Bullous Pemphygoid and Novel Therapeutic Approaches
Abstract
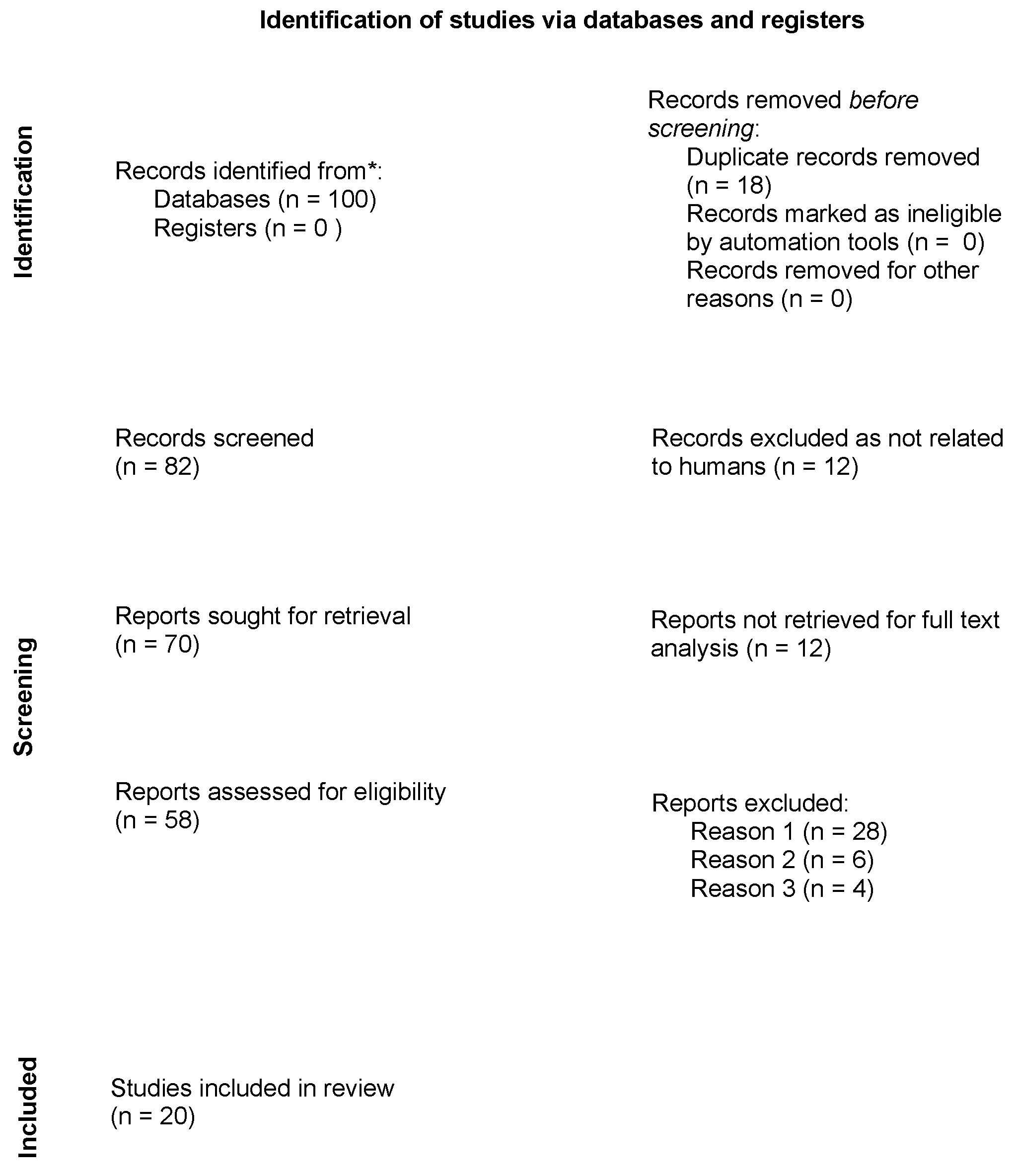
:1. Introduction
2. Materials and Methods
2.1. Identification of the Research Question
2.2. Study Selection Process
2.3. Data Extraction
3. Results
3.1. Rituximab
3.2. Dupilumab
3.3. Omalizumab
3.4. Other Therapies
3.4.1. Complement System Inhibitors
- ○
- Nomacopan (rVA576, a recombinant small protein, formerly known as coversin) is a complement inhibitor with activity against both C5 and LTB4. In a phase 2 nonrandomized, controlled trial (NCT04035733), seven of nine patients with mild-to-moderate new-onset or relapsing BP treated with nomacopan showed no treatment-related AEs (primary endpoint) and a significant decrease in disease activity and improvement of quality of life (secondary endpoint). A randomized, double blind, placebo-controlled clinical trial is expected to enroll 148 participants to evaluate nomacopan’s efficacy regarding the primary endpoint (NCT05061771) [96].
- ○
- Avdoralimab, a specific anti-C5aR1 monoclonal antibody, has already shown a good safety profile in the treatment of solid tumors and rheumatoid arthritis. While C5aR1 mediates anti-BP180 IgG-induced pathogenicity, C5aR2 has a protective effect. Investigators hypothesize that avdoralimab might be a safe and effective treatment in BP patients: an open label, randomized, parallel-group phase 2 clinical trial (NCT04563923) is expected to enroll 40 patients to evaluate the efficacy of avdoralimab in addition to superpotent topical steroids. Complete clinical remission is defined as the primary endpoint [97,98].
- ○
- Sutimlimab (BIVV009, formerly known as TNT009) is a humanized IgG4 monoclonal antibody that targets the C1s component of complement and thus inhibits leukocyte chemoattraction in BP. A phase 1 trial (NCT02502903) was conducted on 122 patients to study the safety, tolerability, and activity of sutimlimab in healthy patients and patients with complement-mediated disorders. Sutimlimab had a good safety profile and predictable and consistent pharmacokinetics and pharmacodynamics in healthy volunteers. In 10 patients with active or past BP, the classical complement pathway was blocked, and C3c deposition along the DEJ was partially or completely abrogated in 4 of 5 patients.
