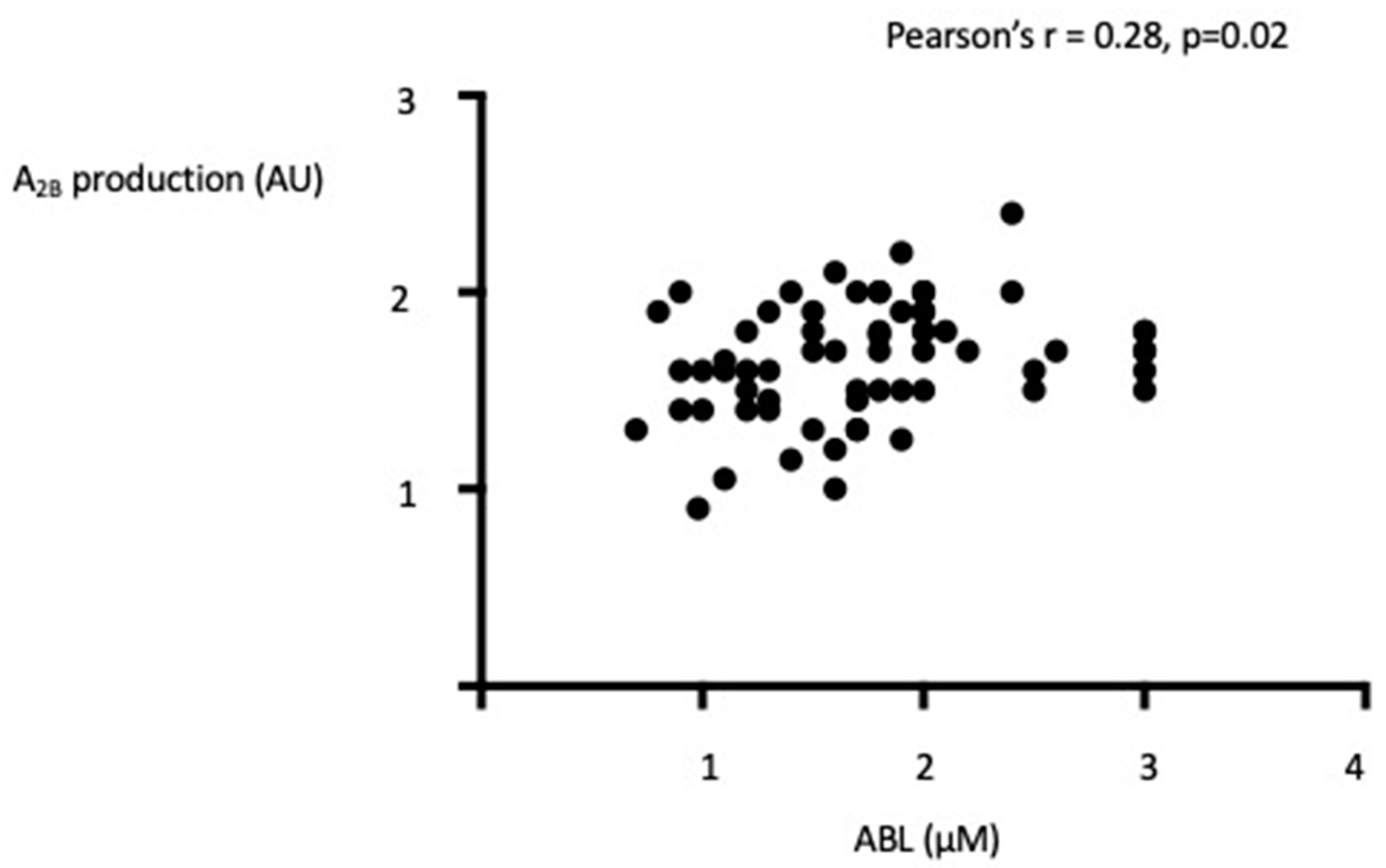
4.2. Adenosine Blood Level (ABL)
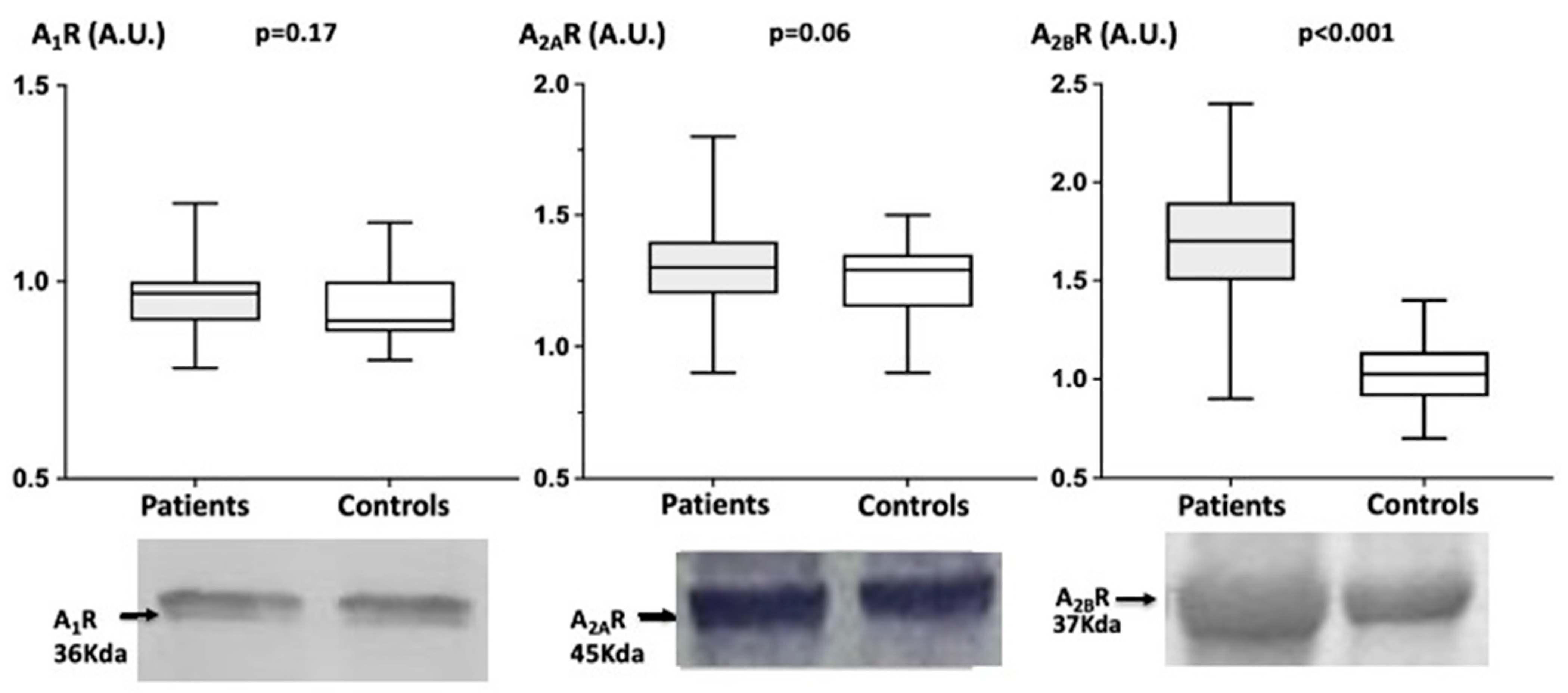
4.3. Adenosine Receptors Production in PBMCs
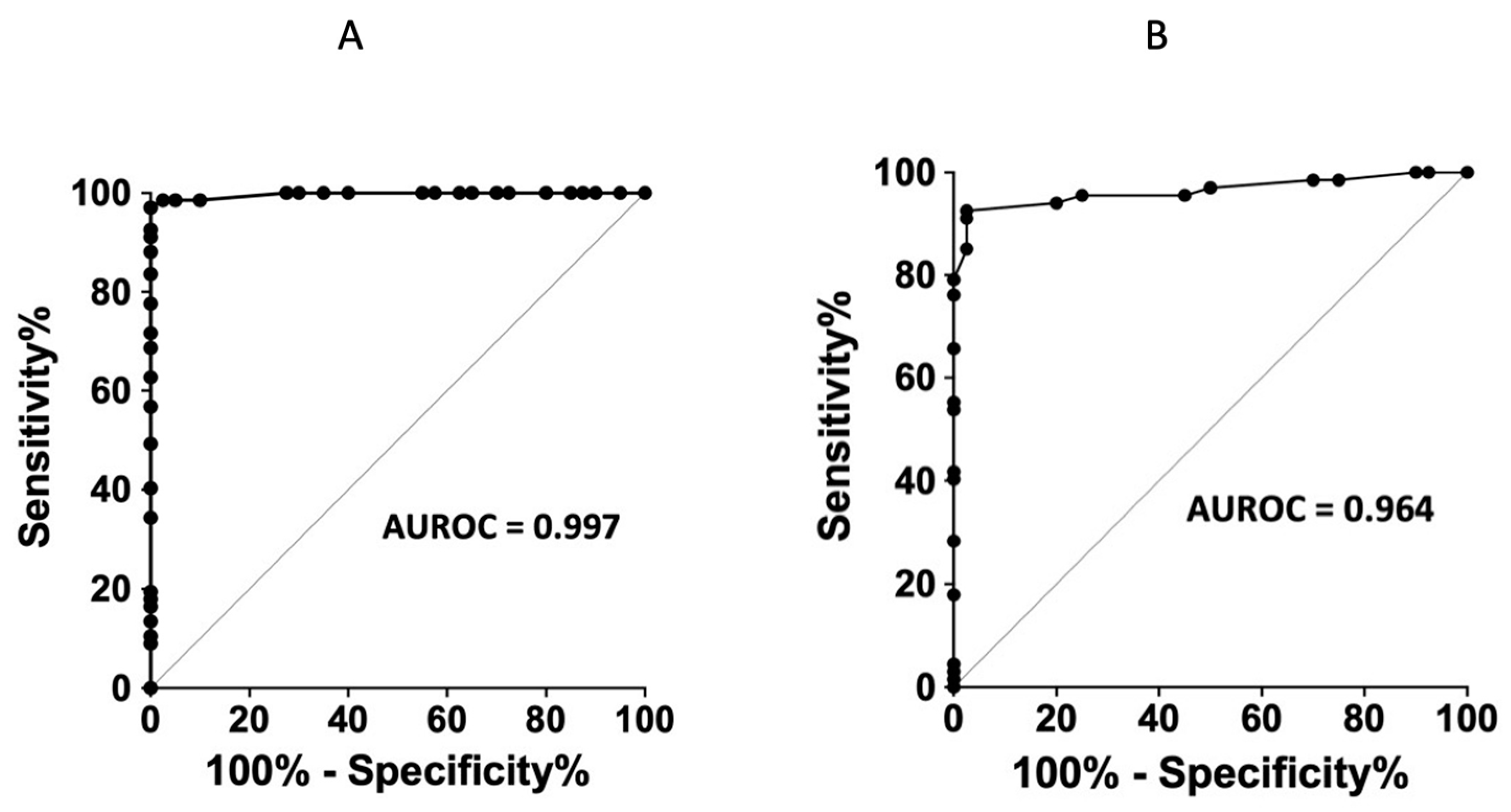
5. Discussion
6. Study Limitation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leckie, A.; Tao, M.J.; Narayanasamy, S.; Khalili, K.; Schieda, N.; Krishna, S. The Renal Vasculature: What the Radiologist Needs to Know. Radiographics 2022, 4, E80. [Google Scholar] [CrossRef] [PubMed]
- Gornik, H.L.; Persu, A.; Adlam, D.; Aparicio, L.S.; Azizi, M.; Boulanger, M.; Bruno, R.M.; de Leeuw, P.; Fendrikova-Mahlay, N.; Froehlich, J.; et al. First International Consensus on the diagnosis and management of fibromuscular dysplasia. Vasc. Med. 2019, 24, 164–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persu, A.; Giavarini, A.; Touzé, E.; Januszewicz, A.; Sapoval, M.; Azizi, M.; Barral, X.; Jeunemaitre, X.; Morganti, A.; Plouin, P.F.; et al. ESH Working Group Hypertension and the Kidney. European consensus on the diagnosis and management of fibromuscular dysplasia. J. Hypertens. 2014, 3, 1367–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olin, J.W.; Froehlich, J.; Gu, X.; Bacharach, J.M.; Eagle, K.; Gray, B.H.; Jaff, M.R.; Kim, E.; Esther, S.H.; Mace, P.; et al. The united states registry for fibromuscular dysplasia: Results in the first 447 patients. Circulation 2012, 125, 3182–3190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigazzi, R.; Bianchi, S.; Quilici, N.; Salvadori, R.; Baldari, G. Bilateral fibromuscular dysplasia in identical twins. Am. J. Kidney 1998, 32, E4. [Google Scholar] [CrossRef]
- Pannier-Moreau, I.; Grimbert, P.; Fiquet-Kempf, B.; Vuagnat, A.; Jeunemaitre, X.; Corvol, P.; Plouin, P.-F. Possible familial origin of multifocal renal artery fibromuscular dysplasia. J. Hypertens. 1997, 15 Pt 2, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Georges, A.; Albuisson, J.; Berrandou, T.; Dupré, D.; Lorthioir, A.; D’Escamard, V.; Di Narzo, A.F.; Kadian-Dodov, D.; Olin, J.W.; Warchol-Celinska, E.; et al. Rare loss-of-function mutations of PTGIR are enriched in fibromuscular dysplasia. Cardiovasc. Res. 2021, 117, 1154–1165. [Google Scholar] [CrossRef]
