Ivermectin Affects Neutrophil-Induced Inflammation through Inhibition of Hydroxylysine but Stimulation of Cathepsin G and Phenylalanine Secretion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Neutrophils
2.3. Quantification of Neutrophil Attachment
2.4. Study of the Morphology of Attached Neutrophils by Scanning Electron Microscopy
2.5. Measuring of Reactive Oxygen Species (ROS) Formation
2.6. Adhesion of Neutrophils to the Fibronectin-Coated Culture Plates and Sampling of Extracellular Medium for Amino Acid and Protein Analysis
2.7. Preparation of Samples for Amino Acid Analysis
2.8. Amino Acid Analysis
2.9. Extraction and Separation Proteins of Extracellular Medium
2.10. Mass Spectrometry Identification of Proteins
2.11. The Effect of Angiotensin II on the Composition of Peptides in the Extracellular Medium of Neutrophils
2.12. Statistics
3. Results
3.1. The Effect of Ivermectin on Neutrophil Morphology and Attachment to the Fibronectin-Coated Substrata
3.2. Reactive Oxygen Species Production by Neutrophils upon Adhesion to Fibronectin in the Presence of Ivermectin
3.3. Effect of Ivermectin on the Composition of Free Amino Acid Secretion by Neutrophils during Adhesion to Fibronectin
3.4. Effect of Ivermectin on the Composition of Protein Secretion by Neutrophils during Adhesion to Fibronectin
3.5. Is the Formation of Phenylalanine in the Extracellular Medium of Neutrophils upon Adhesion to Fibronectin Related to the Processes of Angiotensin Conversation?
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schofield, Z.V.; Woodruff, T.M.; Halai, R.; Wu, M.C.; Cooper, M.A. Neutrophils—A key component of ischemia-reperfusion injury. Shock 2013, 40, 463–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, N. Targeting leukostasis for the treatment of early diabetic retinopathy. Cardiovasc. Hematol. Disord. Drug Targets 2009, 9, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, V.D.; Margaroli, C.; Mall, M.A.; Tirouvanziam, R. Neutrophil Adaptations upon Recruitment to the Lung: New Concepts and Implications for Homeostasis and Disease. Int. J. Mol. Sci. 2020, 21, 851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laval, J.; Ralhan, A.; Hartl, D. Neutrophils in cystic fibrosis. Biol. Chem. 2016, 397, 485–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, D.W.; Gaggar, A.; Solomon, G.M. Neutrophil Fates in Bronchiectasis and Alpha-1 Antitrypsin Deficiency. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S2), S123–S129. [Google Scholar]
- Chiang, C.C.; Korinek, M.; Cheng, W.J.; Hwang, T.L. Targeting Neutrophils to Treat Acute Respiratory Distress Syndrome in Coronavirus Disease. Front. Pharmacol. 2020, 11, 572009. [Google Scholar] [CrossRef]
- Formiga, F.R.; Leblanc, R.; de Souza Reboucas, J.; Farias, L.P.; de Oliveira, R.N.; Pena, L. Ivermectin: An award-winning drug with expected antiviral activity against COVID-19. J. Control. Release Off. J. Control. Release Soc. 2021, 329, 758–761. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, X.; Wu, H.; Li, C.; Zhong, P.; Liu, Z.; Ma, C.; Liu, W.; Wang, C.; Zhang, Y.; et al. Ivermectin contributes to attenuating the severity of acute lung injury in mice. Biomed. Pharmacother. 2022, 155, 113706. [Google Scholar] [CrossRef]
