Evaluation of Lipid Peroxidation in the Saliva of Diabetes Mellitus Type 2 Patients with Periodontal Disease
Abstract
:1. Introduction
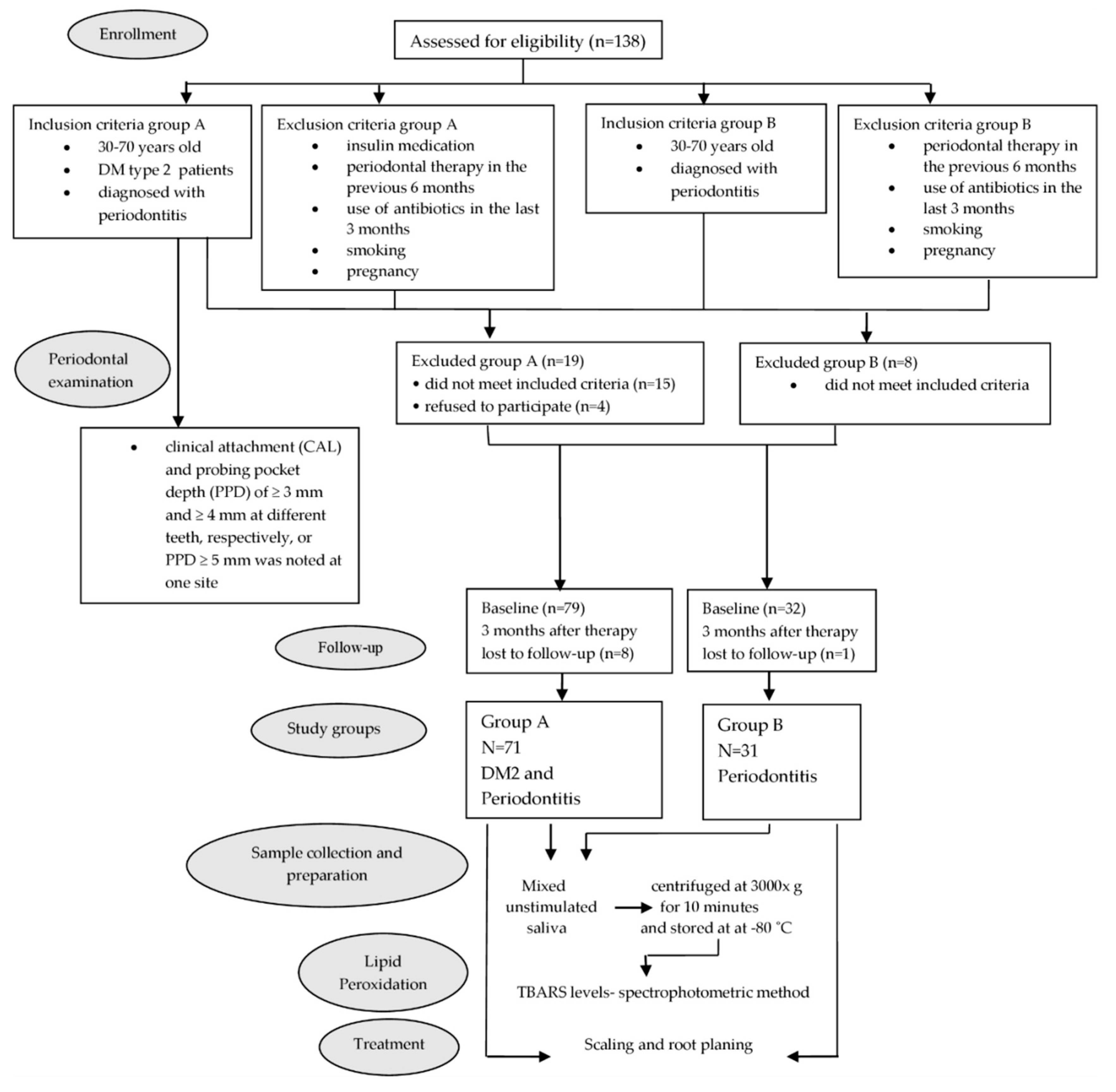
2. Materials and Methods
2.1. Research Participants
2.2. Periodontal Examination
2.3. Sample Collection and Preparation
2.4. Lipid Peroxidation (LP) Determination
2.5. Periodontal Therapy
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Boyko, E.J.; Magliano, D.J.; Karuranga, S.; Piemonte, L.; Riley, P.; Saeedi, P.; Sun, H. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; ISBN 978-2-930229-98-0. Available online: www.diabetesatlas.org (accessed on 2 September 2022).
