Colitis and Colorectal Carcinogenesis: The Focus on Isolated Lymphoid Follicles
Abstract
:1. Introduction
2. Organization and Commensal Bacteria of the Gut-Associated Lymphoid Tissue
3. Innate Lymphoid Cells of the Subepithelial Compartment
4. Innervation and Vascularization of Isolated Lymphoid Follicles
5. Composition and Immune Function of Isolated Lymphoid Follicles
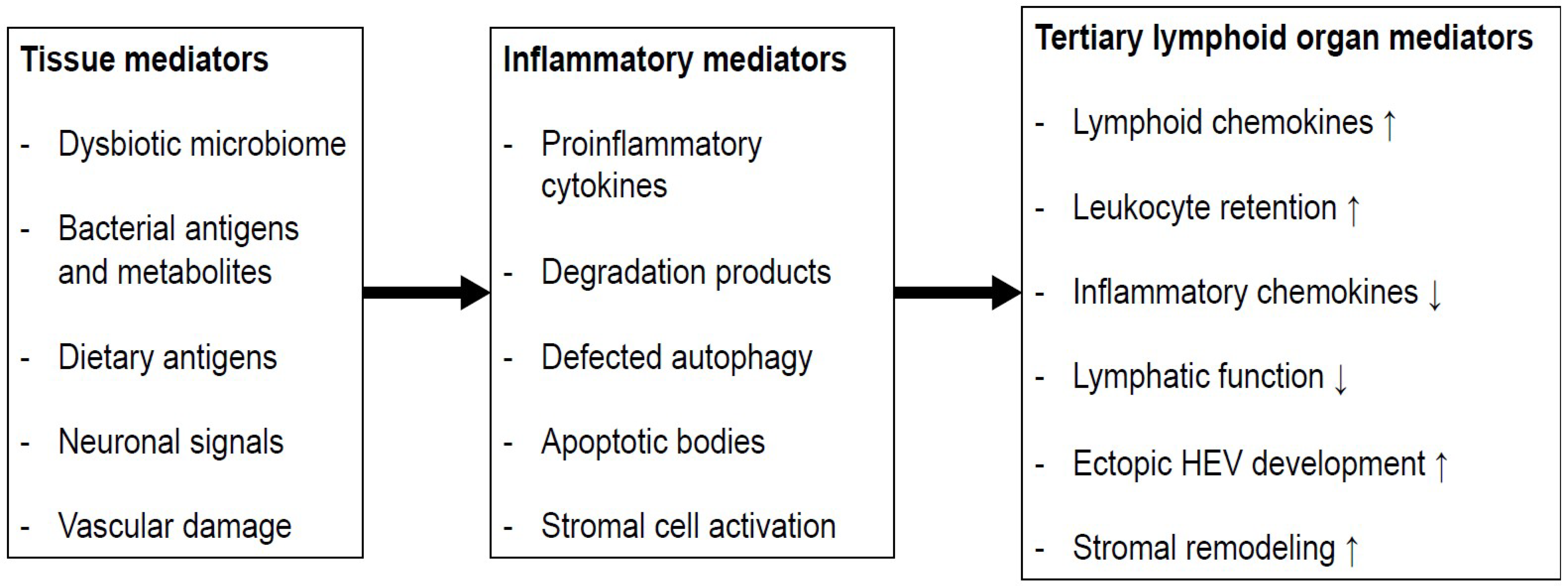
6. Isolated Lymphoid Follicles in Colonic Inflammation
7. Isolated Lymphoid Follicles and Tertiary Lymphoid Organs in Colorectal Carcinogenesis
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heel, K.A.; McCauley, R.D.; Papadimitriou, J.M.; Hall, J.C. Rev. peyer’s patches. J. Gastroenterol. Hepatol. 1997, 12, 122–136. [Google Scholar] [CrossRef]
- Brandtzaeg, P.; Kiyono, H.; Pabst, R.; Russell, M.W. Terminology: Nomenclature of mucosa-associated lymphoid tissue. Mucosal. Immunol. 2008, 1, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Warger, T.; Schild, H.; Rechtsteiner, G. Initiation of adaptive immune responses by transcutaneous immunization. Immunol. Let. 2007, 109, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Kogame, T.; Kabashima, K.; Egawa, G. Putative immunological functions of inducible skin—Associated lymphoid tissue in the context of mucosa-associated lymphoid tissue. Front. Immunol. 2021, 12, 733484. [Google Scholar] [CrossRef] [PubMed]
- Millonig, G.; Schwentner, C.; Mueller, P.; Mayerl, C.; Wick, G. The vascular-associated lymphoid tissue: A new site of local immunity. Curr. Opin. Lipidol. 2001, 12, 547–553. [Google Scholar] [CrossRef]
- Masum, M.A.; Ichii, O.; Elewa, Y.H.A.; Otani, Y.; Namba, T.; Kon, Y. Vasculature-associated lymphoid tissue: A unique tertiary lymphoid tissue correlates with renal lesions in lupus nephritis mouse model. Front. Immunol. 2020, 11, 595672. [Google Scholar] [CrossRef]
- Neutra, M.R.; Mantis, N.J.; Kraehenbuhl, J.P. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 2001, 2, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Senda, T.; Dogra, P.; Granot, T.; Furuhashi, K.; Snyder, M.E.; Carpenter, D.J.; Szabo, P.A.; Thapa, P.; Miron, M.; Farber, D.L. Microanatomical dissection of human intestinal T-cell immunity reveals site-specific changes in gut-associated lymphoid tissues over life. Mucosal Immunol. 2019, 12, 378–389. [Google Scholar] [CrossRef] [Green Version]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.M.; Fitzpatrick, E.; Baird, A.W.; Campion, D.P. Eosinophil-nerve interactions and neuronal plasticity in rat gut associated lymphoid tissue (GALT) in response to enteric parasitism. J. Neuroimmunol. 2008, 197, 1–9. [Google Scholar] [CrossRef]
- Neutra, M.R.; Frey, A.; Kraehenbuhl, J.P.; Epithelial, M. Cells: Gateways for mucosal infection and immunization. Cell 1996, 86, 345–348. [Google Scholar] [CrossRef] [Green Version]
- Sauls, R.S.; Taylor, B.N. Histology, M Cell; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Niedergang, F.; Kraehenbuhl, J.P. Much ado about M cells. Trends Cell Biol. 2000, 10, 137–141. [Google Scholar] [CrossRef]
- Azzali, G. Structure, lymphatic vascularization and lymphocyte migration in mucosa-associated lymphoid tissue. Immunol. Rev. 2003, 195, 178–189. [Google Scholar] [CrossRef]
- Fenton, T.M.; Jørgensen, P.B.; Niss, K.; Rubin, S.J.S.; Mörbe, U.M.; Riis, L.B.; Da Silva, C.; Plumb, A.; Vandamme, J.; Jakobsen, H.L.; et al. Immune profiling of human gut-associated lymphoid tissue identifies a role for isolated lymphoid follicles in priming of region-specific immunity. Immunity 2020, 52, 557–570.e6. [Google Scholar] [CrossRef]
