Allogenic Perinatal Tissue for Musculoskeletal Regenerative Medicine Applications: A Systematic Review
Abstract
1. Introduction
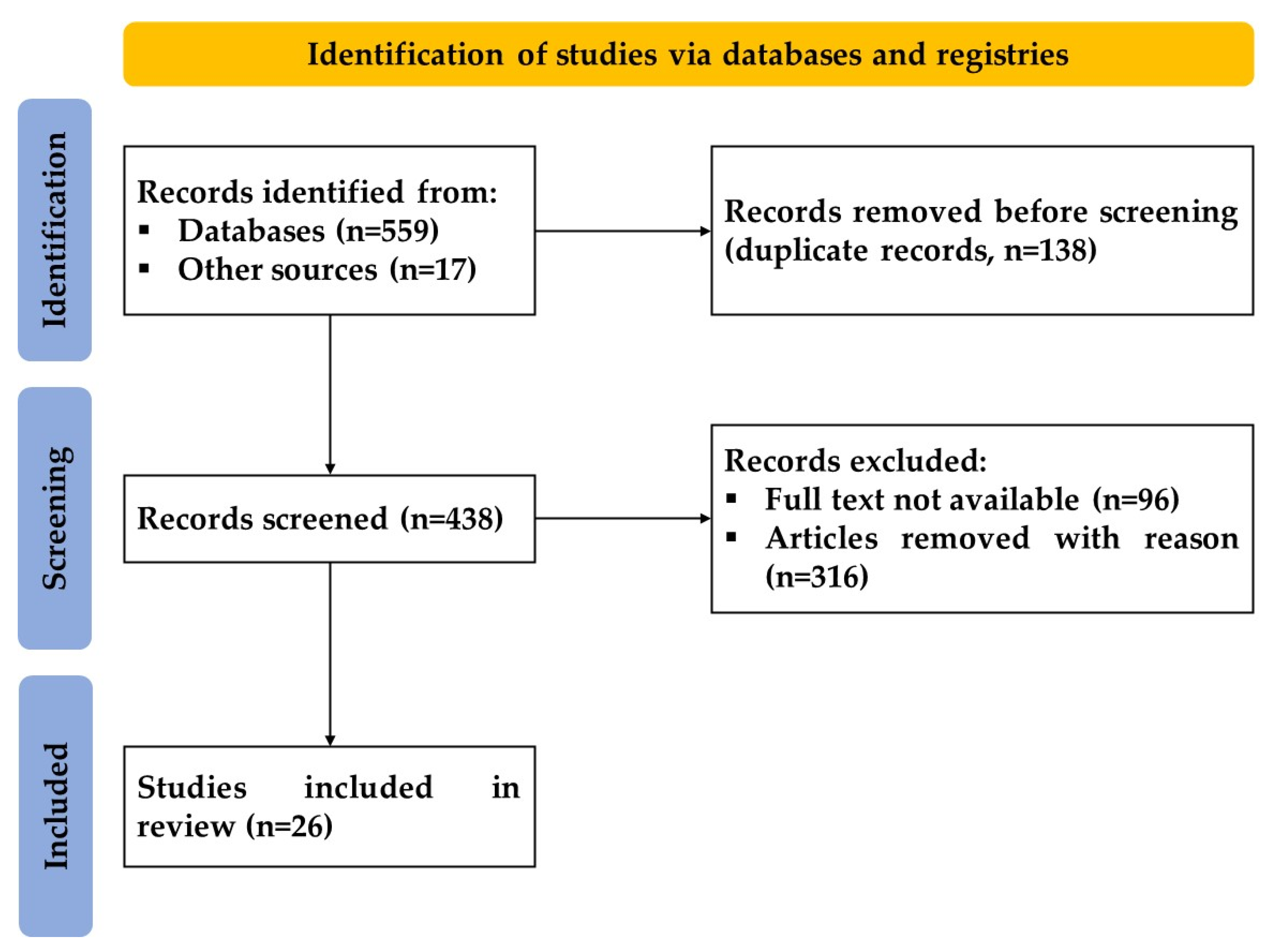
2. Materials and Methods
3. Results
3.1. Umbilical Cord
3.1.1. Preclinical Studies
3.1.2. Clinical Studies
3.2. Amniotic Suspension Allograft
3.2.1. Preclinical Studies
3.2.2. Clinical Studies
3.3. Amniotic Membrane
3.3.1. Preclinical Studies
3.3.2. Clinical Studies
3.4. Amniotic Fluid
3.4.1. Preclinical Studies
3.4.2. Clinical Studies
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Musculoskeletal Pain: Types, Causes, Symptoms & Treatment. Available online: https://my.clevelandclinic.org/health/diseases/14526-musculoskeletal-pain (accessed on 9 November 2022).