- Silhol, F.; Sarlon-Bartoli, G.; Daniel, L.; Bartoli, J.M.; Cohen, S.; Lepidi, H.; Piquet, P.; Bartoli, M.A.; Vaisse, B. Intranuclear expression of progesterone receptors in smooth muscle cells of renovascular fibromuscular dysplasia: A pilot study. Ann. Vasc. Surg. 2015, 29, 830–835. [Google Scholar] [CrossRef]
- Olin, J.W.; Di Narzo, A.F.; d’Escamard, V.; Kadian-Dodov, D.; Cheng, H.; Georges, A.; King, A.; Thomas, A.; Barwari, T.; Michelis, K.C.; et al. A plasma proteogenomic signature for fibromuscular dysplasia. Cardiovasc. Res. 2020, 116, 63–77. [Google Scholar] [CrossRef]
- Silhol, F.; Marlinge, M.; Guiol, C.; Chefrour, M.; Mace, P.; Criado, C.; Kipson, N.; Vaisse, B.; Vairo, D.; Sarlon, G.; et al. Characterization of adenosine A2 receptors in peripheral blood mononuclear cells of patients with fibromuscular dysplasia. Hypertens. Res. 2020, 43, 466–469. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guieu, R.; Deharo, J.-C.; Maille, B.; Crotti, L.; Torresani, E.; Brignole, M.; Parati, G. Adenosine and the Cardiovascular System: The Good and the Bad. J. Clin. Med. 2020, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, H.E.; Schnermann, J.; Oldenburg, P.J.; Mustafa, S.J. Role of A1 adenosine receptors in regulation of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1411–H1416. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, T.; Umemura, S.; Toya, Y.; Uchibori, T.; Kogi, K.; Takagi, N.; Ishii, M. Identification of Adenosine A2 Receptor-cAMP System in Human Aortic Endothelial Cells. Biochem. Biophys. Res. Commun. 1994, 199, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Ponnoth, D.S.; Sanjani, M.S.; Ledent, C.; Roush, K.; Krahn, T.; Mustafa, S.J. Absence of adenosine-mediated aortic relaxation in A2A adenosine receptor knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1655–H1660. [Google Scholar] [CrossRef] [Green Version]
- Kusano, Y.; Echeverry, G.; Miekisiak, G.; Kulik, T.B.; Aronhime, S.N.; Chen, J.F.; Winn, H.R. Role of Adenosine A2 Receptors in Regulation of Cerebral Blood Flow during Induced Hypotension. J. Cereb. Blood Flow Metab. 2010, 30, 808–815. [Google Scholar] [CrossRef]
