Axonal Regeneration: Underlying Molecular Mechanisms and Potential Therapeutic Targets
Abstract
:1. Introduction
2. CNS vs. PNS Regeneration
3. Barriers to Regeneration
4. Epigenetic Modifications and Axonal Regeneration
5. Molecular Pathways and Therapeutic Targets
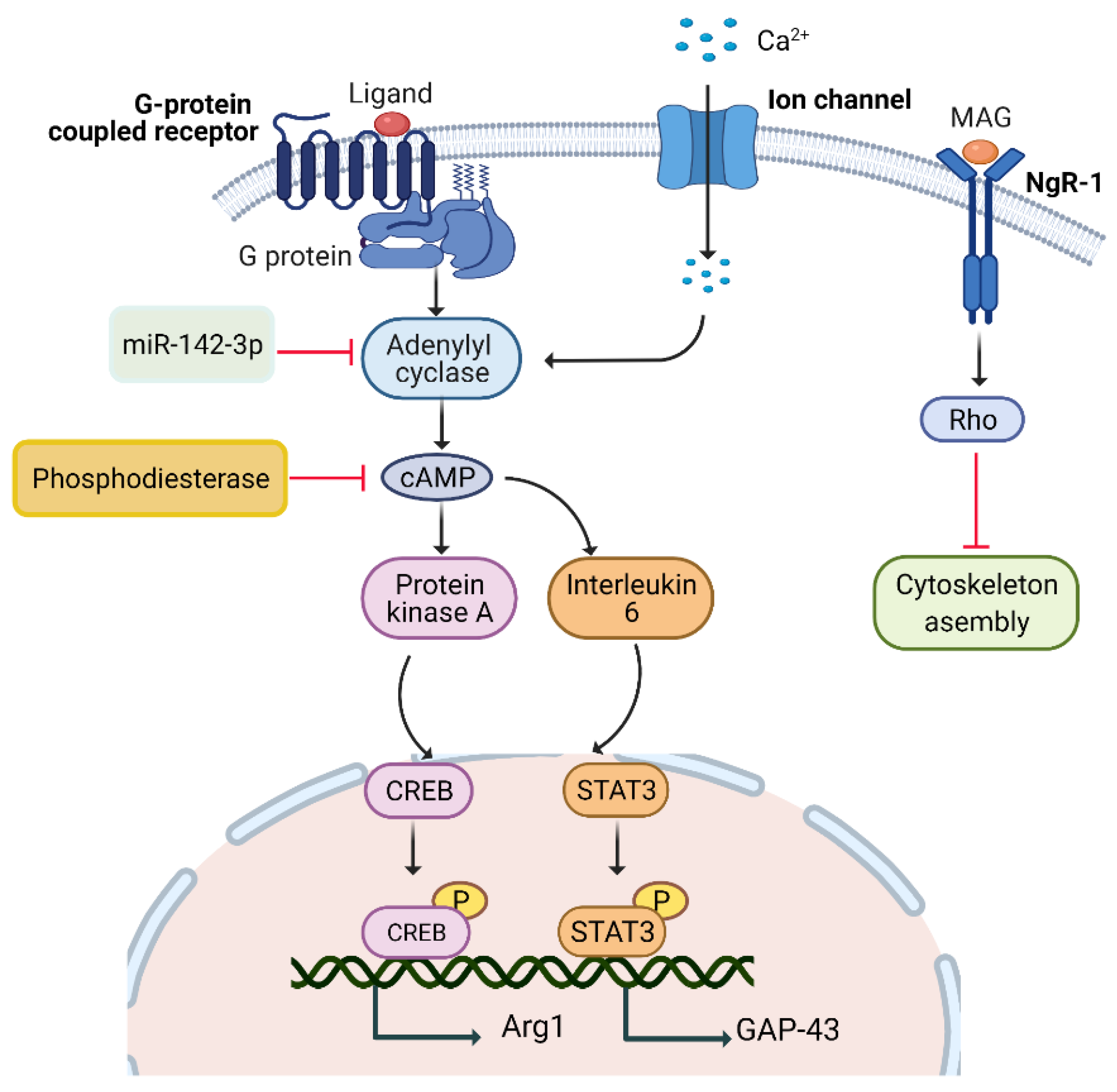
5.1. Rapid Injury Signals
5.1.1. cAMP Pathway
5.1.2. Calcium/cAMP Pathway
5.1.3. IL-6/cAMP Pathway
5.1.4. PKA/cAMP Pathway
5.1.5. miR-142-3p/AC9/cAMP Pathway
5.1.6. Pharmacological Agents
5.2. Delayed Injury Signals
5.2.1. DLK-JNK MAPK Pathway
5.2.2. Pharmacological Agents
5.3. Transcriptional Factors’ Mediated Pathways
5.3.1. JAK/STAT Pathway
5.3.2. ATF3/CREB Pathway
5.3.3. BMP/SMAD Pathway
5.3.4. Pharmacological Agents
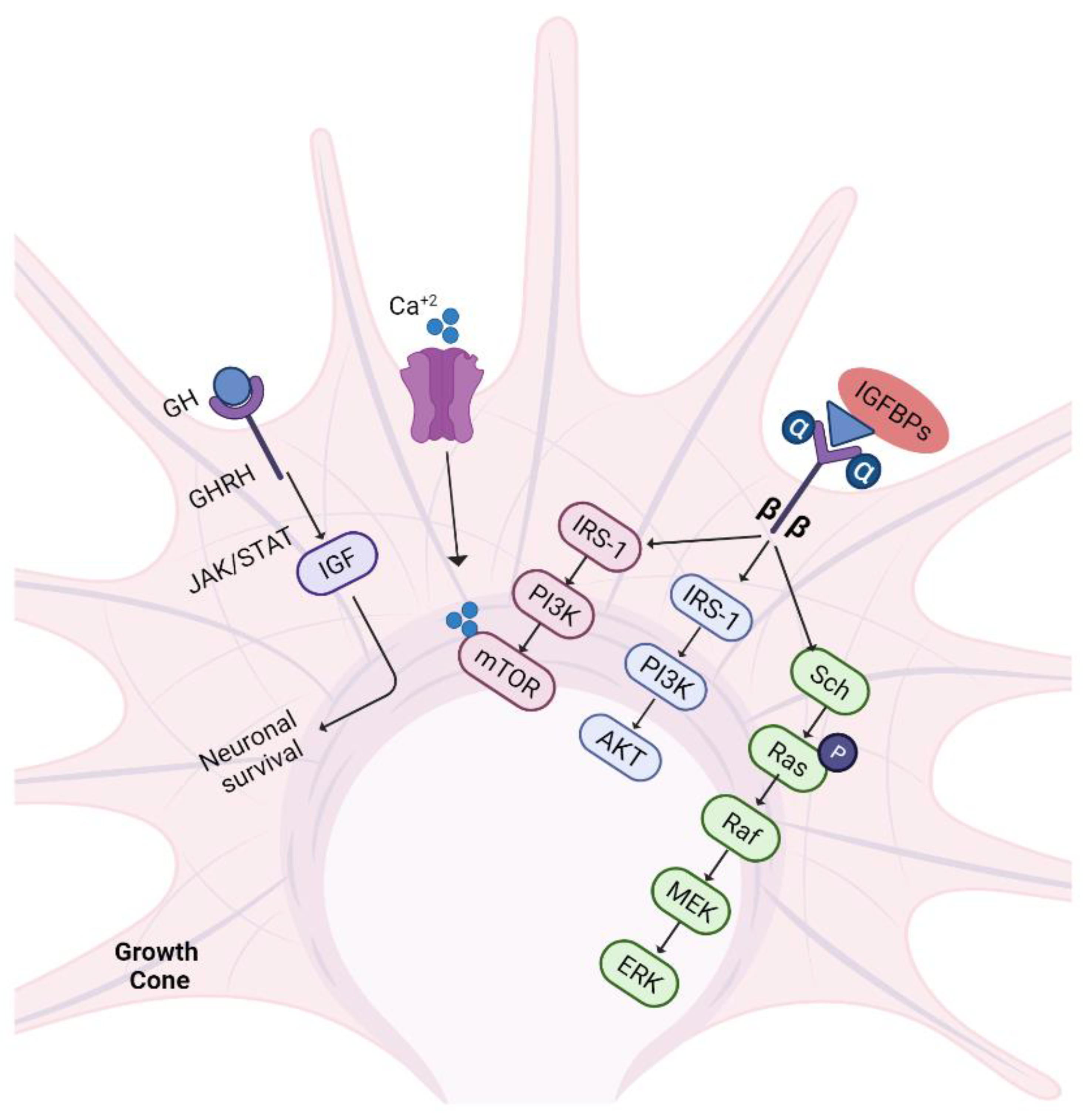
5.4. Neurotrophins
5.4.1. Ras/ERK Pathway
5.4.2. PI3K/AKT Pathway
5.4.3. Dock/BDNF Pathway
5.4.4. Pharmacological Agents
| Regulator | Studied in | Axonal Regeneration | Studied by |
|---|---|---|---|
| Regeneration-associated transcription factors | |||
| STAT3 | Dorsal root ganglion (DRG) neurons |  | [79] |
| cJUN | DRG neurons |  | [80,81,82] |
| KLF4 | Retinal ganglion cells (RGC) neurons | 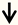 | [4,83] |
| KLF6 | RGC neurons |  | [11,84,85] |
| KLF7 | Corticospinal tract (CST) neurons | 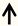 | [11,84,85] |
| KLF9 | RGC neurons | 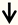 | [86] |
| SMAD1 | DRG neurons |  | [87] |
| ATF3 | DRG neurons | * | [46,88] |
| CREB | DRG neurons | 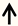 | [89] |
| AP-1 | DRG neurons |  | [90] |
| Intrinsic growth-mediating molecules | |||
| cAMP | DRG neurons |  | [9,22,28,91] |
| mTOR | CST neurons RGC neurons DRG neurons |  | [92,93,94,95] |
| PTEN | CST neurons RGC neurons | 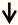 | [96,97] |
| SOCS3 | RGC neurons |  | [98,99] |
| TSC1 (hamartin) | RGC neurons | 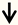 | [100,101] |
| TSC2 (tuberin) | RGC neurons | 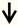 | [102,103] |
| GSK-3β | DRG neurons |  | [104] |
| LIF | Sensory neurons ** |  | [15] |
| IL6 | Sensory neurons, Schwann cells | 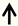 | [105,106] |
| CNTF | Distal nerve stump of DRG neurons | 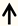 | [106,107,108] |
| IGF-1 | RGC neurons |  | [109] |
| PI3K | DRG neurons | 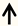 | [110] |
| ERK | DRG neurons | 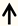 | [111,112,113] |
| FAK | RGC neurons | 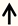 | [114] |
| DLK | DRG neurons |  | [115,116] |
| Regeneration-associated genes (RAGs) | |||
| Galanin | DRG neurons |  | [117,118] |
| Integrin | DRG neurons |  | [119] |
| Gap-43 | DRG neurons |  | [19,120] |
| Cap23 | DRG neurons |  | [121] |
| Hsp27 | DRG neurons |  | [121,122,123] |
| P21/cip1/Waf1 | DRG neurons |  | [41] |
| Sprr1a | DRG neurons |  | [4] |
| Crmp2 | Motor neurons |  | [124] |
| Map1b | Growth cone |  | [125] |
| Spry2 | Sensory axons |  | [126,127] |
| Pdcd4 | Spinal cord neurons |  | [128] |
| Dickkopf1 | DRG neurons |  | [129] |
| Rho GTPases | |||
| Rho-A | DRG neurons |  | [130] |
| Rac1 | RGC neurons |  | [131] |
| ROCK | RGC neurons |  | [132] |
| miRNAs | |||
| miR-142-3p | DRG neurons |  | [133] |
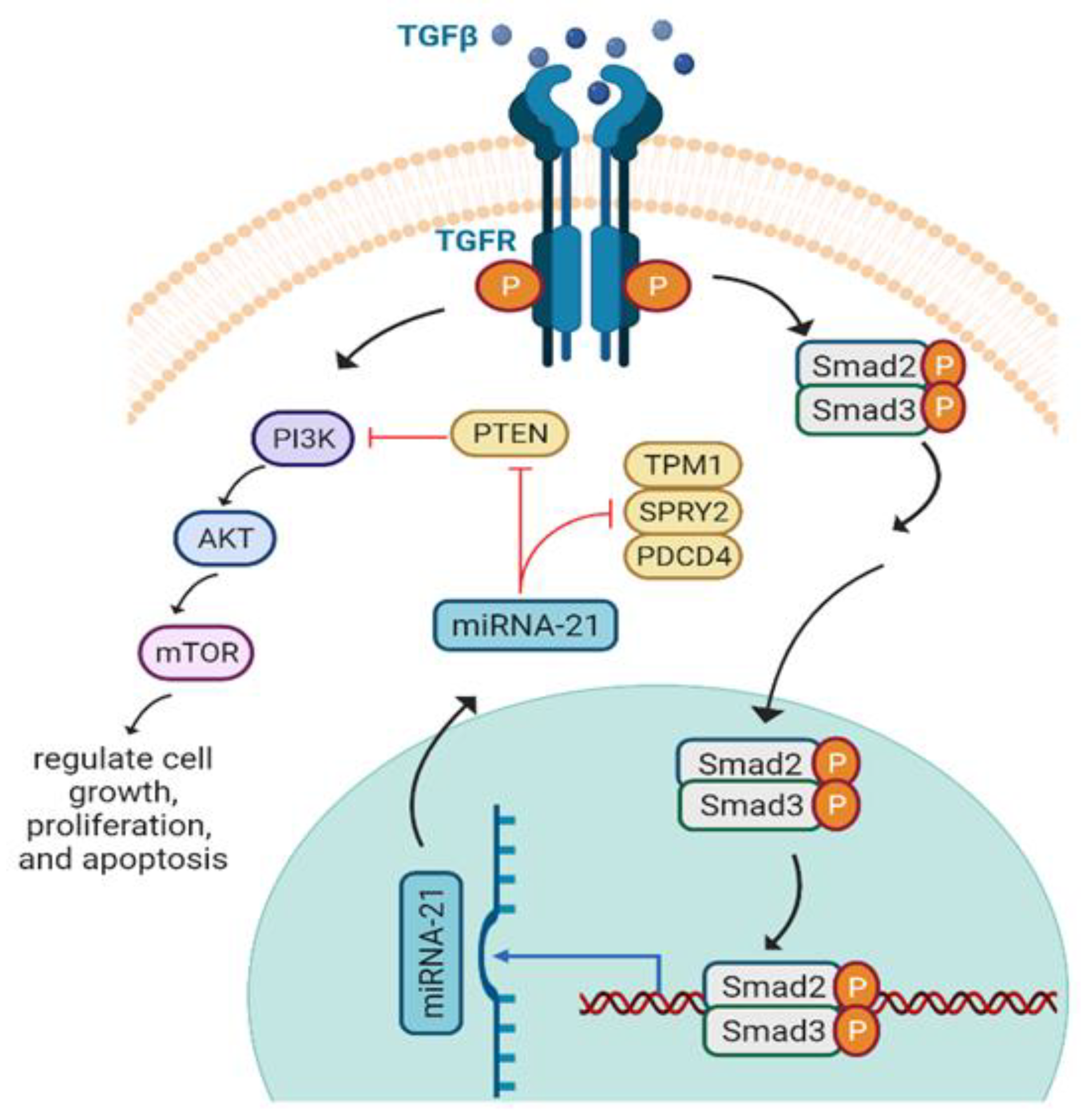
| miR-21 | DRG neurons |  | [134] |
| miRNA-431 | DRG neurons |  | [135] |
| CNS inhibitors | |||
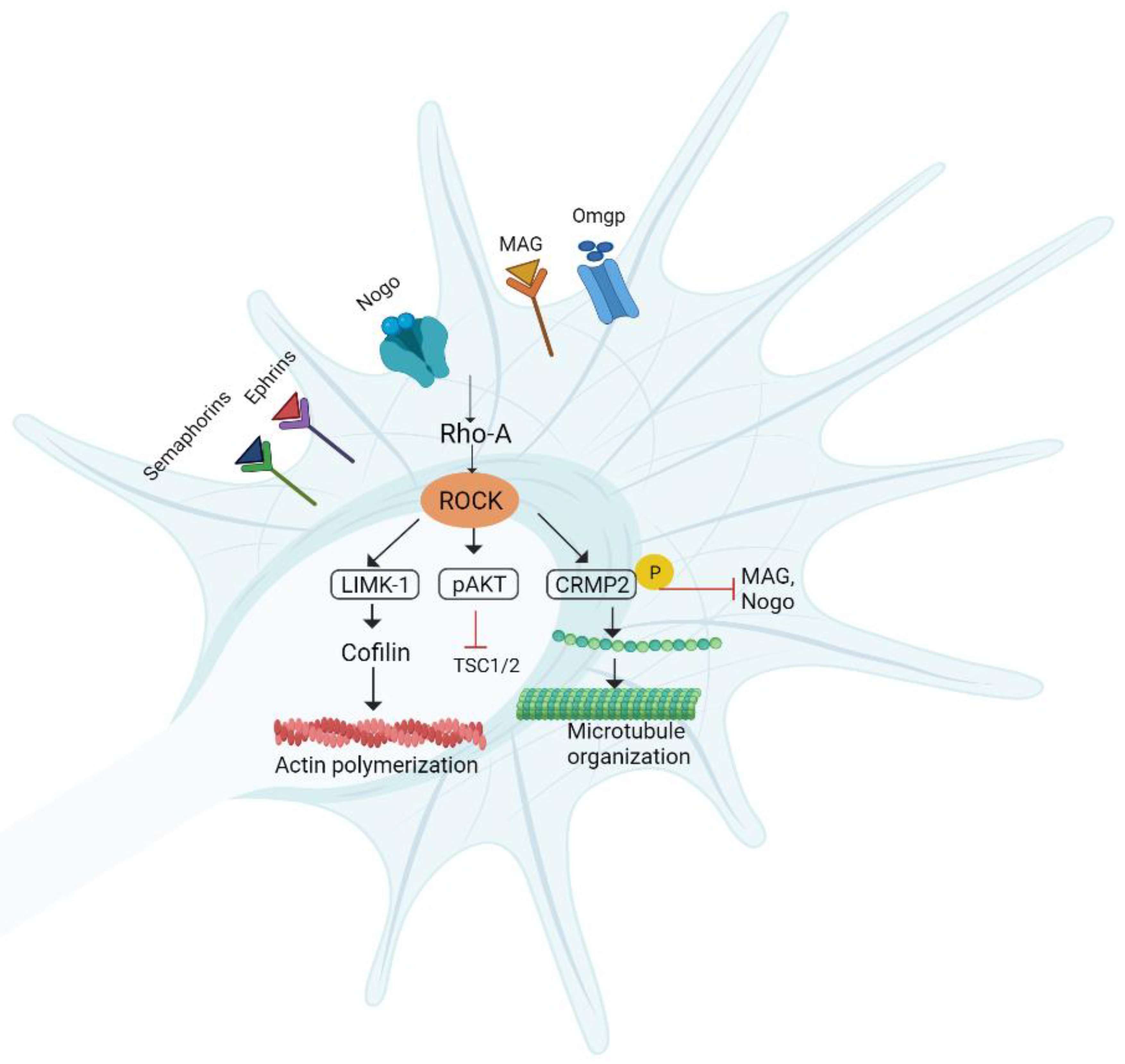
| Nogo | RGC neurons |  | [136] |
| MAG | Central nervous system (CNS) neurons |  | [137] |
| Omgp | CNS neurons |  | [137] |
| CSPGs | CNS neurons |  | [15] |
| Semaphorins | CNS neurons |  | [138] |
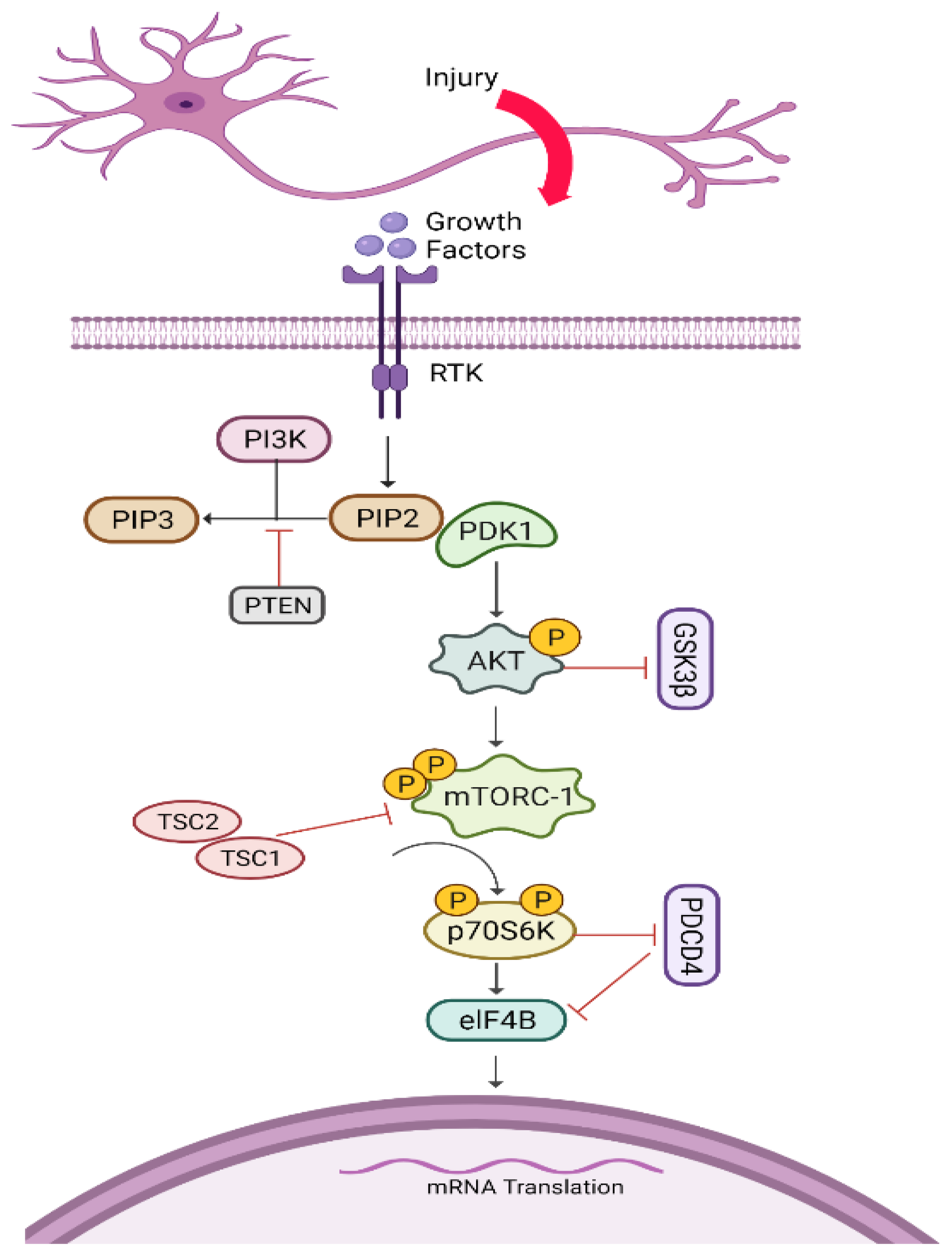
5.5. AKT/mTORC1/p70S6K Pathway
Pharmacological Agents
5.6. IGF-1/GH Pathway
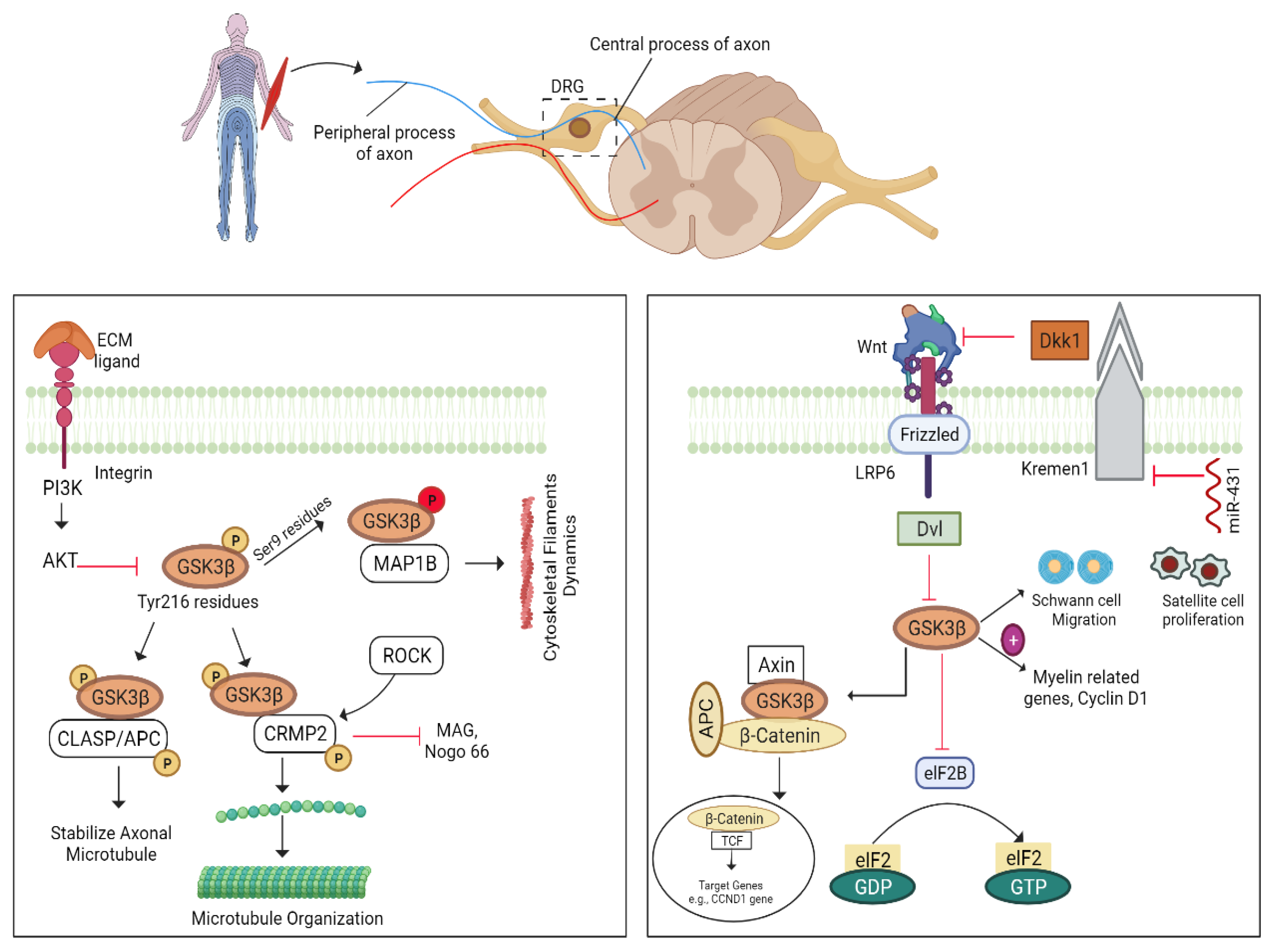
5.7. GSK3β–CLASP/APC Pathway
5.7.1. GSK-3β/Wnt Pathway
5.7.2. miRNA-431/Kremen/Wnt Pathway
6. Other Injury Signals
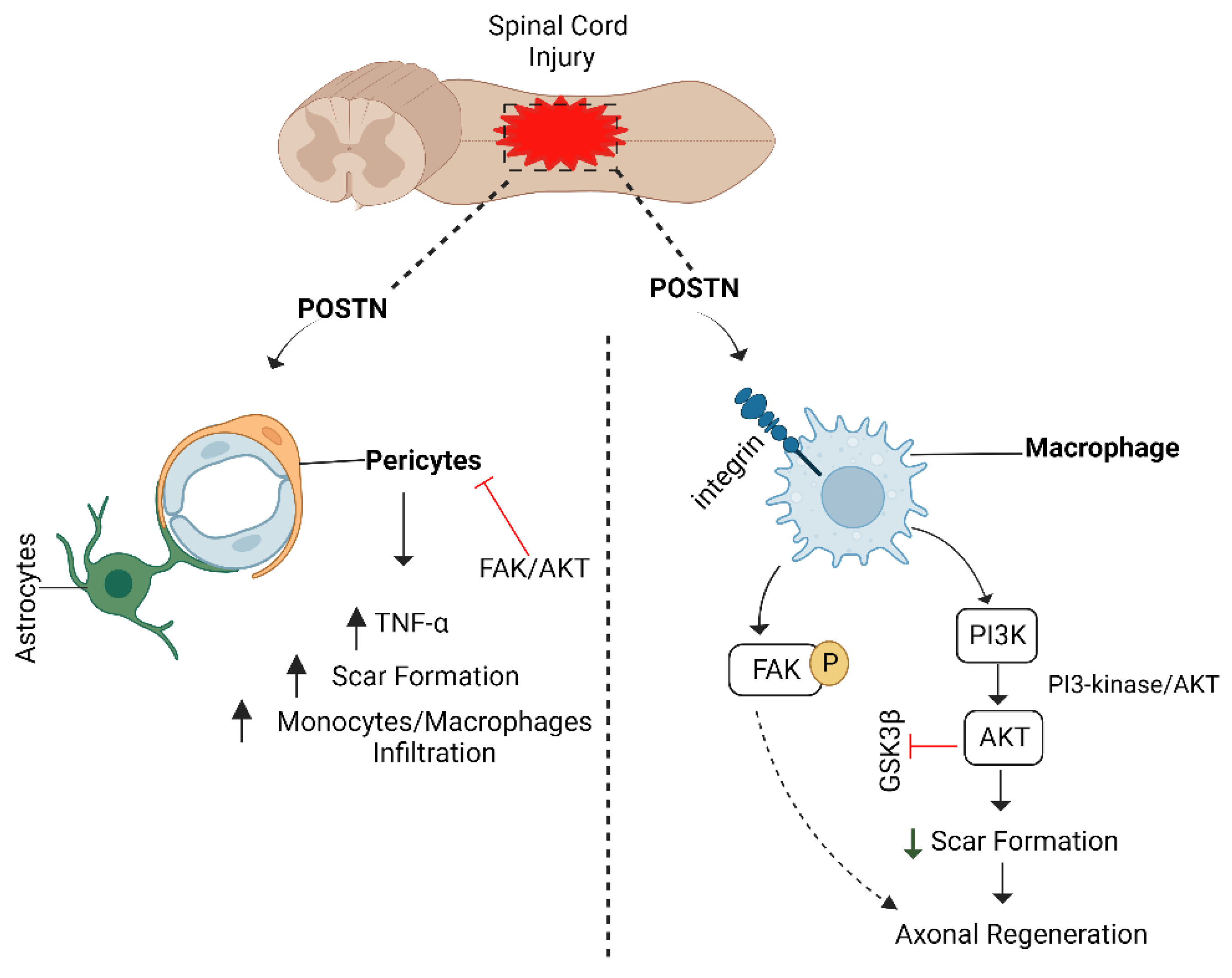
6.1. Regeneration by Astrocytes/Inflammatory Mediators
6.2. miRNA-21 and Axon Regeneration
6.3. Integrin/FAK Pathway
6.4. RhoA/ROCK/LIMK Pathway
Pharmacological Agents
6.5. POSTN/Integrin Pathway and Axon Regeneration
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lingor, P.; Koch, J.C.; Tönges, L.; Bähr, M. Axonal Degeneration as a Therapeutic Target in the CNS. Cell Tissue Res. 2012, 349, 289–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, T.C.; Geoffroy, C.G. The Influence of Neuron-Extrinsic Factors and Aging on Injury Progression and Axonal Repair in the Central Nervous System. Front. Cell Dev. Biol. 2020, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Farah, M.H.; Pan, B.H.; Hoffman, P.N.; Ferraris, D.; Tsukamoto, T.; Nguyen, T.; Wong, P.C.; Price, D.L.; Slusher, B.S.; Griffin, J.W. Reduced BACE1 Activity Enhances Clearance of Myelin Debris and Regeneration of Axons in the Injured Peripheral Nervous System. J. Neurosci. 2011, 31, 5744–5754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahar, M.; Cavalli, V. Intrinsic Mechanisms of Neuronal Axon Regeneration. Nat. Rev. Neurosci. 2018, 19, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.L.G.; et al. Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int. J. Biol. Sci. 2020, 16, 116–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafar, S.; Rasul, A.; Iqbal, J.; Anwar, H.; Imran, A.; Jabeen, F.; Shabbir, A.; Akram, R.; Maqbool, J.; Sajid, F.; et al. Calotropis Procera (Leaves) Supplementation Exerts Curative Effects on Promoting Functional Recovery in a Mouse Model of Peripheral Nerve Injury. Food Sci. Nutr. 2021, 9, 5016–5027. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Morquette, B.; Kroner, A.; Leong, S.Y.; Madwar, C.; Sanz, R.; Banerjee, S.L.; Antel, J.; Bisson, N.; David, S.; et al. Small-Molecule Stabilization of 14-3-3 Protein-Protein Interactions Stimulates Axon Regeneration. Neuron 2017, 93, 1082–1093. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.M.; Gordon, T.; Zochodne, D.W.; Power, H.A. Improving Peripheral Nerve Regeneration: From Molecular Mechanisms to Potential Therapeutic Targets. Exp. Neurol. 2014, 261, 826–835. [Google Scholar] [CrossRef]
- Jara, J.S.; Agger, S.; Hollis, E.R. Functional Electrical Stimulation and the Modulation of the Axon Regeneration Program. Front. Cell Dev. Biol. 2020, 8, 736. [Google Scholar] [CrossRef]
- Nagappan, P.G.; Chen, H.; Wang, D.Y. Neuroregeneration and Plasticity: A Review of the Physiological Mechanisms for Achieving Functional Recovery Postinjury. Mil. Med. Res. 2020, 7, 30. [Google Scholar] [CrossRef]
- He, Z.; Jin, Y. Intrinsic Control of Axon Regeneration. Neuron 2016, 90, 437–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.; Yoon, B.C.; Holt, C.E. Axonal MRNA Localization and Local Protein Synthesis in Nervous System Assembly, Maintenance and Repair. Nat. Rev. Neurosci. 2012, 13, 308. [Google Scholar] [CrossRef] [Green Version]