3.4.2. Eotaxin-1 (CCL-11)
3.4.3. Dimethyl Fumarate
3.4.4. IL-5 Inhibitors
3.4.5. IL-17 and IL-23 Inhibitors
3.4.6. Inflammasome Inhibition
3.4.7. AC-203
3.4.8. Ligelizumab
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Beek, N.; Schulze, F.S.; Zillikens, D.; Schmidt, E. IgE-mediated mechanisms in bullous pemphigoid and other autoimmune bullous diseases. Expert Rev. Clin. Immunol. 2016, 12, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Zillikens, D. Pemphigoid diseases. Lancet 2013, 381, 320–332. [Google Scholar] [CrossRef]
- Miyamoto, D.; Santi, C.G.; Aoki, V.; Maruta, C.W. Bullous pemphigoid. An. Bras. Dermatol. 2019, 94, 133–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beek, N.; Zillikens, D.; Schmidt, E. Bullous autoimmune dermatoses–clinical features, diagnostic evaluation, and treatment options. Dtsch. Ärztebl. Int. 2021, 118, 413–420. [Google Scholar] [CrossRef]
- Saniklidou, A.H.; Tighe, P.J.; Fairclough, L.C.; Todd, I. IgE autoantibodies and their association with the disease activity and phenotype in bullous pemphigoid: A systematic review. Arch. Dermatol. Res. 2018, 310, 11–28. [Google Scholar] [CrossRef] [Green Version]
- Messingham, K.N.; Srikantha, R.; DeGueme, A.M.; Fairley, J.A. FcR-independent effects of IgE and IgG autoantibodies in bullous pemphigoid. J. Immunol. 2011, 187, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Atzmony, L.; Mimouni, I.; Reiter, O.; Leshem, Y.A.; Taha, O.; Gdalevich, M.; Hodak, E.; Mimouni, D. Association of bullous pemphigoid with malignancy: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 691–699. [Google Scholar] [CrossRef]
- Cugno, M.; Tedeschi, A.; Borghi, A.; Bucciarelli, P.; Asero, R.; Venegoni, L.; Griffini, S.; Grovetti, E.; Berti, E.; Marzano, A.V. Activation of Blood Coagulation in Two Prototypic Autoimmune Skin Diseases: A Possible Link with Thrombotic Risk. PLoS ONE 2015, 10, e0129456. [Google Scholar] [CrossRef] [Green Version]
- Kridin, K.; Shihade, W.; Bergman, R. Mortality in patients with bullous pemphigoid: A retrospective cohort study, systematic review and meta-analysis. Acta Derm. Venereol. 2018, 99, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, J.; Joly, P.; Roujeau, J.C. Treatment of bullous pemphigoid. J. Dermatol. 2003, 30, 83–90. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.H.; Tanweer, F.; Sakagiannis, G.; Mair, M.; Mahmood, S.; Ashokkumar, S. Pemphigus Vulgaris and Bullous Pemphigoid of the Upper Aerodigestive Tract: A Review Article and Novel Approaches to Management. ORL 2021, 83, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Garrido, P.M.; Queirós, C.S.; Travassos, A.R.; Borges-Costa, J.; Filipe, P. Emerging treatments for bullous pemphigoid. J. Dermatol. Treat. 2022, 33, 649–661. [Google Scholar] [CrossRef]
- Alaibac, M. Biological therapy of autoimmune blistering diseases. Expert Opin. Biol. Ther. 2019, 19, 149–156. [Google Scholar] [CrossRef]
- Mahévas, M.; Michel, M.; Weill, J.C.; Reynaud, C.A. Long-lived plasma cells in autoimmunity: Lessons from B-cell depleting therapy. Front. Immunol. 2013, 4, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kridin, K.; Ahn, C.; Huang, W.C.; Ansari, A.; Sami, N. Treatment Update of Autoimmune Blistering Diseases. Dermatol. Clin. 2019, 37, 215–228. [Google Scholar] [CrossRef]
- Schmidt, E.; Seitz, C.S.; Benoit, S.; Bröcker, E.B.; Goebeler, M. Rituximab in autoimmune bullous diseases: Mixed responses and adverse effects. Br. J. Dermatol. 2007, 156, 352–356. [Google Scholar] [CrossRef]
- Izumi, K.; Bieber, K.; Ludwig, R.J. Current Clinical Trials in Pemphigus and Pemphigoid. Front. Immunol. 2019, 10, 978. [Google Scholar] [CrossRef]
- Schmidt, E.; Hunzelmann, N.; Zillikens, D.; Bröcker, E.B.; Goebeler, M. Rituximab in refractory autoimmune bullous diseases. Clin. Exp. Dermatol. 2006, 31, 503–508. [Google Scholar] [CrossRef]
- Cho, Y.T.; Chu, C.Y.; Wang, L.F. First-line combination therapy with rituximab and corticosteroids provides a high complete remission rate in moderate-to-severe bullous pemphigoid. Br. J. Dermatol. 2015, 173, 302–304. [Google Scholar] [CrossRef]
- Schmidt, E.; Goebeler, M.; Hertl, M.; Sárdy, M.; Sitaru, C.; Eming, R.; Hofmann, S.C.; Hunzelmann, N.; Kern, J.S.; Kramer, H.; et al. S2k guideline for the diagnosis of pemphigus vulgaris/foliaceus and bullous pemphigoid. J. Dtsch. Dermatol. Ges. 2015, 13, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Polansky, M.; Eisenstadt, R.; DeGrazia, T.; Zhao, X.; Liu, Y.; Feldman, R. Rituximab therapy in patients with bullous pemphigoid: A retrospective study of 20 patients. J. Am. Acad. Dermatol. 2019, 81, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Tovanabutra, N.; Payne, A.S. Clinical outcome and safety of rituximab therapy for pemphigoid diseases. J. Am. Acad. Dermatol. 2020, 82, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, A.; Euverman, H.I.; Terra, J.B.; Jonkman, M.F.; Horváth, B. Effectiveness and Safety of Rituximab in Recalcitrant Pemphigoid Diseases. Front. Immunol. 2018, 9, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amber, K.T.; Maglie, R.; Solimani, F.; Eming, R.; Hertl, M. Targeted Therapies for Autoimmune Bullous Diseases: Current Status. Drugs 2018, 78, 1527–1548. [Google Scholar] [CrossRef]
- Hall, R.P., 3rd; Streilein, R.D.; Hannah, D.L.; McNair, P.D.; Fairley, J.A.; Ronaghy, A.; Edhegard, K.D.; Levesque, M.C. Association of serum B-cell activating factor level and proportion of memory and transitional B cells with clinical response after rituximab treatment of bullous pemphigoid patients. J. Investig. Dermatol. 2013, 133, 2786–2788. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.R.; Shetty, S.; Kaveri, S.; Spigelman, Z.S. Treatment of recalcitrant bullous pemphigoid (BP) with a novel protocol: A retrospective study with a 6-year follow-up. J. Am. Acad. Dermatol. 2016, 74, 700–708.e3. [Google Scholar] [CrossRef]