- Arsyad, A.; Dobson, G.P. Adenosine relaxation in isolated rat aortic rings and possible roles of smooth muscle Kv channels, KATP channels and A2a receptors. BMC Pharmacol. Toxicol. 2016, 17, 23. [Google Scholar] [CrossRef] [Green Version]
- Ray, C.J.; Marshall, J.M. The cellular mechanisms by which adenosine evokes release of nitric oxide from rat aortic endothelium. J. Physiol. 2006, 570 Pt 1, 85–96. [Google Scholar] [CrossRef]
- Paganelli, F.; Gaudry, M.; Ruf, J.; Guieu, R. Recent advances in the role of the adenosinergic system in coronary artery disease. Cardiovasc. Res. 2021, 117, 1284–1294. [Google Scholar] [CrossRef]
- Gariboldi, V.; Vairo, D.; Guieu, R.; Marlingue, M.; Ravis, E.; Lagier, D.; Mari, A.; Thery, E.; Collart, F.; Gaudry, M.; et al. Expressions of adenosine A2A receptors in coronary arteries and peripheral blood mononuclear cells are correlated in coronary artery disease patients. Int. J. Cardiol. 2017, 230, 427–431. [Google Scholar] [CrossRef]
- Gaudry, M.; Marlinge, M.; Deharo, P.; Vairo, D.; Bottone, S.; Mottola, G.; Kipson, N.; Criado, C.; Mace, P.; Chefrour, M.; et al. Pharmacological profile of adenosine A2A receptors in patients with lower extremity peripheral artery disease and associated coronary artery disease: A pilot study. Int. J. Cardiol. 2019, 285, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Laghi-Pasini, F.; Camurri, A.; Capecchi, P.L.; Maccherini, M.; Diciolla, F.; Ceccatelli, L.; Lazzerini, P.E.; Ulouglu, C.; Cattabeni, F.; et al. Changes of peripheral A 2A adenosine receptors in chronic heart failure and cardiac transplantation. FASEB J. 2003, 17, 280–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marlinge, M.; Vairo, D.; Marolda, V.; Bruzzese, L.; Adjriou, N.; Guiol, C.; Kipson, N.; Bonnardel, A.; Gastaldi, M.; Kerbaul, F.; et al. Rapid Measurement of Adenosine Concentration in Human Blood Using Fixed Potential Amperometry: Comparison with Mass Spectrometry and High- Performance Liquid Chromatography. J. Anal. Bioanal. Tech. 2017, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Ruf, J.; Vairo, D.; Paganelli, F.; Guieu, R. Extracellular vesicles with ubiquitinated adenosine A 2A receptor in plasma of patients with coronary artery disease. J. Cell. Mol. Med. 2019, 23, 6805–6811. [Google Scholar] [CrossRef] [Green Version]