- Middlesworth, M. The Cost of Musculoskeletal Disorders (MSDs) [Infographic]. Available online: https://ergo-plus.com/cost-of-musculoskeletal-disorders-infographic/ (accessed on 9 November 2022).
- Gupta, A. Allogenic Amniotic Tissue for Treatment of Knee and Hip Osteoarthritis. Pharmaceuticals 2022, 15, 404. [Google Scholar] [CrossRef]
- Gupta, A.; Maffulli, N.; Rodriguez, H.C.; Mistovich, R.J.; Delfino, K.; Cady, C.; Fauser, A.-M.; Cundiff, E.D.; Martinez, M.A.; Potty, A.G. Cell-Free Stem Cell-Derived Extract Formulation for Treatment of Knee Osteoarthritis: Study Protocol for a Preliminary Non-Randomized, Open-Label, Multi-Center Feasibility and Safety Study. J. Orthop. Surg. Res. 2021, 16, 514. [Google Scholar] [CrossRef]
- Viscosupplementation Treatment for Knee Arthritis-OrthoInfo-AAOS. Available online: https://orthoinfo.aaos.org/en/treatment/viscosupplementation-treatment-for-knee-arthritis/#:~:text=In%20this%20procedure%2C%20a%20gel,shock%20absorber%20for%20joint%20loads (accessed on 9 November 2022).
- Gupta, A.; Maffulli, N. Allogenic umbilical cord tissue treatment of knee osteoarthritis. Sports Med. Arthrosc. Rev. 2022, 30, 162–165. [Google Scholar] [CrossRef]
- Gupta, A.; Jeyaraman, M.; Maffulli, N. Common Medications Which Should Be Stopped Prior to Platelet-Rich Plasma Injection. Biomedicines 2022, 10, 2134. [Google Scholar] [CrossRef]
- Oliva, F. Viscosupplementation with Intra-Articular Hyaluronic Acid for Hip Disorders. A Systematic Review and Meta-Analysis. Muscles Ligaments Tendons J. 2016, 16, 631–640. [Google Scholar] [CrossRef]
- Williams, R.S. NSAIDs: When to Use Them, and How They Help Inflammation. Available online: https://www.coastalorthoteam.com/blog/nsaids-when-to-use-them-and-how-they-help-inflammation (accessed on 9 November 2022).
- Martin, C.L.; Browne, J.A. Intra-Articular Corticosteroid Injections for Symptomatic Knee Osteoarthritis. J. Am. Acad. Orthop. Surg. 2019, 27, e758–e766. [Google Scholar] [CrossRef]
- Orchard, J.W. Pay attention to the evidence: In the longer term, intraarticular corticosteroid injections offer only harm for knee osteoarthritis. Osteoarthr. Cartil. 2022, S1063-4584(22)00891-3. [Google Scholar] [CrossRef]
- Burnett, R.A.; Khalid, S.; DeBenedetti, A.; Terhune, E.B.; Angotti, M.L.; Valle, C.J.D. Intra-articular corticosteroid injections are associated with a dose-dependent risk of total knee arthroplasty at 5 years. Knee Surg. Sport. Traumatol. Arthrosc. 2022. [Google Scholar] [CrossRef]
- Trasolini, N.A.; McKnight, B.M.; Dorr, L.D. The Opioid Crisis and the Orthopedic Surgeon. J. Arthroplast. 2018, 33, 3379–3382.e1. [Google Scholar] [CrossRef]
- Gupta, A.; El-Amin, S.F.; Levy, H.J.; Sze-Tu, R.; Ibim, S.E.; Maffulli, N. Umbilical Cord-Derived Wharton’s Jelly for Regenerative Medicine Applications. J. Orthop. Surg. Res. 2020, 15, 49. [Google Scholar] [CrossRef]
- Navani, A.; Manchikanti, L.; Albers, S.L.; Latchaw, R.E.; Sanapati, J.; Kaye, A.D.; Atluri, S.; Jordan, S.; Gupta, A.; Cedeno, D.; et al. Responsible, Safe, and Effective Use of Biologics in the Management of Low Back Pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines. Pain Physician 2019, 22, S1–S74. [Google Scholar] [PubMed]
- Center for Regenerative Biotherapeutics-about Regenerative Medicine. Available online: https://www.mayo.edu/research/centers-programs/center-regenerative-biotherapeutics/about/about-regenerative-medicine (accessed on 9 November 2022).