- Cooke, P.; Janowitz, H.; Dougherty, S.E. Neuronal Redevelopment and the Regeneration of Neuromodulatory Axons in the Adult Mammalian Central Nervous System. Front. Cell. Neurosci. 2022, 16, 183. [Google Scholar] [CrossRef] [PubMed]
- Van Niekerk, E.A.; Tuszynski, M.H.; Lu, P.; Dulin, J.N. Molecular and Cellular Mechanisms of Axonal Regeneration after Spinal Cord Injury. Mol. Cell. Proteom. 2016, 15, 394–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawcett, J.W. The Struggle to Make CNS Axons Regenerate: Why Has It Been so Difficult? Neurochem. Res. 2020, 45, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Mironets, E.; Wu, D.; Tom, V.J. Manipulating Extrinsic and Intrinsic Obstacles to Axonal Regeneration after Spinal Cord Injury. Neural Regen. Res. 2016, 11, 224. [Google Scholar] [CrossRef]
- Wahane, S.; Halawani, D.; Zhou, X.; Zou, H. Epigenetic Regulation of Axon Regeneration and Glial Activation in Injury Responses. Front. Genet. 2019, 10, 640. [Google Scholar] [CrossRef]
- Ma, T.C.; Willis, D.E. What Makes a RAG Regeneration Associated? Front. Mol. Neurosci. 2015, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.E.; Cho, Y. Epigenetic Regulation of Axon Regeneration after Neural Injury. Mol. Cells 2017, 40, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Gao, L.N.; Cui, Y.L.; Zhang, Y.; Zhou, X. The Cyclic AMP Signaling Pathway: Exploring Targets for Successful Drug Discovery (Review). Mol. Med. Rep. 2016, 13, 3715. [Google Scholar] [CrossRef]
- Ifegwu, O.C.; Awale, G.; Rajpura, K.; Lo, K.W.H.; Laurencin, C.T. Harnessing CAMP Signaling in Musculoskeletal Regenerative Engineering. Drug Discov. Today 2017, 22, 1027. [Google Scholar] [CrossRef] [PubMed]
- Blesch, A.; Lu, P.; Tsukada, S.; Alto, L.T.; Roet, K.; Coppola, G.; Geschwind, D.; Tuszynski, M.H. Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: Superiority to camp-mediated effects. Exp. Neurol. 2012, 235, 162–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, W.; Wu, H.; Yu, X.; Song, T.; Xu, X.; Xu, F. The Calcium Channel A2δ1 Subunit: Interactional Targets in Primary Sensory Neurons and Role in Neuropathic Pain. Front. Cell. Neurosci. 2021, 15, 397. [Google Scholar] [CrossRef]
- Steuer Costa, W.; Yu, S.-c.; Liewald, J.F.; Gottschalk, A. Fast CAMP Modulation of Neurotransmission via Neuropeptide Signals and Vesicle Loading. Curr. Biol. 2017, 27, 495–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, L.; LeBel, R.P.; Storm, D.R.; Chen, X. Type 3 Adenylyl Cyclase: A Key Enzyme Mediating the CAMP Signaling in Neuronal Cilia. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 95. [Google Scholar] [PubMed]
- Kummer, K.K.; Zeidler, M.; Kalpachidou, T.; Kress, M. Role of IL-6 in the Regulation of Neuronal Development, Survival and Function. Cytokine 2021, 144, 155582. [Google Scholar] [CrossRef]
- Knott, E.P.; Assi, M.; Pearse, D.D. Cyclic AMP Signaling: A Molecular Determinant of Peripheral Nerve Regeneration. Biomed Res. Int. 2014, 2014, 651625. [Google Scholar] [CrossRef]
- Niemi, J.P.; Defrancesco-Oranburg, T.; Cox, A.; Lindborg, J.A.; Echevarria, F.D.; McCluskey, J.; Simmons, D.D.; Zigmond, R.E. The Conditioning Lesion Response in Dorsal Root Ganglion Neurons Is Inhibited in Oncomodulin Knock-Out Mice. eNeuro 2022, 9, 1–13. [Google Scholar] [CrossRef]
- Li, Z.H.; Cui, D.; Qiu, C.J.; Song, X.J. Cyclic Nucleotide Signaling in Sensory Neuron Hyperexcitability and Chronic Pain after Nerve Injury. Neurobiol. Pain 2019, 6, 100028. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Yang, X.; Chu, H. MiR-142-3p Targets AC9 to Regulate Sciatic Nerve Injury-Induced Neuropathic Pain by Regulating the CAMP/AMPK Signalling Pathway. Int. J. Mol. Med. 2021, 47, 561. [Google Scholar] [CrossRef]
- Gao, J.; Gu, J.; Pan, X.; Gan, X.; Ju, Z.; Zhang, S.; Xia, Y.; Lu, L.; Wang, X. Blockade of MiR-142-3p Promotes Anti-Apoptotic and Suppressive Function by Inducing KDM6A-Mediated H3K27me3 Demethylation in Induced Regulatory T Cells. Cell Death Dis. 2019, 10, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, K.; Golbakhsh, M.; Haghighi, F.; Afshari, K. International Immunopharmacology Inhibition of Phosphodiesterase IV Enzyme Improves Locomotor and Sensory Complications of Spinal Cord Injury via Altering Microglial Activity: Introduction of Ro Fl Umilast as an Alternative Therapy. Int. Immunopharmacol. 2020, 86, 106743. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, B.; Wang, Z.; Wang, X.; Xia, Z.; Ning, G.; Wang, X.; Yuan, X.; Feng, S.; Chen, X. Sorafenib Promotes Sensory Conduction Function Recovery via MiR-142-3p/AC9/CAMP Axis Post Dorsal Column Injury. Neuropharmacology 2019, 148, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Watkins, T.A.; Wang, B.; Huntwork-Rodriguez, S.; Yang, J.; Jiang, Z.; Eastham-Anderson, J.; Modrusan, Z.; Kaminker, J.S.; Tessier-Lavigne, M.; Lewcock, J.W. DLK Initiates a Transcriptional Program That Couples Apoptotic and Regenerative Responses to Axonal Injury. Proc. Natl. Acad. Sci. USA 2013, 110, 4039–4044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valakh, V.; Frey, E.; Babetto, E.; Walker, L.J.; DiAntonio, A. Cytoskeletal Disruption Activates the DLK/JNK Pathway, Which Promotes Axonal Regeneration and Mimics a Preconditioning Injury. Neurobiol. Dis. 2015, 77, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.E.; Ha, H.; Kim, Y.K.; Cho, Y.; DiAntonio, A. DLK Regulates a Distinctive Transcriptional Regeneration Program after Peripheral Nerve Injury. Neurobiol. Dis. 2019, 127, 178. [Google Scholar] [CrossRef]
- Holland, S.M.; Collura, K.M.; Ketschek, A.; Noma, K.; Ferguson, T.A.; Jin, Y. Palmitoylation Controls DLK Localization, Interactions and Activity to Ensure Effective Axonal Injury Signaling. Proc. Natl. Acad. Sci. USA 2016, 113, 763–768. [Google Scholar] [CrossRef] [Green Version]
- Schellino, R.; Boido, M.; Vercelli, A. JNK Signaling Pathway Involvement in Spinal Cord Neuron Development and Death. Cells 2019, 8, 1576. [Google Scholar] [CrossRef] [Green Version]
- Asghari Adib, E.; Smithson, L.J.; Collins, C.A. An Axonal Stress Response Pathway: Degenerative and Regenerative Signaling by DLK. Curr. Opin. Neurobiol. 2018, 53, 110–119. [Google Scholar] [CrossRef]
- Summers, D.W.; Milbrandt, J.; Diantonio, A. Palmitoylation Enables MAPK-Dependent Proteostasis of Axon Survival Factors. Proc. Natl. Acad. Sci. USA 2018, 115, E8746–E8754. [Google Scholar] [CrossRef]
