Lactobacillus Strains for Vegetable Juice Fermentation—Quality and Health Aspects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Antibiotic Susceptibility
2.3. DNA Extraction
2.4. PCR for Antibiotic Resistance Genes
2.5. Multiplex PCR for Functional Genes
2.6. Testing the Fermentation Qualities of Selected LAB Strains
2.7. Analyzing the Juice for Antibacterial Activity
2.8. Organic Acid Analysis by HPLC Method
3. Results and Discussions
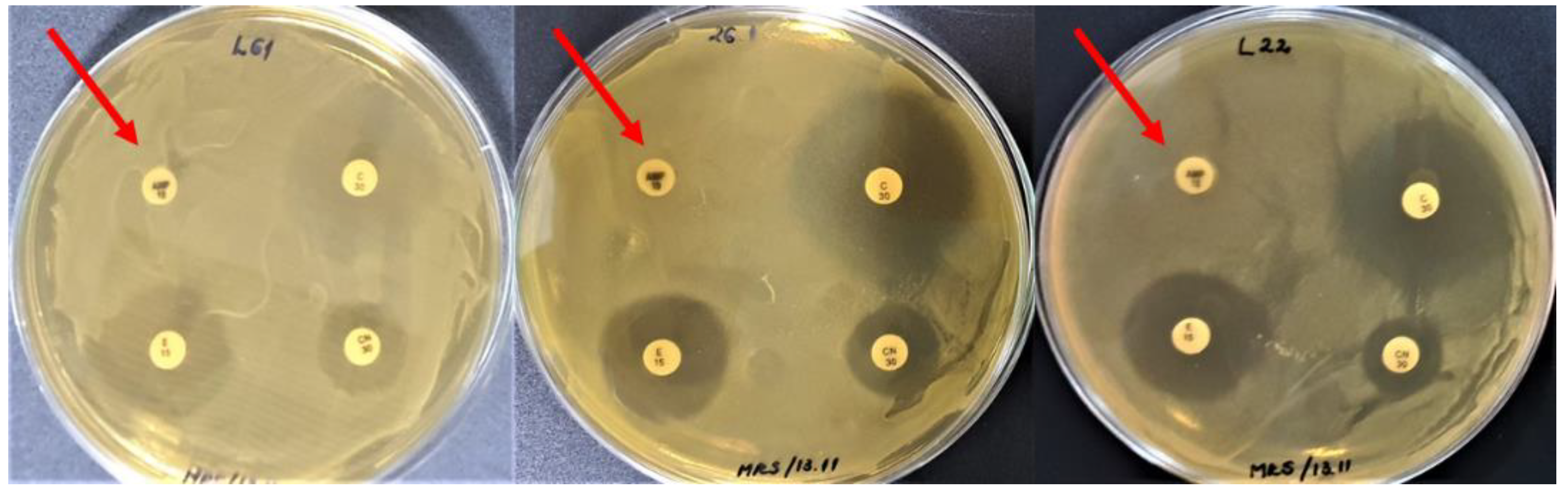
3.1. Susceptibility of LAB to Antibiotics
3.2. LAB Molecular Characterization
3.2.1. Antibiotic Resistance Genes
3.2.2. Functional Genes in LAB
3.3. Suppressive Effects of LAB Fermented Juice
3.4. Organic Acids in LAB Fermented Vegetable Juices
- —
- in the case of MTS (carrot-celery-beet) juice, the fermentation with LAB mix gave the most balanced taste and aroma; such vegetable juice, when fermented, can be used as salad dressing
- —
- in the case of SF (beet) juice, the most appreciated experimental variants were those fermented with L58 and 26.1, respectively. The respondents appreciated that these two fermented juices taste good, have a pleasant, refined aroma, and can be used as salad dressing
- —
- in the case of fermented TM (tomato) juice, the best variant from the organoleptic point of view was the one fermented with the 26.1 strain; the taste was likened to green and the smell to that of a sour fruit (such as vax cherry), in a pleasant, appreciative way.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, N.; Zhang, J.X.; Fan, M.T.; Wang, J.; Guo, G.; Wei, X.Y. Antibiotic resistance of lactic acid bacteria isolated from Chinese yogurts. J. Dairy Sci. 2012, 95, 4775–4783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celik, O.F.; Con, A.H.; Saygin, H.; Sahin, N.; Temiz, H. Isolation and identification of lactobacilli from traditional yogurts as potential starter cultures. LWT-Food Sci. Technol. 2021, 148, 111774. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordónez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; et al. Statement on the update of the list of QPS-recommended microbiological agents intentionally added to food or feed as notified to EFSA. EFSA J. 2022, 20, 38. [Google Scholar] [CrossRef]
- Worku, K.F.; Kurabachew, H.; Hassen, Y. Probiotication of Fruit Juices by Supplemented Culture of Lactobacillus acidophilus. Int. J. Food Sci. Nutr. Eng. 2019, 9, 45–48. [Google Scholar] [CrossRef]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef]
- Da, L.; Nguyen, T.K.C.; Ho, P.H.; Tan, R.; Licandro, H.; Wach, Y. Screening of lactic acid bacteria for their potential use as aromatic starters in fermented vegetables. Int. J. Food Microbiol. 2021, 350, 109242. [Google Scholar]
- Rakin, M.; Vukasinovic, M.; Siler-Marinkovic, S.; Maksimovic, M. Contribution of lactic acid fermentation to improved nutritive quality vegetable juices enriched with brewers yeast autolysate. Food Chem. 2007, 100, 599–602. [Google Scholar] [CrossRef]