- Bigagli, E.; Raimondi, L.; Mannucci, E.; Colombi, C.; Bardini, G.; Rotella, C.M.; Lodovici, M. Lipid and protein oxidation products, antioxidant status and vascular complications in poorly controlled type 2 diabetes. Br. J. Diabetes Vasc. Dis. 2011, 12, 33–39. [Google Scholar] [CrossRef]
- Löe, H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 1993, 16, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yuan, Y.; Liu, H.; Li, S.; Zhang, B.; Chen, W.; An, Z.; Chen, S.; Wu, Y.; Han, B.; et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health 2020, 20, 204. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monea, A.; Mezei, T.; Popsor, S.; Monea, M. Oxidative stress: A link between diabetes mellitus and periodontal disease. Int. J. Endocrinol. 2014, 2014, 917631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Tothova, L.; Celec, P. Oxidative Stress and Antioxidants in the Diagnosis and Therapy of Periodontitis. Front. Physiol. 2017, 8, 1055. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi-Motamayel, F.; Goodarzi, M.T.; Jamshidi, Z.; Kebriaei, R. Evaluation of Salivary and Serum Antioxidant and Oxidative Stress Statuses in Patients with Chronic Periodontitis: A Case-Control Study. Front. Physiol. 2017, 8, 189. [Google Scholar] [CrossRef] [Green Version]
- Veljović, T.; Ðurić, M.; Gušić, I.; Mirnić, J.; Čakić, S.; Maletin, A.; Brkić, S. The Influence of Periodontal Disease Treatment on 8-Hydroxy-Deoxyguanosine Concentrations in Saliva and Plasma of Chronic Periodontitis Patients. Acta Clin. Croat. 2020, 59, 615–622. [Google Scholar] [CrossRef]
- Trivedi, S.; Lal, N.; Mahdi, A.A.; Singh, B.; Pandey, S. Association of salivary lipid peroxidation levels, antioxidant enzymes, and chronic periodontitis. Int. J. Periodontics Restor. Dent. 2015, 35, e14–e19. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Milward, M.R.; Dietrich, T. The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J. Nutr. 2007, 137, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Veljovic, T.; Djuric, M.; Mirnic, J.; Gusic, I.; Maletin, A.; Ramic, B.; Neskovic, I.; Vukoje, K.; Brkic, S. Lipid Peroxidation Levels in Saliva and Plasma of Patients Suffering from Periodontitis. J. Clin. Med. 2022, 11, 3617. [Google Scholar] [CrossRef]
- Tonguç, M.O.; Öztürk, O.; Sütçü, R.; Ceyhan, B.M.; Kılınç, G.; Sönmez, Y.; Ay, Z.Y.; Sahin, U.; Baltacıoğlu, E.; Kırzıoğlu, F.Y. The impact of smoking status on antioxidant enzyme activity and malondialdehyde levels in chronic periodontitis. J. Periodontol. 2011, 82, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Panjamurthy, K.; Manoharan, S.; Ramachandran, C.R. Lipid peroxidation and antioxidant status in patients with periodontitis. Cell Mol. Biol. Lett. 2005, 10, 255–264. [Google Scholar]
- da Silva, J.C.; Gomes Muniz, F.W.M.; Oballe, H.J.R.; Andrades, M.; Rösing, C.K.; Cavagni, J. The effect of periodontal therapy on oxidative stress biomarkers: A systematic review. J Clin. Periodontol. 2018, 45, 1222–1237. [Google Scholar] [CrossRef]
- Mirnic, J.; Djuric, M.; Gusic, I.; Veljovic, T.; Cakic, S.; Katanic, J.; Vukoje, K.; Ramic, B.; Brkic, S. Effects of Nonsurgical Periodontal Therapy on Salivary 8-Hydroxy-Deoxyguanosine Levels and Glycemic Control in Diabetes Mellitus Type 2 Patients. Biomedicines 2022, 10, 2269. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Al-Rawi, N.H. Oxidative stress, antioxidant status and lipid profile in the saliva of type 2 diabetics. Diab. Vasc. Dis. Res. 2011, 8, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Bastos, A.S.; Graves, D.T.; de Melo Loureiro, A.P.; Júnior, C.R.; Abdalla, D.S.P.; Faulin, T.E.S.; Câmara, N.O.; Andriankaja, O.M.; Orrico, S.R.P. Lipid peroxidation is associated with the severity of periodontal disease and local inflammatory markers in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2012, 97, E1353–E1362. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, S.; Lal, N.; Mahdi, A.A.; Mittal, M.; Singh, B.; Pandey, S. Evaluation of antioxidant enzymes activity and malondialdehyde levels in patients with chronic periodontitis and diabetes mellitus. J. Periodontol. 2014, 85, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Page, R.C.; Wei, L.; Thornton-Evans, G.; Genco, R.J. Update of the case definitions for population based surveillance of periodontitis. J. Periodontol. 2012, 83, 1449–1454. [Google Scholar] [CrossRef]
- Silness, J.; Löe, H. Periodontal disease in pregnancy (II). Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Löe, H.; Silness, P. Periodontal disease in pregnancy I. Acta. Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Saxer, U.; Turconi, B.; Elsässer, C. Patient motivation with the papillary bleeding index. J. Prev. Dent. 1977, 4, 20–22. [Google Scholar] [PubMed]
- Mirnić, J.; Đurić, M.; Nikolić, N.; Veljović, T.; Gušić, I.; Petrović, Đ.; Milašin, J. Clinical and microbiological assessment of non-surgical treatment of chronic periodontitis in controlled and uncontrolled type 2 diabetic patients. Acta Clin. Croat. 2021, 60, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Velly, A.M.; Salah, M.H.; Benarroch, M.; Trifiro, M.; Schipper, H.M.; Gornitsky, M. Altered redox homeostasis in human diabetes saliva. J. Oral. Pathol. Med. 2012, 41, 235–241. [Google Scholar] [CrossRef]
- Samojlik, I.; Lakic, N.S.; Mimica-Dukić, N.M.; Djaković-Svajcer, K.; Bozin, B.N. Antioxidant and Hepatoprotective Potential of Essential Oils of Coriander (Coriandrum sativum L.) and Caraway (Carum carvi L.) (Apiaceae). J. Agr. Food Chem. 2010, 58, 8848–8853. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Eid, S.; Sas, K.M.; Abcouwer, S.F.; Feldman, E.L.; Gardner, T.W.; Pennathur, S.; Fort, P.E. New insights into the mechanisms of diabetic complications: Role of lipids and lipid metabolism. Diabetologia 2019, 62, 1539–1549. [Google Scholar] [CrossRef] [Green Version]
- Panigrahy, S.K.; Bhatt, R.; Kumar, A. Reactive oxygen species: Sources, consequences and targeted therapy in type 2 diabetes. J. Drug Target 2017, 25, 93–101. [Google Scholar] [CrossRef]
- Southerland, J.H.; Taylor, G.W.; Moss, K.; Beck, J.D.; Offenbacher, S. Commonality in chronic inflammatory diseases: Periodontitis, diabetes, and coronary artery disease. Periodontol. 2000 2006, 40, 130–143. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Weidman, E.; Lalla, E.; Yan, S.D.; Hori, O.; Cao, R.; Brett, J.G.; Lamster, I.B. Advanced glycation endproducts induce oxidant stress in the gingiva: A potential mechanism underlying accelerated periodontal disease associated with diabetes. J. Periodontal. Res. 1996, 31, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Naruse, K.; Kobayashi, Y.; Miyajima, S.; Mizutani, M.; Kikuchi, T.; Soboku, K.; Nakamura, N.; Sokabe, A.; Tosaki, T.; et al. Involvement of nitrosative stress in experimental periodontitis in diabetic rats. J. Clin. Periodontol. 2012, 39, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, D.; Correa, F.O.B.; Khalil, N.M.; de Faria Oliveira, O.M.M.; Orrico, S.R.P. The effect of non-surgical periodontal therapy on peroxidase activity in diabetic patients: A case-control pilot study. J. Clin. Periodontol. 2008, 35, 799–806. [Google Scholar] [CrossRef]