- Fischer, D. Hyper-IL-6: A Potent and Efficacious Stimulator of RGC Regeneration. Eye 2016, 31, 173–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kole, C.; Brommer, B.; Nakaya, N.; Sengupta, M.; Bonet-Ponce, L.; Zhao, T.; Wang, C.; Li, W.; He, Z.; Tomarev, S. Activating Transcription Factor 3 (ATF3) Protects Retinal Ganglion Cells and Promotes Functional Preservation After Optic Nerve Crush. Invest. Ophthalmol. Vis. Sci. 2020, 61, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gey, M.; Wanner, R.; Schilling, C.; Pedro, M.T.; Sinske, D.; Knöll, B. Atf3 Mutant Mice Show Reduced Axon Regeneration and Impaired Regeneration-Associated Gene Induction after Peripheral Nerve Injury. Open Biol. 2016, 6, 160091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheib, J.; Höke, A. Advances in Peripheral Nerve Regeneration. Nat. Rev. Neurol. 2013, 9, 668–676. [Google Scholar] [CrossRef]
- Ousman, S.S.; Frederick, A.; Lim, E.M.F. Chaperone Proteins in the Central Nervous System and Peripheral Nervous System after Nerve Injury. Front. Neurosci. 2017, 11, 79. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.C.; Snavely, A.; Barrett, L.B.; Zhang, X.; Herman, C.; Frost, D.J.; Riva, P.; Tochitsky, I.; Kawaguchi, R.; Singh, B.; et al. Topoisomerase I Inhibition and Peripheral Nerve Injury Induce DNA Breaks and ATF3-Associated Axon Regeneration in Sensory Neurons. Cell Rep. 2021, 36, 109666. [Google Scholar] [CrossRef]
- Perry, R.B.T.; Hezroni, H.; Goldrich, M.J.; Ulitsky, I. Regulation of Neuroregeneration by Long Noncoding RNAs. Mol. Cell 2018, 72, 553–567.e5. [Google Scholar] [CrossRef] [Green Version]
- Higashi, T.; Tanaka, S.; Iida, T.; Okabe, S. Synapse Elimination Triggered by BMP4 Exocytosis and Presynaptic BMP Receptor Activation. Cell Rep. 2018, 22, 919–929. [Google Scholar] [CrossRef] [Green Version]
- Podkowa, M.; Zhao, X.; Chow, C.-W.; Coffey, E.T.; Davis, R.J.; Attisano, L. Microtubule Stabilization by Bone Morphogenetic Protein Receptor-Mediated Scaffolding of c-Jun N-Terminal Kinase Promotes Dendrite Formation. Mol. Cell. Biol. 2010, 30, 2241–2250. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Ho, C.; Wong, K.; Tessier-Lavigne, M. Axotomy-Induced Smad1 Activation Promotes Axonal Growth in Adult Sensory Neurons. J. Neurosci. 2009, 29, 7116–7123. [Google Scholar] [CrossRef]
- Parikh, P.; Hao, Y.; Hosseinkhani, M.; Patil, S.B.; Huntley, G.W.; Tessier-Lavigne, M.; Zou, H. Regeneration of Axons in Injured Spinal Cord by Activation of Bone Morphogenetic Protein/Smad1 Signaling Pathway in Adult Neurons. Proc. Natl. Acad. Sci. USA 2011, 108, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedeschi, A. Tuning the Orchestra: Transcriptional Pathways Controlling Axon Regeneration. Front. Mol. Neurosci. 2012, 4, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollis, E.R.; Zou, Y. Expression of the Wnt Signaling System in Central Nervous System Axon Guidance and Regeneration. Front. Mol. Neurosci. 2012, 5, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, J.; Zou, H. BMP Signaling in Axon Regeneration. Curr. Opin. Neurobiol. 2014, 27, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsaeidi, F.; Bemben, M.A.; Zhao, X.-F.; Goldman, D. Jak/Stat Signaling Stimulates Zebrafish Optic Nerve Regeneration and Overcomes the Inhibitory Actions of Socs3 and Sfpq. J. Neurosci. 2014, 34, 2632. [Google Scholar] [CrossRef] [Green Version]
- Stanga, S.; Boido, M.; Kienlen-Campard, P. How to Build and to Protect the Neuromuscular Junction: The Role of the Glial Cell Line-Derived Neurotrophic Factor. Int. J. Mol. Sci. 2020, 22, 136. [Google Scholar] [CrossRef]
- Allodi, I.; Udina, E.; Navarro, X. Specificity of Peripheral Nerve Regeneration: Interactions at the Axon Level. Prog. Neurobiol. 2012, 98, 16–37. [Google Scholar] [CrossRef]
- Bo, X.; Wu, D.; Yeh, J.; Zhang, Y. Gene Therapy Approaches for Neuroprotection and Axonal Regeneration after Spinal Cord and Spinal Root Injury. Curr. Gene Ther. 2011, 11, 101–115. [Google Scholar] [CrossRef]
- Aloe, L.; Rocco, M.L.; Bianchi, P.; Manni, L. Nerve Growth Factor: From the Early Discoveries to the Potential Clinical Use. J. Transl. Med. 2012, 10, 239. [Google Scholar] [CrossRef] [Green Version]
- Napoli, I.; Noon, L.A.; Ribeiro, S.; Kerai, A.P.; Parrinello, S.; Rosenberg, L.H.; Collins, M.J.; Harrisingh, M.C.; White, I.J.; Woodhoo, A.; et al. A Central Role for the ERK-Signaling Pathway in Controlling Schwann Cell Plasticity and Peripheral Nerve Regeneration In Vivo. Neuron 2012, 73, 729–742. [Google Scholar] [CrossRef]
- Duraikannu, A.; Krishnan, A.; Chandrasekhar, A.; Zochodne, D.W. Beyond Trophic Factors: Exploiting the Intrinsic Regenerative Properties of Adult Neurons. Front. Cell. Neurosci. 2019, 13, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Cao, F.J.; Xu, D.D.; Xu, Y.Q.; Feng, S.Q. Upregulated Ras/Raf/ERK1/2 Signaling Pathway: A New Hope in the Repair of Spinal Cord Injury. Neural Regen. Res. 2015, 10, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Christie, K.J.; Webber, C.A.; Martinez, J.A.; Singh, B.; Zochodne, D.W. PTEN Inhibition to Facilitate Intrinsic Regenerative Outgrowth of Adult Peripheral Axons. J. Neurosci. 2010, 30, 9306–9315. [Google Scholar] [CrossRef]
- Nieuwenhuis, B.; Barber, A.C.; Evans, R.S.; Pearson, C.S.; Fuchs, J.; MacQueen, A.R.; van Erp, S.; Haenzi, B.; Hulshof, L.A.; Osborne, A.; et al. PI 3-Kinase Delta Enhances Axonal PIP3 to Support Axon Regeneration in the Adult CNS. EMBO Mol. Med. 2020, 12, e11674. [Google Scholar] [CrossRef]
- Auer, M.; Schweigreiter, R.; Hausott, B.; Thongrong, S.; Höltje, M.; Just, I.; Bandtlow, C.; Klimaschewski, L. Rho-Independent Stimulation of Axon Outgrowth and Activation of the ERK and AKT Signaling Pathways by C3 Transferase in Sensory Neurons. Front. Cell. Neurosci. 2012, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Overman, J.J.; Katsman, D.; Kozlov, S.V.; Donnelly, C.J.; Twiss, J.L.; Giger, R.J.; Coppola, G.; Geschwind, D.H.; Carmichael, S.T. An Age-Related Sprouting Transcriptome Provides Molecular Control of Axonal Sprouting after Stroke. Nat. Neurosci. 2010, 13, 1496–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christie, K.J.; Zochodne, D. Peripheral Axon Regrowth: New Molecular Approaches. Neuroscience 2013, 240, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D. The Neurotrophin Family of Neurotrophic Factors: An Overview. Methods Mol. Biol. 2012, 1–12. [Google Scholar] [CrossRef]
- Phuyal, S.; Farhan, H. Multifaceted Rho GTPase Signaling at the Endomembranes. Front. Cell Dev. Biol. 2019, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Namekata, K.; Kimura, A.; Kawamura, K.; Harada, C.; Harada, T. Dock GEFs and Their Therapeutic Potential: Neuroprotection and Axon Regeneration. Prog. Retin. Eye Res. 2014, 43, 1–16. [Google Scholar] [CrossRef]