- Kumar, B.V.; Vijayendra, S.V.N.; Reddy, O.V.S. Trends in dairy and non-dairy probiotic products-a review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Nie, S.P.; Zhua, K.X.; Xiong, T.; Xie, M.Y. Lactobacillus plantarum NCU116 fermented carrot juice evokes changes of metabolites in serum from type 2 diabetic rats. Food Res. Int. 2016, 80, 36–40. [Google Scholar] [CrossRef]
- Kim, S.Y. Production of fermented kale juices with Lactobacillus strains and nutritional composition. Prev. Nutr. Food Sci. 2017, 22, 231–236. [Google Scholar] [CrossRef]
- Patel, A.; Sv, A.; Shah, N.; Verma, D.K. Lactic acid bacteria as metal quenchers to improve food safety and quality. AgroLife Sci. J. 2017, 6, 146–154. [Google Scholar]
- Tenea, G.N.; Guana, J.M.; Ortega, C.; Hurtado, P.; Delgado, T.; Yepez, L. Potential Of Bacteriocin-Like Substances Produced By Lactobacillus Plantarum Utncys5-4 To Inhibit Food Pathogens In Raw Meat. Sci. Bull. Ser. F Biotechnol. 2018, XXII, 130–135. [Google Scholar]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic Fermented Fruit or Vegetable Juices: Past, Present and Future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef]
- Badea, F.; Diguta, C.F.; Matei, F. The use of lactic acid bacteria and their metabolites to improve the shelf life of perishable fruits and vegetables. Sci. Bull. Ser. F Biotechnol. 2022, XXVI, 117–125. [Google Scholar]
- Jankovic, I.; Sybesma, W.; Phothirath, P.; Ananta, E.; Mercenier, A. Application of probiotics in food products-challenges and new approaches. Curr. Opin. Biotechnol. 2010, 21, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; Gonzalez-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Abu-Ghannam, N. Probiotic fermentation of plant based products: Possibilities and opportunities. Crit. Rev. Food Sci. Nutr. 2012, 52, 183–199. [Google Scholar] [CrossRef]
- Peres, C.M.; Peres, C.; Hernández-Mendoza, A.; Malcata, F.X. Review on fermented plant materials as carriers and sources of potentially probiotic lactic acid bacteria with an emphasis on table olives. Trends Food Sci. Technol. 2012, 26, 31–42. [Google Scholar] [CrossRef]
- Ayed, L.; M’hir, S.; Hamdi, M. Microbiological, Biochemical, and Functional Aspects of Fermented Vegetable and Fruit Beverages. J. Chem. 2020, 2020, 5790432. [Google Scholar] [CrossRef]
- Kesa, A.L.; Pop, C.R.; Mudura, E.; Salant, L.C.; Pasqualone, A.; Darab, C.; Burja-Udrea, C.; Zhao, H.; Coldea, T.E. Strategies to Improve the Potential Functionality of Fruit-Based Fermented Beverages. Plants 2021, 10, 2263. [Google Scholar] [CrossRef]
- Ilango, S.; Antony, U. Probiotic microorganisms from non-dairy traditional fermented foods. Trends Food Sci. Technol. 2021, 118, 617–638. [Google Scholar] [CrossRef]
- Guan, Q.; Xiong, T.; Xie, M. Influence of probiotic fermented fruit and vegetables on human health and the related industrial development trend. Engineering 2021, 7, 212–218. [Google Scholar] [CrossRef]
- Torres, S.; Veróna, H.; Contrerasa, L.; Islaa, M.I. An overview of plant-autochthonous microorganisms and fermented vegetable foods. Food Sci. & Human Well. 2020, 9, 112–123. [Google Scholar]