- Ridpath, A.V.; Rzepka, P.V.; Shearer, S.M.; Scrape, S.R.; Olencki, T.E.; Kaffenberger, B.H. Novel use of combination therapeutic plasma exchange and rituximab in the treatment of nivolumab-induced bullous pemphigoid. Int. J. Dermatol. 2018, 57, 1372–1374. [Google Scholar] [CrossRef]
- Kremer, N.; Snast, I.; Cohen, E.S.; Hodak, E.; Mimouni, D.; Lapidoth, M.; Mazor, S.; Levi, A. Rituximab and Omalizumab for the Treatment of Bullous Pemphigoid: A Systematic Review of the Literature. Am. J. Clin. Dermatol. 2019, 20, 209–216. [Google Scholar] [CrossRef]
- Berkani, N.; Joly, P.; Golinski, M.-L.; Colliou, N.; Lim, A.; Larbi, A.; Riou, G.; Caillot, F.; Bernard, P.; Bedane, C.; et al. B-cell depletion induces a shift in self antigen specific B-cell repertoire and cytokine pattern in patients with bullous pemphigoid. Sci. Rep. 2019, 9, 3525, Erratum in Sci. Rep. 2019, 9, 18991.. [Google Scholar] [CrossRef]
- Tavakolpour, S. Dupilumab: A revolutionary emerging drug in atopic dermatitis and its possible role in pemphigus. Dermatol. Ther. 2016, 29, 299. [Google Scholar] [CrossRef] [PubMed]
- Takamura, S.; Teraki, Y. Treatment of bullous pemphigoid with dupilumab: Dupilumab exerts its effect by primarily suppressing T-helper 2 cytokines. J. Dermatol. 2022, 49, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Messingham, K.N.; Holahan, H.M.; Frydman, A.S.; Fullenkamp, C.; Srikantha, R.; Fairley, J.A. Human eosinophils express the high affinity IgE receptor, FcεRI, in bullous pemphigoid. PLoS ONE 2014, 9, e107725. [Google Scholar] [CrossRef] [PubMed]
- Cozzani, E.; Gasparini, G.; Di Zenzo, G.; Parodi, A. Immunoglobulin E and bullous pemphigoid. Eur. J. Dermatol. 2018, 28, 440–448. [Google Scholar] [CrossRef]
- Gounni Abdelilah, S.; Wellemans, V.; Agouli, M.; Guenounou, M.; Hamid, Q.; Beck, L.A.; Lamkhioued, B. Increased expression of Th2-associated chemokines in bullous pemphigoid disease. Role of eosinophils in the production and release of these chemokines. Clin. Immunol. 2006, 120, 220–231. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kursewicz, C.D.; Fayne, R.A.; Nanda, S.; Shah, S.M.; Nattkemper, L.; Yokozeki, H.; Yosipovitch, G. Pathophysiologic mechanisms of itch in bullous pemphigoid. J. Am. Acad. Dermatol. 2020, 83, 53–62. [Google Scholar] [CrossRef]
- Afarideh, M.; Borucki, R.; Werth, V.P. A Review of the Immunologic Pathways Involved in Bullous Pemphigoid and Novel Therapeutic Targets. J. Clin. Med. 2022, 11, 2856. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study to Evaluate the Efficacy and Safety of Dupilumab in Adult Patients with Bullous Pemphigoid, NCT04206553. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT04206553 (accessed on 2 November 2022).
- Kaye, A.; Gordon, S.C.; Deverapalli, S.C.; Her, M.J.; Rosmarin, D. Dupilumab for the Treatment of Recalcitrant Bullous Pemphigoid. JAMA Dermatol. 2018, 154, 1225–1226. [Google Scholar] [CrossRef]
- Seyed Jafari, S.M.; Feldmeyer, L.; Bossart, S.; Simon, D.; Schlapbach, C.; Borradori, L. Case Report: Combination of Omalizumab and Dupilumab for Recalcitrant Bullous Pemphigoid. Front. Immunol. 2021, 11, 611549. [Google Scholar] [CrossRef]
- Seidman, J.S.; Eichenfield, D.Z.; Orme, C.M. Dupilumab for bullous pemphigoid with intractable pruritus. Dermatol. Online J. 2019, 25, 12. [Google Scholar] [CrossRef]
- Abdat, R.; Waldman, R.A.; de Bedout, V.; Czernik, A.; Mcleod, M.; King, B.; Gordon, S.; Ahmed, R.; Nichols, A.; Rothe, M.; et al. Dupilumab as a novel therapy for bullous pemphigoid: A multicenter case series. J. Am. Acad. Dermatol. 2020, 83, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Q.; Chen, L.; Chen, J.; Zhang, J.; Zou, Y.; Gong, T.; Ji, C. Efficacy and Safety of Dupilumab in Moderate-to-Severe Bullous Pemphigoid. Front. Immunol. 2021, 12, 738907. [Google Scholar] [CrossRef] [PubMed]
- Kalowska, M.; Ciepiela, O.; Kowalewski, C.; Demkow, U.; Schwartz, R.A.; Wozniak, K. Enzyme-linked Immunoassay Index for Anti-NC16a IgG and IgE Auto-antibodies Correlates with Severity and Activity of Bullous Pemphigoid. Acta Derm. Venereol. 2016, 96, 191–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriuchi, R.; Nishie, W.; Ujiie, H.; Natsuga, K.; Shimizu, H. In vivo analysis of IgE autoantibodies in bullous pemphigoid: A study of 100 cases. J. Dermatol. Sci. 2015, 78, 21–25. [Google Scholar] [CrossRef]
- Van Beek, N.; Lüttmann, N.; Huebner, F.; Recke, A.; Karl, I.; Schulze, F.S.; Zillikens, D.; Schmidt, E. Correlation of Serum Levels of IgE Autoantibodies Against BP180 with Bullous Pemphigoid Disease Activity. JAMA Dermatol. 2017, 153, 30–38. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Chen, X.; Jin, H.; Li, L. Factors associated with the activity and severity of bullous pemphigoid: A review. Ann. Med. 2020, 52, 55–62. [Google Scholar] [CrossRef]
- Fairley, J.A.; Baum, C.L.; Brandt, D.S.; Messingham, K.A. Pathogenicity of IgE in autoimmunity: Successful treatment of bullous pemphigoid with omalizumab. J. Allergy Clin. Immunol. 2009, 123, 704–705. [Google Scholar] [CrossRef] [Green Version]
- Menzinger, S.; Kaya, G.; Schmidt, E.; Fontao, L.; Laffitte, E. Biological and Clinical Response to Omalizumab in a Patient with Bullous Pemphigoid. Acta Derm. Venereol. 2018, 98, 284–286. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, M.; Bohelay, G.; Gille, T.; Le Roux-Villet, C.; Soued, I.; Morin, F.; Caux, F.; Grootenboer-Mignot, S.; Prost-Squarcioni, C. Rapid Disease Control in First-Line Therapy-Resistant Mucous Membrane Pemphigoid and Bullous Pemphigoid with Omalizumab as Add-On Therapy: A Case Series Of 13 Patients. Front. Immunol. 2022, 13, 874108. [Google Scholar] [CrossRef]