- Namekata, K.; Harada, C.; Taya, C.; Guo, X.; Kimura, H.; Parada, L.F.; Harada, T. Dock3 Induces Axonal Outgrowth by Stimulating Membrane Recruitment of the WAVE Complex. Proc. Natl. Acad. Sci. USA 2010, 107, 7586–7591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, A.; Namekata, K.; Guo, X.; Harada, C.; Harada, T. Neuroprotection, Growth Factors and BDNF-TrkB Signalling in Retinal Degeneration. Int. J. Mol. Sci. 2016, 17, 1584. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Xiang, X.; Liang, C.; Shi, L. Regulating Rac in the Nervous System: Molecular Function and Disease Implication of Rac GEFs and GAPs. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, C.L.; Xu, X.M. PTEN Inhibitor Bisperoxovanadium Protects Oligodendrocytes and Myelin and Prevents Neuronal Atrophy in Adult Rats Following Cervical Hemicontusive Spinal Cord Injury. Neurosci. Lett. 2014, 573, 64–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Tierney, L.; Mann, R.; Lonsway, T.; Walker, C.L. Bisperoxovanadium Promotes Motor Neuron Survival and Neuromuscular Innervation in Amyotrophic Lateral Sclerosis. Mol. Brain 2021, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- de Souza Aarão, T.L.; de Sousa, J.R.; Falcão, A.S.C.; Falcão, L.F.M.; Quaresma, J.A.S. Nerve Growth Factor and Pathogenesis of Leprosy: Review and Update. Front. Immunol. 2018, 9, 939. [Google Scholar] [CrossRef]
- Biernacki, K.; Antel, J.P.; Blain, M.; Narayanan, S.; Arnold, D.L.; Prat, A. Interferon Beta Promotes Nerve Growth Factor Secretion Early in the Course of Multiple Sclerosis. Arch. Neurol. 2005, 62, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Sartor, G.C.; Malvezzi, A.M.; Kumar, A.; Andrade, N.S.; Wiedner, H.J.; Vilca, S.J.; Janczura, K.J.; Bagheri, A.; Al-Ali, H.; Powell, S.K.; et al. Enhancement of BDNF Expression and Memory by HDAC Inhibition Requires BET Bromodomain Reader Proteins. J Neurosci. 2019, 39, 612–626. [Google Scholar] [CrossRef] [Green Version]
- Bareyre, F.M.; Garzorz, N.; Lang, C.; Misgeld, T.; Büning, H.; Kerschensteiner, M. In Vivo Imaging Reveals a Phase-Specific Role of Stat3 during Central and Peripheral Nervous System Axon Regeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 6282–6287. [Google Scholar] [CrossRef] [Green Version]
- Ruff, C.A.; Staak, N.; Patodia, S.; Kaswich, M.; Rocha-Ferreira, E.; Da Costa, C.; Brecht, S.; Makwana, M.; Fontana, X.; Hristova, M.; et al. Neuronal C-Jun Is Required for Successful Axonal Regeneration, but the Effects of Phosphorylation of Its N-Terminus Are Moderate. J. Neurochem. 2012, 121, 607–618. [Google Scholar] [CrossRef]
- Simpson, M.T.; Venkatesh, I.; Callif, B.L.; Thiel, L.K.; Winsor, K.N.; Wang, Z.; Kramer, A.A.; Lerch, J.K.; Repair, S.C.; Ohio, T. The Tumor Suppressor HHEX Inhibits Axon Growth When Prematurely Expressed in Developing Central Nervous System Neurons. Mol Cell Neurosci. 2015, 68, 272–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandran, V.; Coppola, G.; Nawabi, H.; Omura, T.; Huebner, E.A.; Zhang, A.; Costigan, M.; Yekkirala, A.; Barrett, L.; Blesch, A.; et al. A Systems-Level Analysis of the Peripheral Nerve Intrinsic Axonal Growth Program. Neuron 2016, 89, 956–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.H.; Qin, X.Z.; Zhang, H.N.; Ma, Y.X.; Qi, S.B.; Zhang, H.C.; Ma, J.J.; Fu, X.Y.; Xie, J.L.; Saijilafu. Deletion of Krüppel-like Factor-4 Promotes Axonal Regeneration in Mammals. Neural Regen. Res. 2021, 16, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, M.G.; Moore, D.L.; Smith, R.P.; Goldberg, J.L.; Bixby, J.L.; Lemmon, V.P. High Content Screening of Cortical Neurons Identifies Novel Regulators of Axon Growth. Mol. Cell. Neurosci. 2010, 44, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, D.L.; Blackmore, M.G.; Hu, Y.; Kaestner, K.H.; Bixby, J.L.; Lemmon, V.P.; Goldberg, J.L. KLF Family Members Regulate Intrinsic Axon Regeneration Ability. Science 2009, 326, 298–301. [Google Scholar] [CrossRef] [Green Version]
- Apara, X.A.; Galvao, X.J.; Wang, Y.; Blackmore, M.; Trillo, X.A.; Iwao, X.K.; Brown, D.P.; Fernandes, K.A.; Huang, X.A.; Nguyen, T.; et al. KLF9 and JNK3 Interact to Suppress Axon Regeneration in the Adult CNS. J. Neurosci. 2017, 37, 9632–9644. [Google Scholar] [CrossRef] [Green Version]
- Finelli, M.J.; Wong, J.K.; Zou, H. Epigenetic Regulation of Sensory Axon Regeneration after Spinal Cord Injury. J. Neurosci. 2013, 33, 19664–19676. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Lee, J.; Jeon, Y.; Jang, E.; Oh, Y.; Kim, H.; Kwon, M.; Shin, J.E.; Cho, Y. Promoting Axon Regeneration by Enhancing the Non-Coding Function of the Injury-Responsive Coding Gene Gpr151. bioRxiv 2021. [Google Scholar] [CrossRef]
- Tao, T.; Wei, M.Y.; Guo, X.W.; Zhang, J.; Yang, L.Y.; Zheng, H. Modulating CAMP Responsive Element Binding Protein 1 Attenuates Functional and Behavioural Deficits in Rat Model of Neuropathic Pain. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2602–2611. [Google Scholar] [CrossRef]
- Ma, T.C.; Barco, A.; Ratan, R.R.; Willis, D.E. CAMP-Responsive Element-Binding Protein (CREB) and CAMP Co-Regulate Activator Protein 1 (AP1)-Dependent Regeneration-Associated Gene Expression and Neurite Growth. J. Biol. Chem. 2014, 289, 32914–32925. [Google Scholar] [CrossRef]
- Fischer, D.; Leibinger, M. Promoting Optic Nerve Regeneration. Prog. Retin. Eye Res. 2012, 31, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.; Ahmed, Z.; Morgan-Warren, P.; Fulton, D.; Logan, A. Prospects for MTOR-Mediated Functional Repair after Central Nervous System Trauma. Neurobiol. Dis. 2016, 85, 99–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Das, S.; Losert, W.; Parent, C.A. mTORC2 regulates neutrophil chemotaxis in a camp- and rhoa-dependent fashion. Dev. Cell 2010, 19, 845–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Jin, Y.; Shapiro, T.M.; Hinduja, A.; Baas, P.W.; Tom, V.J. Chronic Neuronal Activation Increases Dynamic Microtubules to Enhance Functional Axon Regeneration after Dorsal Root Crush Injury. Nat. Commun. 2020, 11, 6131. [Google Scholar] [CrossRef]
- Chen, W.; Lu, N.; Ding, Y.; Wang, Y.; Chan, L.T.; Wang, X.; Gao, X.; Jiang, S.; Liu, K. Rapamycin-Resistant MTOR Activity Is Required for Sensory Axon Regeneration Induced by a Conditioning Lesion. eNeuro 2017, 3, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Lu, Y.; Lee, J.K.; Samara, R.; Willenberg, R.; Sears-Kraxberger, I.; Tedeschi, A.; Park, K.K.; Jin, D.; Cai, B.; et al. PTEN Deletion Enhances the Regenerative Ability of Adult Corticospinal Neurons. Nat. Neurosci. 2010, 13, 1075–1081. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Qiao, M.; Bei, F.; Kim, I.-J.; He, Z.; Sanes, J.R. Subtype-specific regeneration of retinal ganglion cells following axotomy: Effects of osteopontin and mtor signaling. Neuron 2015, 85, 1244–1256. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.D.; Sun, F.; Park, K.K.; Cai, B.; Wang, C.; Martinez-carrasco, I.; Connolly, L.; He, Z. SOCS3 Deletion Promotes Optic Nerve Regeneration in Vivo. Neuron 2009, 64, 617–623. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Park, K.K.; Belin, S.; Wang, D.; Lu, T.; Chen, G.; Yeung, C.; Feng, G.; Yankner, B.A.; He, Z.; et al. Sustained Axon Regeneration Induced by Co-Deletion of PTEN and SOCS3. Nature 2011, 480, 372–375. [Google Scholar] [CrossRef] [Green Version]
