Expanding Quality by Design Principles to Support 3D Printed Medical Device Development Following the Renewed Regulatory Framework in Europe
Abstract
:1. Introduction
1.1. What Does 3DP or AM Mean?
1.2. Scope of 3D Bioprinting
| Type of Use | Examples | Advantages | Ref |
|---|---|---|---|
| Personalized therapy | Personalized compounding | Suitable for geriatric use, pediatric use, or for patients with Alzheimer’s disease | [98,99,100] |
| Wearable devices | 3DP-ed electronics for MD | Enables real-time monitoring of chronic medical conditions and transmission of data to a patient’s mobile device | [101,102] |
| Alternative drug delivery system | Topical masks and wound dressings, transdermal needles | Drug-eluting patches for transdermal application | [100,103] |
| Personalized MD | Orthodontics, prosthetics, implants vascular stents, orthopedic implants, artificial joints, and heart valves | Drug-eluting implants, resorbable or permanent implants | [73,74,104] |
| Tissue engineering | Scaffold-based bioprinting for tissues and organs | Potential alternative to transplantation due to lack of human donors | [100,105] |
| Surgical models | Pre-operative planning and intra-operative guides | Enhances success of surgeries, reduces complications such as blood loss or even patient loss | [106] |
| Drug Discovery | Drug screening on printed tumor cell lines or other cell lines | Alternative to human or animal models | [107,108] |