- Arevalo, A.P.; Pagotto, R.; Porfido, J.L.; Daghero, H.; Segovia, M.; Yamasaki, K.; Varela, B.; Hill, M.; Verdes, J.M.; Duhalde Vega, M.; et al. Ivermectin reduces in vivo coronavirus infection in a mouse experimental model. Sci. Rep. 2021, 11, 7132. [Google Scholar] [CrossRef]
- Ahsan, T.; Rani, B.; Siddiqui, R.; Glenis, D.S.; Memon, R.; Lutfi, I.; Hasan, O.I.; Javed, R.; Khan, F.; Hassan, M. Clinical Variants, Characteristics, and Outcomes among COVID-19 Patients: A Case Series Analysis at a Tertiary Care Hospital in Karachi, Pakistan. Cureus 2021, 13, e14761. [Google Scholar] [CrossRef]
- Yang, S.; Shen, S.; Hou, N. Is Ivermectin Effective in Treating COVID-19? Front. Pharmacol. 2022, 13, 858693. [Google Scholar] [CrossRef]
- Angkasekwinai, N.; Rattanaumpawan, P.; Chayakulkeeree, M.; Phoompoung, P.; Koomanachai, P.; Chantarasut, S.; Wangchinda, W.; Srinonprasert, V.; Thamlikitkul, V. Safety and Efficacy of Ivermectin for the Prevention and Treatment of COVID-19: A Double-Blinded Randomized Placebo-Controlled Study. Antibiotics 2022, 11, 796. [Google Scholar] [CrossRef]
- Rezai, M.S.; Ahangarkani, F.; Hill, A.; Ellis, L.; Mirchandani, M.; Davoudi, A.; Eslami, G.; Roozbeh, F.; Babamahmoodi, F.; Rouhani, N.; et al. Non-effectiveness of Ivermectin on Inpatients and Outpatients with COVID-19; Results of Two Randomized, Double-Blinded, Placebo-Controlled Clinical Trials. Front. Med. 2022, 9, 919708. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Hu, X.; Wang, Y.; Yao, X.; Zhang, W.; Yu, C.; Cheng, F.; Li, J.; Fang, Q. Ivermectin, a potential anticancer drug derived from an antiparasitic drug. Pharmacol. Res. 2021, 163, 105207. [Google Scholar] [CrossRef] [PubMed]
- Galkina, S.I.; Fedorova, N.V.; Ksenofontov, A.L.; Stadnichuk, V.I.; Baratova, L.A.; Sud’Ina, G.F. Neutrophils as a source of branched-chain, aromatic and positively charged free amino acids. Cell Adhes. Migr. 2019, 13, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galkina, S.I.; Fedorova, N.V.; Ksenofontov, A.L.; Serebryakova, M.V.; Golenkina, E.A.; Stadnichuk, V.I.; Baratova, L.A.; Sud’ina, G.F. Neutrophil Adhesion and the Release of the Free Amino Acid Hydroxylysine. Cells 2021, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Salo, A.M.; Wang, C.; Sipila, L.; Sormunen, R.; Vapola, M.; Kervinen, P.; Ruotsalainen, H.; Heikkinen, J.; Myllyla, R. Lysyl hydroxylase 3 (LH3) modifies proteins in the extracellular space, a novel mechanism for matrix remodeling. J. Cell. Physiol. 2006, 207, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, H.; Terajima, M.; Banerjee, P.; Liu, X.; Yu, J.; Momin, A.A.; Katayama, H.; Hanash, S.M.; Burns, A.R.; et al. Lysyl Hydroxylase 2 Is Secreted by Tumor Cells and Can Modify Collagen in the Extracellular Space. J. Biol. Chem. 2016, 291, 25799–25808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Ristiluoma, M.M.; Salo, A.M.; Eskelinen, S.; Myllyla, R. Lysyl hydroxylase 3 is secreted from cells by two pathways. J. Cell. Physiol. 2012, 227, 668–675. [Google Scholar] [CrossRef]