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z. Mesenchymal Stem Cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Andia, I.; Zumstein, M.A.; Zhang, C.-Q.; Pinto, N.R.; Bielecki, T. Classification of Platelet Concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for Topical and Infiltrative Use in Orthopedic and Sports Medicine: Current Consensus, Clinical Implications and Perspectives. Muscles Ligaments Tendons J. 2014, 4, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Filomeno, P.; Dayan, V.; Touriño, C. Stem Cell Research and Clinical Development in Tendon Repair. Muscles Ligaments Tendons J. 2012, 2, 204–211. [Google Scholar]
- Mardones, R. Cell Therapy for Cartilage Defects of the Hip. Muscles Ligaments Tendons J. 2016, 6, 361–366. [Google Scholar] [CrossRef]
- Middleton, K.K. Evaluation of the Effects of Platelet-Rich Plasma (PRP) Therapy Involved in the Healing of Sports-Related Soft Tissue Injuries. Iowa Orthop. J. 2012, 32, 150. [Google Scholar]
- Kapucu, S.; Karacan, Y. Physiological Problems in Patients Undergoing Autologous and Allogeneic Hematopoietic Stem Cell Transplantation. Asia-Pac. J. Oncol. Nurs. 2014, 1, 50–54. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Boyd, N.L. The Adipose Stromal Vascular Fraction as a Complex Cellular Source for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2018, 24, 289–299. [Google Scholar] [CrossRef]
- Senesi, L.; De Francesco, F.; Farinelli, L.; Manzotti, S.; Gagliardi, G.; Papalia, G.F.; Riccio, M.; Gigante, A. Mechanical and Enzymatic Procedures to Isolate the Stromal Vascular Fraction from Adipose Tissue: Preliminary Results. Front. Cell Dev. Biol. 2019, 7, 88. [Google Scholar] [CrossRef]
- Pak, J.; Lee, J.H.; Park, K.S.; Park, M.; Kang, L.-W.; Lee, S.H. Current Use of Autologous Adipose Tissue-Derived Stromal Vascular Fraction Cells for Orthopedic Applications. J. Biomed. Sci. 2017, 24, 9. [Google Scholar] [CrossRef]
- Malogolowkin, M.H.; Hemmer, M.T.; Le-Rademacher, J.; Hale, G.A.; Mehta, P.A.; Smith, A.R.; Kitko, C.; Abraham, A.; Abdel-Azim, H.; Dandoy, C.; et al. Outcomes Following Autologous Hematopoietic Stem Cell Transplant for Patients with Relapsed Wilms’ Tumor: A CIBMTR Retrospective Analysis. Bone Marrow Transplant. 2017, 52, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Autologous Stem Cell Transplant: A Guide for Patients & Caregivers. Available online: https://www.mskcc.org/cancer-care/patient-education/autologous-stem-cell-transplant-guide-patients-caregivers (accessed on 9 November 2022).
- Andersen, B.L.; Golden-Kreutz, D.M. Cancer. In Comprehensive Clinical Psychology; Elsevier: Amsterdam, The Netherlands, 1998; pp. 217–236. [Google Scholar]
- Zhang, Y.; Ni, M.; Zhou, C.; Wang, Y.; Wang, Y.; Shi, Y.; Jin, J.; Zhang, R.; Jiang, B. Autologous Adipose-Derived Stem Cells for the Treatment of Complex Cryptoglandular Perianal Fistula: A Prospective Case-Control Study. Stem Cell Res. Ther. 2020, 11, 475. [Google Scholar] [CrossRef]
- Aratikatla, A.; Maffulli, N.; Rodriguez, H.C.; Gupta, M.; Potty, A.G.; El-Amin, S.F.; Gupta, A. Allogenic Perinatal Tissue for Musculoskeletal Regenerative Medicine Applications: A Systematic Review Protocol. J. Orthop. Surg. Res. 2022, 17, 307. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zhang, Y.; Gao, L.; Jiang, S.; Ruan, D. Human Wharton’s Jelly Cells Activate Degenerative Nucleus Pulposus Cells in Vitro. Tissue Eng. Part A 2018, 24, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, X.; Qiao, S.; Liu, X.; Liu, C.; Zhu, D.; Su, J.; Wang, Z. Effects of Wharton’s Jelly Cells of the Human Umbilical Cord on Acute Spinal Cord Injury in Rats, and Expression of Interleukin-1β and Nerve Growth Factor in Spinal Cord Tissues. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1254–1258. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, H.; Gu, T.; Zhou, M.; Jia, Z.; Jiang, G.; Chen, C.; Han, Z.; Xu, C.; Wang, D.; et al. The Effects of Human Wharton’s Jelly Cell Transplantation on the Intervertebral Disc in a Canine Disc Degeneration Model. Stem Cell Res. Ther. 2015, 6, 154. [Google Scholar] [CrossRef]