- Jørgensen, P.B.; Fenton, T.M.; Mörbe, U.M.; Riis, L.B.; Jakobsen, H.L.; Nielsen, O.H.; Agace, W.W. Identification, isolation and analysis of human gut-associated lymphoid tissues. Nat. Protoc. 2021, 16, 2051–2067. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Zuo, T.; Yu, J. The composition of colonic commensal bacteria according to anatomical localization in colorectal cancer. Engineering 2017, 3, 90–97. [Google Scholar] [CrossRef]
- Ignacio, A.; Breda, C.N.S.; Camara, N.O.S. Innate lymphoid cells in tissue homeostasis and diseases. World, J. Hepatol. 2017, 9, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Gomez-Bris, R.; Herrero-Fernandez, B.; Mingorance, C.; Rius, C.; Gonzalez-Granado, J.M. Innate lymphoid cells in intestinal homeostasis and inflammatory bowel disease. Int. J. Mol. Sci. 2021, 22, 7618. [Google Scholar] [CrossRef]
- Savage, A.K.; Liang, H.E.; Locksley, R.M. The development of steady-state activation hubs between adult LTi ILC3s and primed macrophages in small intestine. J. Immunol. 2017, 199, 1912–1922. [Google Scholar] [CrossRef]
- Castellanos, J.G.; Longman, R.S. The balance of power: Innate lymphoid cells in tissue inflammation and repair. J. Clin. Invest. 2019, 129, 2640–2650. [Google Scholar] [CrossRef] [Green Version]
- Lim, A.I.; Verrier, T.; Vosshenrich, C.A.; Di Santo, J.P. Developmental options and functional plasticity of innate lymphoid cells. Curr. Opin. Immunol. 2017, 44, 61–68. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, K.; Germain, R.N. Thinking differently about ILCs—Not just tissue resident and not just the same as CD4+ T-cell effectors. Immunol Rev. 2018, 286, 160–171. [Google Scholar] [CrossRef]
- Trabanelli, S.; Gomez-Cadena, A.; Salomé, B.; Michaud, K.; Mavilio, D.; Landis, B.N.; Jandus, P.; Jandus, C. Human innate lymphoid cells (ILCs): Toward a uniform immune-phenotyping. Cytom. B Clin. Cytom. 2018, 94, 392–399. [Google Scholar] [CrossRef]
- Panda, S.K.; Colonna, M. Innate Lymphoid cells in mucosal immunity. Front. Immunol. 2019, 10, 861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherrier, D.E.; Serafini, N.; Di Santo, J.P. Innate lymphoid cell development: A T cell perspective. Immunity 2018, 48, 1091–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate lymphoid cells: 10 years on. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [Green Version]
- Serafini, N.; Klein Wolterink, R.G.; Satoh-Takayama, N.; Xu, W.; Vosshenrich, C.A.; Hendriks, R.W.; Di Santo, J.P. Gata3 drives development of RORγt+ group 3 innate lymphoid cells. J. Exp. Med. 2014, 211, 199–208. [Google Scholar] [CrossRef]
- Hoyler, T.; Klose, C.S.; Souabni, A.; Turqueti-Neves, A.; Pfeifer, D.; Rawlins, E.L.; Voehringer, D.; Busslinger, M.; Diefenbach, A. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 2012, 37, 634–648. [Google Scholar] [CrossRef] [Green Version]
- Bernink, J.H.; Peters, C.P.; Munneke, M.; te Velde, A.A.; Meijer, S.L.; Weijer, K.; Hreggvidsdottir, H.S.; Heinsbroek, S.E.; Legrand, N.; Buskens, C.J.; et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 2013, 14, 221–229. [Google Scholar] [CrossRef]