- Risteli, M.; Ruotsalainen, H.; Salo, A.M.; Sormunen, R.; Sipila, L.; Baker, N.L.; Lamande, S.R.; Vimpari-Kauppinen, L.; Myllyla, R. Reduction of lysyl hydroxylase 3 causes deleterious changes in the deposition and organization of extracellular matrix. J. Biol. Chem. 2009, 284, 28204–28211. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.H.; Yun, H.S.; Kwon, G.T.; Kim, J.Y.; Lee, C.W.; Song, J.Y.; Um, H.D.; Kang, C.M.; Park, J.K.; Kim, J.S.; et al. PLOD3 promotes lung metastasis via regulation of STAT3. Cell Death Dis. 2018, 9, 1138. [Google Scholar] [CrossRef]
- Baek, J.H.; Yun, H.S.; Kwon, G.T.; Lee, J.; Kim, J.Y.; Jo, Y.; Cho, J.M.; Lee, C.W.; Song, J.Y.; Ahn, J.; et al. PLOD3 suppression exerts an anti-tumor effect on human lung cancer cells by modulating the PKC-delta signaling pathway. Cell Death Dis. 2019, 10, 156. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.K.; Huang, L.C.; Tsai, W.C.; Huang, S.M.; Lee, J.T.; Hueng, D.Y. Overexpression of PLOD3 promotes tumor progression and poor prognosis in gliomas. Oncotarget 2018, 9, 15705–15720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Parag-Sharma, K.; Terajima, M.; Musicant, A.M.; Murphy, R.M.; Ramsey, M.R.; Hibi, H.; Yamauchi, M.; Amelio, A.L. Lysyl hydroxylase 2-induced collagen cross-link switching promotes metastasis in head and neck squamous cell carcinomas. Neoplasia 2021, 23, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Mitsui, A.; Sumardika, I.W.; Yokoyama, Y.; Sakaguchi, M.; Kondo, E. PLOD2-driven IL-6/STAT3 signaling promotes the invasion and metastasis of oral squamous cell carcinoma via activation of integrin beta1. Int. J. Oncol. 2021, 58, 29. [Google Scholar] [CrossRef] [PubMed]
- Cheriyamundath, S.; Kumar, A.; Gavert, N.; Brabletz, T.; Ben-Ze’ev, A. The Collagen-Modifying Enzyme PLOD2 Is Induced and Required during L1-Mediated Colon Cancer Progression. Int. J. Mol. Sci. 2021, 22, 3552. [Google Scholar] [CrossRef]
- Kresse, N.; Schroder, H.; Stein, K.P.; Wilkens, L.; Mawrin, C.; Sandalcioglu, I.E.; Dumitru, C.A. PLOD2 Is a Prognostic Marker in Glioblastoma That Modulates the Immune Microenvironment and Tumor Progression. Int. J. Mol. Sci. 2022, 23, 6037. [Google Scholar] [CrossRef] [PubMed]
- Galkina, S.I.; Fedorova, N.V.; Serebryakova, M.V.; Romanova, J.M.; Golyshev, S.A.; Stadnichuk, V.I.; Baratova, L.A.; Sud’ina, G.F.; Klein, T. Proteome analysis identified human neutrophil membrane tubulovesicular extensions (cytonemes, membrane tethers) as bactericide trafficking. Biochim. Biophys. Acta 2012, 1820, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Galkina, S.I.; Fedorova, N.V.; Serebryakova, M.V.; Arifulin, E.A.; Stadnichuk, V.I.; Baratova, L.A.; Sud’ina, G.F. Mold Alkaloid Cytochalasin D Modifies the Morphology and Secretion of fMLP-, LPS-, or PMA-Stimulated Neutrophils upon Adhesion to Fibronectin. Mediat. Inflamm. 