| Microfluidic bioprinting for organ-on-a-chip models | They reflect the structural, microenvironmental and physiological function of human organs | Drug validation testing as an alternative to animal and human models | [109] |
| Surgical tools | Forceps, hemostats, scalpel and clamps | Low-cost production | [110] |
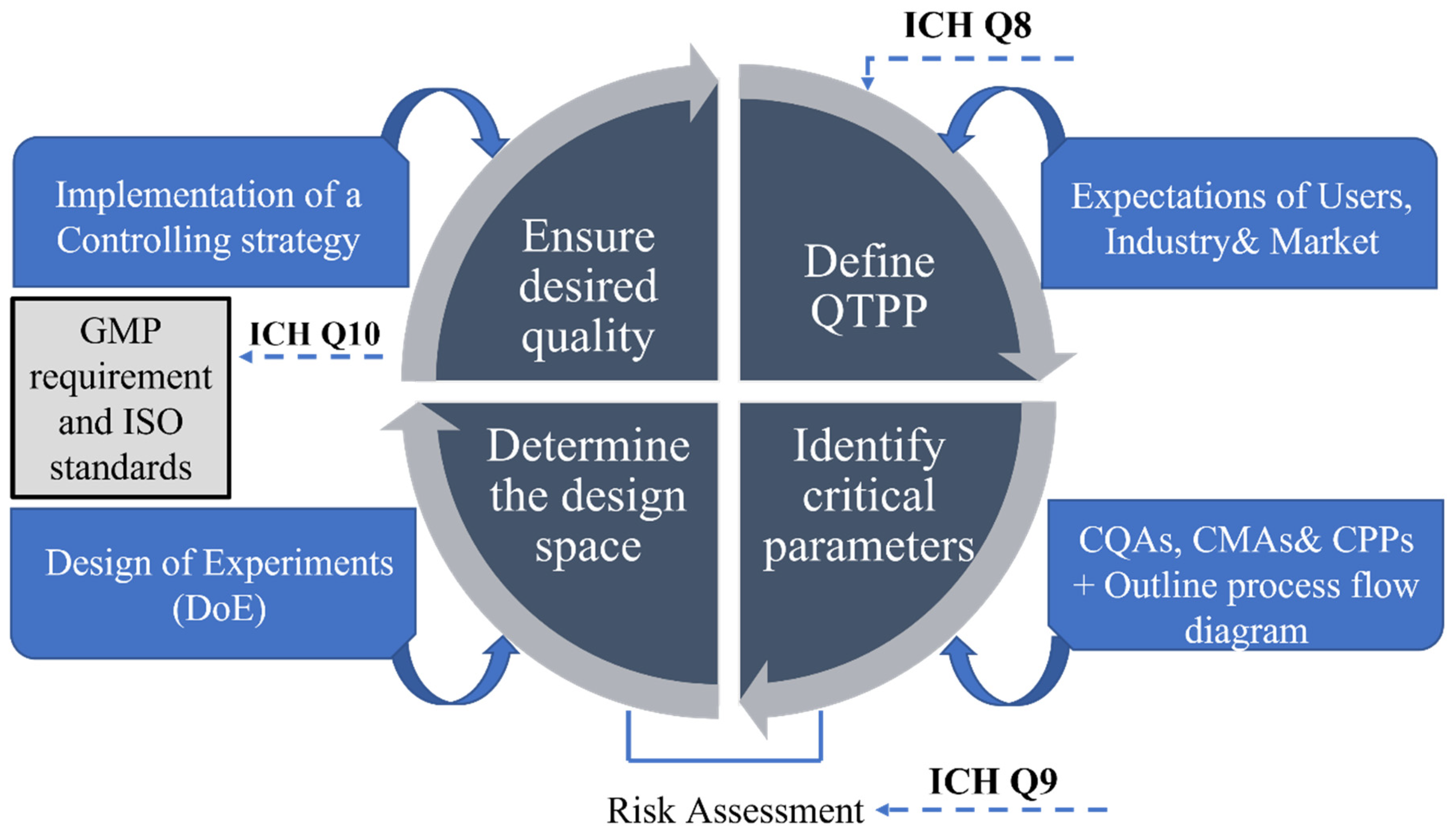
1.3. The Advent of Adapting a Quality by Design Strategy
1.4. Steps of Quality by Design
1.5. New MDR in the EU
1.5.1. Major Improvements in New Regulation
1.5.2. Reclassification to a Stricter Medical Device Classification
1.5.3. Increased Traceability
1.5.4. Heightened Attention on the Quality Management System
1.5.5. Tighter Clinical Evaluation Requirements
1.5.6. Supervision of Notified Bodies
1.5.7. Introduction of an Independent Expert Panel
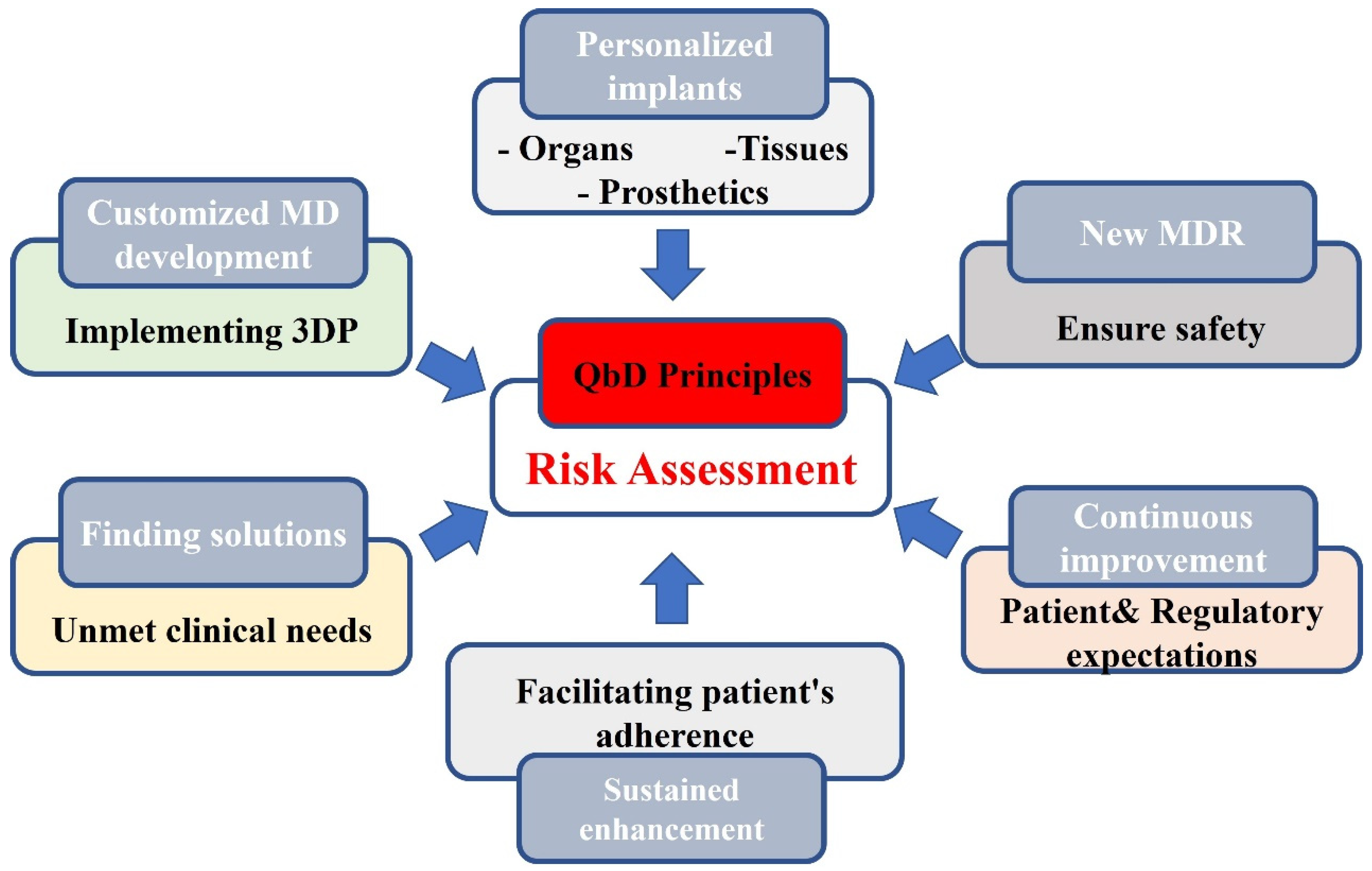
1.5.8. Flexibility to Allow Innovation in the Renewed Regulatory Framework within the EU
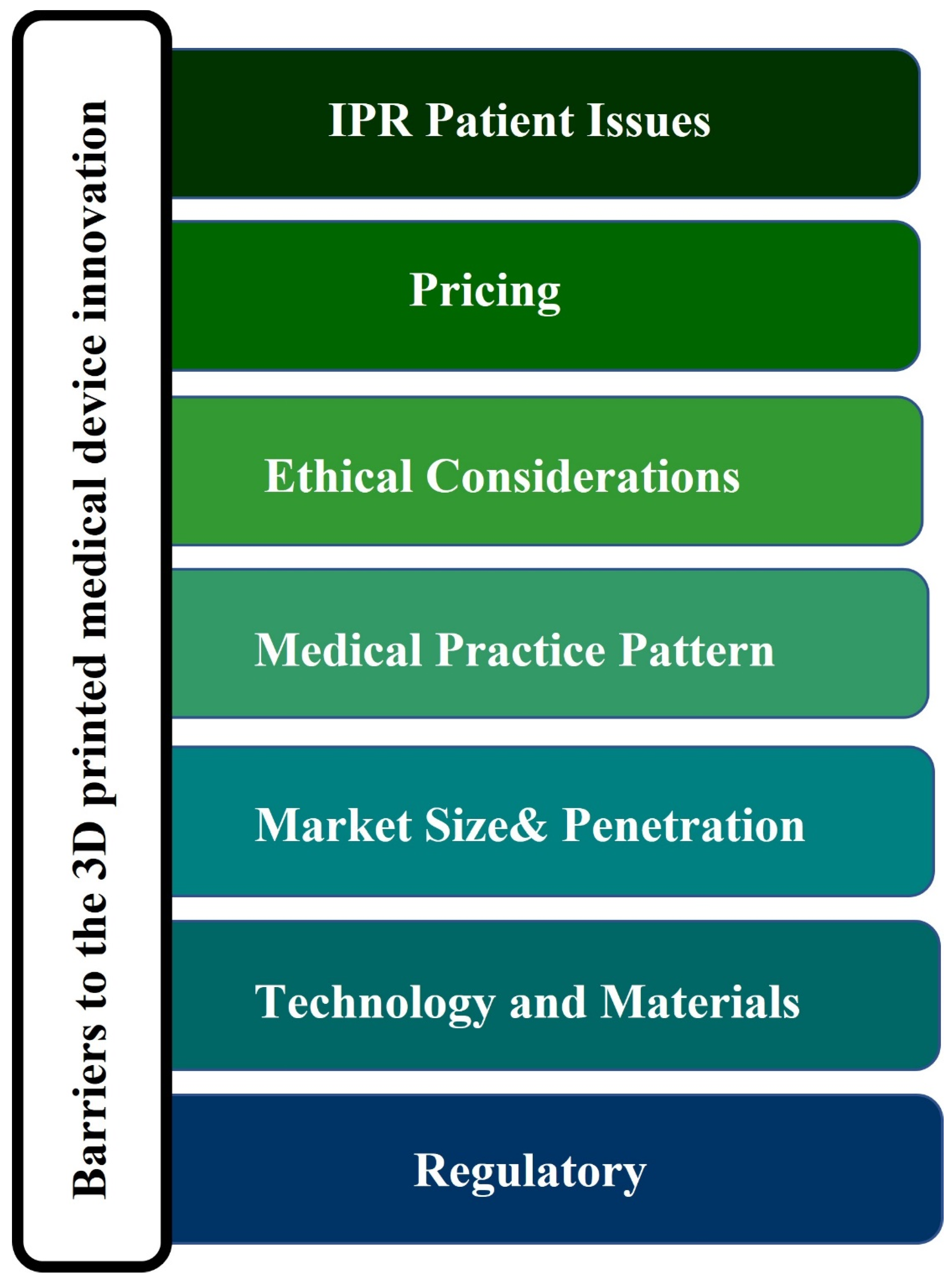
1.6. Barriers to 3D Printed Medical Device Innovation
1.7. Emerging Technologies and Devices Facilitated by the COVID-Pandemic
2. Conclusions
- -
- Quality and safety aspects have been partly remedied by the MDR introduced in the EU in May 2021. The new MDR allows application of this technology for unmet clinical needs, which serves as a first step in the evolution and wider use of this profoundly innovative, customized production method. Regulations are harmonized within the EU that may facilitate the wider use of 3D implants in clinics.