- Mjösberg, J.; Bernink, J.; Golebski, K.; Karrich, J.J.; Peters, C.P.; Blom, B.; te Velde, A.A.; Fokkens, W.J.; van Drunen, C.M.; Spits, H. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity 2012, 37, 649–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nussbaum, J.C.; Van Dyken, S.J.; von Moltke, J.; Cheng, L.E.; Mohapatra, A.; Molofsky, A.B.; Thornton, E.E.; Krummel, M.F.; Chawla, A.; Liang, H.E.; et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013, 502, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Eberl, G.; Marmon, S.; Sunshine, M.J.; Rennert, P.D.; Choi, Y.; Littman, D.R. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 2004, 5, 64–73. [Google Scholar] [CrossRef]
- Emgård, J.; Kammoun, H.; García-Cassani, B.; Chesné, J.; Parigi, S.M.; Jacob, J.M.; Cheng, H.W.; Evren, E.; Das, S.; Czarnewski, P. Oxysterol sensing through the receptor GPR183 promotes the lymphoid-tissue-inducing function of innate lymphoid cells and colonic inflammation. Immunity 2018, 48, 120–132.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mebius, R.E. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 2003, 3, 292–303. [Google Scholar] [CrossRef]
- Cella, M.; Fuchs, A.; Vermi, W.; Facchetti, F.; Otero, K.; Lennerz, J.K.; Doherty, J.M.; Mills, J.C.; Colonna, M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2009, 457, 722–725. [Google Scholar] [CrossRef]
- Al-Shalan, H.A.M.; Hu, D.; Nicholls, P.K.; Greene, W.K.; Ma, B. Innervation and nerve-immune cell contacts in mouse Peyer’s patches. Histol. Histopathol. 2020, 35, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.Y.; Musser, M.A.; Pinho-Ribeiro, F.A.; Baral, P.; Jacobson, A.; Ma, P.; Potts, D.E.; Chen, Z.; Paik, D.; Soualhi, S.; et al. Gut-innervating nociceptor neurons regulate peyer’s patch microfold cells and SFB levels to mediate salmonella host defense. Cell 2020, 180, 33–49.e22. [Google Scholar] [CrossRef]
- Chiocchetti, R.; Mazzuoli, G.; Albanese, V.; Mazzoni, M.; Clavenzani, P.; Lalatta-Costerbosa, G.; Lucchi, M.L.; Di Guardo, G.; Marruchella, G.; Furness, J.B. Anatomical evidence for ileal Peyer’s patches innervation by enteric nervous system: A potential route for prion neuroinvasion? Cell Tissue Res. 2008, 332, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Populin, L.; Stebbing, M.J.; Furness, J.B. Neuronal regulation of the gut immune system and neuromodulation for treating inflammatory bowel disease. FASEB Bioadv. 2021, 3, 953–966. [Google Scholar] [CrossRef]
- Breslin, J.W.; Yang, Y.; Scallan, J.P.; Sweat, R.S.; Adderley, S.P.; Murfee, W.L. Lymphatic vessel network structure and physiology. Compr Physiol. 2018, 9, 207–299. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, Y.; Qi, J.; Wu, W. An update on oral drug delivery via intestinal lymphatic transport. Acta Pharm. Sin. B 2021, 11, 2449–2468. [Google Scholar] [CrossRef]
- Jakovija, A.; Chtanova, T. Neutrophil interactions with the lymphatic system. Cells 2021, 10, 2106. [Google Scholar] [CrossRef] [PubMed]
- Kvietys, P.R.; Sandig, M. Neutrophil diapedesis: Paracellular or transcellular? News Physiol Sci. 2001, 16, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.D. Mechanism of diapedesis: Importance of the transcellular route. Adv. Immunol. 2016, 129, 25–53. [Google Scholar] [CrossRef] [Green Version]
- Sackstein, R. Directing stem cell trafficking via GPS. Methods Enzymol. 2010, 479, 93–105. [Google Scholar] [CrossRef]
- Layhadi, J.A.; Shamji, M.H. Uncovering the immunological properties of isolated lymphoid follicles. Allergy 2021, 76, 1292–1293. [Google Scholar] [CrossRef]
- Barone, F.; Patel, P.; Sanderson, J.D.; Spencer, J. Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal. Immunol. 2009, 2, 495–503. [Google Scholar] [CrossRef]