- Shalaby, S.M.; El-Shal, A.S.; Ahmed, F.E.; Shaban, S.F.; Wahdan, R.A.; Kandel, W.A.; Senger, M.S. Combined Wharton’s Jelly Derived Mesenchymal Stem Cells and Nerve Guidance Conduit: A Potential Promising Therapy for Peripheral Nerve Injuries. Int. J. Biochem. Cell Biol. 2017, 86, 67–76. [Google Scholar] [CrossRef]
- Sofia, V.; Nasrul, E.; Manjas, M.; Revilla, G. The Influence of Wharton Jelly Mesenchymal Stem Cell toward Matrix Metalloproteinase-13 and RELA Synoviocyte Gene Expression on Osteoarthritis. Open Access Maced. J. Med. Sci. 2019, 7, 701–706. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Guo, W.; Wang, M.; Hao, C.; Gao, S.; Zhang, X.; Li, X.; Chen, M.; Jing, X.; et al. Human Umbilical Cord Wharton’s Jelly Mesenchymal Stem Cells Combined with an Acellular Cartilage Extracellular Matrix Scaffold Improve Cartilage Repair Compared with Microfracture in a Caprine Model. Osteoarthr. Cartil. 2018, 26, 954–965. [Google Scholar] [CrossRef]
- Shim, J.; Kim, K.-T.; Kim, K.G.; Choi, U.-Y.; Kyung, J.W.; Sohn, S.; Lim, S.H.; Choi, H.; Ahn, T.-K.; Choi, H.J.; et al. Safety and Efficacy of Wharton’s Jelly-Derived Mesenchymal Stem Cells with Teriparatide for Osteoporotic Vertebral Fractures: A Phase I/IIa Study. Stem Cells Transl. Med. 2020, 10, 554–567. [Google Scholar] [CrossRef]
- Günay, A.E.; Karaman, I.; Guney, A.; Karaman, Z.F.; Demirpolat, E.; Gonen, Z.B.; Dogan, S.; Yerer, M.B. Assessment of Clinical, Biochemical, and Radiological Outcomes Following Intra-Articular Injection of Wharton Jelly-Derived Mesenchymal Stromal Cells in Patients with Knee Osteoarthritis: A Prospective Clinical Study. Medicine 2022, 101, e30628. [Google Scholar] [CrossRef] [PubMed]
- Samara, O.; Jafar, H.; Hamdan, M.; Al-Ta’mari, A.; Rahmeh, R.; Hourani, B.; Mandalawi, N.; Awidi, A. Ultrasound-Guided Intra-Articular Injection of Expanded Umbilical Cord Mesenchymal Stem Cells in Knee Osteoarthritis: A Safety/Efficacy Study with MRI Data. Regen. Med. 2022, 17, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rodriguez, H.C.; Potty, A.G.; Levy, H.J.; El-Amin III, S.F. Treatment of Knee Osteoarthritis with Intraarticular Umbilical Cord-Derived Wharton’s Jelly: A Case Report. Pharmaceuticals 2021, 14, 883. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-C.; Park, Y.-B.; Ha, C.-W.; Cole, B.J.; Lee, B.-K.; Jeong, H.-J.; Kim, M.-K.; Bin, S.-I.; Choi, C.-H.; Choi, C.H.; et al. Allogeneic Umbilical Cord Blood–Derived Mesenchymal Stem Cell Implantation versus Microfracture for Large, Full-Thickness Cartilage Defects in Older Patients: A Multicenter Randomized Clinical Trial and Extended 5-Year Clinical Follow-Up. Orthop. J. Sport. Med. 2021, 9, 232596712097305. [Google Scholar] [CrossRef] [PubMed]
- Mead, O.G.; Mead, L.P. Intra-Articular Injection of Amniotic Membrane and Umbilical Cord Particulate for the Management of Moderate to Severe Knee Osteoarthritis. Orthop. Res. Rev. 2020, 12, 161–170. [Google Scholar] [CrossRef]
- Castellanos, R.; Tighe, S. Injectable Amniotic Membrane/Umbilical Cord Particulate for Knee Osteoarthritis: A Prospective, Single-Center Pilot Study. Pain Med. 2019, 20, 2283–2291. [Google Scholar] [CrossRef]
- Kimmerling, K.A.; Gomoll, A.H.; Farr, J.; Mowry, K.C. Amniotic Suspension Allograft Modulates Inflammation in a Rat Pain Model of Osteoarthritis. J. Orthop. Res. 2019, 38, 1141–1149. [Google Scholar] [CrossRef]
- Vines, J.; Aliprantis, A.; Gomoll, A.; Farr, J. Cryopreserved Amniotic Suspension for the Treatment of Knee Osteoarthritis. J. Knee Surg. 2015, 29, 443–450. [Google Scholar] [CrossRef]
- Farr, J.; Gomoll, A.H.; Yanke, A.B.; Strauss, E.J.; Mowry, K.C. A Randomized Controlled Single-Blind Study Demonstrating Superiority of Amniotic Suspension Allograft Injection over Hyaluronic Acid and Saline Control for Modification of Knee Osteoarthritis Symptoms. J. Knee Surg. 2019, 32, 1143–1154. [Google Scholar] [CrossRef]