- -
- The current standardization methods used for traditional production are not appropriate for 3DP technology. Additive manufacturing incorporates new technologies and continuously emerging new biomaterials in the biomedical field. Therefore, 3DP technology still remains to complement rather than replace traditional manufacturing techniques in the near future.
- -
- The design and fabrication of customized implants requires multiple steps that might lead to imperceptible errors, affecting the final product and, consequently, patient safety. Therefore, triumphant knowledge transfers of this new design and manufacturing method in the industry requires more integrative technology transfer, which is concurrent with multi-disciplinary cooperation.
- -
- The Quality by Design quality management framework offers an integrative tool to build quality and safety into the product development processes, facilitating the incorporation of changes, iterations and improvements. Based on a risk assessment evaluation, it allows some modification, yet following the appropriate steps in the required roadmap, it ensures a target product with the associated quality requirements. QbD has already preferably and successfully been used in the pharmaceutical industry, and it has the proven capability to successfully foster the wider integration and acceptance of 3DP in MD development and manufacturing.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chung, D.S.; Park, P.S.; Jeon, S.; Shin, S.M.; Han, J.W.; Lee, C.; Mun, A.; Mun, W.; Shin, Y.J.; Kim, L. Common Methods of Contraception Used at Monkey Bay Community Hospital in Mangochi District, Malawi. Adv. Infect. Dis. 2021, 1, 13. [Google Scholar] [CrossRef]
- Martinez-Marquez, D.; Mirnajafizadeh, A.; Carty, C.P.; Stewart, R.A. Application of quality by design for 3D printed bone prostheses and scaffolds. PLoS ONE 2018, 13, e0195291. [Google Scholar] [CrossRef] [PubMed]
- Sukanya, V.S.; Panigrahy, N.; Rath, S.N. Recent approaches in clinical applications of 3D printing in neonates and pediatrics. Eur. J. Pediatr. 2021, 180, 323–332. [Google Scholar]
- Lamprou, D.A. Emerging technologies for diagnostics and drug delivery in the fight against COVID-19 and other pandemics. Expert Rev. Med. Devices 2020, 17, 1007–1012. [Google Scholar] [CrossRef]
- European Medicines Agencies Network Strategy to 2025 Protecting Public Health at a Time of Rapid Change. European-Union-Medicines-Agencies-Network-Strategy-2025-Protecting-Public-Health-Time-Rapid-Change_en.pdf. 2020. Available online: https://www.ema.europa.eu/en/documents/report/european-union-medicines-agencies-network-strategy-2025-protecting-public-health-time-rapid-change_en.pdf (accessed on 9 November 2022).
- Martin, J.L.; Norris, B.J.; Murphy, E.; Crowe, J.A. Medical device development: The challenge for ergonomics. Appl. Ergon. 2008, 39, 271–283. [Google Scholar] [CrossRef] [Green Version]
- Mohurle, M.S.M.; Asnani, M.D.A.J.; Chaple, D.R.; Kurian, M.J.; Bais, M.A.G. Quality by Design (QbD): An Emerging Trend in Improving Quality & Development of Pharmaceuticals. Saudi J. Med. Pharm. Sci. 2019, 5, 1132–1138. [Google Scholar]
- Csóka, I.; Pallagi, E.; Paál, T.L. Extension of quality-by-design concept to the early development phase of pharmaceutical R&D processes. Drug Discov. Today 2018, 23, 1340–1343. [Google Scholar]
- Akel, H.; Ismail, R.; Katona, G.; Sabir, F.; Ambrus, R.; Csóka, I. A comparison study of lipid and polymeric nanoparticles in the nasal delivery of meloxicam: Formulation, characterization, and in vitro evaluation. Int. J. Pharm. 2021, 604, 120724. [Google Scholar] [CrossRef]
- Migliore, A. On the new regulation of medical devices in Europe. Expert Rev. Med. Devices 2017, 14, 921–923. [Google Scholar] [CrossRef]
- Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Kondiah, P.P.; Pillay, V. 3D-printing and the effect on medical costs: A new era? Expert Rev. Pharmacoecon. Outcomes Res. 2016, 16, 23–32. [Google Scholar] [CrossRef]
- Gupta, V.; Nesterenko, P.; Paull, B. 3D Printing in Chemical Sciences; The Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Palo, M.; Holländer, J.; Suominen, J.; Yliruusi, J.; Sandler, N. 3D printed drug delivery devices: Perspectives and technical challenges. Expert Rev. Med. Devices 2017, 14, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.; Madurawe, R.D.; Moore, C.M.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- ISO/ASTM 52900:2021(en); Additive Manufacturing—General Principles—Fundamentals and Vocabulary. ISO/Online Browsing Platform (OBP): Geneva, Switzerland, 2021.
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Additive Manufacturing Research Group. About Additive Manufacturing—Binder Jetting. 2022. Available online: https://www.lboro.ac.uk/research/amrg/about/the7categoriesofadditivemanufacturing/binderjetting/ (accessed on 4 November 2022).
- Ian Gibson, I.G. Additive Manufacturing Technologies 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- ASTM F2792-12a; Standard Terminology for Additive Manufacturing Technologies. ASTM International: West Conshohocken, PA, USA, 2012.
- Trombetta, R.; Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D Printing of Calcium Phosphate Ceramics for Bone Tissue Engineering and Drug Delivery. Ann. Biomed. Eng. 2017, 45, 23–44. [Google Scholar] [CrossRef] [Green Version]
- Additive Manufacturing Research Group. About Additive Manufacturing—Directed Energy Deposition. 2022. Available online: https://www.lboro.ac.uk/research/amrg/about/the7categoriesofadditivemanufacturing/directedenergydeposition (accessed on 4 November 2022).
- Chen, Y.; Zhang, X.; Parvez, M.M.; Liou, F. A Review on Metallic Alloys Fabrication Using Elemental Powder Blends by Laser Powder Directed Energy Deposition Process. Materials 2020, 13, 3562. [Google Scholar] [CrossRef]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Kort-Mascort, J.; Kinsella, J.M. Extrusion bioprinting of soft materials: An emerging technique for biological model fabrication. Appl. Phys. Rev. 2019, 6, 011310. [Google Scholar] [CrossRef]
- Zhuang, P.; Ng, W.L.; An, J.; Chua, C.K.; Tan, L.P. Layer-by-layer ultraviolet assisted extrusion-based (UAE) bioprinting of hydrogel constructs with high aspect ratio for soft tissue engineering applications. PLoS ONE 2019, 14, e0216776. [Google Scholar] [CrossRef] [Green Version]
- Chua, C.K.; Leong, K.F.; Lim, C.S. Rapid Prototyping: Principles and Applications (with Companion CD-ROM); World Scientific Publishing Company: Singapore, 2010. [Google Scholar]
- Additive Manufacturing Research Group. About Additive Manufacturing—Material Extrusion. 2022. Available online: https://www.lboro.ac.uk/research/amrg/about/the7categoriesofadditivemanufacturing/materialextrusion (accessed on 4 November 2022).