- Iwata, Y.; Komura, K.; Kodera, M.; Usuda, T.; Yokoyama, Y.; Hara, T.; Muroi, E.; Ogawa, F.; Takenaka, M.; Sato, S. Correlation of IgE autoantibody to BP180 with a severe form of bullous pemphigoid. Arch. Dermatol. 2008, 144, 41–48. [Google Scholar] [CrossRef]
- Döpp, R.; Schmidt, E.; Chimanovitch, I.; Leverkus, M.; Bröcker, E.B.; Zillikens, D. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in Bullous pemphigoid: Serum levels of these immunoglobulins reflect disease activity. J. Am. Acad. Dermatol. 2000, 42, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Nieboer, C. Serum IgE levels in patients with bullous pemphigoid. Acta Derm. Venereol. 1985, 65, 273–274. [Google Scholar] [PubMed]
- Soh, H.; Hosokawa, H.; Asada, Y. IgE and its related phenomena in bullous pemphigoid. Br. J. Dermatol. 1993, 128, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ohzono, A.; Teye, K.; Numata, S.; Hiroyasu, S.; Tsuruta, D.; Hachiya, T.; Kuroda, K.; Hashiguchi, M.; Kawakami, T.; et al. Detection of IgE autoantibodies to BP180 and BP230 and their relationship to clinical features in bullous pemphigoid. Br. J. Dermatol. 2017, 177, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Ghohestani, R.F.; Cozzani, E.; Delaporte, E.; Nicolas, J.F.; Parodi, A.; Claudy, A. IgE antibodies in sera from patients with bullous pemphigoid are autoantibodies preferentially directed against the 230-kDa epidermal antigen (BP230). J. Clin. Immunol. 1998, 18, 202–209. [Google Scholar] [CrossRef]
- Messingham, K.A.; Noe, M.H.; Chapman, M.A.; Giudice, G.J.; Fairley, J.A. A novel ELISA reveals high frequencies of BP180-specific IgE production in bullous pemphigoid. J. Immunol. Methods. 2009, 346, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Ishiura, N.; Fujimoto, M.; Watanabe, R.; Nakashima, H.; Kuwano, Y.; Yazawa, N.; Echigo, T.; Okochi, H.; Tamaki, K. Serum levels of IgE anti-BP180 and anti-BP230 autoantibodies in patients with bullous pemphigoid. J. Dermatol. Sci. 2008, 49, 153–161. [Google Scholar] [CrossRef]
- Bernard, P.; Venot, J.; Constant, F.; Bonnetblane, J.-M. Blood eosinophilia as a severity marker for bullous pemphigoid. J. Am. Acad. Dermatol. 1987, 16, 879–881. [Google Scholar] [CrossRef]
- Yayli, S.; Pelivani, N.; Beltraminelli, H.; Wirthmüller, U.; Beleznay, Z.; Horn, M.; Borradori, L. Detection of linear IgE deposits in bullous pemphigoid and mucous membrane pemphigoid: A useful clue for diagnosis. Br. J. Dermatol. 2011, 165, 1133–1137. [Google Scholar] [CrossRef]
- Engineer, L.; Bhol, K.; Kumari, S.; Ahmed, A.R. Bullous pemphigoid: Interaction of interleukin 5, anti-basement membrane zone antibodies and eosinophils. A preliminary observation. Cytokine 2001, 13, 32–38. [Google Scholar] [CrossRef]
- Crotty, C.; Pittelkow, M.; Muller, S.A. Eosinophilic spongiosis: A clinicopathologic review of seventy-one cases. J. Am. Acad. Dermatol. 1983, 8, 337–343. [Google Scholar] [CrossRef]
- Kridin, K. Peripheral eosinophilia in bullous pemphigoid: Prevalence and influence on the clinical manifestation. Br. J. Dermatol. 2018, 179, 1141. [Google Scholar] [CrossRef] [PubMed]
- Giusti, D.; Gatouillat, G.; Le Jan, S.; Plée, J.; Bernard, P.; Antonicelli, F.; Pham, B.N. Eosinophil Cationic Protein (ECP), a predictive marker of bullous pemphigoid severity and outcome. Sci. Rep. 2017, 7, 4833. [Google Scholar] [CrossRef] [Green Version]
- Amber, K.T.; Valdebran, M.; Kridin, K.; Grando, S.A. The Role of Eosinophils in Bullous Pemphigoid: A Developing Model of Eosinophil Pathogenicity in Mucocutaneous Disease. Front. Med. 2018, 5, 201. [Google Scholar] [CrossRef] [PubMed]
- Amber, K.T.; Chernyavsky, A.; Agnoletti, A.F.; Cozzani, E.; Grando, S.A. Mechanisms of pathogenic effects of eosinophil cationic protein and eosinophil-derived neurotoxin on human keratinocytes. Exp. Dermatol. 2018, 27, 1322–1327. [Google Scholar] [CrossRef]
- Verraes, S.; Hornebeck, W.; Bernard, P.; Polette, M.; Borradori, L. Respective contribution of neutrophil elastase and matrix metalloproteinase 9 in the degradation of BP180 (type XVII collagen) in human bullous pemphigoid. J. Investig. Dermatol. 2001, 117, 1091–1096. [Google Scholar] [CrossRef]
- Simon, D.; Hoesli, S.; Roth, N.; Staedler, S.; Yousefi, S.; Simon, H.-U. Eosinophil extracellular DNA traps in skin diseases. J. Allergy Clin. Immunol. 2011, 127, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Messingham, K.N.; Crowe, T.; Fairley, J.A. The Intersection of IgE Autoantibodies and Eosinophilia in the Pathogenesis of Bullous Pemphigoid. Front. Immunol. 2019, 10, 2331. [Google Scholar] [CrossRef]
- Kawakami, T.; Blank, U. From IgE to Omalizumab. J. Immunol. 2016, 197, 4187–4192. [Google Scholar] [CrossRef] [Green Version]
- Hochhaus, G.; Brookman, L.; Fox, H.; Johnson, C.; Matthews, J.; Ren, S.; Deniz, Y. Pharmacodynamics of omalizumab: Implications for optimised dosing strategies and clinical efficacy in the treatment of allergic asthma. Curr. Med. Res. Opin. 2003, 19, 491–499. [Google Scholar] [CrossRef]
- Schulman, E.S. Development of a monoclonal anti-immunoglobulin E antibody (omalizumab) for the treatment of allergic respiratory disorders. Am. J. Respir. Crit. Care Med. 2001, 164, S6–S11. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Boesel, K.M.; Griffith, D.T.; Prussin, C.; Foster, B.; Romero, F.; Townley, R.; Casale, T.B. Omalizumab rapidly decreases nasal allergic response and FcepsilonRI on basophils. J. Allergy Clin. Immunol. 2004, 113, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Seyed Jafari, S.M.; Gadaldi, K.; Feldmeyer, L.; Yawalkar, N.; Borradori, L.; Schlapbach, C. Effects of Omalizumab on FcεRI and IgE Expression in Lesional Skin of Bullous Pemphigoid. Front. Immunol. 2019, 10, 1919. [Google Scholar] [CrossRef] [Green Version]
