Colocalization Analysis of Cytoplasmic Actin Isoforms Distribution in Endothelial Cells
Abstract
1. Introduction
2. Results and Discussion
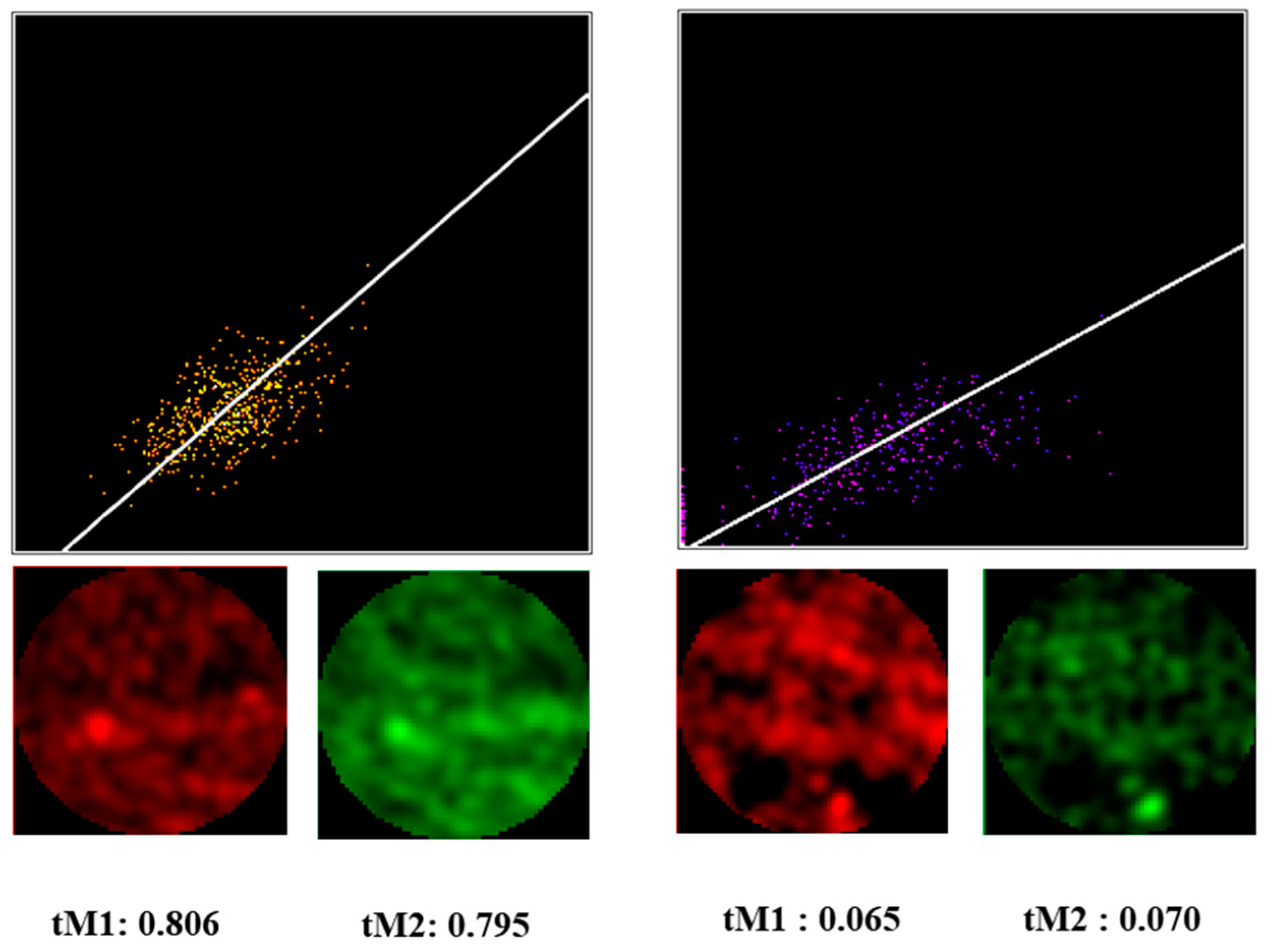
2.1. Selection of a Correlation Analysis Method
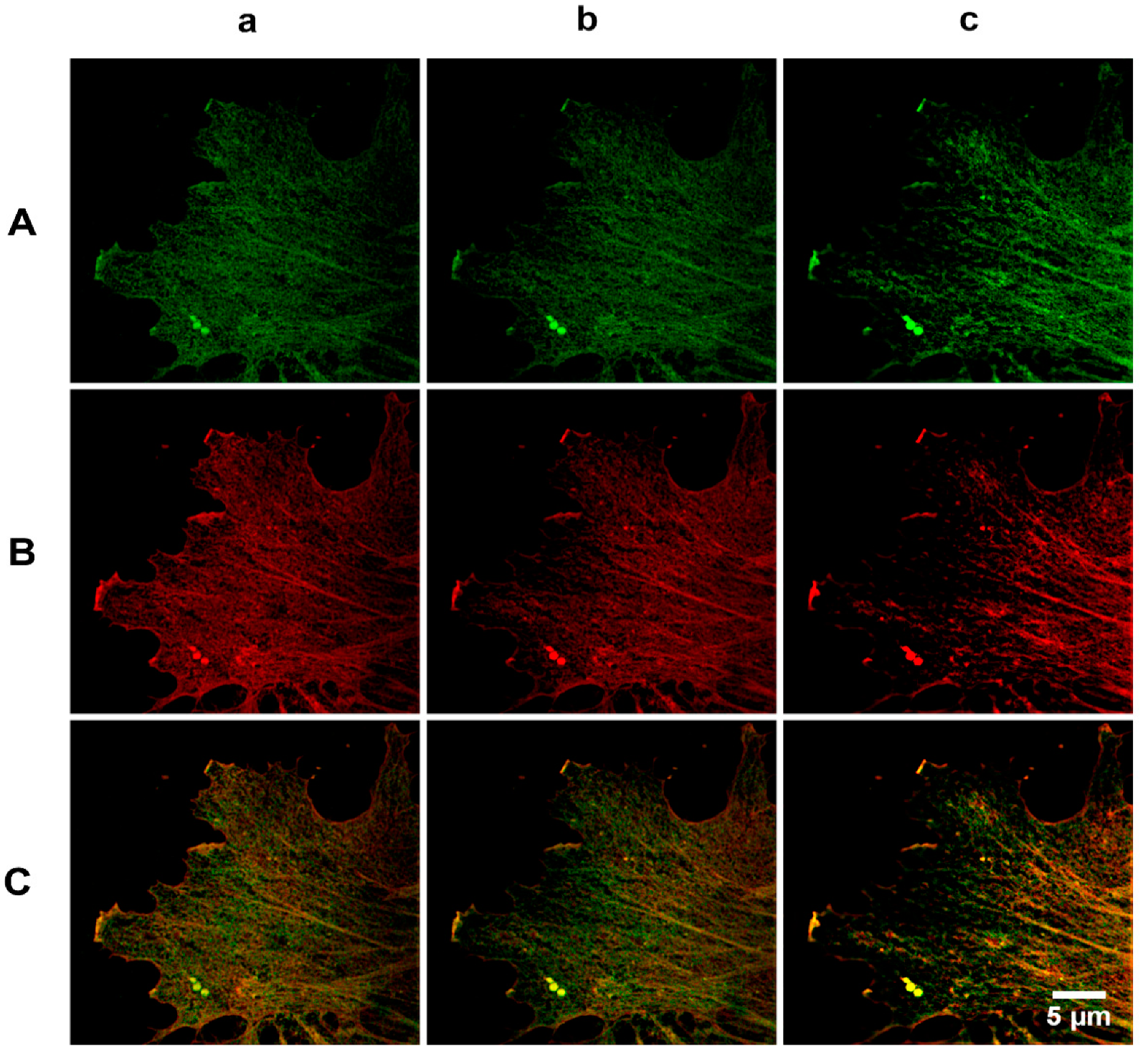
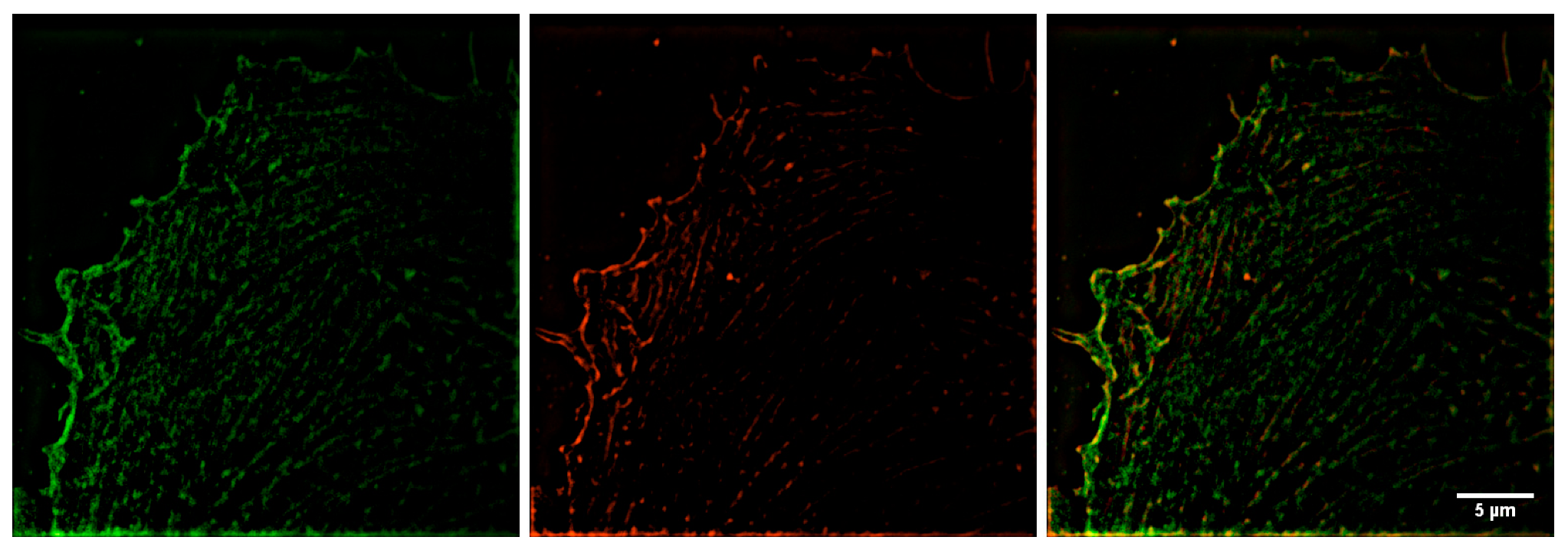
2.2. Analysis of β- and γ-Actin Colocalization at the Cell Edge
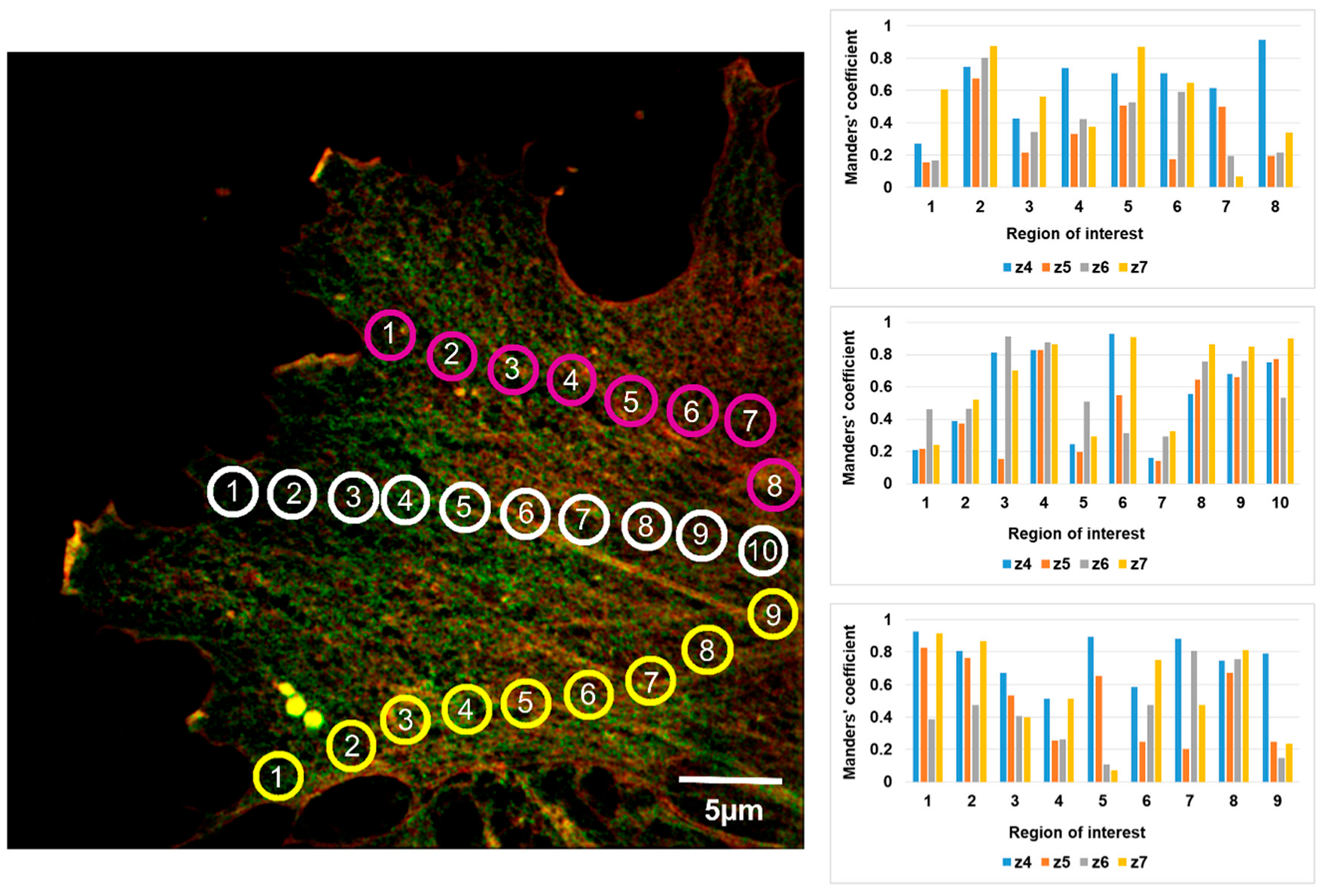
2.3. Analysis of β- and γ-Actin Colocalization at Different Optical Sections of Artery and Vein Endothelial Cells
2.4. Segregation of β- and γ-Actin in the Nocodazole-Induced Endothelial Barrier Dysfunction
3. Materials and Methods
3.1. Cell Cultures and Treatment
3.2. Immunofluorescence
3.3. Correlation Analysis of Cytoplasmic Actin Isoforms Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dugina, V.; Zwaenepoel, I.; Gabbiani, G.; Clement, S.; Chaponnier, C. β- and γ-cytoplasmic actins display distinct distribution and functional diversity. J. Cell Sci. 2009, 122, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Latham, S.L.; Chaponnier, C.; Dugina, V.; Couraud, P.-O.; Grau, G.E.R.; Combes, V. Cooperation between β- and γ-cytoplasmic actins in the mechanical regulation of endothelial microparticle formation. FASEB J. 2013, 27, 672–683. [Google Scholar] [CrossRef]
- Shakhov, A.S.; Verin, A.D.; Alieva, I.B. Reorganization of endothelial cells cytoskeleton during formation of functional monolayer in vitro. Cell Tissue Biol. 2014, 8, 138–151. [Google Scholar] [CrossRef]
- Shakhov, A.S.; Dugina, V.B.; Alieva, I.B. Reorganization of actin and microtubule systems in human vein endothelial cells during intercellular contact formation. Cell Tissue Biol. 2015, 9, 299–309. [Google Scholar] [CrossRef]