- Grosu-Tudor, S.S.; Stancu, M.M.; Pelinescu, D.; Zamfir, M. Characterization of some bacteriocins produced by lactic acid bacteria isolated from fermented foods. World J. Microbiol. Biotechnol. 2014, 30, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Cornea, C.P.; Israel-Roming, F.; Sicuia, O.A.; Voaides, C.; Zamfir, M.; Grosu-Tudor, S.S. Biosurfactant production by Lactobacillus spp. strains isolated from Romanian traditional fermented food products. Rom. Biotechnol. Lett. 2016, 21, 11312–11320. [Google Scholar]
- Voaides, M.C.; Sicuia, O.; Zamfir, M.; Cornea, P. Rapid detection of resistance to pH and bile salts of selected LAB strains by molecular techniques. J. Biotechnol. 2016, 231, S52. [Google Scholar] [CrossRef]
- Ștefan, I.R.; Cornea, C.P.; Grosu-Tudor, S.S.; Zamfir, M. Physiological and metabolic responses of functional lactic acid bacteria to stress factors. AgroLife Sci. J. 2018, 7, 139–148. [Google Scholar]
- Angelescu, I.R.; Zamfir, M.; Stancu, M.M.; Grosu-Tudor, S.S. Identification and probiotic properties of lactobacilli isolated from two different fermented beverages. Ann. Microbiol. 2019, 69, 1557–1565. [Google Scholar] [CrossRef]
- Zamfir, M.; Callewaert, R.; Cornea, C.P.; Savu, L.; Vatafu, I.; De Vuyst, L. Purification and characterization of a bacteriocin produced by Lactobacillus acidophilus IBB801. J. Appl. Microbiol. 1999, 87, 923–931. [Google Scholar] [CrossRef]
- Zamfir, M.; Vancanneyt, M.; Makras, L.; Vaningelgem, F.; Lefebvre, K.; Pot, B.; Swings, J.; De Vuyst, L. Biodiversity of lactic acid bacteria in Romanian dairy products. Syst. Appl. Microbiol. 2006, 29, 487–495. [Google Scholar]
- De Vuyst, L.; Zamfir, M.; Mozzi, F.; Adriany, T.; Marshall, V.; Degeest, B.; Vaningelgem, F. Exopolysaccharide-producing Streptococcus thermophilus strains as functional starter cultures in the production of fermented milks. Int. Dairy J. 2003, 13, 707–717. [Google Scholar] [CrossRef]
- Van der Meulen, R.; Grosu-Tudor, S.S.; Mozzi, F.; Vaningelgem, F.; Zamfir, M.; Font de Valdez, G.; De Vuyst, L. Screening of lactic acid bacteria isolates from dairy and cereal products for exopolysaccharide production and genes involved. Int. J. Food Microbiol. 2007, 118, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Grosu-Tudor, S.S.; Stancu, M.M.; Ştefan, I.R.; Cornea, C.P.; Zamfir, M. Physicochemical and rheological properties of some exopolysaccharides produced by lactic acid bacteria isolated from plant origin materials. Rom. Biotechnol. Lett. 2017, 22, 12694–12705. [Google Scholar]
- Wouters, D.; Grosu-Tudor, S.; Zamfir, M.; De Vuyst, L. Bacterial community dynamics, lactic acid bacteria species diversity and metabolite kinetics of traditional Romanian vegetable fermentations. J. Sci. Food Agric. 2013, 93, 749–760. [Google Scholar] [CrossRef]
- Rojo-Bezares, B.; Sáenz, Y.; Poeta, P.; Zarazaga, M.; Ruiz-Larrea, F.; Torres, C. Assessment of antibiotic susceptibility within lactic acid bacteria strains isolated from wine. Int. J. Food Microbiol. 2006, 111, 234–240. [Google Scholar] [CrossRef]