- Meadows, M.C.; Elisman, K.; Nho, S.J.; Mowry, K.; Safran, M.R. A Single Injection of Amniotic Suspension Allograft Is Safe and Effective for Treatment of Mild to Moderate Hip Osteoarthritis: A Prospective Study. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 325–331. [Google Scholar] [CrossRef]
- Willett, N.J.; Thote, T.; Lin, A.S.; Moran, S.; Raji, Y.; Sridaran, S.; Stevens, H.Y.; Guldberg, R.E. Intra-Articular Injection of Micronized Dehydrated Human Amnion/Chorion Membrane Attenuates Osteoarthritis Development. Arthritis Res. Ther. 2014, 16, R47. [Google Scholar] [CrossRef] [PubMed]
- Marino-Martinez, I.; Martinez-Castro, A.; Pena-Martinez, V.; Acosta-Olivo, C.; Vilchez-Cavazos, F.; Guzman-Lopez, A.; Perez Rodriguez, E.; Romero-Diaz, V.; Ortega-Blanco, J.; Lara-Arias, J. Human Amniotic Membrane Intra-Articular Injection Prevents Cartilage Damage in an Osteoarthritis Model. Exp. Ther. Med. 2018, 17, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Reece, D.S.; Burnsed, O.A.; Parchinski, K.; Marr, E.E.; White, R.M.; Salazar-Noratto, G.E.; Lin, A.S.P.; Willett, N.J.; Guldberg, R.E. Reduced Size Profile of Amniotic Membrane Particles Decreases Osteoarthritis Therapeutic Efficacy. Tissue Eng. Part A 2020, 26, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Tabet, S.K.; Kimmerling, K.A.; Hale, G.J.; Munson, N.R.; Mowry, K.C. Hypothermically Stored Amniotic Membrane for the Treatment of Cartilage Lesions: A Single-Arm Prospective Study with 2-Year Follow-Up. CARTILAGE 2022, 13, 194760352110722. [Google Scholar] [CrossRef] [PubMed]
- Scala, V.A.; Kikuchi, C.K. Sesamoid Avascular Necrosis and Stress Fracture Treated with Core Decompression and Biologic Augmentation. Hawai’i J. Health Soc. Welf. 2022, 81, 16–18. [Google Scholar]
- Liu, C.; Bai, J.; Yu, K.; Liu, G.; Tian, S.; Tian, D. Biological Amnion Prevents Flexor Tendon Adhesion in Zone II: A Controlled, Multicentre Clinical Trial. BioMed Res. Int. 2019, 2019, 2354325. [Google Scholar] [CrossRef]
- Basile, M.; Marchegiani, F.; Novak, S.; Kalajzic, I.; Di Pietro, R. Human Amniotic Fluid Stem Cells Attract Osteoprogenitor Cells in Bone Healing. J. Cell. Physiol. 2019, 235, 4643–4654. [Google Scholar] [CrossRef]
- Oner, M.; Dulgeroglu, T.C.; Karaman, I.; Guney, A.; Kafadar, I.H.; Erdem, S. The Effects of Human Amniotic Fluid and Different Bone Grafts on Vertebral Fusion in an Experimental Rat Model. Curr. Ther. Res. 2015, 77, 35–39. [Google Scholar] [CrossRef][Green Version]
- Maraldi, T.; Riccio, M.; Pisciotta, A.; Zavatti, M.; Carnevale, G.; Beretti, F.; La Sala, G.B.; Motta, A.; De Pol, A. Human Amniotic Fluid-Derived and Dental Pulp-Derived Stem Cells Seeded into Collagen Scaffold Repair Critical-Size Bone Defects Promoting Vascularization. Stem Cell Res. Ther. 2013, 4, 53. [Google Scholar] [CrossRef]
- Gupta, A. Amniotic Suspension Allograft for Treatment of Knee Osteoarthritis. Biomedicines 2022, 10, 2658. [Google Scholar] [CrossRef]
- Lei, J.; Priddy, L.B.; Lim, J.J.; Massee, M.; Koob, T.J. Identification of Extracellular Matrix Components and Biological Factors in Micronized Dehydrated Human Amnion/Chorion Membrane. Adv. Wound Care 2017, 6, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Kimmerling, K.A.; McQuilling, J.P.; Staples, M.C.; Mowry, K.C. Tenocyte Cell Density, Migration, and Extracellular Matrix Deposition with Amniotic Suspension Allograft. J. Orthop. Res. 2018, 37, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.R. The Collagens of Articular Cartilage. Semin. Arthritis Rheum. 1991, 21, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, S.; Huang, J.; Guo, W.; Chen, J.; Zhang, L.; Zhao, B.; Peng, J.; Wang, A.; Wang, Y.; et al. The ECM-Cell Interaction of Cartilage Extracellular Matrix on Chondrocytes. BioMed Res. Int. 2014, 2014, 648459. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.D.; Hambardzumyan, V.; Patel, N.G.; Giacobbe, S.D.; Gesheff, M.G. Immunological Evaluation of Patients with Orthopedic Infections: Taking the Cierny–Mader Classification to the next Level. J. Bone Jt. Infect. 2021, 6, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Gomoll, A.H.; Farr, J.; Cole, B.J.; Flanigan, D.C.; Lattermann, C.; Mandelbaum, B.R.; Strickland, S.M.; Zaslav, K.R.; Kimmerling, K.A.; Mowry, K.C. Safety and Efficacy of an Amniotic Suspension Allograft Injection over 12 Months in a Single-Blinded, Randomized Controlled Trial for Symptomatic Osteoarthritis of the Knee. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 2246–2257. [Google Scholar] [CrossRef]