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet bioprinting of biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef]
- Ng, W.L.; Huang, X.; Shkolnikov, V.; Goh, G.L.; Suntornnond, R.; Yeong, W.Y. Controlling droplet impact velocity and droplet volume: Key factors to achieving high cell viability in sub-nanoliter droplet-based bioprinting. Int. J. Bioprint. 2022, 8, 424. [Google Scholar] [CrossRef]
- Additive Manufacturing Research Group. About Additive Manufacturing—Material Jetting. 2022. Available online: https://www.lboro.ac.uk/research/amrg/about/the7categoriesofadditivemanufacturing/materialjetting (accessed on 4 November 2022).
- Gibson, I.; Rosen, D.W.; Stucker, B.; Khorasani, M.; Rosen, D.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies; Springer: Berlin/Heidelberg, Germany, 2021; Volume 17. [Google Scholar]
- Additive Manufacturing Research Group. About Additive Manufacturing—Powder Bed Fusion. 2022. Available online: https://www.lboro.ac.uk/research/amrg/about/the7categoriesofadditivemanufacturing/powderbedfusion/ (accessed on 4 November 2022).
- Ng, W.L.; Lee, J.M.; Zhou, M.; Chen, Y.-W.; Lee, K.-X.A.; Yeong, W.Y.; Shen, Y.-F. Vat polymerization-based bioprinting—Process, materials, applications and regulatory challenges. Biofabrication 2020, 12, 022001. [Google Scholar] [CrossRef]
- Additive Manufacturing Research Group. About Additive Manufacturing—VAT Photopolymerisation. Available online: https://www.lboro.ac.uk/research/amrg/about/the7categoriesofadditivemanufacturing/vatphotopolymerisation (accessed on 4 November 2022).
- Li, W.; Mille, L.S.; Robledo, J.A.; Uribe, T.; Huerta, V.; Zhang, Y.S. Recent advances in formulating and processing biomaterial inks for vat polymerization-based 3D printing. Adv. Healthc. Mater. 2020, 9, 2000156. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, J.; Zhu, W.; Tang, M.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv. Drug Deliv. Rev. 2018, 132, 235–251. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The role of 3D printing in medical applications: A state of the art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [PubMed]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.-W.; Ahmed, W.; Arafat, B. Emergence of 3D printed dosage forms: Opportunities and challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.A. 3D Printing in Drug Delivery and Biomedical Applications: A State-of-the-Art Review. Compounds 2021, 1, 94–115. [Google Scholar] [CrossRef]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D printing in pharmaceutical and medical applications–recent achievements and challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto, E.B.; Campos, J.; Filho, S.; Teixeira, M.; Martins-Gomes, C.; Zielinska, A.; Carbone, C.; Silva, A. 3D printing in the design of pharmaceutical dosage forms. Pharm. Dev. Technol. 2019, 24, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.A.; O’Mahony, C.; Cronin, M.; O’Mahony, T.; Moore, A.C.; Crean, A.M. Dissolvable microneedle fabrication using piezoelectric dispensing technology. Int. J. Pharm. 2016, 500, 1–10. [Google Scholar] [CrossRef]
- Willings, A. 38 Amazing Examples of 3D Printing in the Medical World. 2022. Available online: https://www.pocket-lint.com/gadgets/news/142506-medical-marvels-how-3d-printing-is-improving-our-lives (accessed on 5 October 2022).
- Gao, G.; Ahn, M.; Cho, W.-W.; Kim, B.-S.; Cho, D.-W. 3D Printing of Pharmaceutical Application: Drug Screening and Drug Delivery. Pharmaceutics 2021, 13, 1373. [Google Scholar] [CrossRef]
- Tagami, T.; Hayashi, N.; Sakai, N.; Ozeki, T. 3D printing of unique water-soluble polymer-based suppository shell for controlled drug release. Int. J. Pharm. 2019, 568, 118494. [Google Scholar] [CrossRef]
- Sun, Y.; Ruan, X.; Li, H.; Kathuria, H.; Du, G.; Kang, L. Fabrication of non-dissolving analgesic suppositories using 3D printed moulds. Int. J. Pharm. 2016, 513, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alamán, J.; Alicante, R.; Peña, J.I.; Sánchez-Somolinos, C. Inkjet printing of functional materials for optical and photonic applications. Materials 2016, 9, 910. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 2009, 30, 6221–6227. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Dean, D.; Ruggeri, Z.M.; Boland, T. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol. Bioeng. 2010, 106, 963–969. [Google Scholar] [CrossRef]
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci. Rep. 2020, 10, 14023. [Google Scholar] [CrossRef]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B. In Situ Printing of Mesenchymal Stromal Cells, by Laser-Assisted Bioprinting, for in Vivo Bone Regeneration Applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef] [Green Version]
- Barron, J.A.; Ringeisen, B.R.; Kim, H.; Spargo, B.J.; Chrisey, D.B. Application of laser printing to mammalian cells. Thin Solid Films 2004, 453, 383–387. [Google Scholar] [CrossRef]
- Barron, J.A.; Krizman, D.B.; Ringeisen, B.R. Laser printing of single cells: Statistical analysis, cell viability, and stress. Ann. Biomed. Eng. 2005, 33, 121–130. [Google Scholar] [CrossRef]
- Ge, Q.; Li, Z.; Wang, Z.; Kowsari, K.; Zhang, W.; He, X.; Zhou, J.; Fang, N.X. Projection micro stereolithography based 3D printing and its applications. Int. J. Extreme Manuf. 2020, 2, 022004. [Google Scholar] [CrossRef]
- Mukhtarkhanov, M.; Perveen, A.; Talamona, D. Application of Stereolithography Based 3D Printing Technology in Investment Casting. Micromachines 2020, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater. Today Bio. 2019, 1, 100008. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y. Application of 3D Printing in Implantable Medical Devices. BioMed Res. Int. 2021, 2021, 6653967. [Google Scholar] [CrossRef] [PubMed]
- Toh, E.M.S.; Thenpandiyan, A.A.; Foo, A.S.C.; Zhang, J.J.Y.; Lim, M.J.R.; Goh, C.P.; Dinesh, N.; Vedicherla, S.V.; Yang, M.; Teo, K.; et al. Clinical Outcomes of 3D-Printed Bioresorbable Scaffolds for Bone Tissue Engineering—A Pilot Study on 126 Patients for Burrhole Covers in Subdural Hematoma. Biomedicines 2022, 10, 2702. [Google Scholar] [PubMed]
- Auricchio, F.; Marconi, S. 3D printing: Clinical applications in orthopaedics and traumatology. EFORT Open Rev. 2017, 1, 121–127. [Google Scholar] [CrossRef]
- Pillai, S.; Upadhyay, A.; Khayambashi, P.; Farooq, I.; Sabri, H.; Tarar, M.; Lee, K.T.; Harb, I.; Zhou, S.; Wang, Y.; et al. Dental 3D-Printing: Transferring Art from the Laboratories to the Clinics. Polymers 2021, 13, 157. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Emadi, R.; Valiani, A.; Kharaziha, M.; Poursamar, S.A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Three-dimensional printing constructs based on the chitosan for tissue regeneration: State of the art, developing directions and prospect trends. Materials 2020, 13, 2663. [Google Scholar] [CrossRef]
- Sangiovanni, A.; Tibaldi, E.C.; Bortolotto, C.; Eshja, E.; Peroni, C.; Rossi, L.; Orlandi, M.; Poggi, P. The Growth of 3d Printing in Biomedicine: Applications in Radiology. Available online: https://www.dieurope.com/pdf/125682.pdf (accessed on 9 November 2022).
