Dienogest May Reduce Estradiol- and Inflammatory Cytokine-Induced Cell Viability and Proliferation and Inhibit the Pathogenesis of Endometriosis: A Cell Culture- and Mouse Model-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Primary Cell Isolation and Culture
2.2. Experimental Setups for Estradiol, TNF-α, IL-1β, IL-32, and DNG Treatment
2.3. Cell Viability Assay
2.4. Invasion Assay
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Western Blot Analysis
2.7. Allotransplantation of Endometrial Tissues in C57BL/6 Mice
2.8. Immunohistochemistry
2.9. Statistical Analysis
3. Results
3.1. Changes in ESCs in Response to Treatment with Estradiol Alone or in Combination with DNG
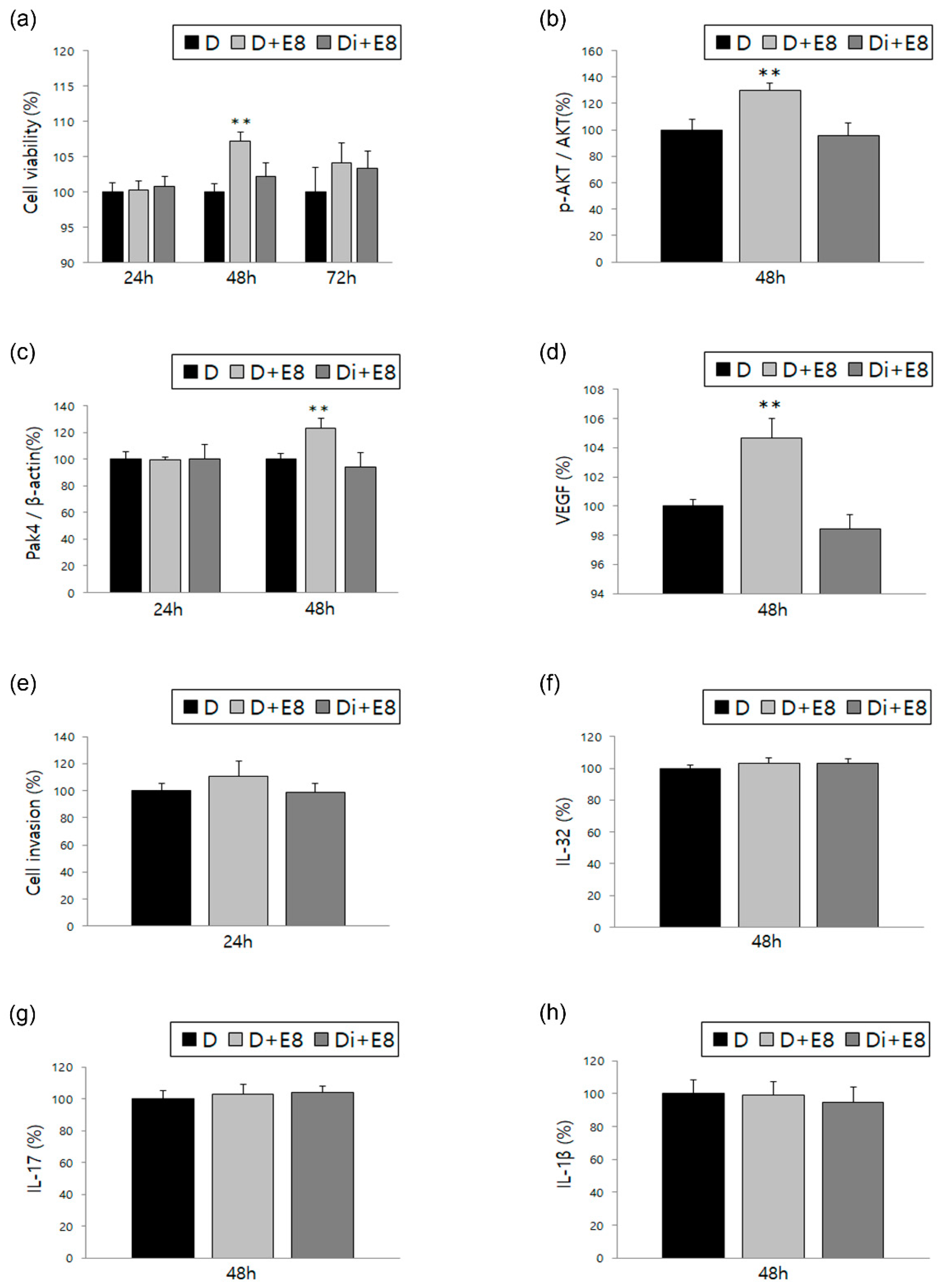
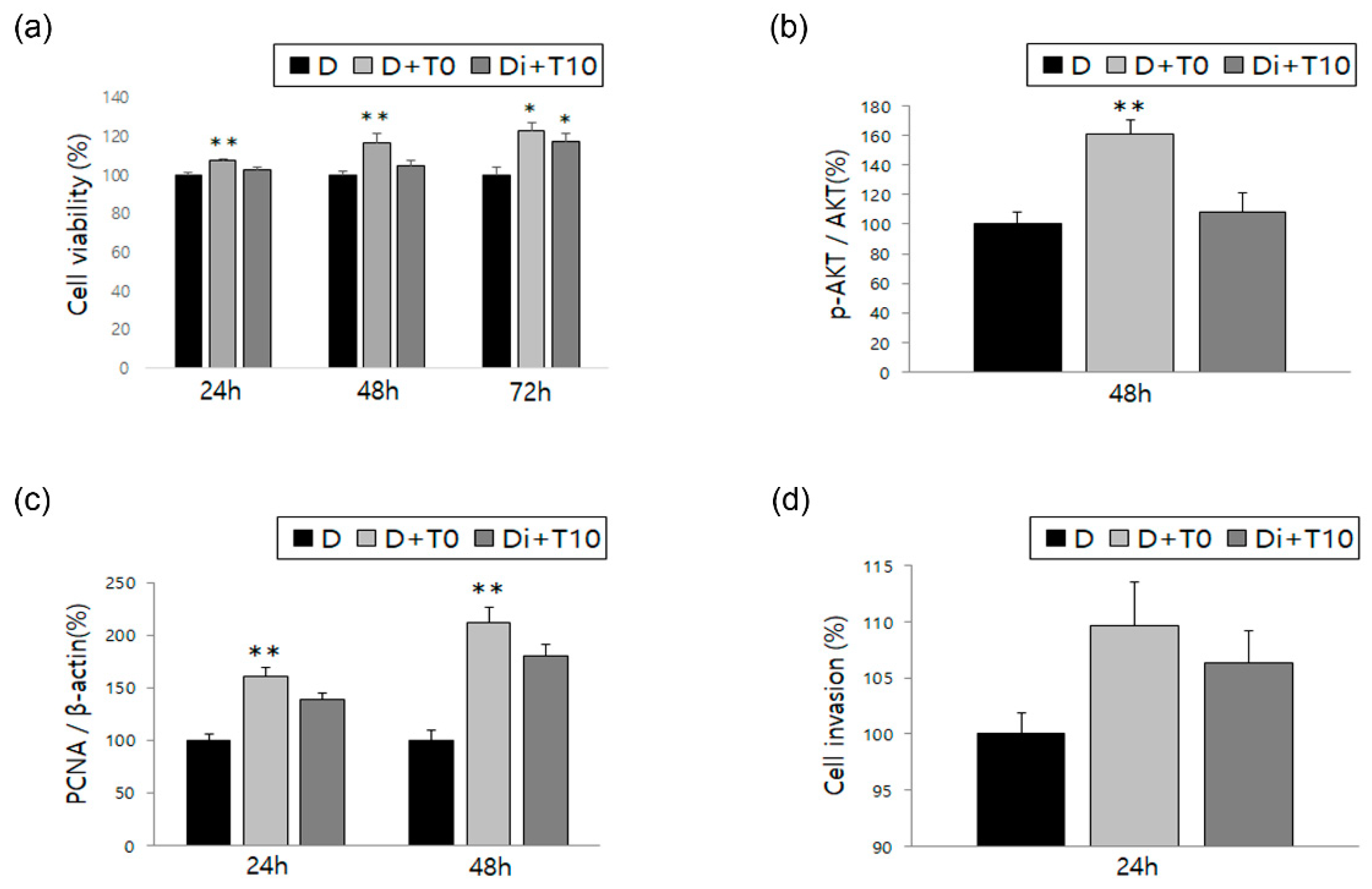
3.2. Changes in ESCs in Response to Treatment with TNF-α and IL-1β, Alone or in Combination with DNG
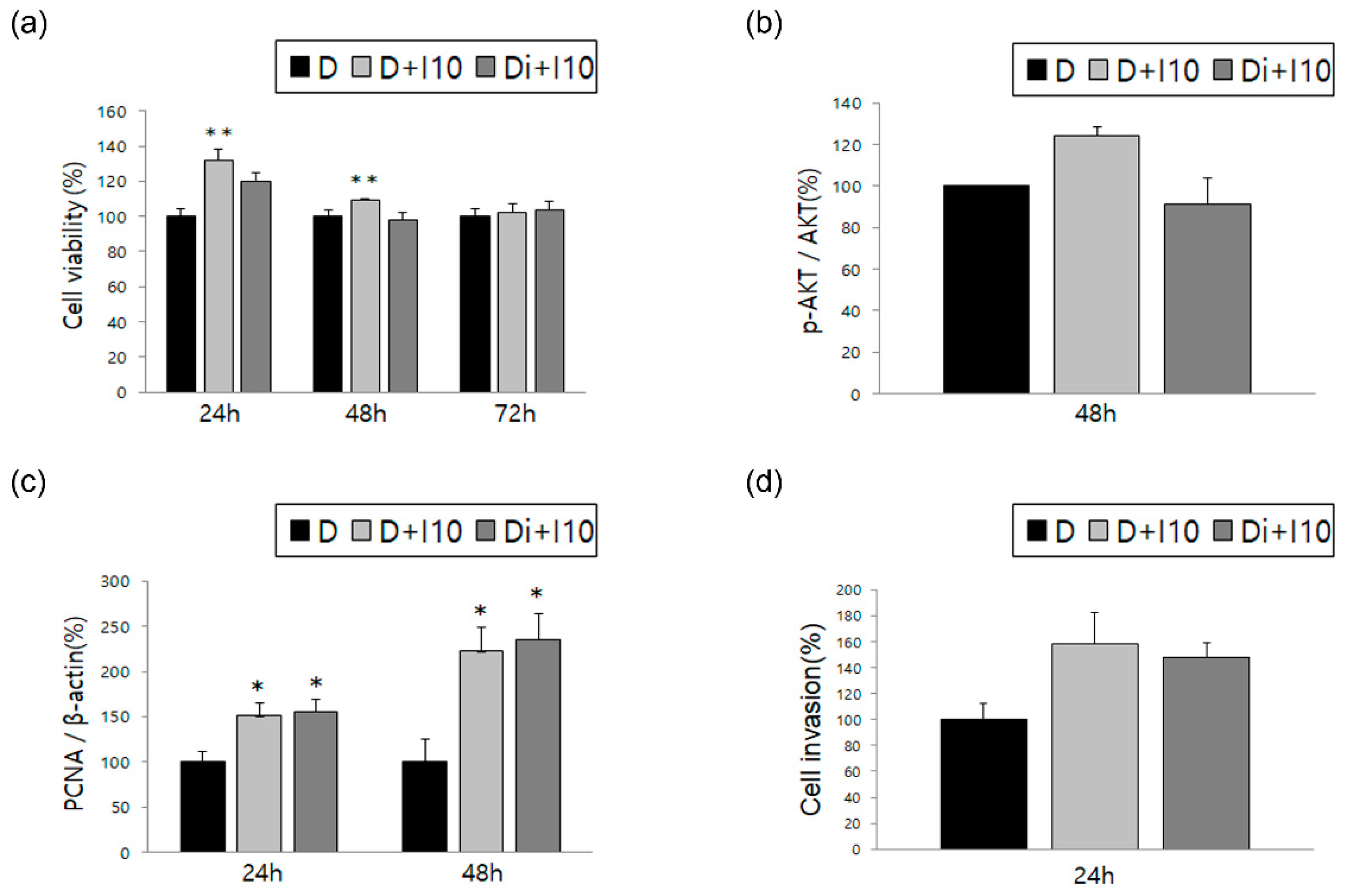
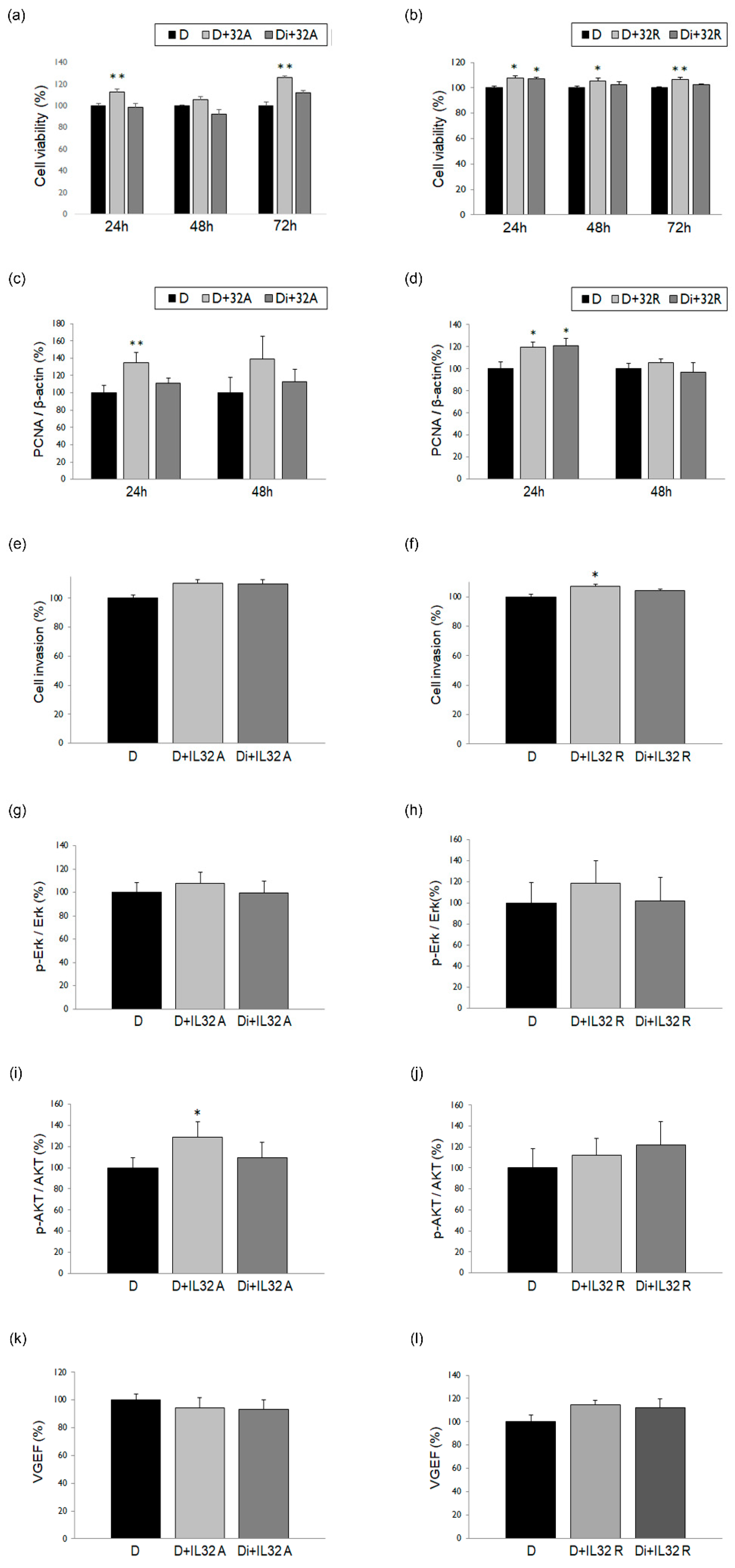
3.3. Changes in ESCs in Response to Treatment with IL-32α/γ Alone or in Combination with DNG
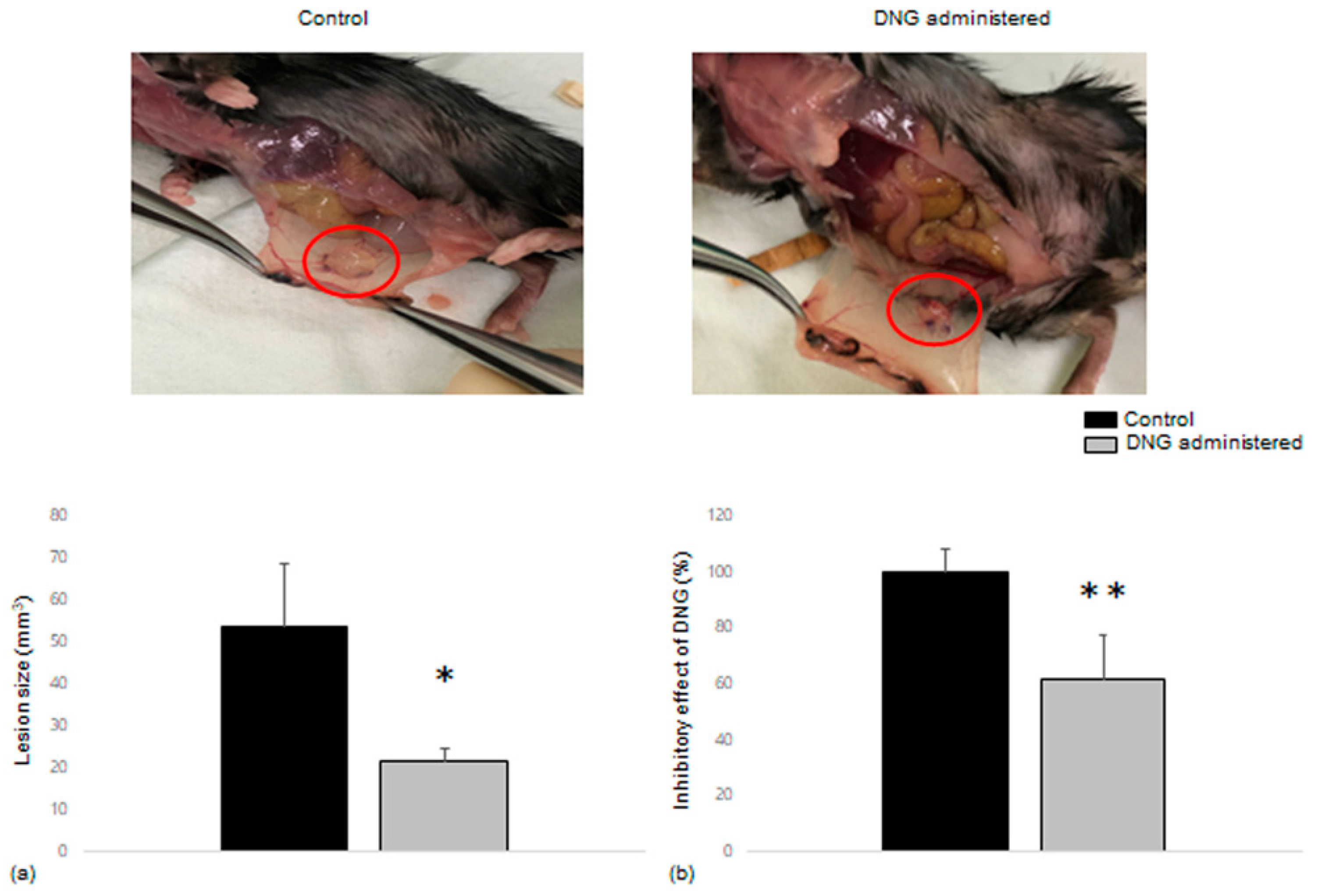
3.4. Comparison of Endometrial Tissue Implants in Mice with and without DNG Treatment
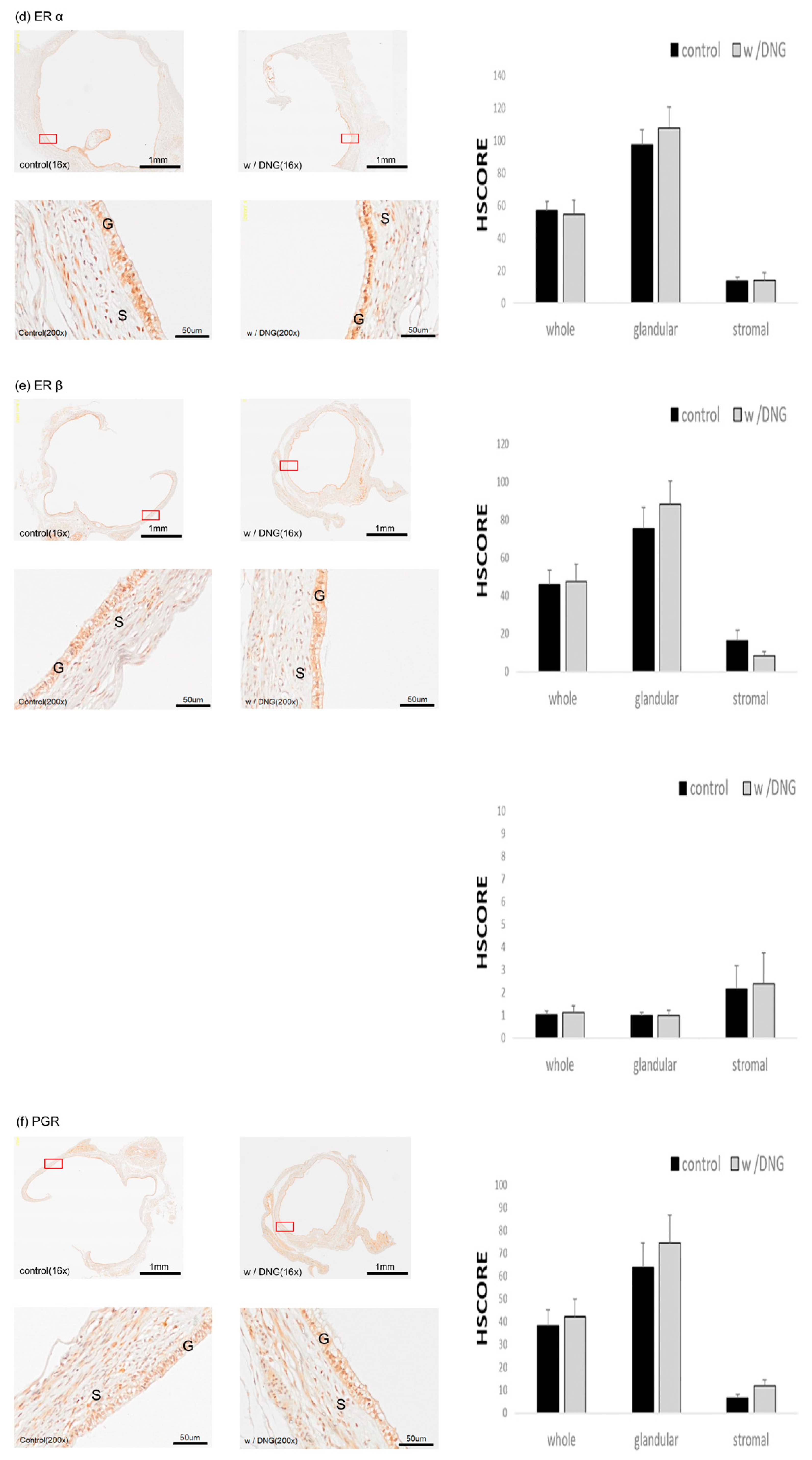
3.5. Expression of Several Markers in Endometrial Tissues Implanted in Mice with and without DNG Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, M.-H.; Hsiao, K.-Y.; Tsai, S.-J. Endometriosis and possible inflammation markers. Gynecol. Minim. Invasive Ther. 2015, 4, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Dunselman, G.A.J.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef]
- Mounsey, A.L.; Wilgus, A.; Slawson, D.C. Diagnosis and management of endometriosis. Am. Fam. Physician 2006, 74, 594–600. [Google Scholar] [CrossRef]
- Sinaii, N.; Plumb, K.; Cotton, L.; Lambert, A.; Kennedy, S.; Zondervan, K.; Stratton, P. Differences in characteristics among 1,000 women with endometriosis based on extent of disease. Fertil. Steril. 2008, 89, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Eskenazi, B.; Warner, M.L. Epidemiology of endometriosis. Obstet. Gynecol. Clin. N. Am. 1997, 24, 235–258. [Google Scholar] [CrossRef]