- Guo, H.; Pan, L.; Li, L.; Lu, J.; Kwok, L.; Menghe, B.; Zhang, H.; Zhang, W. Characterization of Antibiotic Resistance Genes from Lactobacillus Isolated from Traditional Dairy Products. J. Food Sci. 2017, 82, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Gad, F.M.; Abdel-Hamid, A.M.; Farag, Z.S.H. Antibiotic resistance in lactic acid bacteria isolated from some pharmaceutical and dairy products. Braz. J. Microbiol. 2014, 45, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Turpin, W.; Humblot, C.; Guyot, J.P. Genetic Screening of Functional Properties of Lactic Acid Bacteria in a Fermented Pearl Millet Slurry and in the Metagenome of Fermented Starchy Foods. Appl. Environ. Microb. 2011, 77, 8722–8734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Zhang, H.; Feng, J.; Maa, L.; de la Fuente-Núnez, C.; Wang, S.; Lu, X. Antibiotic resistance of lactic acid bacteria isolated from dairy products in Tianjin, China. J. Agric. Food Res. 2019, 1, 100006. [Google Scholar] [CrossRef]
- Danielsen, M.; Wind, A. Susceptibility of Lactobacillus spp. to antimicrobial agents. Int. J. Food Microbiol. 2003, 82, 1–11. [Google Scholar] [CrossRef]
- Coppola, R.; Succi, M.; Tremonte, P.; Reale, A.; Salzano, G.; Sorrentino, E. Antibiotic susceptibility of Lactobacillus rhamnosus strains isolated from Parmigiano Reggiano cheese. Lait 2005, 85, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Delgado, S.; Florez, A.B.; Mayo, B. Antibiotic susceptibility of Lactobacillus and Bifidobacterium species from the human gastrointestinal tract. Curr. Microbiol. 2005, 50, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Ammor, M.S.; Florez, A.B.; van Hoek, A.H.; de Los Reyes-Gavilan, C.G.; Aarts, H.J.; Margolles, A.; Mayo, B. Molecular characterization of intrinsic and acquired antibiotic resistance in lactic acid bacteria and bifidobacteria. J. Mol. Microbiol. Biotechnol. 2008, 14, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Genes and Molecules of Lactobacilli Supporting Probiotic Action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef] [Green Version]
- Licandro-Seraut, H.; Scornec, H.; Pédron, T.; Cavin, J.F.; Sansonetti, P.J. Functional genomics of Lactobacillus casei establishment in the gut. Proc. Natl. Acad. Sci. USA 2014, 111, 3101–3109. [Google Scholar] [CrossRef] [Green Version]
- Douillard, F.P.; de Vos, W.M. Functional genomics of lactic acid bacteria: From food to health. Microb. Cell Fact. 2014, 13 (Suppl. 1), S8. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Yu, J.; Sun, X.; Song, Y.; Wang, X.; Wang, H.; Wuren, T.; Zha, M.; Menghe, B.; Heping, Z. Relationships between functional genes in Lactobacillus delbrueckii ssp. bulgaricus isolates and phenotypic characteristics associated with fermentation time and flavor production in yogurt elucidated using multilocus sequence typing. J. Dairy Sci. 2016, 99, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Syrokou, M.K.; Paramithiotis, S.; Drosinos, E.H.; Bosnea, L.; Mataragas, M. A Comparative Genomic and Safety Assessment of Six Lactiplantibacillus plantarum subsp. argentoratensis Strains Isolated from Spontaneously Fermented Greek Wheat Sourdoughs for Potential Biotechnological Application. Int. J. Mol. Sci. 2022, 23, 2487. [Google Scholar] [CrossRef]