- Gustafson, C.E.; Higbee, D.; Yeckes, A.R.; Wilson, C.C.; De Zoeten, E.F.; Jedlicka, P.; Janoff, E.N. Limited expression of APRIL and its receptors prior to intestinal IgA plasma cell development during human infancy. Mucosal Immunol. 2014, 7, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Van Nierop, K.; de Groot, C. Human follicular dendritic cells: Function, origin and development. Semin. Immunol. 2002, 14, 251–257. [Google Scholar] [CrossRef]
- Park, C.S.; Choi, Y.S. How do follicular dendritic cells interact intimately with B cells in the germinal centre? Immunology 2005, 114, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, A.D.; von Boehmer, L.; Gazumyan, A.; Shulman, Z.; Oliveira, T.Y.; Nussenzweig, M.C. Independent roles of switching and hypermutation in the development and persistence of B lymphocyte memory. Immunity 2016, 44, 769–781. [Google Scholar] [CrossRef] [Green Version]
- Meier, D.; Docena, G.H.; Ramisch, D.; Toscanini, U.; Berardi, G.; Gondolesi, G.E.; Rumbo, M. Immunological status of isolated lymphoid follicles after intestinal transplantation. Am. J. Transplant. 2014, 14, 2148–2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, J.; Siu, J.H.Y.; Montorsi, L. Human intestinal lymphoid tissue in time and space. Mucosal Immunol. 2019, 12, 296–298. [Google Scholar] [CrossRef] [Green Version]
- Magri, G.; Comerma, L.; Pybus, M.; Sintes, J.; Lligé, D.; Segura-Garzón, D.; Bascones, S.; Yeste, A.; Grasset, E.K.; Gutzeit, C.; et al. Human secretory IgM emerges from plasma cells clonally related to gut memory B cells and targets highly diverse commensals. Immunity 2017, 47, 118–134.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; Tian, L.; Tan, B.; Shen, Z.; Xiao, M.; Wu, S.; Meng, X.; Wu, X.; Wang, X. Update: Innate lymphoid cells in inflammatory bowel disease. Dig. Dis Sci. 2022, 67, 56–66. [Google Scholar] [CrossRef]
- Giuffrida, P.; Corazza, G.R.; Di Sabatino, A. Old and new lymphocyte players in inflammatory bowel disease. Dig. Dis Sci. 2018, 63, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Drayton, D.L.; Liao, S.; Mounzer, R.H.; Ruddle, N.H. Lymphoid organ development: From ontogeny to neogenesis. Nat. Immunol. 2006, 7, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Sepahi, A.; Liu, Q.; Friesen, L.; Kim, C.H. Dietary fiber metabolites regulate innate lymphoid cell responses. Mucosal Immunol. 2021, 14, 317–330. [Google Scholar] [CrossRef]
- Zhou, W.; Sonnenberg, G.F. Activation and suppression of group 3 innate lymphoid cells in the gut. Trends Immunol. 2020, 41, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shen, J. Innate lymphoid cells in Crohn’s disease. Front. Immunol. 2020, 11, 554880. [Google Scholar] [CrossRef]
- Diefenbach, A.; Gnafakis, S.; Shomrat, O. Innate lymphoid cell-epithelial cell modules sustain intestinal homeostasis. Immunity 2020, 52, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Forkel, M.; van Tol, S.; Höög, C.; Michaëlsson, J.; Almer, S.; Mjösberg, J. Distinct alterations in the composition of mucosal innate lymphoid cells in newly diagnosed and established Crohn’s disease and ulcerative colitis. J. Crohns Colitis. 2019, 13, 67–78. [Google Scholar] [CrossRef]
- Zitti, B.; Bryceson, Y.T. Natural killer cells in inflammation and autoimmunity. Cytokine Growth Factor Rev. 2018, 42, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, S.; Grootjans, J.; Tschurtschenthaler, M.; Krupka, N.; Matute, J.D.; Flak, M.B.; Martinez-Naves, E.; Gomez Del Moral, M.; Glickman, J.N.; Ohira, M.; et al. Intestinal epithelial cell endoplasmic reticulum stress promotes MULT1 up-regulation and NKG2D-mediated inflammation. J. Exp. Med. 2017, 214, 2985–2997. [Google Scholar] [CrossRef]