- Dominguez, R.; Holmes, K.C. Actin structure and function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Rottner, K.; Faix, J.; Bogdan, S.; Linder, S.; Kerkhoff, E. Actin assembly mechanisms at a glance. J. Cell Sci. 2017, 130, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Vandekerckhove, J.; Weber, K. At least six different actins are expressed in a higher mammal: An analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J. Mol. Biol. 1978, 126, 783–802. [Google Scholar] [CrossRef]
- Ampe, C.; Van Troys, M. Mammalian Actins: Isoform-Specific Functions and Diseases. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–37. ISBN 978-3-319-29806-1. [Google Scholar]
- Müller, M.; Diensthuber, R.P.; Chizhov, I.; Claus, P.; Heissler, S.M.; Preller, M.; Taft, M.H.; Manstein, D.J. Distinct Functional Interactions between Actin Isoforms and Nonsarcomeric Myosins. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Pasquier, E.; Tuset, M.-P.; Sinnappan, S.; Carnell, M.; Macmillan, A.; Kavallaris, M. γ-Actin plays a key role in endothelial cell motility and neovessel maintenance. Vasc. Cell 2015, 7, 2. [Google Scholar] [CrossRef]
- Peñarrubia, P.G.; Ruiz, X.F.; Gálvez, J. Quantitative analysis of the factors that affect the determination of colocalization coefficients in dual-color confocal images. IEEE Trans. Image Process. 2005, 14, 1151–1158. [Google Scholar] [CrossRef]
- Oheim, M.; Li, D. Quantitative Colocalisation Imaging: Concepts, Measurements, and Pitfalls. In Quantitative Colocalisation Imaging: Concepts, Measurements, and Pitfalls; Shorte, S.L., Frischknecht, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 117–155. ISBN 978-3-540-71330-2. [Google Scholar]