- Velázquez, J.S.; Cavas, F.; Bolarín, J.M.; Alió, J.L. 3D printed personalized corneal models as a tool for improving patient’s knowledge of an asymmetric disease. Symmetry 2020, 12, 151. [Google Scholar] [CrossRef] [Green Version]
- Xie, P.; Hu, Z.; Zhang, X.; Li, X.; Gao, Z.; Yuan, D.; Liu, Q. Application of 3-dimensional printing technology to construct an eye model for fundus viewing study. PLoS ONE 2014, 9, e109373. [Google Scholar] [CrossRef] [Green Version]
- Zada, M.; Shah, I.A.; Basir, A.; Yoo, H. Ultra-compact implantable antenna with enhanced performance for leadless cardiac pacemaker system. IEEE Trans. Antennas Propag. 2020, 69, 1152–1157. [Google Scholar] [CrossRef]
- Lueders, C.; Jastram, B.; Hetzer, R.; Schwandt, H. Rapid manufacturing techniques for the tissue engineering of human heart valves. Eur. J. Cardio-Thorac. Surg. 2014, 46, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [Green Version]
- Tappa, K.; Jammalamadaka, U. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawrat, A. 3D printing in the medical field: Four major applications revolutionizing the industry. Med. Device Netw. 2020. Available online: https://www.medicaldevice-network.com/analysis/3d-printing-in-the-medical-field-applications/ (accessed on 9 November 2022).
- Skardal, A.; Atala, A. Biomaterials for integration with 3-D bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746. [Google Scholar] [CrossRef]
- Ng, W.L.; Chua, C.K.; Shen, Y.-F. Print me an organ! Why we are not there yet. Prog. Polym. Sci. 2019, 97, 101145. [Google Scholar] [CrossRef]
- Davoodi, E.; Sarikhani, E.; Montazerian, H.; Ahadian, S.; Costantini, M.; Swieszkowski, W.; Willerth, S.M.; Walus, K.; Mofidfar, M.; Toyserkani, E.; et al. Extrusion and microfluidic-based bioprinting to fabricate biomimetic tissues and organs. Adv. Mater. Technol. 2020, 5, 1901044. [Google Scholar] [CrossRef]
- Parak, A.; Pradeep, P.; du Toit, L.C.; Kumar, P.; Choonara, Y.E.; Pillay, V. Functionalizing bioinks for 3D bioprinting applications. Drug Discov. Today 2019, 24, 198–205. [Google Scholar] [CrossRef]
- Masri, S.; Maarof, M.; Mohd, N.F.; Hiraoka, Y.; Tabata, Y.; Fauzi, M.B. Injectable Crosslinked Genipin Hybrid Gelatin-PVA Hydrogels for Future Use as Bioinks in Expediting Cutaneous Healing Capacity: Physicochemical Characterisation and Cytotoxicity Evaluation. Biomedicines 2022, 10, 2651. [Google Scholar] [CrossRef] [PubMed]
- Shahrubudin, N.; Koshy, P.; Alipal, J.; Kadir, M.H.A.; Lee, T.C. Challenges of 3D printing technology for manufacturing biomedical products: A case study of Malaysian manufacturing firms. Heliyon 2020, 6, e03734. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Guo, Y.; Liu, T.; Xu, R.; Mao, S.; Mo, X.; Zhang, T.; Ouyang, L.; Xiong, Z.; Sun, W. Advances in 3D Bioprinting. Chin. J. Mech. Eng. Addit. Manuf. Front. 2022, 1, 100011. [Google Scholar] [CrossRef]
- Datta, P.; Barui, A.; Wu, Y.; Ozbolat, V.; Moncal, K.K.; Ozbolat, I.T. Essential steps in bioprinting: From pre- to post-bioprinting. Biotechnol. Adv. 2018, 36, 1481–1504. [Google Scholar] [CrossRef]
- Birla, R.K.; Williams, S.K. 3D bioprinting and its potential impact on cardiac failure treatment: An industry perspective. APL Bioeng. 2020, 4, 010903. [Google Scholar] [CrossRef] [Green Version]
- Kankala, R.K.; Zhu, K.; Li, J.; Wang, C.-S.; Wang, S.-B.; Chen, A.-Z. Fabrication of arbitrary 3D components in cardiac surgery: From macro-, micro- to nanoscale. Biofabrication 2017, 9, 032002. [Google Scholar] [CrossRef]
- Xu, X.; Wang, T.; Zeng, D.; Shi, Y.; Jia, Q.; Yuan, H.; Huang, M.; Zhuang, J. Accurate Congenital Heart Disease Model Generation for 3D Printing. In Proceedings of the 2019 IEEE International Workshop on Signal Processing Systems (SiPS), Nanjing, China, 20–23 October 2019. [Google Scholar]
- Morrison, R.J.; Hollister, S.J.; Niedner, M.F.; Mahani, M.G.; Park, A.H.; Mehta, D.K.; Ohye, R.G.; Green, G.E. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci. Transl. Med. 2015, 7, 285ra64. [Google Scholar] [CrossRef] [Green Version]
- Youssef, R.F.; Spradling, K.; Yoon, R.; Dolan, B.; Chamberlin, J.; Okhunov, Z.; Clayman, R.; Landman, J. Applications of three-dimensional printing technology in urological practice. BJU Int. 2015, 116, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Eggbeer, D. 3—Computational Design of Biostructures, in 3D Bioprinting for Reconstructive Surgery; Thomas, D.J., Jessop, Z.M., Whitaker, I.S., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 33–73. [Google Scholar]
- Martinez-Marquez, D.; Mirnajafizadeh, A.; Carty, C.P.; Stewart, R.A. Facilitating industry translation of custom 3d printed bone prostheses and scaffolds through Quality by Design. Procedia Manuf. 2019, 30, 284–291. [Google Scholar] [CrossRef]
- Wixted, C.M.; Peterson, J.R.; Kadakia, R.J.; Adams, S.B. Three-dimensional Printing in Orthopaedic Surgery: Current Applications and Future Developments. Journal of the American Academy of Orthopaedic Surgeons. Glob. Res. Rev. 2021, 5, e20.00230. [Google Scholar]