2017, 2017, 4308684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedorova, N.V.; Ksenofontov, A.L.; Serebryakova, M.V.; Stadnichuk, V.I.; Gaponova, T.V.; Baratova, L.A.; Sud’ina, G.F.; Galkina, S.I. Neutrophils Release Metalloproteinases during Adhesion in the Presence of Insulin, but Cathepsin G in the Presence of Glucagon. Mediat. Inflamm. 2018, 2018, 1574928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Cen, J.; Huang, Y.; Shen, H.; Yao, L.; Wang, Y.; Chen, Z. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS ONE 2011, 6, e20599. [Google Scholar] [CrossRef]
- Dejonckheere, E.; Vandenbroucke, R.E.; Libert, C. Matrix metalloproteinases as drug targets in ischemia/reperfusion injury. Drug Discov. Today 2011, 16, 762–778. [Google Scholar]
- Hamada, T.; Fondevila, C.; Busuttil, R.W.; Coito, A.J. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology 2008, 47, 186–198. [Google Scholar] [CrossRef]
- Moon, A.; Gil, S.; Gill, S.E.; Chen, P.; Matute-Bello, G. Doxycycline impairs neutrophil migration to the airspaces of the lung in mice exposed to intratracheal lipopolysaccharide. J. Inflamm. 2012, 9, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galkina, S.I.; Fedorova, N.V.; Serebryakova, M.V.; Arifulin, E.A.; Stadnichuk, V.I.; Gaponova, T.V.; Baratova, L.A.; Sud’ina, G.F. Inhibition of the GTPase dynamin or actin depolymerisation initiates outward plasma membrane tubulation/vesiculation (cytoneme formation) in neutrophils. Biol. Cell Auspices Eur. Cell Biol. Organ. 2015, 107, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Burster, T.; Mustafa, Z.; Myrzakhmetova, D.; Zhanapiya, A.; Zimecki, M. Hindrance of the Proteolytic Activity of Neutrophil-Derived Serine Proteases by Serine Protease Inhibitors as a Management of Cardiovascular Diseases and Chronic Inflammation. Front. Chem. 2021, 9, 784003. [Google Scholar] [CrossRef]
- Mambole, A.; Baruch, D.; Nusbaum, P.; Bigot, S.; Suzuki, M.; Lesavre, P.; Fukuda, M.; Halbwachs-Mecarelli, L. The cleavage of neutrophil leukosialin (CD43) by cathepsin G releases its extracellular domain and triggers its intramembrane proteolysis by presenilin/gamma-secretase. J. Biol. Chem. 2008, 283, 23627–23635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reilly, C.F.; Tewksbury, D.A.; Schechter, N.M.; Travis, J. Rapid conversion of angiotensin I to angiotensin II by neutrophil and mast cell proteinases. J. Biol. Chem. 1982, 257, 8619–8622. [Google Scholar] [CrossRef] [PubMed]
- Ramaha, A.; Patston, P.A. Release and degradation of angiotensin I and angiotensin II from angiotensinogen by neutrophil serine proteinases. Arch. Biochem. Biophys. 2002, 397, 77–83. [Google Scholar] [CrossRef]
- Owen, C.A.; Campbell, E.J. Angiotensin II generation at the cell surface of activated neutrophils: Novel cathepsin G-mediated catalytic activity that is resistant to inhibition. J. Immunol. 1998, 160, 1436–1443. [Google Scholar] [PubMed]