- Paganelli, F.; Resseguier, N.; Marlinge, M.; Laine, M.; Malergue, F.; Kipson, N.; Armangau, P.; Pezzoli, N.; Kerbaul, F.; Bonello, L.; et al. Specific Pharmacological Profile of A2A Adenosine Receptor Predicts Reduced Fractional Flow Reserve in Patients with Suspected Coronary Artery Disease. J. Am. Heart Assoc. 2018, 7, e008290. [Google Scholar] [CrossRef]
- Ruf, J.; Paganelli, F.; Bonello, L.; Kipson, N.; Mottola, G.; Fromonot, J.; Condo, J.; Boussuges, A.; Bruzzese, L.; Kerbaul, F.; et al. Spare Adenosine A2a Receptors Are Associated with Positive Exercise Stress Test in Coronary Artery Disease. Mol. Med. 2016, 22, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Guieu, R.; Kipson, N.; Ruf, J.; Fournier, N.; Laine, M.; Foucher, M.C.; Fromonot, J.; Mottola, G.; Bruzzese, L.; Boussuges, A.; et al. Low basal expression of A2A adenosine receptors and increase in adenosine plasma concentration are associated with positive exercise stress testing. Int. J. Cardiol. 2015, 180, 15–17. [Google Scholar] [CrossRef]
- By, Y.; Durand-Gorde, J.-M.; Condo, J.; Lejeune, P.-J.; Mallet, B.; Carayon, P.; Guieu, R.; Ruf, J. Production of an agonist-like monoclonal antibody to the human A2A receptor of adenosine for clinical use. Mol. Immunol. 2009, 46, 400–405. [Google Scholar] [CrossRef]
- Togna, A.R.; Latina, V.; Orlando, R.; Togna, G.I. Cigarette smoke inhibits adenine nucleotide hydrolysis by human platelets. Platelets 2008, 19, 537–542. [Google Scholar] [CrossRef]
- Franceschi, F.; Deharo, J.-C.; Giorgi, R.; By, Y.; Monserrat, C.; Condo, J.; Ibrahim, Z.; Saadjian, A.; Guieu, R. Peripheral plasma adenosine release in patients with chronic heart failure. Heart 2009, 95, 651–655. [Google Scholar] [CrossRef]