- Nakamura, K.; Watakabe, A.; Hioki, H.; Fujiyama, F.; Tanaka, Y.; Yamamori, T.; Kaneko, T. Transiently increased colocalization of vesicular glutamate transporters 1 and 2 at single axon terminals during postnatal development of mouse neocortex: A quantitative analysis with correlation coefficient. Eur. J. Neurosci. 2007, 26, 3054–3067. [Google Scholar] [CrossRef] [PubMed]
- Zinchuk, V.; Zinchuk, O.; Okada, T. Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: Pushing pixels to explore biological phenomena. Acta Histochem. Cytochem. 2007, 40, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.; Pagakis, S.N.; Parmryd, I. Replicate-based noise corrected correlation for accurate measurements of colocalization. J. Microsc. 2008, 230, 121–133. [Google Scholar] [CrossRef]
- French, A.P.; Mills, S.; Swarup, R.; Bennett, M.J.; Pridmore, T.P. Colocalization of fluorescent markers in confocal microscope images of plant cells. Nat. Protoc. 2008, 3, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Zinchuk, V.; Grossenbacher-Zinchuk, O. Recent advances in quantitative colocalization analysis: Focus on neuroscience. Prog. Histochem. Cytochem. 2009, 44, 125–172. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.; Parmryd, I. Quantifying colocalization by correlation: The pearson correlation coefficient is superior to the Mander’s overlap coefficient. Cytom. Part A 2010, 77, 733–742. [Google Scholar] [CrossRef]
- Theart, R.P.; Loos, B.; Niesler, T.R. Virtual reality assisted microscopy data visualization and colocalization analysis. BMC Bioinform. 2017, 18, 1–16. [Google Scholar] [CrossRef]