- Ngo, T.T.; Lenhoff, H.M. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal. Biochem. 1980, 105, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Paola, D.D.; Iaria, C.; Marino, F.; Gugliandolo, E.; Piras, C.; Crupi, R.; Cuzzocrea, S.; Spano, N.; Britti, D.; Peritore, A.F. Environmental Impact of Pharmaceutical Pollutants: Synergistic Toxicity of Ivermectin and Cypermethrin. Toxics 2022, 10, 388. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Canga, A.; Sahagun Prieto, A.M.; Jose Diez Liebana, M.; Martinez, N.F.; Vega, M.S.; Vieitez, J.J. The pharmacokinetics and metabolism of ivermectin in domestic animal species. Vet. J. 2009, 179, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Zahner, R.; Busetto, S.; Decleva, E.; Cramer, R.; Dri, P.; Patriarca, P. Triggering of chloride ion efflux from human neutrophils as a novel function of leukocyte beta 2 integrins: Relationship with spreading and activation of the respiratory burst. J. Immunol. 1999, 162, 423–434. [Google Scholar] [PubMed]
- Menegazzi, K.; Robinson, C.; Cave, C.; Williams, M.A.; Lentsch, A.B.; Cuschieri, J.; Solomkin, J.S. NADPH oxidase activation in fibronectin adherent human neutrophils: A potential role for beta1 integrin ligation. Surgery 2003, 134, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Umansky, H.; Schmidtchen, D.; Mutasa, J.A. Ivermectin-induced killing of microfilariae in vitro by neutrophils mediated by NO. Exp. Parasitol. 1997, 86, 110–117. [Google Scholar] [CrossRef]
- Damascena, H.L.; Silveira WA, A.; Castro, M.S.; Fontes, W. Neutrophil Activated by the Famous and Potent PMA (Phorbol Myristate Acetate). Cells 2022, 11, 2889. [Google Scholar] [CrossRef]
- Galkina, S.I.; Fedorova, N.V.; Ksenofontov, A.L.; Golenkina, E.A.; Serebryakova, M.V.; Stadnichuk, V.I.; Baratova, L.A.; Sud’ina, G.F. Inhibitor of Hyaluronic Acid Synthesis 4-Methylumbelliferone Suppresses the Secretory Processes That Ensure the Invasion of Neutrophils into Tissues and Induce Inflammation. Biomedicines 2022, 10, 314. [Google Scholar] [CrossRef]
- Galkina, S.I.; Golenkina, E.A.; Fedorova, N.V.; Ksenofontov, A.L.; Serebryakova, M.V.; Arifulin, E.A.; Stadnichuk, V.I.; Baratova, L.A.; Sud’ina, G.F. Inhibition of Neutrophil Secretion Upon Adhesion as a Basis for the Anti-Inflammatory Effect of the Tricyclic Antidepressant Imipramine. Front. Pharmacol. 2021, 12, 709719. [Google Scholar] [CrossRef]
- Avalos-Gomez, C.; Ramirez-Rico, G.; Ruiz-Mazon, L.; Sicairos, N.L.; Serrano-Luna, J.; de la Garza, M. Lactoferrin: An Effective Weapon in the Battle Against Bacterial Infections. Curr. Pharm. Des. 2022, 28, 3243–3260. [Google Scholar] [PubMed]
- Krzyzowska, M.; Janicka, M.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Celichowski, G.; Grobelny, J.; Szymanski, P. Lactoferrin-Conjugated Nanoparticles as New Antivirals. Pharmaceutics 2022, 14, 1862. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.C.; Girard, D.; Tessier, P.A. Induction of neutrophil degranulation by S100A9 via a MAPK-dependent mechanism. J. Leukoc. Biol. 2010, 87, 905–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, H.M.; Lee, H.J.; Kim, D.C. High expression of S100A8 and S100A9 is associated with poor disease-free survival in patients with cancer: A systematic review and meta-analysis. Transl. Cancer Res. 2021, 10, 3225–3235. [Google Scholar] [CrossRef]