- Serrano, C.; Perez, C.; Rodríguez, M. Periodontal conditions in a group of Colombian type 2 diabetic patients with different degrees of metabolic control. Acta Odontol. Latinoam. 2012, 25, 130–137. [Google Scholar]
- Vitkov, L.; Muñoz, L.E.; Knopf, J.; Schauer, C.; Oberthaler, H.; Minnich, B.; Hannig, M.; Herrmann, M. Connection between Periodontitis-Induced Low-Grade Endotoxemia and Systemic Diseases: Neutrophils as Protagonists and Targets. Int. J. Mol. Sci. 2021, 22, 4647. [Google Scholar] [CrossRef] [PubMed]
- Arana, C.; Moreno-Fernandez, A.M.; Gomez-Moreno, G.; Morales-Portillo, C.; Serrano-Olmedo, I.; de la Cuesta Mayor, M.C.; Hernandez, T.M. Increased salivary oxidative stress parameters in patients with type 2 diabetes: Relation with periodontal disease. Endocrinol. Diabetes Nutr. 2017, 64, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Latha, N.; Uppoor, A.; Nayak, S.U.; Naik, D.G. Effect of non-surgical therapy on salivary nitric oxide and lipid peroxidation levels in type II diabetic and non diabetic patients with periodontal disease. Asian J. Pharm. Clin. Res. 2018, 11, 330–336. [Google Scholar] [CrossRef]
- Patil, V.S.; Patil, V.P.; Gokhale, N.; Acharya, A.; Kangokar, P. Chronic Periodontitis in Type 2 Diabetes Mellitus: Oxidative Stress as a Common Factor in Periodontal Tissue Injury. J. Clin. Diagn. Res. 2016, 10, BC12–BC16. [Google Scholar] [CrossRef] [PubMed]
- Michalowicz, B.S.; Hyman, L.; Hou, W.; Oates, T.W., Jr.; Reddy, M.; Paquette, D.W.; Katancik, J.A.; Engebretson, S.P. Diabetes and Periodontal Therapy Trial Study Team. Factors associated with the clinical response to nonsurgical periodontal therapy in people with type 2 diabetes mellitus. JADA 2014, 145, 1227–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirnić, J.; Đurić, M.; Predin, T.; Gušić, I.; Petrović, Đ.; Anđelković, A.; Bajkin, B. Impact of the level of metabolic control on the non-surgical periodontal therapy outcomes in diabetes mellitus type 2 patients—Clinical effects. Srp. Arh. Celok. Lek. 2013, 141, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Chen, H.S.; Chen, S.L.; Ho, Y.P.; Ho, K.Y.; Wu, Y.M.; Hung, C.C. Lipid peroxidation: A possible role in the production and progression of chronic periodontitis. J. Periodontal. Res. 2005, 40, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhang, X.L.; Wang, Y.Z.; Yang, C.X.; Chen, G. Lipid peroxidation levels, total oxidant status and superoxide dismutase in serum, saliva and gingival crevicular fluid in chronic periodontitis patients before and after periodontal therapy. Aust. Dent. J. 2010, 55, 70–78. [Google Scholar] [CrossRef]
- Costa, K.L.; Taboza, Z.A.; Angelino, G.B.; Silveira, V.R.; Montenegro, R., Jr.; Haas, A.N.; Rego, R.O. Influence of Periodontal Disease on Changes of Glycated Hemoglobin Levels in Patients with Type 2 Diabetes Mellitus: A Retrospective Cohort Study. J. Periodontol. 2017, 88, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.J.; Presaw, P.M.; Lalla, E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J. Clin. Periodontol. 2013, 40 (Suppl. S14), S113–S134. [Google Scholar] [CrossRef]
- Allen, E.M.; Matthews, J.B.; O’Halloran, D.J.; Griffiths, H.R.; Chapple, I.L. Oxidative and inflammatory status in type 2 diabetes patients with periodontitis. J. Clin. Periodontol. 2011, 38, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Ramakrishnan, T.; Harinath, P.; Moses, J.; Shankarram, V.; Raj, S. Effect of non-surgical periodontal therapy on plasma level of reactive oxygen metabolites and glycemic status in type 2 diabetic patients with chronic periodontitis. Biosci. Biotechnol. Res. Asia 2017, 14, 357–365. [Google Scholar] [CrossRef]