- Pritts, E.A.; Taylor, R.N. An evidence-based evaluation of endometriosis-associated infertility. Endocrinol. Metab. Clin. N. Am. 2003, 32, 653–667. [Google Scholar] [CrossRef]
- Zanelotti, A.; Decherney, A.H. Surgery and Endometriosis. Clin. Obstet. Gynecol. 2017, 60, 477–484. [Google Scholar] [CrossRef]
- Abbott, J.; Hawe, J.; Hunter, D.; Holmes, M.; Finn, P.; Garry, R. Laparoscopic excision of endometriosis: A randomized, placebo-controlled trial. Fertil. Steril. 2004, 82, 878–884. [Google Scholar] [CrossRef]
- Guo, S.W. Recurrence of endometriosis and its control. Hum. Reprod. Update 2009, 15, 441–461. [Google Scholar] [CrossRef]
- Kondo, W.; Bourdel, N.; Tamburro, S.; Cavoli, D.; Jardon, K.; Rabischong, B.; Botchorishvili, R.; Pouly, J.L.; Mage, G.; Canis, M. Complications after surgery for deeply infiltrating pelvic endometriosis. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 292–298. [Google Scholar] [CrossRef]
- Brown, J.; Pan, A.; Hart, R.J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst. Rev. 2010, 2010, CD008475. [Google Scholar] [CrossRef]
- Della Corte, L.; Barra, F.; Mercorio, A.; Evangelisti, G.; Rapisarda, A.M.C.; Ferrero, S.; Bifulco, G.; Giampaolino, P. Tolerability considerations for gonadotropin-releasing hormone analogues for endometriosis. Expert Opin. Drug Metab. Toxicol. 2020, 16, 759–768. [Google Scholar] [CrossRef]
- Irahara, M.; Harada, T.; Momoeda, M.; Tamaki, Y. Hormonal and histological study on irregular genital bleeding in patients with endometriosis during treatment with dienogest, a novel progestational therapeutic agent. Reprod. Med. Biol. 2007, 6, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Schweppe, K.W. Current place of progestins in the treatment of endometriosis-related complaints. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2001, 15 (Suppl. S6), 22–28. [Google Scholar] [CrossRef]