- Park, K.K.; Liu, K.; Hu, Y.; Smith, P.D.; Wang, C.; Cai, B.; Xu, B.; Connolly, L.; Kramvis, I.; Sahin, M.; et al. Promoting Axon Regeneration in the Adult CNS by Modulation of the PTEN/MTOR Pathway. Science 2008, 323, 963–966. [Google Scholar] [CrossRef]
- Yang, G.; Sau, C.; Lai, W.; Cichon, J.; Li, W. The MTORC1 Effectors S6K1 and 4E-BP Play Different Roles in CNS Axon Regeneration. Nat. Community 2015, 5, 5416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doron-Mandel, E.; Fainzilber, M.; Terenzio, M. Growth Control Mechanisms in Neuronal Regeneration. FEBS Lett. 2015, 589, 1669–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laplante, M.; Sabatini, D.M. MTOR Signaling in Growth Control and Disease. Cell 2012, 149, 1–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saijilafu; Zhang, B.Y.; Zhou, F.Q. Signaling Pathways That Regulate Axon Regeneration. Neurosci. Bull. 2013, 29, 411–420. [Google Scholar] [CrossRef] [Green Version]
- O’Donovan, K.J. Intrinsic Axonal Growth and the Drive for Regeneration. Front. Neurosci. 2016, 10, 486. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Deng, N.; Liu, K.; Zhou, N.; Sun, Y.; Zeng, W. CNTF-STAT3-IL-6 Axis Mediates Neuroinflammatory Cascade across Schwann Cell-Neuron-Microglia. Cell Rep. 2020, 31, 107657. [Google Scholar] [CrossRef]
- Homs, J.; Ariza, L.; Pagès, G.; Udina, E.; Navarro, X.; Chillón, M.; Bosch, A. Schwann Cell Targeting via Intrasciatic Injection of AAV8 as Gene Therapy Strategy for Peripheral Nerve Regeneration. Gene Ther. 2011, 18, 622–630. [Google Scholar] [CrossRef] [Green Version]
- Gordon, T. The Role of Neurotrophic Factors in Nerve Regeneration. Neurosurg Focus 2009, 26, E3. [Google Scholar] [CrossRef] [Green Version]
- Apel, P.J.; Jun, J.; Callahan, M.; Northam, C.N.; Alton, T.B.; Sonntag, W.E.; Li, Z. Effect of locally delivered igf-1 on nerve regeneration during aging: An experimental study in rats. Muscle Nerve 2010, 41, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Saijilafu; Hur, E.M.; Liu, C.M.; Jiao, Z.; Xu, W.L.; Zhou, F.Q. PI3K-GSK3 Signaling Regulates Mammalian Axon Regeneration by Inducing the Expression of Smad1. Nat. Commun. 2013, 4, 2690. [Google Scholar] [CrossRef]
- Jin, L.Q.; John, B.H.; Hu, J.; Selzer, M.E. Activated Erk Is an Early Retrograde Signal After Spinal Cord Injury in the Lamprey. Front. Neurosci. 2020, 14, 1166. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J. RAFting the Rapids of Axon Regeneration Signaling. Neural Regen. Res. 2015, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Hausott, B.; Klimaschewski, L. Promotion of Peripheral Nerve Regeneration by Stimulation of the Extracellular Signal-Regulated Kinase (ERK) Pathway. Anat. Rec. 2019, 302, 1261–1267. [Google Scholar] [CrossRef] [Green Version]
- Onofrio, P.M.D.; Shabanzadeh, A.P.; Choi, B.K.; Mathias, B.; Paulo, D. MMP Inhibition Preserves Integrin Ligation and FAK Activation to Induce Survival and Regeneration in RGCs Following Optic Nerve Damage. Investig. Ophthalmol. Vis. Sci. 2019, 60, 634–649. [Google Scholar] [CrossRef] [Green Version]
- Valakh, V.; Walker, L.J.; Skeath, J.B.; Diantonio, A.; Mapkkk, T. Loss of the Spectraplakin Short Stop Activates the DLK Injury Response Pathway in Drosophila. J. Neurosci. 2013, 33, 17863–17873. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Frey, E.; Yoon, C.; Wong, H.; Nestorovski, D.; Holzman, L.B.; Giger, R.J.; DiAntonio, A.; Collins, C. An Evolutionarily Conserved Mechanism for CAMP Elicited Axonal Regeneration Involves Direct Activation of the Dual Leucine Zipper Kinase DLK. eLife 2016, 5, e14048. [Google Scholar] [CrossRef]
- Xu, X.F.; Zhang, D.D.; Liao, J.C.; Xiao, L.; Wang, Q.; Qiu, W. Galanin and Its Receptor System Promote the Repair of Injured Sciatic Nerves in Diabetic Rats. Neural Regen. Res. 2016, 11, 1517. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Lyu, G.W.; Mulder, J.; Uhlén, M.; Cai, X.H.; Hökfelt, T.; Sten Shi, T.J. Expression and Regulation of FRMD6 in Mouse DRG Neurons and Spinal Cord after Nerve Injury. Sci. Rep. 2020, 10, 1880. [Google Scholar] [CrossRef] [Green Version]
- Cheah, M.; Andrews, M.R.; Chew, D.J.; Moloney, E.B.; Verhaagen, J.; Fässler, R.; Fawcett, J.W. Expression of an Activated Integrin Promotes Long-Distance Sensory Axon Regeneration in the Spinal Cord. J. Neurosci. 2016, 36, 7283–7297. [Google Scholar] [CrossRef]
- Chung, D.; Shum, A.; Caraveo, G. GAP-43 and BASP1 in Axon Regeneration: Implications for the Treatment of Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 890. [Google Scholar] [CrossRef]
- Ma, C.H.E.; Omura, T.; Cobos, E.J.; Latrémolière, A.; Ghasemlou, N.; Brenner, G.J.; Van Veen, E.; Barrett, L.; Sawada, T.; Gao, F.; et al. Accelerating Axonal Growth Promotes Motor Recovery after Peripheral Nerve Injury in Mice. J. Clin. Investig. 2011, 121, 4332–4347. [Google Scholar] [CrossRef] [Green Version]
- Höke, A. Commentaries A (Heat) Shock to the System Promotes Peripheral Nerve Regeneration. J. Clin. Investig. 2011, 121, 4231–4234. [Google Scholar] [CrossRef] [PubMed]
- Lerch, J.K.; Alexander, J.K.; Madalena, K.M.; Motti, D.; Quach, T.; Dhamija, A.; Zha, A.; Gensel, J.C.; Marketon, J.W.; Lemmon, V.P.; et al. Stress Increases Peripheral Axon Growth and Regeneration through Glucocorticoid Receptor-Dependent Transcriptional Programs. eNeuro 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Kondo, S.; Takahashi, K.; Kinoshita, Y.; Nagai, J.; Wakatsuki, S.; Araki, T.; Goshima, Y.; Ohshima, T. Genetic Inhibition of CRMP2 Phosphorylation at Serine 522 Promotes Axonal Regeneration after Optic Nerve Injury. Sci. Rep. 2019, 9, 7188. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, Y.; Okada, M.; Honda, A.; Ito, Y.; Tamada, A.; Endo, N.; Igarashi, M. Phosphorylation Sites of Microtubule-Associated Protein 1B (MAP 1B) Are Involved in Axon Growth and Regeneration. Mol. Brain 2019, 12, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marvaldi, L.; Thongrong, S.; Kozłowska, A.; Irschick, R.; Pritz, C.O.; Bäumer, B.; Ronchi, G.; Geuna, S.; Hausott, B.; Klimaschewski, L. Enhanced Axon Outgrowth and Improved Long-Distance Axon Regeneration in Sprouty2 Deficient Mice. Dev. Neurobiol. 2015, 75, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Hausott, B.; Vallant, N.; Auer, M.; Yang, L.; Dai, F.; Brand-saberi, B.; Klimaschewski, L. Sprouty2 Down-Regulation Promotes Axon Growth by Adult Sensory Neurons. Mol. Cell. Neurosci. 2009, 42, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, S.; Ding, Y.; Nong, L.; Li, H.; Gao, G.; Zhou, D.; Xu, N. MicroRNA-21 Promotes Neurite Outgrowth by Regulating PDCD4 in a Rat Model of Spinal Cord Injury. Mol. Med. Rep. 2017, 16, 2522–2528. [Google Scholar] [CrossRef] [Green Version]
- Ross, S.P.; Baker, K.E.; Fisher, A.; Hoff, L.; Pak, E.S.; Murashov, A.K. MiRNA-431 Prevents Amyloid-β-Induced Synapse Loss in Neuronal Cell Culture Model of Alzheimer’s Disease by Silencing Kremen1. Front. Cell. Neurosci. 2018, 12, 87. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Peng, X.; Chiang, P.; Kim, J.; Sun, X.; Fink, D.J.; Mata, M. HSV-Mediated Gene Transfer of C3 Transferase Inhibits Rho to Promote Axonal Regeneration. Exp. Neurol. 2012, 237, 126–133. [Google Scholar] [CrossRef]