- Sanam, M.; Detha, A.; Rohi, N.K. Detection of antibacterial activity of lactic acid bacteria, isolated from Sumba mare’s milk, against Bacillus cereus, Staphylococcus aureus, and Escherichia coli. J. Adv. Vet. Anim. Res. 2022, 9, 53–58. [Google Scholar] [CrossRef]
- Agius, C.; Tucher, S.; Poppenberger, B.; Rozhon, W. Quantification of sugars and organic acids in tomato fruits. MethodsX 2018, 5, 537–550. [Google Scholar] [CrossRef]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Bergquist, S.W.; Sandberg, A.S.; Carlsson, N.G.; Andlid, T. Improved iron solubility in carrot juice fermented by homo- and hetero-fermentative lactic acid bacteria. Food Microbiol. 2005, 21, 53–61. [Google Scholar] [CrossRef]
- Sew, S.W.; Lu, Y.; Taniasuri, F.; Liu, S.Q. Chemical analysis and flavour compound changes of vegetable blend slurry fermented with selected probiotic bacteria. Food Biosci. 2022, 47, 101784. [Google Scholar] [CrossRef]
- Buruleanu, L.C.; Bratu, M.G.; Manea, I.; Avram, D.; Nicolescu, C.L. Fermentation of Vegetable Juices by Lactobacillus acidophilus LA-5. In Lactic Acid Bacteria—R & D for Food, Health, and Livestock Purposes; Kongo, M., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Vilela, A.; Cosme, F.; Inês, A. Wine and non-dairy fermented beverages: A novel source of pro- and prebiotics. Fermentation 2020, 6, 113. [Google Scholar] [CrossRef]






| Species | Strain Designation (Collection Number) | Isolation Source | Reference |
|---|---|---|---|
| Lactococcus lactis | 19.3 (R19316) b | Milk | [24] |
| Lactobacillus acidophilus | IBB (ICCF 416) a | Yogurt | [29] |
| Lactobacillus amylolyticus | P40 (OP524193) c | Bors | [24] |
| P50 (OP524194) c | |||
| Lactobacillus helveticus | 34.9 (R19426) b | Fermented milk | [30] |
| Lactobacillus plantarum | BR9 | Braga | [28] |
| CR1 | Water kefir | ||
| 26.1 | Fermented milk | [24] | |
| L26 | Bors | [25] | |
| L35 | |||
| L22 | |||
| L61 | Pickled cabbage | ||
| L58 | |||
| Streptococcus thermophilus | ST111 | Yogurt | [31] |
| Leuconostoc mesenteroides | 21.2 (R24209) b | Milk | [32] |
| P109 | Pepper | [33] | |
| P124 (OP546102) c | Beans | ||
| Leuconostoc citreum | Fv177 | Pickles | [34] |
| Fv52 |
| Antibiotic | Concentration /Disc | Code | Producer | Class | Target |
|---|---|---|---|---|---|
| Ampicillin | 10 µg | AMP 10 | RightChoice Diagnostics, Yavne 70650, Israel | Β-Lactam | Cell wall |
| Chloramphenicol | 30 µg | C 30 | Liofilchem®, 64026 Roseto degli Abruzzi (TE), Italy | Amphenicol | Protein synthesis, 50S |
| Erythromycin | 15 µg | E15 | Liofilchem®, 64026 Roseto degli Abruzzi (TE), Italy | Macrolide | Protein synthesis, 50S |
| Gentamicin | 30 µg | CN 30 | Liofilchem®, 64026 Roseto degli Abruzzi (TE), Italy | Aminoglycoside | Protein synthesis, 30S |
| Kanamycin | 30 µg | K 30 | Liofilchem®, 64026 Roseto degli Abruzzi (TE), Italy | Aminoglycoside | Protein synthesis, 30S |