- Shimizu, Y.; Takeuchi, T.; Mita, S.; Mizuguchi, K.; Kiyono, T.; Inoue, M.; Kyo, S. Dienogest, a synthetic progestin, inhibits the proliferation of immortalized human endometrial epithelial cells with suppression of cyclin D1 gene expression. Mol. Hum. Reprod. 2009, 15, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, M.; Koga, K.; Takamura, M.; Izumi, G.; Nagai, M.; Harada, M.; Hirata, T.; Hirota, Y.; Fujii, T.; Osuga, Y. Dienogest reduces proliferation, aromatase expression and angiogenesis, and increases apoptosis in human endometriosis. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2014, 30, 644–648. [Google Scholar] [CrossRef]
- Iwabe, T.; Harada, T.; Tsudo, T.; Nagano, Y.; Yoshida, S.; Tanikawa, M.; Terakawa, N. Tumor Necrosis Factor-Promotes Proliferation of Endometriotic Stromal Cells by Inducing Interleukin-8 Gene and Protein Expression. J. Clin. Endocrinol. Metab. 2000, 85, 824–829. [Google Scholar]
- Horie, S.; Harada, T.; Mitsunari, M.; Taniguchi, F.; Iwabe, T.; Terakawa, N. Progesterone and progestational compounds attenuate tumor necrosis factor alpha-induced interleukin-8 production via nuclear factor kappaB inactivation in endometriotic stromal cells. Fertil. Steril. 2005, 83, 1530–1535. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kim, S.H.; Oh, Y.S.; Heo, S.H.; Kim, K.H.; Chae, H.D.; Kim, C.H.; Kang, B.M. Role of interleukin-32 in the pathogenesis of endometriosis: In vitro, human and transgenic mouse data. Hum. Reprod. 2018, 33, 807–816. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.W.; Kim, Y.H.; Koo, Y.H.; Chae, H.D.; Kim, C.H.; Lee, P.R.; Kang, B.M. Down-regulation of p21-activated kinase 1 by progestin and its increased expression in the eutopic endometrium of women with endometriosis. Hum. Reprod. 2009, 24, 1133–1141. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R.; Kim, S.H.; Lee, H.W.; Kim, Y.H.; Chae, H.D.; Kim, C.H.; Kang, B.M. Increased expression of glutathione by estradiol, tumor necrosis factor-alpha, and interleukin 1-beta in endometrial stromal cells. Am. J. Reprod. Immunol. 2009, 62, 352–356. [Google Scholar] [CrossRef]