- Lorenzetto, E.; Ettorre, M.; Pontelli, V.; Bolomini-vittori, M.; Bolognin, S.; Zorzan, S.; Laudanna, C.; Buffelli, M. Rac1 Selective Activation Improves Retina Ganglion Cell Survival and Regeneration. PLoS ONE 2013, 8, e64350. [Google Scholar] [CrossRef] [PubMed]
- Tönges, L. ROCKing Regeneration: Rho Kinase Inhibition as Molecular Target for Neurorestoration. Front. Mol. Neurosci. 2011, 4, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Yu, B.; Qian, T.; Yao, D.; Wang, Y.; Ding, F.; Gu, X. Early Changes of MicroRNAs Expression in the Dorsal Root Ganglia Following Rat Sciatic Nerve Transection. Neurosci. Lett. 2011, 494, 89–93. [Google Scholar] [CrossRef]
- Strickland, I.T.; Richards, L.; Holmes, F.E.; Wynick, D.; Uney, J.B.; Wong, L.F. Axotomy-Induced Mir-21 Promotes Axon Growth in Adult Dorsal Root Ganglion Neurons. PLoS ONE 2011, 6, e23423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Murashov, A.K. MicroRNA-431 Regulates Axon Regeneration in Mature Sensory Neurons by Targeting the Wnt Antagonist Kremen1. Front. Mol. Neurosci. 2013, 6, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Sun, Z.; Yang, X.; Zhu, L.; Feng, D. Exploring Optic Nerve Axon Regeneration. Curr. Neuropharmacol. 2017, 15, 861–873. [Google Scholar] [CrossRef] [Green Version]
- McKerracher, L.; Rosen, K.M. MAG, Myelin and Overcoming Growth Inhibition in the CNS. Front. Mol. Neurosci. 2015, 8, 51. [Google Scholar] [CrossRef] [Green Version]
- Giger, R.J.; Hollis, E.R.; Tuszynski, M.H. Guidance Molecules in Axon Regeneration. Cold Spring Harb. Perspect. Biol. 2010, 2, a001867. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Qi, Y.; Li, Y.; Xu, K. PI3 Kinase Regulation of Neural Regeneration and Muscle Hypertrophy after Spinal Cord Injury. Mol. Biol. Rep. 2012, 39, 3541–3547. [Google Scholar] [CrossRef]
- Dan, H.C.; Antonia, R.J.; Baldwin, A.S.; Dan, H.C.; Antonia, R.J.; Baldwin, A.S. PI3K/AKT Promotes Feedforward MTORC2 Activation through IKKα. Oncotarget 2016, 7, 21064–21075. [Google Scholar] [CrossRef]
- Switon, K.; Kotulska, K.; Janusz-Kaminska, A.; Zmorzynska, J.; Jaworski, J. Molecular Neurobiology of MTOR. Neuroscience 2017, 341, 112–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, L.; Yang, L.; Huang, H.; Liang, F.; Ling, C.; Hu, Y. MTORC1 Is Necessary but MTORC2 and GSK3β Are Inhibitory for AKT3-Induced Axon Regeneration in the Central Nervous System. eLife 2016, 5, e14908. [Google Scholar] [CrossRef] [PubMed]
- Tungsukruthai, S.; Sritularak, B.; Chanvorachote, P. Cycloartobiloxanthone? Nhibits Μigration and Nvasion of Lung Cancer Cells. Anticancer Res. 2017, 37, 6311–6319. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D. MTOR Signaling in Growth, Metabolism and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curatolo, P.; Moavero, R. MTOR Inhibitors in Tuberous Sclerosis Complex. Curr. Neuropharmacol. 2012, 10, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Rabinovsky, E.D. The Multifunctional Role of IGF-1 in Peripheral Nerve Regeneration. Neurol. Res. 2004, 26, 204–210. [Google Scholar] [CrossRef]
- Tuffaha, S.H.; Singh, P.; Budihardjo, J.D.; Means, K.R.; Higgins, J.P.; Shores, J.T.; Salvatori, R.; Höke, A.; Lee, W.P.A.; Brandacher, G. Therapeutic Augmentation of the Growth Hormone Axis to Improve Outcomes Following Peripheral Nerve Injury. Expert Opin. Ther. Targets 2016, 20, 1259–1265. [Google Scholar] [CrossRef]
- Yang, X.; Wei, A.; Liu, Y.; He, G.; Zhou, Z.; Yu, Z. IGF-1 Protects Retinal Ganglion Cells from Hypoxia-Induced Apoptosis by Activating the Erk-1/2 and AKT Pathways. Mol. Vis. 2013, 19, 1901–1912. [Google Scholar]
- Dupraz, S.; Grassi, D.; Karnas, D.; Nieto Guil, A.F.; Hicks, D.; Quiroga, S. The Insulin-Like Growth Factor 1 Receptor Is Essential for Axonal Regeneration in Adult Central Nervous System Neurons. PLoS ONE 2013, 8, e54462. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Cho, K.S.; Li, Y.; Tchedre, K.; Antolik, C.; Ma, J.; Chew, J.; Utheim, T.P.; Huang, X.A.; Yu, H.; et al. IGFBPL1 Regulates Axon Growth through IGF-1-Mediated Signaling Cascades. Sci. Rep. 2018, 8, 2054. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Bianchi, V.E.; Locatelli, V.; Rizzi, L. Neurotrophic and Neuroregenerative Effects of GH/IGF1. Int. J. Mol. Sci. 2017, 18, 2441. [Google Scholar] [CrossRef] [Green Version]
- Slavin, B.R.; Sarhane, K.A.; von Guionneau, N.; Hanwright, P.J.; Qiu, C.; Mao, H.Q.; Höke, A.; Tuffaha, S.H. Insulin-Like Growth Factor-1: A Promising Therapeutic Target for Peripheral Nerve Injury. Front. Bioeng. Biotechnol. 2021, 9, 549. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, E.; Chen, D.F. Emerging Roles for Insulin-like Growth Factor Binding Protein like Protein 1. Neural Regen. Res. 2019, 14, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Quan, A.; Budihardjo, J.; Xiang, S.; Wang, H.; Koshy, K.; Cashman, C.; Lee, W.P.A.; Hoke, A.; Tuffaha, S.; et al. Growth Hormone Improves Nerve Regeneration, Muscle Re-Innervation, and Functional Outcomes After Chronic Denervation Injury. Sci. Rep. 2019, 9, 3117. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.M.; Hur, E.M.; Zhou, F.Q. Coordinating Gene Expression and Axon Assembly to Control Axon Growth: Potential Role of GSK3 Signaling. Front. Mol. Neurosci. 2012, 5, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martelli, A.M.; Paganelli, F.; Evangelisti, C.; Chiarini, F.; McCubrey, J.A. Pathobiology and Therapeutic Relevance of GSK-3 in Chronic Hematological Malignancies. Cells 2022, 11, 1812. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen Synthase Kinase-3 (GSK3): Regulation, Actions, and Diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liz, M.A.; Mar, F.M.; Santos, T.E.; Pimentel, H.I.; Marques, A.M.; Morgado, M.M.; Vieira, S.; Sousa, V.F.; Pemble, H.; Wittmann, T.; et al. Neuronal Deletion of GSK3β Increases Microtubule Speed in the Growth Cone and Enhances Axon Regeneration via CRMP-2 and Independently of MAP1B and CLASP2. BMC Biol. 2014, 12, 47. [Google Scholar] [CrossRef] [Green Version]
- Xing, H.; Lim, Y.A.; Chong, J.R.; Lee, J.H.; Aarsland, D.; Ballard, C.G.; Francis, P.T.; Chen, C.P.; Lai, M.K.P. Increased Phosphorylation of Collapsin Response Mediator Protein-2 at Thr514 Correlates with β-Amyloid Burden and Synaptic Deficits in Lewy Body Dementias. Mol. Brain 2016, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Leibinger, M.; Andreadaki, A.; Golla, R.; Levin, E.; Hilla, A.M.; Diekmann, H.; Fischer, D. Boosting CNS Axon Regeneration by Harnessing Antagonistic Effects of GSK3 Activity. Proc. Natl. Acad. Sci. USA 2017, 114, E5454–E5463. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.I.; Min, J.; Choi, K.H.; Kim, S.W.; Kim, K.S.; Jeon, S.R. Axonal Regeneration Effects of Wnt3a-Secreting Fibroblast Transplantation in Spinal Cord-Injured Rats. Acta Neurochir. 2011, 153, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.L.; Udeh, A.; Kalahasty, K.; Hackam, A.S. A Growing Field: The Regulation of Axonal Regeneration by Wnt Signaling. Neural Regen. Res. 2018, 13, 43–52. [Google Scholar]