- Ren, L.; Zhou, X.; Song, Z.; Zhao, C.; Liu, Q.; Xue, J.; Li, X. Process Parameter Optimization of Extrusion-Based 3D Metal Printing Utilizing PW–LDPE–SA Binder System. Materials 2017, 10, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Zheng, Q.; Sun, W.; Xu, H.; Yang, X. Levofloxacin implants with predefined microstructure fabricated by three-dimensional printing technique. Int. J. Pharm. 2007, 339, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Singh, S.; Kumar, R.; Parkash, C.; Pruncu, C.; Ramakrishna, S. Tissues and organ printing: An evolution of technology and materials. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2022, 09544119221125084. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, J.; Edelhoff, D.; Güth, J.-F. 3D Printing in Digital Prosthetic Dentistry: An Overview of Recent Developments in Additive Manufacturing. J. Clin. Med. 2021, 10, 2010. [Google Scholar] [CrossRef] [PubMed]
- Dodziuk, H. Applications of 3D printing in healthcare. Kardiochir. Torakochirurgia Polska 2016, 13, 283–293. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [Green Version]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Panja, N.; Maji, S.; Choudhuri, S.; Ali, K.A.; Hossain, C.M. 3D Bioprinting of Human Hollow Organs. AAPS PharmSciTech 2022, 23, 139. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Xu, L.-W.; Zhu, H.-Y.; Liu, S.S.-Y. Technical procedures for template-guided surgery for mandibular reconstruction based on digital design and manufacturing. BioMed. Eng. OnLine 2014, 13, 63. [Google Scholar] [CrossRef] [Green Version]
- Saunders, S. 3D Printing Webinar and Event Roundup: 2 October 2022. 2022. Available online: https://3dprint.com/294659/3d-printing-webinars-events-10-2-22/ (accessed on 5 October 2022).
- Araújo, M.R.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. The digital pharmacies era: How 3D printing technology using fused deposition modeling can become a reality. Pharmaceutics 2019, 11, 128. [Google Scholar] [CrossRef] [Green Version]
- Sandler, N.; Preis, M. Printed drug-delivery systems for improved patient treatment. Trends Pharmacol. Sci. 2016, 37, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Almalki, W.H.; Malik, A.; Farhan, M.; Aatif, M.; Rahman, Z.; Alruwaili, N.K.; Alrobaian, M.; Tarique, M.; Rahman, M. 3D printing for drug delivery and biomedical applications. Drug Discov. Today 2020, 25, 1668–1681. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Park, H.S.; McAlpine, M.C. 3D printed deformable sensors. Sci. Adv. 2020, 6, eaba5575. [Google Scholar] [CrossRef] [PubMed]
- Anadioti, E.; Musharbash, L.; Blatz, M.B.; Papavasiliou, G.; Kamposiora, P. 3D printed complete removable dental prostheses: A narrative review. BMC Oral Health 2020, 20, 343. [Google Scholar] [CrossRef]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef]
- Al-Dulimi, Z.; Wallis, M.; Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. 3D printing technology as innovative solutions for biomedical applications. Drug Discov. Today 2021, 26, 360–383. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Khademhosseini, A.; Mironov, V. The synergy of scaffold-based and scaffold-free tissue engineering strategies. Trends Biotechnol. 2018, 36, 348–357. [Google Scholar] [CrossRef]
- Wang, K.; Ho, C.-C.; Zhang, C.; Wang, B. A Review on the 3D Printing of Functional Structures for Medical Phantoms and Regenerated Tissue and Organ Applications. Engineering 2017, 3, 653–662. [Google Scholar] [CrossRef]
- Mazrouei, R.; Velasco, V.; Esfandyarpour, R. 3D-bioprinted all-inclusive bioanalytical platforms for cell studies. Sci. Rep. 2020, 10, 14669. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014, 6, 035001. [Google Scholar] [CrossRef]
- Yu, F.; Choudhury, D. Microfluidic bioprinting for organ-on-a-chip models. Drug Discov. Today 2019, 24, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Aroom, K.R.; Hawes, H.G.; Gill, B.S.; Love, J. 3D Printed Surgical Instruments: The Design and Fabrication Process. World J. Surg. 2017, 41, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Frankforter, S.A. Strategic Total Quality Management: Corporate Performance and Product Quality. J. Acad. Mark. Sci. 1998, 26, 352. [Google Scholar]
- Yu, L.X. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef]
- Snee, R.D. Quality by design: Building quality into products and processes. In Nonclinical Statistics for Pharmaceutical and Biotechnology Industries; Springer: Berlin/Heidelberg, Germnay, 2016; pp. 461–499. [Google Scholar]
- Vogt, F.G.; Kord, A.S. Development of quality-by-design analytical methods. J. Pharm. Sci. 2011, 100, 797–812. [Google Scholar] [CrossRef]
- Kawashita, Y.; Soutome, S.; Umeda, M.; Saito, T. Predictive Risk Factors Associated with Severe Radiation-Induced Mucositis in Nasopharyngeal or Oropharyngeal Cancer Patients: A Retrospective Study. Biomedicines 2022, 10, 2661. [Google Scholar] [CrossRef]
- Lewis, J.A. Statistical principles for clinical trials (ICH E9): An introductory note on an international guideline. Stat. Med. 1999, 18, 1903–1942. [Google Scholar] [CrossRef]
- EMA-FDA Pilot Program for Parallel Assessment of Quality-by-Design Applications: Lessons Learnt and Q&A Resulting from the First Parallel Assessment. 2013. Available online: European-medicines-agency-food-drug-administration-pilot-programme-parallel-assessment-quality_en[1].pdf (accessed on 10 November 2022).