- Theart, R.P.; Loos, B.; Powrie, Y.S.L.; Niesler, T.R. Improved region of interest selection and colocalization analysis in three-dimensional fluorescence microscopy samples using virtual reality. PLoS ONE 2018, 13, e0201965. [Google Scholar] [CrossRef]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 1993, 169, 375–382. [Google Scholar] [CrossRef]
- Birukova, A.A.; Smurova, K.; Birukov, K.G.; Usatyuk, P.; Liu, F.; Kaibuchi, K.; Ricks-Cord, A.; Natarajan, V.; Alieva, I.; Garcia, J.G.N.; et al. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: Role of Rho-dependent mechanisms. J. Cell Physiol. 2004, 201, 55–70. [Google Scholar] [CrossRef]
- Birukova, A.A.; Birukov, K.G.; Smurova, K.; Adyshev, D.; Kaibuchi, K.; Alieva, I.; Garcia, J.G.N.; Verin, A.D. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J. 2004, 18, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Alieva, I.B.; Zemskov, E.A.; Smurova, K.M.; Kaverina, I.N.; Verin, A.D. The leading role of microtubules in endothelial barrier dysfunction: Disassembly of peripheral microtubules leaves behind the cytoskeletal reorganization. J. Cell Biochem. 2013, 114, 2258–2272. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, S.; Naydenov, N.G.; Harris, G.; Dugina, V.; Morgan, K.G.; Chaponnier, C.; Ivanov, A.I. Nonredundant roles of cytoplasmic β- and γ-actin isoforms in regulation of epithelial apical junctions. Mol. Biol. Cell 2012, 23, 3542–3553. [Google Scholar] [CrossRef] [PubMed]