- Kitakaze, M.; Minamino, T.; Node, K.; Koretsune, Y.; Komamura, K.; Funaya, H.; Kuzuya, T.; Hori, M. levation of Plasma Adenosine Levels May Attenuate the Severity of Chronic Heart Failure. Cardiovasc. Drugs Ther. 1998, 12, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Carrega, L.; Saadjian, A.Y.; Mercier, L.; Zouher, I.; Bergé-Lefranc, J.-L.; Gerolami, V.; Giaime, P.; Sbragia, P.; Paganelli, F.; Fenouillet, E.; et al. Increased expression of adenosine A2A receptors in patients with spontaneous and head-up-tilt-induced syncope. Heart Rhythm 2007, 4, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Deharo, J.C.; Mechulan, A.; Giorgi, R.; Franceschi, F.; Prevot, S.; Peyrouse, E.; Condo, J.; By, Y.; Ruf, J.; Brignole, M.; et al. Adenosine plasma level and A2A adenosine receptor expression: Correlation with laboratory tests in patients with neurally mediated syncope. Heart 2012, 98, 855–859. [Google Scholar] [CrossRef]
- Gaubert, M.; Marlinge, M.; Kerbaul, F.; Resseguier, N.; Laine, M.; Cautella, J.; Cordier, C.; Colomb, B.; Kipson, N.; Thuny, F.; et al. Adenosine Plasma Level and A2A Receptor Expression in Patients with Cardiogenic Shock. Crit. Care Med. 2018, 46, e874–e880. [Google Scholar] [CrossRef] [PubMed]
- Saadjian, A.Y.; Gerolami, V.; Giorgi, R.; Mercier, L.; Berge-Lefranc, J.-L.; Paganelli, F.; Ibrahim, Z.; By, Y.; Guéant, J.L.; Lévy, S.; et al. Head-up tilt induced syncope and adenosine A2A receptor gene polymorphism. Eur. Heart J. 2009, 30, 1510–1515. [Google Scholar] [CrossRef]
- Maille, B.; Fromonot, J.; Guiol, C.; Marlinge, M.; Baptiste, F.; Lim, S.; Colombani, C.; Chaptal, M.C.; Chefrour, M.; Gastaldi, M.; et al. A2 Adenosine Receptor Subtypes Overproduction in Atria of Perioperative Atrial Fibrillation Patients Undergoing Cardiac Surgery: A Pilot Study. Front. Cardiovasc. Med. 2021, 8, 761164. [Google Scholar] [CrossRef] [PubMed]
- Rubino, A.; Ralevic, V.; Burnstock, G. Contribution of P1 (A2b subtype) and P2-purinoceptors to the control of vascular tone in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 1995, 115, 648–652. [Google Scholar] [CrossRef] [Green Version]
- Dubey, R.K.; Gillespie, D.G.; Osaka, K.; Suzuki, F.; Jackson, E.K. Adenosine Inhibits Growth of Rat Aortic Smooth Muscle Cells. Possible role of A2b receptor. Hypertension 1996, 27 Pt 2, 786–793. [Google Scholar] [CrossRef]