- Fakih, H.; Baggett, B.; Holtz, G.; Tsang, K.Y.; Lee, J.C.; Williamson, H.O. Interleukin-1: A possible role in the infertility associated with endometriosis. Fertil. Steril. 1987, 47, 213–217. [Google Scholar] [CrossRef]
- Eisermann, J.; Gast, M.J.; Pineda, J.; Odem, R.R.; Collins, J.L. Tumor necrosis factor in peritoneal fluid of women undergoing laparoscopic surgery. Fertil. Steril. 1988, 50, 573–579. [Google Scholar] [CrossRef]
- Suen, J.L.; Chang, Y.; Chiu, P.R.; Hsieh, T.H.; Hsi, E.; Chen, Y.C.; Chen, Y.F.; Tsai, E.M. Serum level of IL-10 is increased in patients with endometriosis, and IL-10 promotes the growth of lesions in a murine model. Am. J. Pathol. 2014, 184, 464–471. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J.J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Katsuki, Y.; Takano, Y.; Futamura, Y.; Shibutani, Y.; Aoki, D.; Udagawa, Y.; Nozawa, S. Effects of dienogest, a synthetic steroid, on experimental endometriosis in rats. Eur. J. Endocrinol. 1998, 138, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.H.; Edwards, A.K.; Singh, S.S.; Young, S.L.; Lessey, B.A.; Tayade, C. IL-17A Contributes to the Pathogenesis of Endometriosis by Triggering Proinflammatory Cytokines and Angiogenic Growth Factors. J. Immunol. 2015, 195, 2591–2600. [Google Scholar] [CrossRef] [Green Version]
- Cunha, G.R.; Bigsby, R.M.; Cooke, P.S.; Sugimura, Y. Stromal-epithelial interactions in adult organs. Cell Differ. 1985, 17, 137–148. [Google Scholar] [CrossRef]
- Arnold, J.T.; Kaufman, D.G.; Seppälä, M.; Lessey, B.A. Endometrial stromal cells regulate epithelial cell growth in vitro: A new co-culture model. Hum. Reprod. 2001, 16, 836–845. [Google Scholar] [CrossRef]
- Logan, P.C.; Yango, P.; Tran, N.D. Endometrial Stromal and Epithelial Cells Exhibit Unique Aberrant Molecular Defects in Patients with Endometriosis. Reprod. Sci. 2018, 25, 140–159. [Google Scholar] [CrossRef] [Green Version]
- Kao, L.C.; Germeyer, A.; Tulac, S.; Lobo, S.; Yang, J.P.; Taylor, R.N.; Osteen, K.; Lessey, B.A.; Giudice, L.C. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 2003, 144, 2870–2881. [Google Scholar] [CrossRef]