- James, T.; Salman, S.; Stevenson, B.; Bundell, C.; Kelly, G.; Nolan, D.; John, M. IgE blockade in autoimmunity: Omalizumab induced remission of bullous pemphigoid. Clin. Immunol. 2019, 198, 54–56. [Google Scholar] [CrossRef] [PubMed]
- London, V.A.; Kim, G.H.; Fairley, J.A.; Woodley, D.T. Successful treatment of bullous pemphigoid with omalizumab. Arch. Dermatol. 2012, 148, 1241–1243. [Google Scholar] [CrossRef]
- Gönül, M.Z.; Keseroglu, H.O.; Ergin, C.; Özcan, I.; Erdem, Ö. Bullous pemphigoid successfully treated with omalizumab. Indian J. Derm. Venereol. Leprol. 2016, 82, 577–579. [Google Scholar] [CrossRef]
- Sarrazin, M.; Jouen, F.; Duvert-Lehembre, S. Refractory bullous pemphigoid with IgE anti-BP230 and IgG anti-p200 antibodies successfully treated with omalizumab. Ann. Dermatol. Venereol. 2021, 148, 60–62. [Google Scholar] [CrossRef]
- Yu, K.K.; Crew, A.B.; Messingham, K.A.; Fairley, J.A.; Woodley, D.T. Omalizumab therapy for bullous pemphigoid. J. Am. Acad. Dermatol. 2014, 71, 468–474. [Google Scholar] [CrossRef]
- De, D.; Kaushik, A.; Handa, S.; Mahajan, R.; Schmidt, E. Omalizumab: An underutilized treatment option in bullous pemphigoid patients with co-morbidities. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e469–e472. [Google Scholar] [CrossRef]
- Dufour, C.; Souillet, A.; Chaneliere, C.; Jouen, F.; Bodemer, C.; Jullien, D.; Cambazard, F.; Joly, P.; Reix, P. Successful management of severe infant bullous pemphigoid with omalizumab. Br. J. Dermatol. 2012, 166, 1140–1142. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). An Open-Label Study to Evaluate the Efficacy and Safety of Rituximab Combined with Omalizumab in Patients with Bullous Pemphigoid, NCT04128176. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT04128176 (accessed on 2 November 2022).
- Balakirski, G.; Alkhateeb, A.; Merk, H.; Leverkus, M.; Megahed, M. Successful treatment of bullous pemphigoid with omalizumab as corticosteroid-sparing agent: Report of two cases and review of literature. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1778–1782. [Google Scholar] [CrossRef] [PubMed]
- Garrido, P.M.; Alexandre, M.I.; Travassos, A.R.; Filipe, P. Dipeptidyl-peptidase IV inhibitor-associated bullous pemphigoid efficiently treated with omalizumab. Dermatol. Ther. 2020, 33, e14160. [Google Scholar] [CrossRef] [PubMed]
- Vico-Alonso, C.; Calleja-Algarra, A.; Aragón-Miguel, R.; Sánchez-Velázquez, A.; Velasco-Tamariz, V.; Ortiz-Romero, P.L.; Monsálvez-Honrubia, V. Omalizumab as an alternative therapeutic tool in the treatment of bullous pemphigoid: A case report. Dermatol. Ther. 2019, 32, e12829. [Google Scholar] [CrossRef] [PubMed]
- Maglie, R.; Antiga, E.; Quintarelli, L.; Verdelli, A.; Caproni, M. Dramatic exacerbation of bullous pemphigoid following rituximab and successful treatment with omalizumab. Eur. J. Dermatol. 2019, 29, 213–215. [Google Scholar] [CrossRef]
- Uysal, P.I.; Yalcin, B.; Oktem, A. Our clinical experience with the use of omalizumab in the treatment of bullous pemphigoid. Turkderm Turk. Arch. Dermatol. Venereol. 2017, 51, 124–128. [Google Scholar]
- Lonowski, S.; Sachsman, S.; Patel, N.; Truong, A.; Holland, V. Increasing evidence for omalizumab in the treatment of bullous pemphigoid. JAAD Case Rep. 2020, 6, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Mangin, M.-A.; Lienhart, A.; Gouraud, A.; Roux, S.; Hodique, F.; Jouen, F.; Balme, B.; Dalle, S.; Debarbieux, S. Onset of acquired haemophilia A after omalizumab treatment in severe bullous pemphigoid—A report on two cases successfully treated with mycophenolate mofetil. Ann. Dermatol. Venereol. 2021, 148, 57–59. [Google Scholar] [CrossRef]
- Sinha, S.; Agrawal, D.; Sardana, K.; Kulhari, A.; Malhotra, P. Complete Remission in a Patient with Treatment Refractory Bullous Pemphigoid after a Single Dose of Omalizumab. Indian Dermatol. Online J. 2020, 11, 607–611. [Google Scholar] [CrossRef]
- Lessey, E.; Li, N.; Diaz, L.; Liu, Z. Complement and cutaneous autoimmune blistering diseases. Immunol. Res. 2008, 41, 223–232. [Google Scholar] [CrossRef]
- Iwata, H.; Kitajima, Y. Bullous pemphigoid: Role of complement and mechanisms for blister formation within the lamina lucida. Exp. Dermatol. 2013, 22, 381–385. [Google Scholar] [CrossRef]
- Sezin, T.; Murthy, S.; Attah, C.; Seutter, M.; Holtsche, M.M.; Hammers, C.M.; Schmidt, E.; Meshrkey, F.; Mousavi, S.; Zillikens, D.; et al. Dual inhibition of complement factor 5 and leukotriene B4 synergistically suppresses murine pemphigoid disease. JCI Insight 2019, 4, e128239. [Google Scholar] [CrossRef] [PubMed]
- Sadik, C.; Miyabe, Y.; Sezin, T.; Luster, A.D. The critical role of C5a as an initiator of neutrophil-mediated autoimmune inflammation of the joint and skin. Semin. Immunol. 2018, 37, 21–29. [Google Scholar] [CrossRef]
- Sezin, T.; Krajewski, M.; Wutkowski, A.; Mousavi, S.; Chakievska, L.; Bieber, K.; Ludwig, R.; Dahlke, M.; Rades, D.; Schulze, F.S.; et al. The Leukotriene B4 and its Receptor BLT1 Act as Critical Drivers of Neutrophil Recruitment in Murine Bullous Pemphigoid-Like Epidermolysis Bullosa Acquisita. J. Investig. Dermatol. 2017, 137, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Sadik, C.D.; Rashid, H.; Hammers, C.M.; Diercks, G.F.; Weidinger, A.; Beissert, S.; Schauer, F.; Fettiplace, J.; Thaçi, D.; Ngai, Y.; et al. Evaluation of Nomacopan for Treatment of Bullous Pemphigoid: A Phase 2a Nonrandomized Controlled Trial. JAMA Dermatol. 2022, 158, 641–649. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). Treatment of Bullous Pemphigoid with Avdoralimab (IPH5401), an Anti-C5aR1 Monoclonal Antibody, NCT04563923. 2010. Available online: https://clinicaltrials.gov/ct2/show/NCT04563923 (accessed on 1 October 2022).