- Lockhart, J.S.; Sumagin, R. Non-Canonical Functions of Myeloperoxidase in Immune Regulation, Tissue Inflammation and Cancer. Int. J. Mol. Sci. 2022, 23, 12250. [Google Scholar] [CrossRef] [PubMed]
- Burster, T.; Macmillan, H.; Hou, T.; Boehm, B.O.; Mellins, E.D. Cathepsin G: Roles in antigen presentation and beyond. Mol. Immunol. 2010, 47, 658–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Mao, X.; Ye, L.; Cheng, H.; Dai, X. The Role of the S100 Protein Family in Glioma. J. Cancer 2022, 13, 3022–3030. [Google Scholar] [CrossRef]
- Mondet, J.; Chevalier, S.; Mossuz, P. Pathogenic Roles of S100A8 and S100A9 Proteins in Acute Myeloid and Lymphoid Leukemia: Clinical and Therapeutic Impacts. Molecules 2021, 26, 1323. [Google Scholar] [CrossRef]
- Vickers, C.; Hales, P.; Kaushik, V.; Dick, L.; Gavin, J.; Tang, J.; Godbout, K.; Parsons, T.; Baronas, E.; Hsieh, F.; et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002, 277, 14838–14843. [Google Scholar] [CrossRef] [Green Version]
- Campbell, W.C. Lessons from the History of Ivermectin and Other Antiparasitic Agents. Annu. Rev. Anim. Biosci. 2016, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mastrangelo, E.; Pezzullo, M.; De Burghgraeve, T.; Kaptein, S.; Pastorino, B.; Dallmeier, K.; de Lamballerie, X.; Neyts, J.; Hanson, A.M.; Frick, D.N.; et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: New prospects for an old drug. J. Antimicrob. Chemother. 2012, 67, 1884–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA J. Am. Med. Assoc. 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M.; Chen, X.; Montaner, L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020, 108, 17–41. [Google Scholar] [CrossRef]
- Aschenbrenner, A.C.; Mouktaroudi, M.; Kramer, B.; Oestreich, M.; Antonakos, N.; Nuesch-Germano, M.; Gkizeli, K.; Bonaguro, L.; Reusch, N.; Bassler, K.; et al. Disease severity-specific neutrophil signatures in blood transcriptomes stratify COVID-19 patients. Genome Med. 2021, 13, 7. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, G.; Zhu, H.; Yu, L.; She, D.; Wei, Y.; Huang, J.; Li, T.; Zhan, S.; Zhou, S.; et al. PLOD2 high expression associates with immune infiltration and facilitates cancer progression in osteosarcoma. Front. Oncol. 2022, 12, 980390. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, S.; Zhang, X.; Wu, Z.; Li, Z.; Ding, Z.; Huang, X.; Chen, S.; Jing, Y.; Zhang, X.; et al. Identification and Validation of PLOD2 as an Adverse Prognostic Biomarker for Oral Squamous Cell Carcinoma. Biomolecules 2021, 11, 1842. [Google Scholar] [CrossRef]