- Dai, S.M.; Matsuno, H.; Nakamura, H.; Nishioka, K.; Yudoh, K. Interleukin-18 Enhances Monocyte Tumor Necrosis Factor α and Interleukin-1β Production Induced by Direct Contact with T Lymphocytes: Implications in Rheumatoid Arthritis. Arthritis Rheum. 2004, 50, 432–443. [Google Scholar] [CrossRef]
- Mita, S.; Shimizu, Y.; Sato, A.; Notsu, T.; Imada, K.; Kyo, S. Dienogest inhibits nerve growth factor expression induced by tumor necrosis factor-α or interleukin-1β. Fertil. Steril. 2014, 101, 595–601.e1. [Google Scholar] [CrossRef]
- Kim, S.H.; Ihm, H.J.; Oh, Y.S.; Chae, H.D.; Kim, C.H.; Kang, B.M. Increased Nuclear Expression of Nuclear Factor Kappa-B p65 Subunit in the Eutopic Endometrium and Ovarian Endometrioma of Women with Advanced Stage Endometriosis. Am. J. Reprod. Immunol. 2013, 70, 497–508. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, S.Y.; Azam, T.; Yoon, D.Y.; Dinarello, C.A. Interleukin-32: A cytokine and inducer of TNFα. Immunity 2005, 22, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Sun, W.; Liu, L.; Yang, F.; Li, Y.; Chen, Y.; Fang, J.; Zhang, W.; Wu, J.; Zhu, Y. IL-32: A host proinflammatory factor against influenza viral replication is upregulated by aberrant epigenetic modifications during influenza A virus infection. J. Immunol. 2010, 185, 5056–5065. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Kim, S.-H.; Azam, T.; McGibney, M.T.; Huang, H.; Dinarello, C.A.; Chan, E.D. IL-32 is a host protective cytokine against Mycobacterium tuberculosis in differentiated THP-1 human macrophages. J. Immunol. 2010, 184, 3830–3840. [Google Scholar] [CrossRef] [Green Version]
- Joosten, L.A.B.; Heinhuis, B.; Netea, M.G.; Dinarello, C.A. Novel insights into the biology of interleukin-32. Cell. Mol. Life Sci. 2013, 70, 3883–3892. [Google Scholar] [CrossRef]
- Soyka, M.B.; Treis, A.; Eiwegger, T.; Menz, G.; Zhang, S.; Holzmann, D.; Akdis, C.A.; Meyer, N. Regulation and expression of IL-32 in chronic rhinosinusitis. Allergy 2012, 67, 790–798. [Google Scholar] [CrossRef]