- Karsten, C.M.; Beckmann, T.; Holtsche, M.M.; Tillmann, J.; Tofern, S.; Schulze, F.S.; Heppe, E.N.; Ludwig, R.; Zillikens, D.; König, I.R.; et al. Tissue destruction in bullous pemphigoid can be complement independent and may be mitigated by C5aR2. Front. Immunol. 2018, 9, 488. [Google Scholar] [CrossRef] [PubMed]
- Bartko, J.; Schoergenhofer, C.; Schwameis, M.; Firbas, C.; Beliveau, M.; Chang, C.; Marier, J.F.; Nix, D.; Gilbert, J.C.; Panicker, S.; et al. A Randomized, First-in-Human, Healthy Volunteer Trial of sutimlimab, a Humanized Antibody for the Specific Inhibition of the Classical Complement Pathway. Clin. Pharmacol. Ther. 2018, 104, 655–663. [Google Scholar] [CrossRef]
- Freire, P.C.; Muñoz, C.H.; Derhaschnig, U.; Schoergenhofer, C.; Firbas, C.; Parry, G.C.; Panicker, S.; Gilbert, J.C.; Stingl, G.; Jilma, B.; et al. Specific Inhibition of the Classical Complement Pathway Prevents C3 Deposition along the Dermal-Epidermal Junction in Bullous Pemphigoid. J. Investig. Dermatol. 2019, 139, 2417–2424.e2. [Google Scholar] [CrossRef]
- Fiorino, A.; Baum, S.; Czernik, A.; Hall, R.; Zeeli, T.; Baniel, A.; Sinha, A.; Seiffert-Sinha, K.; Kolatch, B.; Zhang, Z.; et al. 570 Safety and efficacy of bertilimumab, a human anti-eotaxin-1 monoclonal antibody, in bullous pemphigoid in a phase 2a study. J. Investig. Dermatol. 2019, 139, S98. [Google Scholar] [CrossRef] [Green Version]
- Müller, S.; Behnen, M.; Bieber, K.; Möller, S.; Hellberg, L.; Witte, M.; Hänsel, M.; Zillikens, D.; Solbach, W.; Laskay, T.; et al. Dimethylfumarate Impairs Neutrophil Functions. J. Investig. Dermatol. 2016, 136, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Bilgic-Temel, A.; Das, S.; Murrell, D.F. Successful management of bullous pemphigoid with dimethyl fumarate therapy: A case report. Int. J. Women Dermatol. 2019, 5, 179–180. [Google Scholar] [CrossRef]
- Flood-Page, P.; Menzies-Gow, A.; Phipps, S.; Ying, S.; Wangoo, A.; Ludwig, M.S.; Barnes, N.; Robinson, D.; Kay, A.B. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J. Clin. Investig. 2003, 112, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Simon, D.; Yousefi, S.; Cazzaniga, S.; Bürgler, C.; Radonjic, S.; Houriet, C.; Heidemeyer, K.; Klötgen, H.; Kozlowski, E.; Borradori, L.; et al. Mepolizumab failed to affect bullous pemphigoid: A randomized, placebo-controlled, double-blind phase 2 pilot study. Allergy 2020, 75, 669–672. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). Ixekizumab in the Treatment of Bullous Pemphigoid, NCT03099538. 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03099538 (accessed on 1 October 2022).
- Loget, J.; Plée, J.; Antonicelli, F.; Bernard, P. A successful treatment with ustekinumab in a case of relapsing bullous pemphigoid associated with psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e228–e230. [Google Scholar] [CrossRef]
- Le Guern, A.; Alkeraye, S.; Vermersch-Langlin, A.; Coupe, P.; Vonarx, M. Bullous pemphigoid during ustekinumab therapy. JAAD Case Rep. 2015, 1, 359–360. [Google Scholar] [CrossRef] [Green Version]
- Onsun, N.; Sallahoglu, K.; Dizman, D.; Su, O.; Tosuner, Z. Bullous pemphigoid during ustekinumab therapy in a psoriatic patient. Eur. J. Dermatol. 2017, 27, 81–82. [Google Scholar] [CrossRef]
- National Library of Medicine (U.S.). Efficacy and Safety of Ustekinumab in Bullous Pemphigoid, NCT04117932. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT04117932 (accessed on 1 October 2022).
- National Library of Medicine (U.S.). The Effects of Tildrakizumab in Treatment of Bullous Pemphigoid, NCT04465292. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04465292 (accessed on 1 October 2022).
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Shao, S.; Cao, T.; Lei, J.; Dang, E.; Zhang, J.; Wang, G. Increased expression of NLRP3 inflammasome components and interleukin-18 in patients with bullous pemphigoid. J. Dermatol. Sci. 2016, 83, 116–123. [Google Scholar] [CrossRef]
- Le Jan, S.; Muller, C.; Plée, J.; Durlach, A.; Bernard, P.; Antonicelli, F. IL-23/IL-17 Axis Activates IL-1β-Associated Inflammasome in Macrophages and Generates an Auto-Inflammatory Response in a Subgroup of Patients with Bullous Pemphigoid. Front. Immunol. 2019, 10, 1972. [Google Scholar] [CrossRef] [Green Version]
- Kridin, K.; Kowalski, E.H.; Kneiber, D.; Laufer-Britva, R.; Amber, K.T. From bench to bedside: Evolving therapeutic targets in autoimmune blistering disease. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2239–2252. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). A Randomized, Open-Label, Controlled Trial of Topical AC-203 in Subjects with Bullous Pemphigoid, NCT03286582. 2017. Available online: https://adisinsight.springer.com/trials/700288760 (accessed on 1 October 2022).