- Gwela, A.; Siddhanathi, P.; Chapman, R.W.; Travis, S.; Powrie, F.; Arancibia-Cárcamo, C.V.; Geremia, A. Th1 and innate lymphoid cells accumulate in primary sclerosing cholangitis-associated inflammatory Bowel disease. J. Crohns Colitis. 2017, 11, 1124–1134. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Tsai, P.Y.; Zhang, B.; He, W.Q.; Zha, J.M.; Odenwald, M.A.; Singh, G.; Tamura, A.; Shen, L.; Sailer, A.; Yeruva, S.; et al. IL-22 upregulates epithelial claudin-2 to drive diarrhea and enteric pathogen clearance. Cell Host Microbe 2017, 21, 671–681.e4. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Shi, W.; Sun, H.; Ji, Y.; Chen, Y.; Guo, X.; Sheng, H.; Shu, J.; Zhou, L.; Cai, T.; et al. Activation of DR3 signaling causes loss of ILC3s and exacerbates intestinal inflammation. Nat. Commun. 2019, 10, 3371. [Google Scholar] [CrossRef] [Green Version]
- Mazzurana, L.; Bonfiglio, F.; Forkel, M.; D’Amato, M.; Halfvarson, J.; Mjösberg, J. Crohn’s disease is associated with activation of circulating innate lymphoid cells. Inflamm Bowel Dis. 2021, 27, 1128–1138. [Google Scholar] [CrossRef]
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef]
- Krauss, E.; Agaimy, A.; Neumann, H.; Schulz, U.; Kessler, H.; Hartmann, A.; Neurath, M.F.; Raithel, M.; Mudter, J. Characterization of lymphoid follicles with red ring signs as first manifestation of early Crohn’s disease by conventional histopathology and confocal laser endomicroscopy. Int. J. Clin. Exp. Pathol. 2012, 5, 411–421. [Google Scholar] [PubMed]
- Gullberg, E.; Söderholm, J.D. Peyer’s patches and M cells as potential sites of the inflammatory onset in Crohn’s disease. Ann. N Y Acad. Sci. 2006, 1072, 218–232. [Google Scholar] [CrossRef]
- Van Kruiningen, H.J.; Colombel, J.F. The forgotten role of lymphangitis in Crohn’s disease. Gut 2008, 57, 1–4. [Google Scholar] [CrossRef]
- Sura, R.; Colombel, J.F.; Van Kruiningen, H.J. Lymphatics, tertiary lymphoid organs and the granulomas of Crohn’s disease: An immunohistochemical study. Aliment. Pharm. Ther. 2011, 33, 930–939. [Google Scholar] [CrossRef] [PubMed]
- McNamee, E.N.; Rivera-Nieves, J. Ectopic tertiary lymphoid tissue in inflammatory Bowel disease: Protective or provocateur? Front. Immunol. 2016, 7, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Smillie, C.S.; Biton, M.; Ordovas-Montanes, J.; Sullivan, K.M.; Burgin, G.; Graham, D.B.; Herbst, R.H.; Rogel, N.; Slyper, M.; Waldman, J.; et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell 2019, 178, 714–730.e22. [Google Scholar] [CrossRef] [PubMed]
- Smids, C.; Horjus Talabur Horje, C.S.; Drylewicz, J.; Roosenboom, B.; Groenen, M.J.M.; van Koolwijk, E.; van Lochem, E.G.; Wahab, P.J. Intestinal T Cell Profiling in Inflammatory Bowel Disease: Linking T Cell Subsets to Disease Activity and Disease Course. J. Crohns Colitis. 2018, 12, 465–475. [Google Scholar] [CrossRef]
- Horjus Talabur Horje, C.S.; Smids, C.; Meijer, J.W.; Groenen, M.J.; Rijnders, M.K.; van Lochem, E.G.; Wahab, P.J. High endothelial venules associated with T cell subsets in the inflamed gut of newly diagnosed inflammatory bowel disease patients. Clin. Exp. Immunol. 2017, 188, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Kinchen, J.; Chen, H.H.; Parikh, K.; Antanaviciute, A.; Jagielowicz, M.; Fawkner-Corbett, D.; Ashley, N.; Cubitt, L.; Mellado-Gomez, E.; Attar, M.; et al. Structural remodeling of the human colonic mesenchyme in inflammatory Bowel disease. Cell 2018, 175, 372–386.e17. [Google Scholar] [CrossRef] [Green Version]