- Ciccia, F.; Rizzo, A.; Accardo-palumbo, A.; Giardina, A.R.; Bombardieri, M.; Guggino, G.; Taverna, S.; Leo, G.D.; Alessandro, R.; Triolo, G. Increased expression of interleukin-32 in the inflamed ileum of ankylosing spondylitis patients. Rheumatology 2012, 51, 1966–1972. [Google Scholar] [CrossRef] [Green Version]
- Shioya, M.; Nishida, A.; Yagi, Y.; Ogawa, A.; Tsujikawa, T.; Kim-Mitsuyama, S.; Takayanagi, A.; Shimizu, N.; Fujiyama, Y.; Andoh, A. Epithelial overexpression of interleukin-32α in inflammatory bowel disease. Clin. Exp. Immunol. 2007, 149, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Kim, S.; Oh, Y.S.; Cho, S.H.; Hoon Kim, S. Elevated serum interleukin-32 levels in patients with endometriosis: A cross-sectional study. Am. J. Reprod. Immunol. 2019, 82, e13149. [Google Scholar] [CrossRef] [PubMed]
- Petraglia, F.; Hornung, D.; Seitz, C.; Faustmann, T.; Gerlinger, C.; Luisi, S.; Lazzeri, L.; Strowitzki, T. Reduced pelvic pain in women with endometriosis: Efficacy of long-term dienogest treatment. Arch. Gynecol. Obstet. 2012, 285, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Köhler, G.; Faustmann, T.A.; Gerlinger, C.; Seitz, C.; Mueck, A.O. A dose-ranging study to determine the efficacy and safety of 1, 2, and 4mg of dienogest daily for endometriosis. Int. J. Gynaecol. Obstet. 2010, 108, 21–25. [Google Scholar] [CrossRef]
- Strowitzki, T.; Faustmann, T.; Gerlinger, C.; Seitz, C. Dienogest in the treatment of endometriosis-associated pelvic pain: A 12-week, randomized, double-blind, placebo-controlled study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 151, 193–198. [Google Scholar] [CrossRef]
- Strowitzki, T.; Faustmann, T.; Gerlinger, C.; Schumacher, U.; Ahlers, C.; Seitz, C. Safety and tolerability of dienogest in endometriosis: Pooled analysis from the European clinical study program. Int. J. Women’s Health 2015, 7, 391–401. [Google Scholar] [CrossRef] [Green Version]
- Cho, B.; Roh, J.-W.; Park, J.; Jeong, K.; Kim, T.-H.; Kim, Y.S.; Kwon, Y.-S.; Cho, C.-H.; Park, S.H.; Kim, S.H. Safety and Effectiveness of Dienogest (Visanne®) for Treatment of Endometriosis: A Large Prospective Cohort Study. Reprod. Sci. 2020, 27, 905–915. [Google Scholar] [CrossRef]