- Dubey, R.K.; Gillespie, D.G.; Jackson, E.K. Adenosine Inhibits Collagen and Protein Synthesis in Cardiac Fibroblasts: Role of A2B receptors. Hypertension 1998, 31, 943–948. [Google Scholar] [CrossRef] [Green Version]
- Dubey, R.K.; Gillespie, D.G.; Shue, H.; Jackson, E.K. A 2B Receptors Mediate Antimitogenesis in Vascular Smooth Muscle Cells. Hypertension 2000, 35 Pt 2, 267–272. [Google Scholar] [CrossRef]
- Hilaire, C.S.; Yang, D.; Schreiber, B.; Ravid, K. B-Myb regulates the A2B adenosine receptor in vascular smooth muscle cells. J. Cell. Biochem. 2008, 103, 1962–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Koupenova, M.; McCrann, D.J.; Kopeikina, K.J.; Kagan, H.M.; Schreiber, B.M.; Ravid, K. The A2b adenosine receptor protects against vascular injury. Proc. Natl. Acad. Sci. USA 2008, 105, 792–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberger, P.; Schwab, J.; Mirakaj, V.; Masekowsky, E.; Mager, A.; Morote-Garcia, J.C.; E Unertl, K.; Eltzschig, H.K. Hypoxia-inducible factor–dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat. Immunol. 2009, 10, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, E.A.; White, P.J.; May, L.T. Targeting Adenosine Receptors for the Treatment of Cardiac Fibrosis. Front. Pharmacol. 2017, 8, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pathological overproduction: The bad side of adenosine. Br. J. Pharmacol. 2017, 174, 1945–1960. [Google Scholar] [CrossRef] [Green Version]
- Peyot, M.L.; Gadeau, A.P.; Dandré, F.; Belloc, I.; Dupuch, F.; Desgranges, C. Extracellular adenosine induces apoptosis of human arterial smooth muscle cellsvia A(2b)-purinoceptor. Circ. Res. 2000, 86, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Beukers, M.W.; den Dulk, H.; van Tilburg, E.W.; Brouwer, J.; Ijzerman, A.P. Why are A(2B) receptors low-affinity adenosine receptors? Mutation of Asn273 to Tyr increases affinity of human A(2B) receptor for 2-(1-Hexynyl)adenosine. Mol. Pharmacol. 2000, 58, 1349–1356. [Google Scholar] [CrossRef] [Green Version]