| Rifampicin | 30 µg | RD 30 | Liofilchem®, 64026 Roseto degli Abruzzi (TE), Italy | Rifamycin | RNA polymerase |
| Streptomycin | 300 µg | S 300 | Liofilchem®, 64026 Roseto degli Abruzzi (TE), Italy | Aminoglycoside | Protein synthesis, 30S |
| Tetracycline | 30 µg | TE30 | Liofilchem®, 64026 Roseto degli Abruzzi (TE), Italy | Tetracycline | Protein synthesis, 30S |
| Antibiotic | Antibiotic Resistance Gene | Primer Sequences (5′–3′) | Amplicon Size (bp) | Annealing T (°C) | References |
|---|---|---|---|---|---|
| Ampicillin | blaZ | Fw: ACTTCAACACCTGCTGCTTTC Rev: TAGGTTCAGATTGGCCCTTAG | 240 pb | 61 °C | [36] |
| bla | Fw: CATARTTCCGATAATASMGCC Rev: CGTSTTTAACTAAGTATSGY | 297 pb | 60 °C | ||
| Chloramphenicol | catA | Fw: GGATATGAAATTTATCCCTC Rev: CAATCATCTACCCTATGAAT | 486 pb | 60 °C | [35] |
| Erythromycin | erm(B)-1 | Fw: CATTTAACGACGAAACTGGC Rev: GGAACATCTGTGGTATGGCG | 405 pb | 54 °C | [37] |
| Gentamicin | aac(6′)-aph(2″) | Fw: CCAAGAGCAATAAGGGCATA Rev: CACTATCATAACCACTACCG | 220 pb | 60 °C | [35] |
| Kanamycin | aph(3′)-IIIa | Fw: GCCGATGTGGATTGCGAAAA Rev: GCTTGATCCCCAGTAAGTCA | 292 pb | 52 °C | |
| Streptomycin | ant(6) | Fw: ACTGGCTTAATCAATTTGGG Rev: GCCTTTCCGCCACCTCACCG | 597 pb | 61 °C | |
| Tetracycline | tet(M) | Fw: GGTGAACATCATAGACACGC Rev: CTTGTTCGAGTTCCAATGC | 401 pb | 52 °C | [37] |
| Functional Feature | Primer | Synthesis Product | Nucleotide Sequence 5′–3′ | Annealing Temperature | Amplicon Size |
|---|---|---|---|---|---|
| pH Survival | LBA1272 | Cyclopropane FA synthase | Fw: GGCCGGTGTTCCACTAGTCC Rev: ACGTTGGGTCGATTTGACGA | 60 °C | 203 bp |
| dltD | D-alanine transfer protein | Fw: TTCGCCTGTTCAAGCCACAT Rev: ACGTGCCCTTCTTTGGTTCC | 283 bp | ||
| Folate synthesis | folP | Dihydropteroate synthase/dihydropteroate pyrophosphorylase | Fw: CCASGRCSGCTTGCATGAC Rev: TKACGCCGGACTCCTTTTWY | 61 °C | 261 bp |
| folK | 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine diphosphokinase | Fw: CCATTTCCAGGTGGGGAATC Rev: GGGGTGGTCCAGCAAACTT | 214 bp | ||
| Starch metabolism | agl | α-glucosidase | Fw: GCSAAAATGCTAGCGACYMT Rev: CCACTGCATYGGYGTACGY | 236 bp | |
| α-amy | α-amylase | Fw: AGATCAGGCGCAAGTTCAGT Rev: TTTTATGGGCACACCACTCA | 62 °C | 220 bp | |
| malL | Oligo-1,6-glucosidase | Fw: TTGCCTAACAACTGGGGTTC Rev: ATCAACGCCTTTGTTCAACC | 177 bp | ||
| Riboflavin synthesis | ribA | 3,4-dihydroxy-2-butanone 4-phosphate synthase/GTP cyclohydrolase II | Fw: TTTACGGGCGATGTTTTAGG Rev: CGACCCTCTTGCCGTAAATA | 121 bp |
| Raw Material | Preparation | Juice Code |
|---|---|---|
| Beet | Freshly squeezed beetroot juice, autoclaved at 121 °C for 15 min | SF |
| Carrots, celery, and beets | Mixture of freshly squeezed juice from carrot, celery, and beet roots, in 2:1:2 (v/v/v) ratio, autoclaved at 121 °C for 15 min | MTS |
| Tomato juice | Fine blended tomato juice, autoclaved at 121 °C for 15 min | TM |
| Strain | Amp10 | C30 | CN30 | E15 | K30 | RD30 | S300 | TE30 |
|---|---|---|---|---|---|---|---|---|
| L22 | R | S | S | S | T | S | S | S |
| L26 | T | S | S | S | T | S | S | T |