- Harada, T.; Taniguchi, F. Dienogest: A new therapeutic agent for the treatment of endometriosis. Women’s Health 2010, 6, 27–35. [Google Scholar] [CrossRef]
- Sasagawa, S.; Shimizu, Y.; Kami, H.; Takeuchi, T.; Mita, S.; Imada, K.; Kato, S.; Mizuguchi, K. Dienogest is a selective progesterone receptor agonist in transactivation analysis with potent oral endometrial activity due to its efficient pharmacokinetic profile. Steroids 2008, 73, 222–231. [Google Scholar] [CrossRef]
- Vannuccini, S.; Clemenza, S.; Rossi, M.; Petraglia, F. Hormonal treatments for endometriosis: The endocrine background. Rev. Endocr. Metab. Disord. 2021, 23, 333–355. [Google Scholar] [CrossRef]
- Bulun, S.E.; Monsavais, D.; Pavone, M.E.; Dyson, M.; Xue, Q.; Attar, E.; Tokunaga, H.; Su, E.J. Role of estrogen receptor-β in endometriosis. Semin. Reprod. Med. 2012, 30, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Tanabe, A.; Kawabe, S.; Hayashi, M.; Yuguchi, H.; Yamashita, Y.; Okuda, K.; Ohmichi, M. Dienogest increases the progesterone receptor isoform B/A ratio in patients with ovarian endometriosis. J. Ovarian Res. 2012, 5, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichioka, M.; Mita, S.; Shimizu, Y.; Imada, K.; Kiyono, T.; Bono, Y.; Kyo, S. Dienogest, a synthetic progestin, down-regulates expression of CYP19A1 and inflammatory and neuroangiogenesis factors through progesterone receptor isoforms A and B in endometriotic cells. J. Steroid Biochem. Mol. Biol. 2015, 147, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Singhal, H.; Greene, M.E.; Zarnke, A.L.; Laine, M.; Al Abosy, R.; Chang, Y.F.; Dembo, A.G.; Schoenfelt, K.; Vadhi, R.; Qiu, X.; et al. Progesterone receptor isoforms, agonists and antagonists differentially reprogram estrogen signaling. Oncotarget 2018, 9, 4282–4300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabona, J.M.P.; Simmen, F.A.; Nikiforov, M.A.; Zhuang, D.Z.; Shankar, K.; Velarde, M.C.; Zelenko, Z.; Giudice, L.C.; Simmen, R.C.M. Krüppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: Implications for the pathogenesis of endometriosis. J. Clin. Endocrinol. Metab. 