- Chiba, M.; Yamano, H.; Fujiwara, K.; Abe, T.; Iizuka, M.; Watanabe, S. Lymph folliculitis in ulcerative colitis. Scand, J. Gastroenterol. 2001, 36, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Lochner, M.; Ohnmacht, C.; Presley, L.; Bruhns, P.; Si-Tahar, M.; Sawa, S.; Eberl, G. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J. Exp. Med. 2011, 208, 125–134. [Google Scholar] [CrossRef]
- Isene, R.; Bernklev, T.; Høie, O.; Munkholm, P.; Tsianos, E.; Stockbrügger, R.; Odes, S.; Palm, Ø.; Småstuen, M.; Moum, B. EC-IBD study group. extraintestinal manifestations in Crohn’s disease and ulcerative colitis: Results from a prospective, population-based European inception cohort. Scand J. Gastroenterol. 2015, 50, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Belvedere, A.; Scoglio, R.; Viola, A.; Costantino, G.; Sitibondo, A.; Muscianisi, M.; Inferrera, S.; Alibrandi, A.; Fries, W. A real world investigation on prevalence, clinical features, and therapy of inflammatory bowel disease in the city of Messina, Italy. Acta Biomed. 2021, 92, e2021161. [Google Scholar] [CrossRef]
- Schaubeck, M.; Clavel, T.; Calasan, J.; Lagkouvardos, I.; Haange, S.B.; Jehmlich, N.; Basic, M.; Dupont, A.; Hornef, M.; von Bergen, M.; et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut 2016, 65, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamee, E.N.; Masterson, J.C.; Jedlicka, P.; Collins, C.B.; Williams, I.R.; Rivera-Nieves, J. Ectopic lymphoid tissue alters the chemokine gradient, increases lymphocyte retention and exacerbates murine ileitis. Gut 2013, 62, 53–62. [Google Scholar] [CrossRef]
- Maoz, A.; Dennis, M.; Greenson, J.K. The Crohn’s-like lymphoid reaction to colorectal cancer-tertiary lymphoid structures with immunologic and potentially therapeutic relevance in colorectal cancer. Front. Immunol. 2019, 10, 1884. [Google Scholar] [CrossRef] [Green Version]
- Väyrynen, J.P.; Sajanti, S.A.; Klintrup, K.; Mäkelä, J.; Herzig, K.H.; Karttunen, T.J.; Tuomisto, A.; Mäkinen, M.J. Characteristics and significance of colorectal cancer associated lymphoid reaction. Int. J. Cancer. 2014, 134, 2126–2135. [Google Scholar] [CrossRef]
- Di Caro, G.; Bergomas, F.; Grizzi, F.; Doni, A.; Bianchi, P.; Malesci, A.; Laghi, L.; Allavena, P.; Mantovani, A.; Marchesi, F. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin. Cancer Res. 2014, 20, 2147–2158. [Google Scholar] [CrossRef] [Green Version]
- Rozek, L.S.; Schmit, S.L.; Greenson, J.K.; Tomsho, L.P.; Rennert, H.S.; Rennert, G.; Gruber, S.B. Tumor-infiltrating lymphocytes, Crohn’s-like lymphoid reaction, and survival from colorectal cancer. J. Natl. Cancer Inst. 2016, 108, djw027. [Google Scholar] [CrossRef] [Green Version]
- Ogino, S.; Nosho, K.; Irahara, N.; Meyerhardt, J.A.; Baba, Y.; Shima, K.; Glickman, J.N.; Ferrone, C.R.; Mino-Kenudson, M.; Tanaka, N.; et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin. Cancer Res. 2009, 15, 6412–6420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, H.; Hashiguchi, Y.; Shimazaki, H.; Shinto, E.; Kajiwara, Y.; Nakanishi, K.; Kato, K.; Maekawa, K.; Miyai, K.; Nakamura, T.; et al. Objective criteria for crohn-like lymphoid reaction in colorectal cancer. Am. J. Clin. Pathol. 2013, 139, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halama, N.; Michel, S.; Kloor, M.; Zoernig, I.; Pommerencke, T.; von Knebel Doeberitz, M.; Schirmacher, P.; Weitz, J.; Grabe, N.; Jäger, D. The localization and density of immune cells in primary tumors of human metastatic colorectal cancer shows an association with response to chemotherapy. Cancer Immun. 2009, 9, 1. [Google Scholar] [PubMed]