- Arm, J.P.; Bottoli, I.; Skerjanec, A.; Floch, D.; Groenewegen, A.; Maahs, S.; Owen, C.E.; Jones, I.; Lowe, P.J. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin. Exp. Allergy 2014, 44, 1371–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauvreau, G.M.; Arm, J.P.; Boulet, L.-P.; Leigh, R.; Cockcroft, D.W.; Davis, B.E.; Mayers, I.; FitzGerald, J.M.; Dahlen, B.; Killian, K.J.; et al. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses. J. Allergy Clin. Immunol. 2016, 138, 1051–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasser, P.; Tarchevskaya, S.S.; Guntern, P.; Brigger, D.; Ruppli, R.; Zbären, N.; Kleinboelting, S.; Heusser, C.; Jardetzky, T.S.; Eggel, A. The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat. Commun. 2020, 11, 165. [Google Scholar] [CrossRef] [Green Version]
- National Library of Medicine (U.S.). A Randomized, Double-blind, Placebo Controlled, Parallel Group Study Evaluating the Efficacy, Safety, Pharmacokinetics and Pharmacodynamics of QGE031 in the Treatment of Patients with Bullous Pemphigoid With Disease Refractory to Oral Steroid Treatment, NCT01688882. 2012. Available online: https://adisinsight.springer.com/trials/700238487 (accessed on 1 October 2022).
- Cozzani, E.; Marzano, A.V.; Caproni, M.; Feliciani, C.; Calzavara-Pinton, P.; Alaibac, M.; Antiga, E.; Arisi, M.; Assalve, D.; Atzori, L. Cutaneous Immunology group of SIDeMaST. Bullous pemphigoid: Italian guidelines adapted from the EDF/EADV guidelines. G. Ital. Dermatol. Venereol. 2018, 153, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Bardazzi, F.; Filippi, F.; Chessa, M.; Iommi, M.; Loi, C.; Campanati, A.; Rizzetto, G.; Tagliati, C.; Atzori, L.; Muratori, S.; et al. Mortality and prognostic factors in patients with bullous pemphigoid: A retrospective multicenter Italian study. J. Eur. Acad. Dermatol. Venereol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Liu, Z.; Shapiro, S.D.; Zhou, X.; Twining, S.S.; Senior, R.M.; Giudice, G.J.; Fairley, J.A.; Diaz, L.A. A critical role for neutrophil elastase in experimental bullous pemphigoid. J. Clin. Investig. 2000, 105, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Hakievska, L.; Holtsche, M.M.; Künstner, A.; Goletz, S.; Petersen, B.-S.; Thaci, D.; Ibrahim, S.M.; Ludwig, R.J.; Franke, A.; Sadik, C.D.; et al. IL-17A is functionally relevant and a potential therapeutic target in bullous pemphigoid. J. Autoimmun. 2019, 96, 104–112. [Google Scholar] [CrossRef]
- Delli, F.S.; Sotiriou, E.; Lazaridou, E.; Apalla, Z.; Lallas, A.; Vakirlis, E.; Gerou, S.; Bougioukas, K.; Ioannides, D. Total IgE, eosinophils, and interleukins 16, 17A, and 23 correlations in severe bullous pemphigoid and treatment implications. Dermatol. Ther. 2020, 33, e13958. [Google Scholar] [CrossRef]
| Authors | Drug | No. of Patients | Dose | Efficacy | Safety | Associated Therapy |
|---|---|---|---|---|---|---|
| Schmidt et al., (2015) [20] | Rituximab | 13 | 500 mg weekly for 4 weeks | >90% | infections | PDSN |
| Polansky et al., (2019) [21] | Rituximab | 20 | 1 g repeated in 2 weeks or 375 mg/m2 weekly for 4 weeks | 75% | infections | PDSN, MFM, AZA, MTX |
| Tovanabutra et al., (2019) [22] | Rituximab | 38 | 1 g repeated in 2 weeks or 375 mg/m2 weekly for 4 weeks | 76% | - | PDN |
| Lamberts et al., (2018) [23] | Rituximab | 28 | 500 or 1000 mg on days 1 and 15 | 67,9 | - | - |
| Ahmed et al., (2015) [26] | Rituximab | 12 | 4 weekly infusions of 375 mg/m2 | 100% | - | IVIg |
| Kremer et al., (2018) [28] | Rituximab | 62 | initial dose of 375 mg/m2 every 1–4 weeks to 500 mg weekly for 2 weeks | 85% | infections, anemia, neutropenia, syndrome of inappropriate antidiuretic hormone secretion (SIADH), drug fever, acute pruritus, peripheral arterial occlusive disease and tachycardia. | - |
| Author | Drug | No. of Patients | Dose | Primary Endpoint | Safety | Associated Therapies | Phase of Study |
|---|---|---|---|---|---|---|---|
| NCT04206553 [37] | Dupilumab | 98 (estimated) | Loading dose administered SC, followed by once every 2 weeks (Q2W) | Proportion of patients achieving sustained remission | - | - | Phase 2/3 |
| Kaye A. et al., (2018) [38] | Dupilumab | 1 | 600 mg week 0, 300 mg every other week | Complete remission | NA | No | Case report |
| Seyed Jafari S. et al., (2020) [39] | Dupilumab | 1 | 600 mg week 0, 300 mg every other week | Complete remission | NA | Omalizumab, MFM, TCS | Case report |
| Seidman S. et al., (2019) [40] | Dupilumab | 1 | 600 mg week 0, 300 mg every other week | Improved pruritus, complete remission | NA | PDN, MFM, DXC, nicotinamide, TCS | Case report |
| Abdat R. et al., (2020) [41] | Dupilumab | 13 | 600 mg week 0, 300 mg every other week; 600 mg week 0, 300 mg weekly. 600 mg week 0, 300 mg every 12 days; | 7/13 Disease clearance 5/13 Satisfactory disease control 1/13 No response | NA | MTX, PDN, IVIg | Case series |
| Zhang Y et al., (2021) [42] | Dupilumab | 8 | 600 mg week 0, 300 mg every other week | Complete remission (62.5%) | NA | AZA, MPDSN | Comparative Study |
| Author | Drug | No. of Patients | Dose | Efficacy | Adverse Effects | Associated Therapies | Failed Treatments |
|---|---|---|---|---|---|---|---|