2012, 97, E376–E392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmen, R.C.M.; Eason, R.R.; McQuown, J.R.; Linz, A.L.; Kang, T.J.; Chatman, L.; Till, S.R.; Fujii-Kuriyama, Y.; Simmen, F.A.; Oh, S.P. Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Kruppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. J. Biol. Chem. 2004, 279, 29286–29294. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Du, H.; Taylor, H.S. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol. Reprod. 2009, 80, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Heard, M.E.; Simmons, C.D.; Simmen, F.A.; Simmen, R.C. Krüppel-like factor 9 deficiency in uterine endometrial cells promotes ectopic lesion establishment associated with activated notch and hedgehog signaling in a mouse model of endometriosis. Endocrinology 2014, 155, 1532–1546. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Kim, S.H.; Oh, Y.S.; Lee, S.R.; Chae, H.D. Dienogest May Reduce Estradiol- and Inflammatory Cytokine-Induced Cell Viability and Proliferation and Inhibit the Pathogenesis of Endometriosis: A Cell Culture- and Mouse Model-Based Study. Biomedicines 2022, 10, 2992. https://doi.org/10.3390/biomedicines10112992
Kim HJ, Kim SH, Oh YS, Lee SR, Chae HD. Dienogest May Reduce Estradiol- and Inflammatory Cytokine-Induced Cell Viability and Proliferation and Inhibit the Pathogenesis of Endometriosis: A Cell Culture- and Mouse Model-Based Study. Biomedicines. 2022; 10(11):2992. https://doi.org/10.3390/biomedicines10112992
Chicago/Turabian StyleKim, Hyun Jin, Sung Hoon Kim, Young Sang Oh, Sa Ra Lee, and Hee Dong Chae. 2022. "Dienogest May Reduce Estradiol- and Inflammatory Cytokine-Induced Cell Viability and Proliferation and Inhibit the Pathogenesis of Endometriosis: A Cell Culture- and Mouse Model-Based Study" Biomedicines 10, no. 11: 2992. https://doi.org/10.3390/biomedicines10112992