- Dayer, C.; Stamenkovic, I. Recruitment of Matrix Metalloproteinase-9 (MMP-9) to the Fibroblast Cell Surface by Lysyl Hydroxylase 3 (LH3) Triggers Transforming Growth Factor-beta (TGF-beta) Activation and Fibroblast Differentiation. J. Biol. Chem. 2015, 290, 13763–13778. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Zhang, Y.; Liu, K.; Liu, B.; Xu, W.; Gao, J.; Ding, L.; Tao, L. Ivermectin induces cell cycle arrest and apoptosis of HeLa cells via mitochondrial pathway. Cell Prolif. 2019, 52, e12543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancion, A.; Tridetti, J.; Nguyen Trung, M.L.; Oury, C.; Lancellotti, P. A Review of the Role of Bradykinin and Nitric Oxide in the Cardioprotective Action of Angiotensin-Converting Enzyme Inhibitors: Focus on Perindopril. Cardiol. Ther. 2019, 8, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Peach, M.J. Renin-angiotensin system: Biochemistry and mechanisms of action. Physiol. Rev. 1977, 57, 313–370. [Google Scholar] [CrossRef] [PubMed]
- Letko, M.; Marzi, A.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]





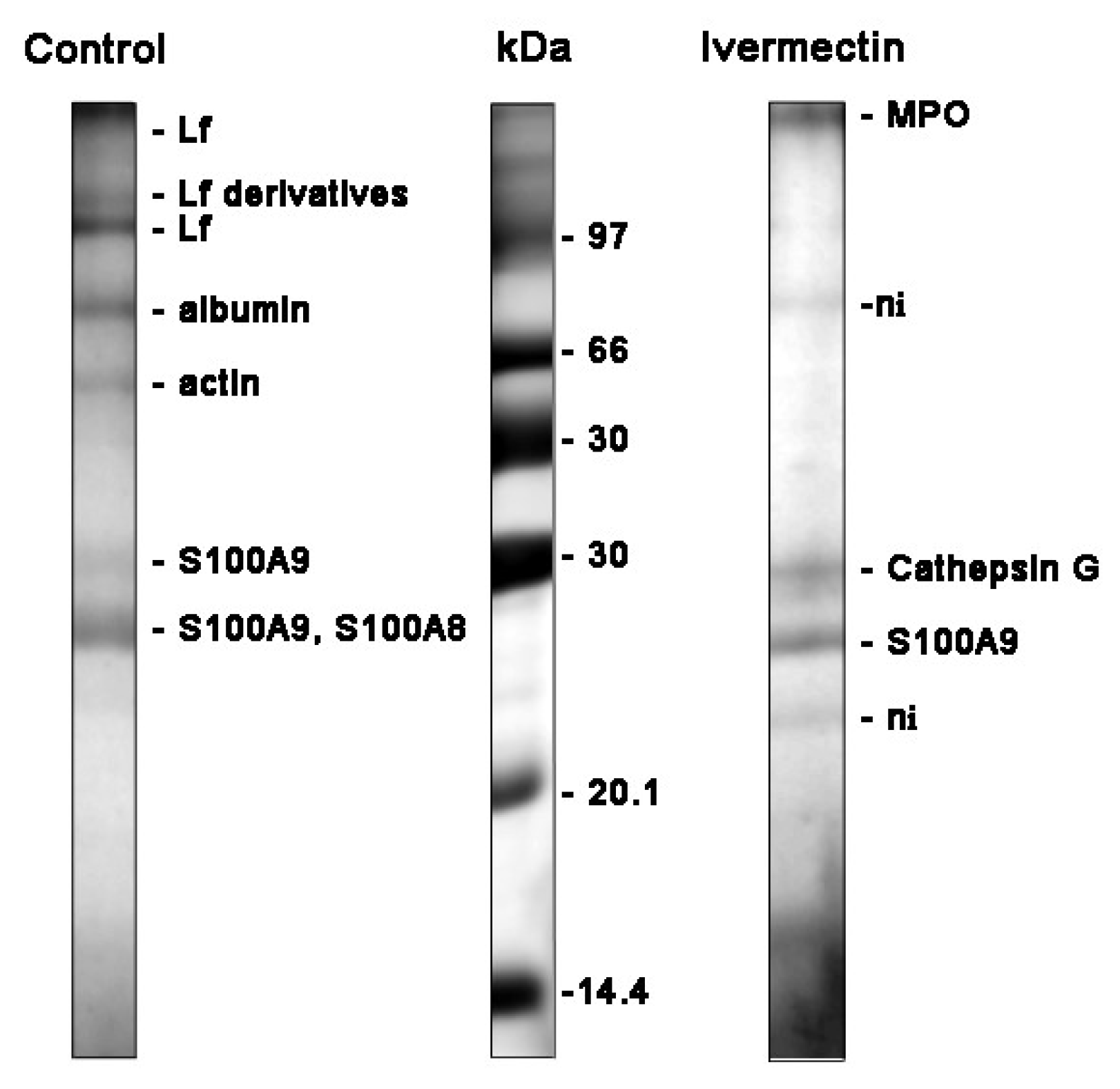

| Treatment | Protein Name | Peptides Matched/ Total | Coverage % | MOWSE Score | |
|---|---|---|---|---|---|
| control | TRFL_HUMAN | LF | 18/28 | 23 | 141 |
| ALBU_HUMAN | albumin | 23/56 | 49 | 115 | |
| AKTB_HUMAN | actin | 2/74 | 27 | 72 | |
| S10A9_HUMAN | S100-A9 | 7/24 | 60 | 96 | |
| S10A8_HUMAN | S100-A8 | 6/24 | 45 | 75 | |
| ivermectin | PERM_HUMAN | MPO | 13/38 | 14 | 79 |
| CATG_HUMAN | cathepsin G | 8/21 | 20 | 72 | |
| S10A9_HUMAN | S100-A9 | 4/50 | 33 | 30 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galkina, S.I.; Golenkina, E.A.; Serebryakova, M.V.; Fedorova, N.V.; Ksenofontov, A.L.; Stadnichuk, V.I.; Sud’ina, G.F. Ivermectin Affects Neutrophil-Induced Inflammation through Inhibition of Hydroxylysine but Stimulation of Cathepsin G and Phenylalanine Secretion. Biomedicines 2022, 10, 3284. https://doi.org/10.3390/biomedicines10123284
Galkina SI, Golenkina EA, Serebryakova MV, Fedorova NV, Ksenofontov AL, Stadnichuk VI, Sud’ina GF. Ivermectin Affects Neutrophil-Induced Inflammation through Inhibition of Hydroxylysine but Stimulation of Cathepsin G and Phenylalanine Secretion. Biomedicines. 2022; 10(12):3284. https://doi.org/10.3390/biomedicines10123284
Chicago/Turabian StyleGalkina, Svetlana I., Ekaterina A. Golenkina, Marina V. Serebryakova, Natalia V. Fedorova, Alexander L. Ksenofontov, Vladimir I. Stadnichuk, and Galina F. Sud’ina. 2022. "Ivermectin Affects Neutrophil-Induced Inflammation through Inhibition of Hydroxylysine but Stimulation of Cathepsin G and Phenylalanine Secretion" Biomedicines 10, no. 12: 3284. https://doi.org/10.3390/biomedicines10123284
APA StyleGalkina, S. I., Golenkina, E. A., Serebryakova, M. V., Fedorova, N. V., Ksenofontov, A. L., Stadnichuk, V. I., & Sud’ina, G. F. (2022). Ivermectin Affects Neutrophil-Induced Inflammation through Inhibition of Hydroxylysine but Stimulation of Cathepsin G and Phenylalanine Secretion. Biomedicines, 10(12), 3284. https://doi.org/10.3390/biomedicines10123284