| Menzinger S. et al., (2018) [49] | OMZ | 1 | 300 mg monthly | Disease was completely controlled (complete remission, no blister and pruritus) | None | CLB tapered and stopped | |
| Dufour C. et al., (2012) [81] | OMZ | 1 | 100 mg E2W and E4W | Disease control | Not reported | Not clear | PDSN, MPDSN, topical betamethasone, DPN, AZA |
| London V.A. et al., (2012) [76] | OMZ | 1 | 300 mg E4-8W | Complete remission (no blister and no pruritus) | None | AZA PDN | PDN, AZA, MFM, MPDSN, CFM |
| Pinar I.U. et al., (2017) [87] | OMZ | 11 | 300 mg E2W-E4W to E8W. | 6 complete clinical responses, 1 partial response, 4 N/A (not applicable). | Elevated liver enzymes, trombocytopenia, 2 myocardial infarctions not directly related to OMZ therapy | MPDSN, AZA, CLB, PDSN. | |
| Balakirski G. et al., (2016) [83] | OMZ | 2 | 300 mg E4W to 300 mg E3W; 300 mg E3W | Free of pruritus and few isolated blisters; almost free of symptoms | None | PDSN; PDSN | PDSN + AZA; PDSN |
| Fairley J.A. et al., (2009) [48] | OMZ | 1 | 300 mg E2W | Small amount of residual disease | N/A | N/A | PDN, AZA, minocycline. |
| Yu K.K. et al., (2014) [79] | OMZ | 6 | 375 mg E2W; 300 mg E8W | 2 disease-free, 3 symptom-free, 1 N/A caused by exacerbated COPD | COPD exacerbation related to termination of PDN; epigastric pain and mild elevation of liver enzymes (that responded to decrease of AZA) | PDN, AZA; PDN; PDN, AZA | PDN, AZA, minocycline; PDN niacinammide DXC; PDN; PDN, plasmapheresis, CFM, AZA; PDN; PDSN, AZA, plasmapheresis |
| Gönül M. et al., (2016) [77] | OMZ | 1 | 300 mg E4W | Complete remission | Thrombocytopenia | PDSN | CLB, tetracycline, PDSN, DPN |
| Author | DRUGS | N° Patients | dose | Primary Endpoint | Safety | Associated Therapies | Phase of Study |
|---|---|---|---|---|---|---|---|
| Pavord ID et al., (2012) [105] Simon D et al. (2020) [106] | Mepolizumab | 30 | 750 mg four times for four months | Cumulative rate of relapse-free patients after initiation of therapy | No mepolizumab-related adverse events | No | Phase 3 |
| FitzGerald JM et al., (2020) [107] | Benralizumab | 120 | Subcutaneously (SC) loading dose followed by repeat dosing | Complete remission at 36 weeks | NA | OCS | Phase 3 |
| Arm JP et al., (2014) [119] | Ligelizumab | 20 | 240 mg Q2W s.c. | Number of Patients That Had a Clinical Global Assessment of Change (CGA-C) Responder Rate by Week 12 | Phase 2 | ||
| NCT03099538 (2017) [108] | Ixekizumab | 4 | SC Ixekizumab 160 at week 0, 80 mg at weeks 2, 4, 6, 8, 10, 12 weeks | Cessation of blister formation | NA | No | Phase 2 |
| NCT04117932 (2019) [112] | Ustekinumab | 18 | SC Ustekinumab 90 mg at weeks 0, 4, 16 | Complete remission | NA | Superpotent TCS | Phase 2 |
| NCT04465292 (2020) [113] | Tildrakizumab | 16 | SC Tildrakizumab 100 mg at weeks 0, 4 e 16 | Change in disease severity | NA | No | Phase 1 |
| Sadik CD et al., (2020) [96] | Nomacopan | 9 | SC Nomacopan 90 mg at day 1 30 mg daily until day 42 | Incidence of grade 3, 4 and 5 adverse events | NA | No | Phase 2 |
| Karsten CM et al., (2018) [98] | Avdoralimab | 40 | 3 SC injections of avdoralimab every week for 12 weeks | Complete clinical remission at 3 months | CLB | Phase 2 underway | |
| Bartko, Johann et al., (2022) [99] | Sutimlimab | 10 | Test dose of 10 mg/kg, followed by 4 weekly doses of 60 mg/kg EV | Drug-related Adverse Event for 6 weeks | NA | No | Phase 1 |
| Fiorino, A.S et al., (2019) [101] | Bertilimumab | 11 | Intravenous 10 mg/kg, 3 doses biweekly | Safety endpoints | PDN 30 mg | Phase 2 | |
| Bilgic-Temel A et al., (2019) [103] | Dimethyl fumarate | 1 | 120 mg/BD for 7 days and then increased to 240 mg/BD | NA | PDSN 25mg/day DXC 100 mg twice per day (BD), and nicotinamide 500 mg/BD | Case report | |
| Topical therapies | |||||||
| NCT03286582 (2017) [118] | AC-203 | 10 | ointment applied twice a day | Incidence of adverse events during the treatment period | NA | No | Phase 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Agostino, G.M.; Rizzetto, G.; Marani, A.; Marasca, S.; Candelora, M.; Gambini, D.; Gioacchini, H.; De Simoni, E.; Maurizi, A.; Campanati, A.; et al. Bullous Pemphygoid and Novel Therapeutic Approaches. Biomedicines 2022, 10, 2844. https://doi.org/10.3390/biomedicines10112844
D’Agostino GM, Rizzetto G, Marani A, Marasca S, Candelora M, Gambini D, Gioacchini H, De Simoni E, Maurizi A, Campanati A, et al. Bullous Pemphygoid and Novel Therapeutic Approaches. Biomedicines. 2022; 10(11):2844. https://doi.org/10.3390/biomedicines10112844
Chicago/Turabian StyleD’Agostino, Giovanni Marco, Giulio Rizzetto, Andrea Marani, Samuele Marasca, Matteo Candelora, Daisy Gambini, Helena Gioacchini, Edoardo De Simoni, Andrea Maurizi, Anna Campanati, and et al. 2022. "Bullous Pemphygoid and Novel Therapeutic Approaches" Biomedicines 10, no. 11: 2844. https://doi.org/10.3390/biomedicines10112844
APA StyleD’Agostino, G. M., Rizzetto, G., Marani, A., Marasca, S., Candelora, M., Gambini, D., Gioacchini, H., De Simoni, E., Maurizi, A., Campanati, A., & Offidani, A. (2022). Bullous Pemphygoid and Novel Therapeutic Approaches. Biomedicines, 10(11), 2844. https://doi.org/10.3390/biomedicines10112844