- Tassew, N.G.; Charish, J.; Shabanzadeh, A.P.; Luga, V.; Harada, H.; Farhani, N.; D’Onofrio, P.; Choi, B.; Ellabban, A.; Nickerson, P.E.B.; et al. Exosomes Mediate Mobilization of Autocrine Wnt10b to Promote Axonal Regeneration in the Injured CNS. Cell Rep. 2017, 20, 99–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wang, X.; Lu, C.C.; Sherman-Kermen, R.; Steward, O.; Xu, X.M.; Zou, Y. Repulsive Wnt Signaling Inhibits Axon Regeneration after CNS Injury. J. Neurosci. 2008, 28, 8376–8382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yam, P.T.; Charron, F. Signaling Mechanisms of Non-Conventional Axon Guidance Cues: The Shh, BMP and Wnt Morphogens. Curr. Opin. Neurobiol. 2013, 23, 965–973. [Google Scholar] [CrossRef]
- Makoukji, J.; Belle, M.; Meffre, D.; Stassart, R.; Grenier, J.; Shackleford, G.; Fledrich, R.; Fonte, C.; Branchu, J.; Goulard, M.; et al. Lithium Enhances Remyelination of Peripheral Nerves. Proc. Natl. Acad. Sci. USA 2012, 109, 3973–3978. [Google Scholar] [CrossRef] [Green Version]
- Weng, J.; Wang, Y.H.; Li, M.; Zhang, D.Y.; Jiang, B.G. GSK3β Inhibitor Promotes Myelination and Mitigates Muscle Atrophy after Peripheral Nerve Injury. Neural Regen. Res. 2018, 13, 324–330. [Google Scholar] [CrossRef]
- Zhao, A.; Yang, L.; Ma, K.; Sun, M.; Li, L.; Huang, J.; Li, Y.; Zhang, C.; Li, H.; Fu, X. Overexpression of Cyclin D1 Induces the Reprogramming of Differentiated Epidermal Cells into Stem Cell-like Cells. Cell Cycle 2016, 15, 644–653. [Google Scholar] [CrossRef]
- Chen, Y.; Weng, J.; Han, D.; Chen, B.; Ma, M.; Yu, Y.; Li, M.; Liu, Z.; Zhang, P.; Jiang, B. GSK3β Inhibition Accelerates Axon Debris Clearance and New Axon Remyelination. Am. J. Transl. Res. 2016, 8, 5410–5420. [Google Scholar]
- Brack, A.S.; Fabienne Murphy-Seiler, J.H.; Deka, J.; Eyckerman, S.; Keller, C.; Aguet, M.; Rando, T.A. BCL9 Is an Essential Component of Canonical Wnt Signaling That Mediates the Differentiation of Myogenic Progenitors during Muscle Regeneration. Dev. Biol. 2009, 335, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Otto, A.; Schmidt, C.; Luke, G.; Allen, S.; Valasek, P.; Muntoni, F.; Lawrence-Watt, D.; Patel, K. Canonical Wnt Signalling Induces Satellite-Cell Proliferation during Adult Skeletal Muscle Regeneration. J. Cell Sci. 2008, 121, 2939–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czeh, M.; Gressens, P.; Kaindl, A.M. The Yin and Yang of Microglia. Dev. Neurosci. 2011, 33, 199–209. [Google Scholar] [CrossRef]
- Zheng, R.; Lee, K.; Qi, Z.; Wang, Z.; Xu, Z.; Wu, X.; Mao, Y. Neuroinflammation Following Traumatic Brain Injury: Take It Seriously or Not. Front. Immunol. 2022, 13, 855701. [Google Scholar] [CrossRef]
- Liu Lee, D.; Edwards-Faret, G.; Tapia, V.S.; Larraín, J. Spinal Cord Regeneration: Lessons for Mammals from Non-Mammalian Vertebrates. Genesis 2013, 51, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Bollaerts, I.; Van Houcke, J.; Andries, L.; De Groef, L.; Moons, L. Neuroinflammation as Fuel for Axonal Regeneration in the Injured Vertebrate Central Nervous System. Mediators Inflamm. 2017, 2017, 9478542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicard, A.; Yvon, M.; Timchenko, T.; Gronenborn, B.; Michalakis, Y.; Gutierrez, S.; Blanc, S. Gene Copy Number Is Differentially Regulated in a Multipartite Virus. Nat. Commun. 2013, 4, 2248. [Google Scholar] [CrossRef] [Green Version]
- Roberts, T.C.; Wood, M.J.A. Therapeutic Targeting of Non-Coding RNAs. Essays Biochem. 2013, 54, 127–145. [Google Scholar] [CrossRef] [Green Version]
- Mak, H.; Leung, C. MicroRNA-Based Therapeutics for Optic Neuropathy: Opportunities and Challenges. Neural Regen. Res. 2021, 16, 1996–1997. [Google Scholar] [CrossRef]
- Jinek, M.; Doudna, J.A. A Three-Dimensional View of the Molecular Machinery of RNA Interference. Nature 2009, 457, 405–412. [Google Scholar] [CrossRef]
- Eacker, S.M.; Dawson, T.M.; Dawson, V.L. Understanding MicroRNAs in Neurodegeneration. Nat. Rev. Neurosci. 2009, 10, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, W.; Wang, S.; Xie, W.; Li, H.; Ning, B. MicroRNA-21 Regulates Astrocytic Reaction Post-Acute Phase of Spinal Cord Injury through Modulating TGF-ß Signaling. Aging 2018, 10, 1474–1488. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elmagd, M.; Goljanek Whysall, K.; Wheeler, G.; Münsterberg, A. Sprouty2 Mediated Tuning of Signalling Is Essential for Somite Myogenesis. BMC Med. Genom. 2015, 8, S8. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.Q.; Yang, X.W.; Chen, Y.B.; Zhang, D.W.; Jiang, X.F.; Xue, P. Exosomal MiR-21 Regulates the TETs/PTENp1/PTEN Pathway to Promote Hepatocellular Carcinoma Growth. Mol. Cancer 2019, 18, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, X.J.; Lu, X.H.; Luo, J.C.; Chen, C.; Gao, Q.; Li, Z.Y.; Wang, H. Molecular Mechanism of MicroRNA-21 Promoting Schwann Cell Proliferation and Axon Regeneration during Injured Nerve Repair. RNA Biol. 2020, 17, 1508–1519. [Google Scholar] [CrossRef]
- Eva, R.; Fawcett, J. Integrin Signalling and Traffic during Axon Growth and Regeneration. Curr. Opin. Neurobiol. 2014, 27, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Cheah, M.; Andrews, M.R. Integrin Activation: Implications for Axon Regeneration. Cells 2018, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Colognato, H.; Tzvetanova, I.D. Glia Unglued: How Signals from the Extracellular Matrix Regulate the Development of Myelinating Glia. Dev. Neurobiol. 2011, 71, 924–955. [Google Scholar] [CrossRef] [Green Version]
- Rognoni, E.; Ruppert, R.; Fässler, R. The Kindlin Family: Functions, Signaling Properties and Implications for Human Disease. J. Cell Sci. 2016, 129, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Tucker, R.P.; Chiquet-Ehrismann, R. Tenascin-C: Its Functions as an Integrin Ligand. Int. J. Biochem. Cell Biol. 2015, 65, 165–168. [Google Scholar] [CrossRef]
- Reinhard, J.; Roll, L.; Faissner, A. Tenascins in Retinal and Optic Nerve Neurodegeneration. Front. Integr. Neurosci. 2017, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.R.; Czvitkovich, S.; Dassie, E.; Vogelaar, C.F.; Faissner, A.; Blits, B.; Gage, F.H.; Ffrench-Constant, C.; Fawcett, J.W. A9 Integrin Promotes Neurite Outgrowth on Tenascin-C and Enhances Sensory Axon Regeneration. J. Neurosci. 2009, 29, 5546–5557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, M.R.; Soleman, S.; Cheah, M.; Tumbarello, D.A.; Mason, M.R.J.; Moloney, E.; Verhaagen, J.; Bensadoun, J.C.; Schneider, B.; Aebischer, P.; et al. Axonal Localization of Integrins in the CNS Is Neuronal Type and Age Dependent. eNeuro 2016, 3, 5546–5557. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W. An Integrin Approach to Axon Regeneration. Eye 2017, 31, 206–208. [Google Scholar] [CrossRef]