- Halama, N.; Michel, S.; Kloor, M.; Zoernig, I.; Benner, A.; Spille, A.; Pommerencke, T.; von Knebel, D.M.; Folprecht, G.; Luber, B.; et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011, 71, 5670–5677. [Google Scholar] [CrossRef] [Green Version]
- Wouters, M.C.A.; Nelson, B.H. Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin. Cancer Res. 2018, 24, 6125–6135. [Google Scholar] [CrossRef] [Green Version]
- Tsou, P.; Katayama, H.; Ostrin, E.J.; Hanash, S.M. The emerging role of B cells in tumor immunity. Cancer Res. 2016, 76, 5597–5601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuen, G.J.; Demissie, E.; Pillai, S. B lymphocytes and cancer: A love-hate relationship. Trends Cancer. 2016, 2, 747–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnebacher, M.; Maletzki, C. Tumor-infiltrating B cells: The ignored players in tumor immunology. Oncoimmunology 2012, 1, 1186–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mungenast, F.; Meshcheryakova, A.; Beer, A.; Salzmann, M.; Tamandl, D.; Gruenberger, T.; Pietschmann, P.; Koperek, O.; Birner, P.; Kirsch, I.; et al. The immune phenotype of isolated lymphoid structures in non-tumorous colon mucosa encrypts the information on pathobiology of metastatic colorectal cancer. Cancers 2020, 12, 3117. [Google Scholar] [CrossRef] [PubMed]
- Gatto, D.; Brink, R. The germinal center reaction. J. Allergy Clin. Immunol. 2010, 126, 898–907. [Google Scholar] [CrossRef]
- Stebegg, M.; Kumar, S.D.; Silva-Cayetano, A.; Fonseca, V.R.; Linterman, M.A.; Graca, L. Regulation of the germinal center response. Front. Immunol. 2018, 9, 2469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyasaka, M.; Tanaka, T. Lymphocyte trafficking across high endothelial venules: Dogmas and enigmas. Nat. Rev. Immunol. 2004, 4, 360–370. [Google Scholar] [CrossRef]
- Mechtcheriakova, D.; Svoboda, M.; Meshcheryakova, A.; Jensen-Jarolim, E. Activation-induced cytidine deaminase (AID) linking immunity, chronic inflammation, and cancer. Cancer Immunol. Immunother. 2012, 61, 1591–1598. [Google Scholar] [CrossRef] [Green Version]
- Bannard, O.; Cyster, J.G. Germinal centers: Programmed for affinity maturation and antibody diversification. Curr. Opin. Immunol. 2017, 45, 21–30. [Google Scholar] [CrossRef]
- Nelson, B.H. CD20+ B cells: The other tumor-infiltrating lymphocytes. J. Immunol. 2010, 185, 4977–4982. [Google Scholar] [CrossRef] [Green Version]
- Boothby, M.R.; Hodges, E.; Thomas, J.W. Molecular regulation of peripheral B cells and their progeny in immunity. Genes Dev. 2019, 33, 26–48. [Google Scholar] [CrossRef]
- Seifert, M.; Küppers, R. Human memory B cells. Leukemia 2016, 30, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.; Guddati, A.K. Secondary mucosa-associated lymphoid tissue (MALT) lymphoma of the colon. Med. Oncol. 2013, 30, 502. [Google Scholar] [CrossRef]
- Buettner, M.; Lochner, M. Development and function of secondary and tertiary lymphoid organs in the small intestine and the colon. Front. Immunol. 2016, 7, 342. [Google Scholar] [CrossRef] [Green Version]