- Natali, S.; Farinelli, L.; Screpis, D.; Trojan, D.; Montagner, G.; Favaretto, F.; Zorzi, C. Human Amniotic Suspension Allograft Improves Pain and Function in Knee Osteoarthritis: A Prospective Not Randomized Clinical Pilot Study. J. Clin. Med. 2022, 11, 3295. [Google Scholar] [CrossRef]
- Serafini, A.; Riello, E.; Trojan, D.; Cogliati, E.; Palù, G.; Manganelli, R.; Paolin, A. Evaluation of New Antibiotic Cocktails against Contaminating Bacteria Found in Allograft Tissues. Cell Tissue Bank. 2016, 17, 619–628. [Google Scholar] [CrossRef]
- Montagner, G.; Trojan, D.; Cogliati, E.; Manea, F.; Vantini, A.; Paolin, A. Stability Analysis of the Antibiotic Cocktail Used by Treviso Tissue Bank Foundation for Tissues Decontamination. Cell Tissue Bank. 2018, 19, 721–726. [Google Scholar] [CrossRef]
- Gaggi, G.; Di Credico, A.; Izzicupo, P.; Sancilio, S.; Di Mauro, M.; Iannetti, G.; Dolci, S.; Amabile, G.; Di Baldassarre, A.; Ghinassi, B. Decellularized Extracellular Matrices and Cardiac Differentiation: Study on Human Amniotic Fluid-Stem Cells. Int. J. Mol. Sci. 2020, 21, 6317. [Google Scholar] [CrossRef]
- Ma, B.; Wang, T.; Li, J.; Wang, Q. Extracellular Matrix Derived from Wharton’s Jelly-Derived Mesenchymal Stem Cells Promotes Angiogenesis via Integrin AVβ3/C-Myc/P300/VEGF. Stem Cell Res. Ther. 2022, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Valiyaveettil, M.; Achur, R.N.; Muthusamy, A.; Gowda, D.C. Characterization of Chondroitin Sulfate and Dermatan Sulfate Proteoglycans of Extracellular Matrices of Human Umbilical Cord Blood Vessels and Wharton’s Jelly. Glycoconj. J. 2004, 21, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Franc, S.; Rousseau, J.-C.; Garrone, R.; van der Rest, M.; Moradi-Améli, M. Microfibrillar Composition of Umbilical Cord Matrix: Characterization of Fibrillin, Collagen vi and Intact Collagen V. Placenta 1998, 19, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Pennati, G. Biomechanical Properties of the Human Umbilical Cord. Biorheology 2014, 38, 355–366. [Google Scholar]
- Yun, M. Changes in Regenerative Capacity through Lifespan. Int. J. Mol. Sci. 2015, 16, 25392–25432. [Google Scholar] [CrossRef]
- Oh, J.; Lee, Y.D.; Wagers, A.J. Stem Cell Aging: Mechanisms, Regulators and Therapeutic Opportunities. Nat. Med. 2014, 20, 870–880. [Google Scholar] [CrossRef]
- Pérez, L.M.; de Lucas, B.; Gálvez, B.G. Unhealthy Stem Cells: When Health Conditions Upset Stem Cell Properties. Cell. Physiol. Biochem. 2018, 46, 1999–2016. [Google Scholar] [CrossRef]
- Oñate, B.; Vilahur, G.; Ferrer-Lorente, R.; Ybarra, J.; Díez-Caballero, A.; Ballesta-López, C.; Moscatiello, F.; Herrero, J.; Badimon, L. The Subcutaneous Adipose Tissue Reservoir of Functionally Active Stem Cells Is Reduced in Obese Patients. FASEB J. 2012, 26, 4327–4336. [Google Scholar] [CrossRef]
- Cramer, C.; Freisinger, E.; Jones, R.K.; Slakey, D.P.; Dupin, C.L.; Newsome, E.R.; Alt, E.U.; Izadpanah, R. Persistent High Glucose Concentrations Alter the Regenerative Potential of Mesenchymal Stem Cells. Stem Cells Dev. 2010, 19, 1875–1884. [Google Scholar] [CrossRef]
| Study Identifier | Tissue Type/Biologic Used | Study Phase; Estimated Enrolment [N] | Condition | Primary Outcome Measures | Recruitment Status | Country |
|---|---|---|---|---|---|---|
| NCT05160831 | Human umbilical cord mesenchymal stem cells | Not Applicable; 50 | Knee OA | VAS score, Kellgren-Lawrence score | Not yet recruiting | USA |