- Vijayamahantesh; Amit, A.; Kumar, S.; Dikhit, M.R.; Jha, P.K.; Singh, A.K.; Sinha, K.K.; Pandey, K.; Das, V.; Das, P.; et al. Up regulation of A2B adenosine receptor on monocytes are crucially required for immune pathogenicity in Indian patients exposed to Leishmania donovani. Cytokine 2016, 79, 38–44. [Google Scholar] [CrossRef]
- Gao, Z.-G.; Jacobson, K.A. A2B Adenosine Receptor and Cancer. Int. J. Mol. Sci. 2019, 20, 5139. [Google Scholar] [CrossRef]
- Xaus, J.; Mirabet, M.; Lloberas, J.; Soler, C.; Lluis, C.; Franco, R.; Celada, A. IFN-gamma up-regulates the A2B adenosine receptor expression in macrophages: A mechanism of macrophage deactivation. J. Immunol. 1999, 162, 3607–3614. [Google Scholar] [PubMed]
- Available online: www.orpha.net/data/patho/Pub/fr/DysplasieFibromusculaireArterielle-FRfrPub5540.pdf (accessed on 4 November 2022).

| FMD n = 67 | Controls n = 40 | |
|---|---|---|
| Age (Years, mean, Range) | 55 (29–77) | 56 (37–70) |
| M/F | 50/17 | 30/10 |
| Race | ||
| White | 67 | 35 |
| Black | 0 | 1 |
| Asian | 0 | 4 |
| Hypertension | 29 (43%) | 0 |
| Current smoker | 25 (37%) | 12 (30%) |
| Hyperlipemia | 32 (47%) | 0 |
| Medications | ||
| Diuretic | 21 (31%) | 0 |
| ARBs | 20 (29%) | 0 |
| Beta-blockers | 8 (12%) | 0 |
| ACE Inhibitors | 15 (22%) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guiol, C.; El Harake, S.; Fromonot, J.; Chefrour, M.; Gastaldi, M.; Alibouch, Y.; Doublier, M.; Deharo, P.; Sarlon, G.; Marlinge, M.; et al. Adenosine Receptors Profile in Fibromuscular Dysplasia. Biomedicines 2022, 10, 2831. https://doi.org/10.3390/biomedicines10112831
Guiol C, El Harake S, Fromonot J, Chefrour M, Gastaldi M, Alibouch Y, Doublier M, Deharo P, Sarlon G, Marlinge M, et al. Adenosine Receptors Profile in Fibromuscular Dysplasia. Biomedicines. 2022; 10(11):2831. https://doi.org/10.3390/biomedicines10112831
Chicago/Turabian StyleGuiol, Claire, Sarah El Harake, Julien Fromonot, Mohamed Chefrour, Marguerite Gastaldi, Yassine Alibouch, Maxime Doublier, Pierre Deharo, Gabrielle Sarlon, Marion Marlinge, and et al. 2022. "Adenosine Receptors Profile in Fibromuscular Dysplasia" Biomedicines 10, no. 11: 2831. https://doi.org/10.3390/biomedicines10112831