- Muthuraj, M.S.; Janakiram, S.; Chithresan, K.; Maradi, A.P.; Maddur, P.K.; Rangaraju, R. Effect of scaling and root planing on levels of 8-hydroxydeoxyguanosine in gingival crevicular fluid of chronic periodontitis patients with and without Type II diabetes mellitus. J. Indian Soc. Periodontol. 2017, 21, 201–206. [Google Scholar] [CrossRef]
- Sonoki, K.; Nakashima, S.; Takata, Y.; Naito, T.; Fujisawa, K.; Ootsubo, T.; Wakisaka, M.; Iwase, M.; Iida, M.; Yokota, M. Decreased lipid peroxidation following periodontal therapy in type 2 diabetic patients. J. Periodontol. 2006, 77, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
| Group | Start of the Study | End of the Study | Change Δ (Baseline—3 Months) | ap | bp | cp | |
|---|---|---|---|---|---|---|---|
| PI | A B | 1.73 ± 0.46 1.32 ± 0.51 | 1.18 ± 0.43 0.66 ± 0.49 | 0.56 ± 0.37 0.66 ± 0.39 | p < 0.001 | p < 0.001 p < 0.001 | p > 0.05 |
| GI | A B | 1.53 ± 0.62 0.94 ± 0.72 | 0.88 ± 0.46 0.37 ± 0.45 | 0.65 ± 0.44 0.57 ± 0.53 | p < 0.001 | p < 0.001 p < 0.001 | p > 0.05 |
| PBI | A B | 1.71 ± 0.75 1.45 ± 0.82 | 0.98 ± 0.62 0.67 ± 0.45 | 0.73 ± 0.61 0.78 ± 0.54 | p > 0.05 | p < 0.001 p < 0.001 | p > 0.05 |
| PPD; mm | A B | 2.02 ± 0.50 2.38 ± 0.60 | 1.93 ± 0.46 2.05 ± 0.52 | 0.09 ± 0.21 0.34 ± 0.23 | p < 0.01 | p < 0.001 p < 0.001 | p < 0.001 |
| PPD ≤ 3 mm (%) | A B | 94.48 ± 8.48 90.85 ± 11.96 | 95.26 ± 8.71 93.69 ± 10.58 | −0.78 ± 4.17 −2.83 ± 5.75 | p > 0.05 | p > 0.05 p < 0.05 | p < 0.05 |
| PPD = 4 or 5 mm (%) | A B | 5.01 ± 7.10 7.49 ± 9.76 | 4.22 ± 7.27 5.61 ± 8.36 | 0.79 ± 3.77 1.89 ± 5.46 | p > 0.05 | p > 0.05 p > 0.05 | p > 0.05 |
| PPD ≥ 6 mm (%) | A B | 0.55 ± 1.73 1.65 ± 3.29 | 0.52 ± 1.86 0.78 ± 2.94 | 0.03 ± 1.42 0.87 ± 2.23 | p > 0.05 | p > 0.05 p < 0.05 | p > 0.05 |
| CAL; mm | A B | 2.57 ± 1.23 2.29 ± 1.44 | 2.33 ± 1.18 1.98 ± 1.31 | 0.24 ± 0.25 0.34 ± 0.31 | p > 0.05 | p < 0.001 p < 0.001 | p > 0.05 |
| Group | Start of the Study | End of the Study | Change Δ (Baseline—3 Months) | ap | bp | cp | |
|---|---|---|---|---|---|---|---|
| LP (%) | A B | 38.4 ± 27.16 36.22 ± 26.10 | 15.33 ± 13.43 15.46 ± 0.40 | 23.07 ± 30.94 20.76 ± 31.95 | p > 0.05 | p < 0.01 p < 0.05 | p > 0.05 |
| PI | GI | PBI | PPD | CAL | HbA1c | |
|---|---|---|---|---|---|---|
| LP (Group A) | 0.204 | 0.575 | 0.146 | 0.044 * | 0.423 | 0.577 |
| LP (Group B) | 0.420 | 0.634 | 0.254 | 0.030 * | 0.248 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirnic, J.; Djuric, M.; Veljovic, T.; Gusic, I.; Katanic, J.; Vukoje, K.; Ramic, B.; Tadic, A.; Brkic, S. Evaluation of Lipid Peroxidation in the Saliva of Diabetes Mellitus Type 2 Patients with Periodontal Disease. Biomedicines 2022, 10, 3147. https://doi.org/10.3390/biomedicines10123147
Mirnic J, Djuric M, Veljovic T, Gusic I, Katanic J, Vukoje K, Ramic B, Tadic A, Brkic S. Evaluation of Lipid Peroxidation in the Saliva of Diabetes Mellitus Type 2 Patients with Periodontal Disease. Biomedicines. 2022; 10(12):3147. https://doi.org/10.3390/biomedicines10123147
Chicago/Turabian StyleMirnic, Jelena, Milanko Djuric, Tanja Veljovic, Ivana Gusic, Jasmina Katanic, Karolina Vukoje, Bojana Ramic, Ana Tadic, and Snezana Brkic. 2022. "Evaluation of Lipid Peroxidation in the Saliva of Diabetes Mellitus Type 2 Patients with Periodontal Disease" Biomedicines 10, no. 12: 3147. https://doi.org/10.3390/biomedicines10123147