- Gurba-Bryśkiewicz, L.; Dawid, U.; Smuga, D.A.; Maruszak, W.; Delis, M.; Szymczak, K.; Stypik, B.; Moroz, A.; Błocka, A.; Mroczkiewicz, M.; et al. Implementation of QbD Approach to the Development of Chromatographic Methods for the Determination of Complete Impurity Profile of Substance on the Preclinical and Clinical Step of Drug Discovery Studies. Int. J. Mol. Sci. 2022, 23, 10720. [Google Scholar] [CrossRef]
- Akel, H.; Ismail, R.; Csóka, I. Progress and perspectives of brain-targeting lipid-based nanosystems via the nasal route in Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2020, 148, 38–53. [Google Scholar] [CrossRef]
- Gandhi, A.; Roy, C. Quality by Design (QbD) in pharmaceutical industry: Tools, perspectives and challenges. PharmaTutor 2016, 4, 12–20. [Google Scholar]
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Crișan, A.G.; Katona, G.; Pallagi, E.; Dobó, D.G.; Akel, H.; Berkesi, D.; Kónya, Z.; Csóka, I. QbD guided development of immediate release FDM-3D printed tablets with customizable API doses. Int. J. Pharm. 2022, 613, 121411. [Google Scholar] [CrossRef] [PubMed]
- Németh, Z.; Fahy, N.; Aissat, D.; Lenormand, M.-C.; Stüwe, L.; Zablit-Schmidt, I.; Delafuys, S.; Le Douarin, Y.-M.; Muscat, N.A. An Updated Risk Assessment as Part of the QbD-Based Liposome Design and Development. Pharmaceutics 2021, 13, 1071. [Google Scholar] [CrossRef]
- Lambert, W.J. Considerations in developing a target product profile for parenteral pharmaceutical products. Aaps Pharmscitech 2010, 11, 1476–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, E. Risk Assessment of 3D Printers and 3D Printed Products; Environmental Protection Agency: Washington, DC, USA, 2018. [Google Scholar]
- Sabir, F.; Katona, G.; Pallagi, E.; Dobó, D.G.; Akel, H.; Berkesi, D.; Kónya, Z.; Csóka, I. Quality-by-Design-Based Development of n-Propyl-Gallate-Loaded Hyaluronic-Acid-Coated Liposomes for Intranasal Administration. Molecules 2021, 26, 1429. [Google Scholar] [CrossRef]
- Williams, G.A.; Fahy, N.; Aissat, D.; Lenormand, M.-C.; Stüwe, L.; Zablit-Schmidt, I.; Delafuys, S.; Le Douarin, Y.-M.; Muscat, N.A. COVID-19 and the use of digital health tools: Opportunity amid crisis that could transform health care delivery. Eurohealth 2022, 28, 1. [Google Scholar]
- Fried, S. 3D Printing Medical Devices: Considerations for Wearables and Implants. 2019. Available online: https://www.nano-di.com/resources/blog/2019-3d-printing-medical-devices-considerations-for-wearables-and-implants (accessed on 26 May 2022).
- ec.europa.eu. The Impact of New European Medical Device Regulations. 2021. Available online: https://www.med-technews.com/medtech-insights/medtech-regulatory-insights/the-impact-of-new-european-medical-device-regulations/ (accessed on 20 May 2022).
- ec.europa.eu. New Regulations. 2022. Available online: https://ec.europa.eu/health/medical-devices-sector/new-regulations_en#:~:text=The%20EU%20revised%20the%20laws,of%20safety%2C%20while%20supporting%20innovation (accessed on 26 May 2022).
- Medical Device Coordination Group. MDCG 2021-24. Guidance on Classification of Medical Devices. 2021. Available online: ec.europa.eu (accessed on 18 October 2022).
- Tetsworth, K.; Block, S.; Glatt, V. Putting 3D modelling and 3D printing into practice: Virtual surgery and preoperative planning to reconstruct complex post-traumatic skeletal deformities and defects. SICOT-J 2017, 3, 16. [Google Scholar] [CrossRef]
- D’Avenio, G.; Daniele, C.; Grigioni, M. Critical issues of the regulatory pathway for nanostructured medical devices. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2021. [Google Scholar]
- Bianchini, E.; Francesconi, M.; Testa, M.; Tanase, M.; Gemignani, V. Unique device identification and traceability for medical software: A major challenge for manufacturers in an ever-evolving marketplace. J. Biomed. Inform. 2019, 93, 103150. [Google Scholar] [CrossRef]
- Malak, T.T. The Investigation of Surrogate Markers for Post-Market Surveillance in Primary Total Hip Arthroplasty; University of Oxford: Oxford, UK, 2021. [Google Scholar]
- Vasiljeva, K.; van Duren, B.H.; Pandit, H. Changing device regulations in the European Union: Impact on research, innovation and clinical practice. Indian J. Orthop. 2020, 54, 123–129. [Google Scholar] [CrossRef]
- Pérez, J.R. Quality Risk Management in the FDA-Regulated Industry; Quality Press: Phoenix, AZ, USA, 2012. [Google Scholar]
- Whittaker, M.A. Impact Assessment of the Quality System Regulations for Medical Devices-ISO 13485: 2003 and 21 CFR 820 and the CAPA System; University of Georgia: Athens, GA, USA, 2009. [Google Scholar]
- Aliverti, A. Wearable technology: Role in respiratory health and disease. Breathe 2017, 13, e27–e36. [Google Scholar] [CrossRef] [Green Version]
- Thienpont, E.; Quaglio, G.; Karapiperis, T.; Kjaersgaard-Andersen, P. Guest Editorial: New Medical Device Regulation in Europe: A Collaborative Effort of Stakeholders to Improve Patient Safety. Clin. Orthop. Relat. Res. 2020, 478, 928. [Google Scholar] [CrossRef] [PubMed]
- Ben-Menahem, S.M.; Nistor-Gallo, R.; Macia, G.; von Krogh, G.; Goldhahn, J. How the new European regulation on medical devices will affect innovation. Nat. Biomed. Eng. 2020, 4, 585–590. [Google Scholar] [CrossRef] [PubMed]
- eur-lex.europa.eu. Commission Implementing Decision (EU) 2019/1396 of 10 September 2019 Laying Down the Rules for the Application of Regulation (EU) 2017/745 of the European Parliament and of the Council as Regards the Designation of Expert Panels in the Field of Medical Devices (Text with EEA relevance). 2019. Available online: https://eur-lex.europa.eu/eli/dec_impl/2019/1396/oj (accessed on 26 May 2022).
- Fraser, A.G.; Byrne, R.A.; Kautzner, J.; Butchart, E.G.; Szymański, P.; Leggeri, I.; de Boer, R.A.; Caiani, E.G.; Van de Werf, F.; Vardas, P.E. Implementing the new European Regulations on medical devices—Clinical responsibilities for evidence-based practice: A report from the Regulatory Affairs Committee of the European Society of Cardiology. Eur. Heart J. 2020, 41, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Commition, E. Guidlines on Medical Devices. Clinical Evaluation: A Guide for Manufacturers and Notified Bodies under 93/42/EEC and 90/385/EEC. 2016. Available online: https://ec.europa.eu/docsroom/documents/17522/attachments/1/translations/en/renditions/native (accessed on 9 November 2022).