- Simiczyjew, A.; Pietraszek-Gremplewicz, K.; Mazur, A.J.; Nowak, D. Are non-muscle actin isoforms functionally equivalent? Histol. Histopathol. 2017, 32, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, S.; Naydenov, N.G.; Feygin, A.; Cruise, M.; Ervasti, J.M.; Ivanov, A.I. Loss of β-Cytoplasmic Actin in the Intestinal Epithelium Increases Gut Barrier Permeability in vivo and Exaggerates the Severity of Experimental Colitis. Front. Cell Dev. Biol. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Shagieva, G.S.; Alieva, I.B.; Chaponnier, C.; Dugina, V.B. Divergent Impact of Actin Isoforms on Division of Epithelial Cells. Biochemistry 2020, 85, 1072–1081. [Google Scholar] [CrossRef]
- Lechuga, S.; Baranwal, S.; Li, C.; Naydenov, N.G.; Kuemmerle, J.F.; Dugina, V.; Chaponnier, C.; Ivanov, A.I. Loss of γ-cytoplasmic actin triggers myofibroblast transition of human epithelial cells. Mol. Biol. Cell 2014, 25, 3133–3146. [Google Scholar] [CrossRef]
- Dugina, V.B.; Shagieva, G.S.; Shakhov, A.S.; Alieva, I.B. The cytoplasmic actins in the regulation of endothelial cell function. Int. J. Mol. Sci. 2021, 22, 7836. [Google Scholar] [CrossRef]
- Dugina, V.; Khromova, N.; Rybko, V.; Blizniukov, O.; Shagieva, G.; Chaponnier, C.; Kopnin, B.; Kopnin, P. Tumor promotion by γ and suppression by β non-muscle actin isoforms. Oncotarget 2015, 6, 14556–14571. [Google Scholar] [CrossRef]
- Dugina, V.; Shagieva, G.; Khromova, N.; Kopnin, P. Divergent impact of actin isoforms on cell cycle regulation. Cell Cycle 2018, 17, 2610–2621. [Google Scholar] [CrossRef]