| L35 | R | T | T | S | T | S | T | T |
| L58 | T | T | S | T | T | S | T | T |
| L61 | R | S | S | S | T | S | T | T |
| 19.3 | R | S | S | S | S | S | S | S |
| 21.2 | R | T | T | T | R | S | T | T |
| 26.1 | R | S | S | S | T | S | T | T |
| 34.9 | R | S | S | S | T | S | S | S |
| P40 | R | S | S | S | R | S | S | S |
| P50 | R | T | S | T | R | S | T | S |
| P109 | R | S | S | S | R | T | T | T |
| P124 | R | T | S | S | R | S | T | T |
| Fv52 | R | S | S | S | T | S | S | S |
| Fv177 | R | S | T | S | R | S | T | T |
| BR9 | T | S | S | S | T | S | S | T |
| CR1 | T | S | S | S | T | S | S | T |
| IBB | R | S | S | T | T | S | S | S |
| ST111 | R | S | S | T | S | S | S | S |
| Strain | Main Phenotype | Antibiotic Resistance Genes Detected by Multiplex PCR |
|---|---|---|
| L22 | AmpR | - |
| L26 | AmpT, KanT | bla |
| L35 | AmpR | - |
| LAB43 | - | - |
| L58 | AmpT, ChlT, EryT, KanT, ST | bla, ant(6) |
| L61 | AmpR | - |
| BR9 | AmpT, KanT | bla, aph(3″)III |
| CR1 | AmpT, KanT | aph(3″)III |
| Fv52 | AmpR | blaZ |
| Fv177 | AmpR, KanR | - |
| P124 | - | aph(3″)III |
| ST111 | AmpR | blaZ |
| 26.1 | AmpR, KanR | blaZ, bla, aph(3″)III |
| 34.9 | AmpR | blaZ |
| Strain/Gene | LBA1272 | dltD | folP | folK | agl | α-amy | malL | ribA |
|---|---|---|---|---|---|---|---|---|
| L22 | + | − | − | − | − | − | − | − |
| L26 | + | − | + | − | + | + | − | + |
| L35 | + | − | + | − | + | + | − | + |
| LAB43 | + | + | + | − | + | + | − | + |
| L58 | + | − | + | − | + | + | + | + |
| L61 | − | − | + | − | + | + | + | + |
| BR9 | + | − | + | − | + | + | + | + |
| CR1 | + | − | + | − | + | + | + | + |
| Fv52 | + | − | + | − | + | + | + | + |
| Fv177 | + | − | + | − | + | + | + | + |
| P124 | + | − | + | − | + | + | + | + |
| ST111 | + | − | + | − | + | + | + | + |
| 26.1 | + | − | + | − | + | + | + | + |
| 34.9 | + | − | + | − | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voaides, C.; Boiu-Sicuia, O.; Israel-Roming, F.; Zamfir, M.; Grosu-Tudor, S.S.; Angelescu, I.R.; Cornea, C.P. Lactobacillus Strains for Vegetable Juice Fermentation—Quality and Health Aspects. Biomedicines 2022, 10, 2867. https://doi.org/10.3390/biomedicines10112867
Voaides C, Boiu-Sicuia O, Israel-Roming F, Zamfir M, Grosu-Tudor SS, Angelescu IR, Cornea CP. Lactobacillus Strains for Vegetable Juice Fermentation—Quality and Health Aspects. Biomedicines. 2022; 10(11):2867. https://doi.org/10.3390/biomedicines10112867
Chicago/Turabian StyleVoaides, Catalina, Oana Boiu-Sicuia, Florentina Israel-Roming, Medana Zamfir, Silvia Simona Grosu-Tudor, Iulia Roxana Angelescu, and Calina Petruta Cornea. 2022. "Lactobacillus Strains for Vegetable Juice Fermentation—Quality and Health Aspects" Biomedicines 10, no. 11: 2867. https://doi.org/10.3390/biomedicines10112867
APA StyleVoaides, C., Boiu-Sicuia, O., Israel-Roming, F., Zamfir, M., Grosu-Tudor, S. S., Angelescu, I. R., & Cornea, C. P. (2022). Lactobacillus Strains for Vegetable Juice Fermentation—Quality and Health Aspects. Biomedicines, 10(11), 2867. https://doi.org/10.3390/biomedicines10112867