- Goshima, Y.; Sasaki, Y.; Yamashita, N.; Nakamura, F. Class 3 Semaphorins as a Therapeutic Target. Expert Opin. Ther. Targets 2012, 16, 933–944. [Google Scholar] [CrossRef]
- Nieuwenhuis, B.; Haenzi, B.; Andrews, M.R.; Verhaagen, J.; Fawcett, J.W. Integrins Promote Axonal Regeneration after Injury of the Nervous System. Biol. Rev. 2018, 93, 1339–1362. [Google Scholar] [CrossRef]
- Rao, S.N.R.; Pearse, D.D. Regulating Axonal Responses to Injury: The Intersection between Signaling Pathways Involved in Axon Myelination and the Inhibition of Axon Regeneration. Front. Mol. Neurosci. 2016, 9, 33. [Google Scholar] [CrossRef]
- Sakai, Y.; Tsunekawa, M.; Ohta, K.; Shimizu, T.; Pastuhov, S.I.; Hanafusa, H.; Hisamoto, N.; Matsumoto, K. The Integrin Signaling Network Promotes Axon Regeneration via the Src–Ephexin–RhoA GTPase Signaling Axis. J. Neurosci. 2021, 41, 4754–4767. [Google Scholar] [CrossRef]
- Wu, X.; Xu, X.M. RhoA/Rho Kinase in Spinal Cord Injury. Neural Regen. Res. 2016, 11, 23–27. [Google Scholar] [CrossRef]
- Govek, E.E.; Newey, S.E.; Van Aelst, L. The Role of the Rho GTPases in Neuronal Development. Genes Dev. 2005, 19, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Joshi, A.R.; Bobylev, I.; Zhang, G.; Sheikh, K.A.; Lehmann, H.C. Inhibition of Rho-Kinase Differentially Affects Axon Regeneration of Peripheral Motor and Sensory Nerves. Exp. Neurol. 2014, 263, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.; Hilton, B.J.; Burnside, E.R.; Dupraz, S.; Handley, E.E.; Gonyer, J.M.; Brakebusch, C.; Bradke, F. RhoA Drives Actin Compaction to Restrict Axon Regeneration and Astrocyte Reactivity after CNS Injury. Neuron 2021, 109, 3436–3455.e9. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.C.; Tatenhorst, L.; Roser, A.E.; Saal, K.A.; Tönges, L.; Lingor, P. ROCK Inhibition in Models of Neurodegeneration and Its Potential for Clinical Translation. Pharmacol. Ther. 2018, 189, 1–21. [Google Scholar] [CrossRef]
- de Sousa, G.R.; Vieira, G.M.; das Chagas, P.F.; Pezuk, J.A.; Brassesco, M.S. Should We Keep Rocking? Portraits from Targeting Rho Kinases in Cancer. Pharmacol. Res. 2020, 160, 105093. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.B.; Zhong, Y.S.; Cheng, Y.; Shen, X. Rho/ROCK Pathway and Neural Regeneration: A Potential Therapeutic Target for Central Nervous System and Optic Nerve Damage. Int. J. Ophthalmol. 2011, 4, 652–657. [Google Scholar] [CrossRef]
- Koch, J.C.; Tönges, L.; Barski, E.; Michel, U.; Bähr, M.; Lingor, P. ROCK2 Is a Major Regulator of Axonal Degeneration, Neuronal Death and Axonal Regeneration in the CNS. Cell Death Dis. 2014, 5, e1225. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Wen, J.; Chen, Z. Distinct Roles of ROCK1 and ROCK2 on the Cerebral Ischemia Injury and Subsequently Neurodegenerative Changes. Pharmacology 2020, 105, 3–8. [Google Scholar] [CrossRef]
- Oka, M.; Fagan, K.A.; Jones, P.L.; McMurtry, I.F. Therapeutic Potential of RhoA/Rho Kinase Inhibitors in Pulmonary Hypertension. Br. J. Pharmacol. 2008, 155, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Joshi, A.R.; Muke, I.; Bobylev, I.; Lehmann, H.C. ROCK Inhibition Improves Axonal Regeneration in a Preclinical Model of Amyotrophic Lateral Sclerosis. J. Comp. Neurol. 2019, 527, 2334–2340. [Google Scholar] [CrossRef]
- Li, R.; Li, D.H.; Zhang, H.Y.; Wang, J.; Li, X.K.; Xiao, J. Growth Factors-Based Therapeutic Strategies and Their Underlying Signaling Mechanisms for Peripheral Nerve Regeneration. Acta Pharmacol. Sin. 2020, 41, 1289–1300. [Google Scholar] [CrossRef]
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.J.; Kudo, A. The Role of Periostin in Tissue Remodeling across Health and Disease. Cell. Mol. Life Sci. 2014, 71, 1279–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, T.; Kii, I.; Kashima, T.G.; Kikuchi, Y.; Ohazama, A.; Shimazaki, M.; Fukayama, M.; Kudo, A. Delayed Re-Epithelialization in Periostin-Deficient Mice during Cutaneous Wound Healing. PLoS ONE 2011, 6, e18410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.Q.; Zheng, H.Y.; Peng, C.X.; Liu, D.; Li, H.A.; Wang, Q.; Wang, J.Z. Protein Phosphatase 2A Facilitates Axonogenesis by Dephosphorylating CRMP2. J. Neurosci. 2010, 30, 3839–3848. [Google Scholar] [CrossRef]
- Shih, C.H.; Lacagnina, M.; Leuer-Bisciotti, K.; Pröschel, C. Astroglial-Derived Periostin Promotes Axonal Regeneration after Spinal Cord Injury. J. Neurosci. 2014, 34, 2438–2443. [Google Scholar] [CrossRef] [Green Version]
- Li, F.Q.; Fowler, K.A.; Neil, J.E.; Colton, C.A.; Vitek, M.P. An Apolipoprotein E-Mimetic Stimulates Axonal Regeneration and Remyelination after Peripheral Nerve Injury. J. Pharmacol. Exp. Ther. 2010, 334, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Kii, I.; Nishiyama, T.; Li, M.; Matsumoto, K.I.; Saito, M.; Amizuka, N.; Kudo, A. Incorporation of Tenascin-C into the Extracellular Matrix by Periostin Underlies an Extracellular Meshwork Architecture. J. Biol. Chem. 2010, 285, 2028–2039. [Google Scholar] [CrossRef] [Green Version]
- Yokota, K.; Kobayakawa, K.; Saito, T.; Hara, M.; Kijima, K.; Ohkawa, Y.; Harada, A.; Okazaki, K.; Ishihara, K.; Yoshida, S.; et al. Periostin Promotes Scar Formation through the Interaction between Pericytes and Infiltrating Monocytes/Macrophages after Spinal Cord Injury. Am. J. Pathol. 2017, 187, 639–653. [Google Scholar] [CrossRef] [Green Version]
- Kawakita, F.; Suzuki, H. Periostin in Cerebrovascular Disease. Neural Regen. Res. 2020, 15, 63–64. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akram, R.; Anwar, H.; Javed, M.S.; Rasul, A.; Imran, A.; Malik, S.A.; Raza, C.; Khan, I.U.; Sajid, F.; Iman, T.; et al. Axonal Regeneration: Underlying Molecular Mechanisms and Potential Therapeutic Targets. Biomedicines 2022, 10, 3186. https://doi.org/10.3390/biomedicines10123186
Akram R, Anwar H, Javed MS, Rasul A, Imran A, Malik SA, Raza C, Khan IU, Sajid F, Iman T, et al. Axonal Regeneration: Underlying Molecular Mechanisms and Potential Therapeutic Targets. Biomedicines. 2022; 10(12):3186. https://doi.org/10.3390/biomedicines10123186
Chicago/Turabian StyleAkram, Rabia, Haseeb Anwar, Muhammad Shahid Javed, Azhar Rasul, Ali Imran, Shoaib Ahmad Malik, Chand Raza, Ikram Ullah Khan, Faiqa Sajid, Tehreem Iman, and et al. 2022. "Axonal Regeneration: Underlying Molecular Mechanisms and Potential Therapeutic Targets" Biomedicines 10, no. 12: 3186. https://doi.org/10.3390/biomedicines10123186
APA StyleAkram, R., Anwar, H., Javed, M. S., Rasul, A., Imran, A., Malik, S. A., Raza, C., Khan, I. U., Sajid, F., Iman, T., Sun, T., Han, H. S., & Hussain, G. (2022). Axonal Regeneration: Underlying Molecular Mechanisms and Potential Therapeutic Targets. Biomedicines, 10(12), 3186. https://doi.org/10.3390/biomedicines10123186