- Morrison, R.J.; Kashlan, K.N.; Flanangan, C.L.; Wright, J.K.; Green, G.E.; Hollister, S.J.; Weatherwax, K.J. Regulatory considerations in the design and manufacturing of implantable 3D-printed medical devices. Clin. Transl. Sci. 2015, 8, 594–600. [Google Scholar] [CrossRef]
- Beg, S.; Hasnain, M.S.; Rahman, M.; Swain, S. Chapter 1—Introduction to Quality by Design (QbD): Fundamentals, Principles, and Applications. In Pharmaceutical Quality by Design; Beg, S., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–17. [Google Scholar]
- Beg, S.; Rahman, M.; Kohli, K. Quality-by-design approach as a systematic tool for the development of nanopharmaceutical products. Drug Discov. Today 2019, 24, 717–725. [Google Scholar] [CrossRef]
- Buttini, F.; Rozou, S.; Rossi, A.; Zoumpliou, V.; Rekkas, D.M. The application of quality by design framework in the pharmaceutical development of dry powder inhalers. Eur. J. Pharm. Sci. 2018, 113, 64–76. [Google Scholar] [CrossRef]
- Ballard, D.H.; Trace, A.P.; Ali, S.; Hodgdon, T.; Zygmont, M.E.; DeBenedectis, C.M.; Smith, S.E.; Richardson, M.L.; Patel, M.J.; Decker, S.J.; et al. Clinical Applications of 3D Printing: Primer for Radiologists. Acad. Radiol. 2018, 25, 52–65. [Google Scholar] [CrossRef]
- Bergsland, J.; Elle, O.J.; Fosse, E. Barriers to medical device innovation. Med. Devices 2014, 7, 205–209. [Google Scholar] [CrossRef]
- Kritikos, M. 3D bio-printing for medical and enhancement purposes: Legal and ethical aspects. In IN-DEPTH ANALYSIS—Science and Technology Options Assessment; European Parliamentary Research Service (EPRS), Scientific Foresight Unit (STOA): Brussels, Belgium, 2018. [Google Scholar]
- Gioumouxouzis, C.I.; Karavasili, C.; Fatouros, D.G. Recent advances in pharmaceutical dosage forms and devices using additive manufacturing technologies. Drug Discov. Today 2019, 24, 636–643. [Google Scholar] [CrossRef]
- Birtchnell, T.; Hoyle, W. 3D Printing for Development in the Global South: The 3D4D Challenge; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Srai, J.S.; Kumar, M.; Graham, G.; Phillips, W.; Tooze, J.; Ford, S.; Beecher, P.; Raj, B.; Gregory, M.; Tiwari, M.K. Distributed manufacturing: Scope, challenges and opportunities. Int. J. Prod. Res. 2016, 54, 6917–6935. [Google Scholar] [CrossRef]
- Mathew, E.; Pitzanti, G.; Larrañeta, E.; Lamprou, D.A. 3D printing of pharmaceuticals and drug delivery devices. Pharmaceutics 2020, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Lessof, S.; Figueras, J.; Palm, W. The European Observatory on Health Systems and Policies: Knowledge brokering for health systems strengthening. Eurohealth 2016, 22, 55–59. [Google Scholar]
- Whitelaw, S.; Mamas, M.A.; Topol, E.; Van Spall, H.G. Applications of digital technology in COVID-19 pandemic planning and response. Lancet Digit. Health 2020, 2, e435–e440. [Google Scholar] [CrossRef]
- Negreiro, M. The Rise of Digital Health Technologies during the Pandemic; European Parliament: Brussels, Belgium, 2021. [Google Scholar]
- Kumpunen, S.; Webb, E.; Permanand, G.; Zheleznyakov, E.; Edwards, N.; van Ginneken, E.; Jakab, M. Transformations in the landscape of primary health care during COVID-19: Themes from the European region. Health Policy 2022, 126, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Kanti, S.Y.; Csóka, I.; Jójárt-Laczkovich, O.; Adalbert, L. Recent Advances in Antimicrobial Coatings and Material Modification Strategies for Preventing Urinary Catheter-Associated Complications. Biomedicines 2022, 10, 2580. [Google Scholar] [CrossRef]
- Floro, J.N.; Dunton, G.E.; Delfino, R.J. Assessing physical activity in children with asthma: Convergent validity between accelerometer and electronic diary data. Res. Q. Exerc. Sport 2009, 80, 153–163. [Google Scholar] [CrossRef]
- Lorkowski, J.; Pokorski, M. Medical Records: A Historical Narrative. Biomedicines 2022, 10, 2594. [Google Scholar] [CrossRef]
- Portnoy, J.M.; Waller, M.; De Lurgio, S.; Dinakar, C. Telemedicine is as effective as in-person visits for patients with asthma. Ann. Allergy Asthma Immunol. 2016, 117, 241–245. [Google Scholar] [CrossRef]
- Vierhile, A.; Tuttle, J.; Adams, H.; tenHoopen, C.; Baylor, E. Feasibility of Providing Pediatric Neurology Telemedicine Care to Youth with Headache. J. Pediatr. Health Care 2018, 32, 500–506. [Google Scholar] [CrossRef]
- Zapata, M.A.; Arcos, G.; Fonollosa, A.; Abraldes, M.; Oleñik, A.; Gutierrez, E.; Garcia-Arumi, J. Telemedicine for a General Screening of Retinal Disease Using Nonmydriatic Fundus Cameras in Optometry Centers: Three-Year Results. Telemed. J. E Health 2017, 23, 30–36. [Google Scholar] [CrossRef]
- Portnoy, J.M.; Pandya, A.; Waller, M.; Elliott, T. Telemedicine and emerging technologies for health care in allergy/immunology. J. Allergy Clin. Immunol. 2020, 145, 445–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pivetta, E.; Ravetti, A.; Paglietta, G.; Cara, I.; Buggè, F.; Scozzari, G.; Maule, M.M.; Morello, F.; Locatelli, S.; Lupia, E. Feasibility of Self-Performed Lung Ultrasound with Remote Teleguidance for Monitoring at Home COVID-19 Patients. Biomedicines 2022, 10, 2569. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-T.; Chen, L.; Yue, W.-W.; Xu, H.-X. Digital Technology-Based Telemedicine for the COVID-19 Pandemic. Front. Med. 2021, 8, 646506. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adalbert, L.; Kanti, S.P.Y.; Jójárt-Laczkovich, O.; Akel, H.; Csóka, I. Expanding Quality by Design Principles to Support 3D Printed Medical Device Development Following the Renewed Regulatory Framework in Europe. Biomedicines 2022, 10, 2947. https://doi.org/10.3390/biomedicines10112947
Adalbert L, Kanti SPY, Jójárt-Laczkovich O, Akel H, Csóka I. Expanding Quality by Design Principles to Support 3D Printed Medical Device Development Following the Renewed Regulatory Framework in Europe. Biomedicines. 2022; 10(11):2947. https://doi.org/10.3390/biomedicines10112947
Chicago/Turabian StyleAdalbert, Livia, S P Yamini Kanti, Orsolya Jójárt-Laczkovich, Hussein Akel, and Ildikó Csóka. 2022. "Expanding Quality by Design Principles to Support 3D Printed Medical Device Development Following the Renewed Regulatory Framework in Europe" Biomedicines 10, no. 11: 2947. https://doi.org/10.3390/biomedicines10112947