- Simiczyjew, A.; Mazur, A.J.; Popow-Woźniak, A.; Malicka-Błaszkiewicz, M.; Nowak, D. Effect of overexpression of β- and γ-actin isoforms on actin cytoskeleton organization and migration of human colon cancer cells. Histochem. Cell Biol. 2014, 142, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Simiczyjew, A.; Mazur, A.J.; Ampe, C.; Malicka-Błaszkiewicz, M.; van Troys, M.; Nowak, D. Active invadopodia of mesenchymally migrating cancer cells contain both β and γ cytoplasmic actin isoforms. Exp. Cell Res. 2015, 339, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Dugina, V.; Alieva, I.; Khromova, N.; Kireev, I.; Gunning, P.W.; Kopnin, P. Interaction of microtubules with the actin cytoskeleton via cross-talk of EB1-containing +TIPs and γ-actin in epithelial cells. Oncotarget 2016, 7, 72699–72715. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakhov, A.S.; Kovaleva, P.A.; Churkina, A.S.; Kireev, I.I.; Alieva, I.B. Colocalization Analysis of Cytoplasmic Actin Isoforms Distribution in Endothelial Cells. Biomedicines 2022, 10, 3194. https://doi.org/10.3390/biomedicines10123194
Shakhov AS, Kovaleva PA, Churkina AS, Kireev II, Alieva IB. Colocalization Analysis of Cytoplasmic Actin Isoforms Distribution in Endothelial Cells. Biomedicines. 2022; 10(12):3194. https://doi.org/10.3390/biomedicines10123194
Chicago/Turabian StyleShakhov, Anton S., Polina A. Kovaleva, Alexandra S. Churkina, Igor I. Kireev, and Irina B. Alieva. 2022. "Colocalization Analysis of Cytoplasmic Actin Isoforms Distribution in Endothelial Cells" Biomedicines 10, no. 12: 3194. https://doi.org/10.3390/biomedicines10123194
APA StyleShakhov, A. S., Kovaleva, P. A., Churkina, A. S., Kireev, I. I., & Alieva, I. B. (2022). Colocalization Analysis of Cytoplasmic Actin Isoforms Distribution in Endothelial Cells. Biomedicines, 10(12), 3194. https://doi.org/10.3390/biomedicines10123194