- Knoop, K.A.; Newberry, R.D. Isolated lymphoid follicles are dynamic reservoirs for the induction of intestinal IgA. Front. Immunol. 2012, 3, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, C.; Uhlig, H.H.; Powrie, F. Lymphoid microenvironments and innate lymphoid cells in the gut. Trends Immunol. 2012, 33, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Pabst, O.; Herbrand, H.; Worbs, T.; Friedrichsen, M.; Yan, S.; Hoffmann, M.W.; Körner, H.; Bernhardt, G.; Pabst, R.; Förster, R. Cryptopatches and isolated lymphoid follicles: Dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur. J. Immunol. 2005, 35, 98–107. [Google Scholar] [CrossRef]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Buchan, S.L.; Rogel, A.; Al-Shamkhani, A. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood 2018, 131, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchan, S.L.; Fallatah, M.; Thirdborough, S.M.; Taraban, V.Y.; Rogel, A.; Thomas, L.J.; Penfold, C.A.; He, L.Z.; Curran, M.A.; Keler, T.; et al. PD-1 blockade and CD27 stimulation activate distinct transcriptional programs that synergize for CD8+ T-Cell-driven antitumor immunity. Clin. Cancer Res. 2018, 24, 2383–2394. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, P.; Gronke, K.; Diefenbach, A. A catch-22: Interleukin-22 and cancer. Eur. J. Immunol. 2018, 48, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Penny, H.A.; Hodge, S.H.; Hepworth, M.R. Orchestration of intestinal homeostasis and tolerance by group 3 innate lymphoid cells. Semin. Immunopathol. 2018, 40, 357–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, H.; Roth, S.; Pechloff, K.; Kiss, E.A.; Kuhn, S.; Heikenwälder, M.; Diefenbach, A.; Greten, F.R.; Ruland, J. Card9-dependent IL-1β regulates IL-22 production from group 3 innate lymphoid cells and promotes colitis-associated cancer. Eur. J. Immunol. 2017, 47, 1342–1353. [Google Scholar] [CrossRef]
- Kokkonen, J.; Karttunen, T.J. Lymphonodular hyperplasia on the mucosa of the lower gastrointestinal tract in children: An indication of enhanced immune response? J. Pediatr. Gastroenterol. Nutr. 2002, 34, 42–46. [Google Scholar] [CrossRef]
- Longman, R.S.; Diehl, G.E.; Victorio, D.A.; Huh, J.R.; Galan, C.; Miraldi, E.R.; Swaminath, A.; Bonneau, R.; Scherl, E.J.; Littman, D.R. CX₃CR1⁺ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J. Exp. Med. 2014, 211, 1571–1583. [Google Scholar] [CrossRef]
- Jiang, L.; Jung, S.; Zhao, J.; Kasinath, V.; Ichimura, T.; Joseph, J.; Fiorina, P.; Liss, A.S.; Shah, K.; Annabi, N.; et al. Simultaneous targeting of primary tumor, draining lymph node, and distant metastases through high endothelial venule-targeted delivery. Nano Today 2021, 36, 101045. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, A.M.; Storkus, W.J. Biosynthesis and functional significance of peripheral node addressin in cancer-associated TLO. Front. Immunol. 2016, 7, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Műzes, G.; Bohusné Barta, B.; Sipos, F. Colitis and Colorectal Carcinogenesis: The Focus on Isolated Lymphoid Follicles. Biomedicines 2022, 10, 226. https://doi.org/10.3390/biomedicines10020226
Műzes G, Bohusné Barta B, Sipos F. Colitis and Colorectal Carcinogenesis: The Focus on Isolated Lymphoid Follicles. Biomedicines. 2022; 10(2):226. https://doi.org/10.3390/biomedicines10020226
Chicago/Turabian StyleMűzes, Györgyi, Bettina Bohusné Barta, and Ferenc Sipos. 2022. "Colitis and Colorectal Carcinogenesis: The Focus on Isolated Lymphoid Follicles" Biomedicines 10, no. 2: 226. https://doi.org/10.3390/biomedicines10020226
APA StyleMűzes, G., Bohusné Barta, B., & Sipos, F. (2022). Colitis and Colorectal Carcinogenesis: The Focus on Isolated Lymphoid Follicles. Biomedicines, 10(2), 226. https://doi.org/10.3390/biomedicines10020226