| NCT04414592 | Human umbilical cord mesenchymal stem cells | Not Applicable; 20 | Lumbar Disc Degeneration and Herniation | Lumbar disc signaling values from magnetic resonance imaging, VAS, ODI | Recruiting | China |
| NCT04719793 | Umbilical cord derived Wharton’s Jelly | Early Phase 1; 12 | Knee OA | Treatment-emergent adverse effects as assessed by Comprehensive Metabolic Profile, creatinine levels, LFT, FBC, CRP, ESR, T, B and NK cell lymphocyte subsets, serum IgG, IgA, Ig M, and IgE at 1 week, 6 weeks, 3 months, 6 months, 1 year. | Not yet recruiting | USA |
| NCT04234412 | Umbilical cord blood-derived stem cell | Not Applicable; 10 | Knee OA | International Cartilage Repair Society (ICRS) grade improvement, VAS, WOMAC, and IKDC score | Not yet recruiting | South Korea |
| NCT05152381 | Umbilical cord derived MSCs (AlloRX) | Phase I; 20 | Osteoporosis | Safety (adverse events) | Recruiting | Antigua and Barbuda |
| NCT04711304 | Umbilical cord derived Wharton’s Jelly | Phase I/II; 168 | Knee OA | Adverse or severe adverse events and patients satisfaction associated with IA administration of UC-WJ, and Change in patient reported outcome measures, NPRS | Not yet recruiting | USA |
| NCT03383081 | Human umbilical cord derived mesenchymal stem cells (hUC-MSCs) | Phase II; 60 | Knee OA | Kellgren-Lawrence Grading Scale, Assessment of Preoperative Cartilage Defect Severity (AMADEUS), and Lysholm scoring | Recruiting | China |
| NCT04339504 | Allogeneic umbilical cord blood-derived mesenchymal stem cells | Phase I; 12 | Knee OA | Change of total score in WOMAC (Western Ontario and McMaster University) and its subscales, VAS | Recruiting | South Korea |
| NCT05234489 | Human Umbilical Cord (Signature Cord Prime) | Phase 1; 10 | Knee OA | Evaluate the safety and tolerability of Signature Cord Prime as defined per CTCAE v. Stopping criteria as defined in 6.11.2.8 Exploratory objective to observe for early data suggestive of efficacy by estimating and comparing changes from baseline | Not yet recruiting | USA |
| NCT04971980 | Human umbilical cord-mesenchymal stem cell infusion | Phase I/II; 9 | Rheumatoid Arthritis | Number and frequency of adverse events, changes of vital signs from 1 h after infusion to day 28 ± 3, changes of complete blood count (CBC), blood biochemical, and coagulation function, from day 1 to day 28 ± 3. Additionally, routine urine analysis, urine pregnancy test, and cardiac rate measured by 12-lead ECG. | Recruiting | China |
| NCT05147675 | Umbilical cord derived MSCs (AlloRX) | Phase I; 20 | Osteoarthritis, Spinal Arthritis | Safety (adverse events) Efficacy: Single Assessment Numeric Evaluation Score (SANE) | Recruiting | Antigua and Barbuda |
| NCT03828344 | Human umbilical cord-mesenchymal stem cells suspension | Phase I; 16 | Rheumatoid Arthritis | Percentage of participants achieving ACR20, ACR50, and ACR 70 from Baseline at Week 12 and Week 24. Change from Baseline in the DAS28-CRP, HAQ-DI, rheumatoid factor, and anti-CCP at Week 12 and 24. Percentage of participants acquiring remission by SDAI based criteria at Week 12 and 24. | Not yet recruiting | USA |
| NCT05000593 | Umbilical Cord Blood-Mononuclear Cells | Not Applicable; 60 | Knee OA | Lysholm, American knee society knee, the knee injury and osteoarthritis, and VAS for pain scores | Recruiting | China |
| NCT04863183 | UC-WJ’s MSCs | Phase I/II; 30 | Knee OA | Decrease in joint pain, Increased joint functionality, Improvement in the quality of life, and Imaging improvement of articular cartilage | Not yet recruiting | Colombia |
| NCT03390920 | Umbilical Allograft | Not Applicable; 200 | OA, Tendinitis, Sports Injury | Short Musculoskeletal Function Assessment Questionnaire (SMFA), Work Status, and (VAS) | Not yet recruiting | USA |
| NCT02963727 | Wharton Jelly Derived MSC | Phase I; 10 | Knee OA | Evaluation of the safety and tolerability of the intra articular injection and assessment of the efficacy of intra-articular injection of WJMSC | Recruiting | Jordan |
| NCT05016011 | Human Umbilical cord derived mesenchymal stem cells (hUC-MSCs) | Phase II; 50 | Knee OA | Recording of Adverse Events and Serious Adverse Events, International Knee Documentation Committee (IKDC) score, (KOOS) | Recruiting | Malaysia |
| Study Identifier | Tissue Type/Biologic Used | Study Phase; Estimated Enrolment [N] | Condition | Primary Outcome Measures | Recruitment Status | Country |
|---|---|---|---|---|---|---|
| NCT04636229 | Amniotic Suspension Allograft | Phase III; 474 | Knee OA | The difference in change from baseline to Week 26 in WOMAC Pain scale between ASA- and placebo-treated patients. | Recruiting | USA |
| NCT04698265 | Amniotic Suspension Allograft | Not Applicable; >150 | Knee OA | Change of the Western Ontario and McMaster Universities Arthritis Index (WOMAC) between baseline, 1 week, and 1, 3, 6, 12 months. | Not yet recruiting | Taiwan |
| Study Identifier | Tissue Type/Biologic Used | Study Phase; Estimated Enrolment [N] | Condition | Primary Outcome Measures | Recruitment Status | Country |
|---|---|---|---|---|---|---|
| NCT05092646 | Amniotic Membrane | Phase I/II; 48 | Ankle Osteoarthritis | Proportion of patients achieving Composite Clinical Success at 4 weeks, 3 months, and 6 months | Recruiting | USA |
| NCT03899298 | Amniotic and Umbilical Cord Tissue | Phase I; 5000 | Orthopedic Disorders and Arthritis | Disabilities of Arm, Shoulder, Hand (DASH), O’Leary/Sant, and Oswestry Low Back Pain Disability Questionnaires, and WOMAC Osteoarthritis Index | Not yet recruiting | Mexico and Pakistan |
| NCT04612023 | Acellular Amniotic Membrane Derived Allograft Injection | Phase II; 90 | Knee OA | KOOS, WOMAC, and VAS | Recruiting | USA |
| NCT04967963 | Amniotic Membrane | Not Applicable; 14 | Medication Related, Osteonecrosis of the Jaw | Change in mucosal coverage | Active, not recruiting | Turkiye |
| NCT05320419 | Amniotic Membrane | Not Applicable; 40 | Rotator Cuff Tear and Tendinopathy | VAS | Not yet recruiting | Taiwan |
| NCT05079035 | Lyophilized and Micronized Particulate Human Amniotic and Umbilical Cord | Phase II; 90 | Knee OA, Chronic Pain, and Arthritis | Pain Relief and/or Functional Improvement | Recruiting | USA |
| Study Identifier | Tissue Type/Biologic Used | Study Phase; Estimated Enrolment [N] | Condition | Primary Outcome Measures | Recruitment Status | Country |
|---|---|---|---|---|---|---|
| NCT04886960 | Amniotic Fluid | Phase I/II; 60 | Knee OA | Whether or not participants in either group may require a repeat injection within 6 months. | Recruiting | USA |
| NCT04537026 | Amniotic Fluid | phase I/II; 112 | Lumbar Spinal Stenosis | Number of adverse reactions, the percentage of patients stating a >50% improvement in NRS pain scores, and percentage of patients stating a >30% improvement in SSSQ scores. | Recruiting | USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aratikatla, A.; Maffulli, N.; Rodriguez, H.C.; Gupta, M.; Potty, A.G.; Gupta, A. Allogenic Perinatal Tissue for Musculoskeletal Regenerative Medicine Applications: A Systematic Review. Biomedicines 2022, 10, 3173. https://doi.org/10.3390/biomedicines10123173
Aratikatla A, Maffulli N, Rodriguez HC, Gupta M, Potty AG, Gupta A. Allogenic Perinatal Tissue for Musculoskeletal Regenerative Medicine Applications: A Systematic Review. Biomedicines. 2022; 10(12):3173. https://doi.org/10.3390/biomedicines10123173
Chicago/Turabian StyleAratikatla, Adarsh, Nicola Maffulli, Hugo C. Rodriguez, Manu Gupta, Anish G. Potty, and Ashim Gupta. 2022. "Allogenic Perinatal Tissue for Musculoskeletal Regenerative Medicine Applications: A Systematic Review" Biomedicines 10, no. 12: 3173. https://doi.org/10.3390/biomedicines10123173
APA StyleAratikatla, A., Maffulli, N., Rodriguez, H. C., Gupta, M., Potty, A. G., & Gupta, A. (2022). Allogenic Perinatal Tissue for Musculoskeletal Regenerative Medicine Applications: A Systematic Review. Biomedicines, 10(12), 3173. https://doi.org/10.3390/biomedicines10123173









