Carbon Monoxide (CO) as a Retinal Regulator of Heme Oxygenases -1, and -2 (HO’s) Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. RNA Analysis
2.3. Protein Analysis (Western Blot Method)
2.4. Statistical Analysis
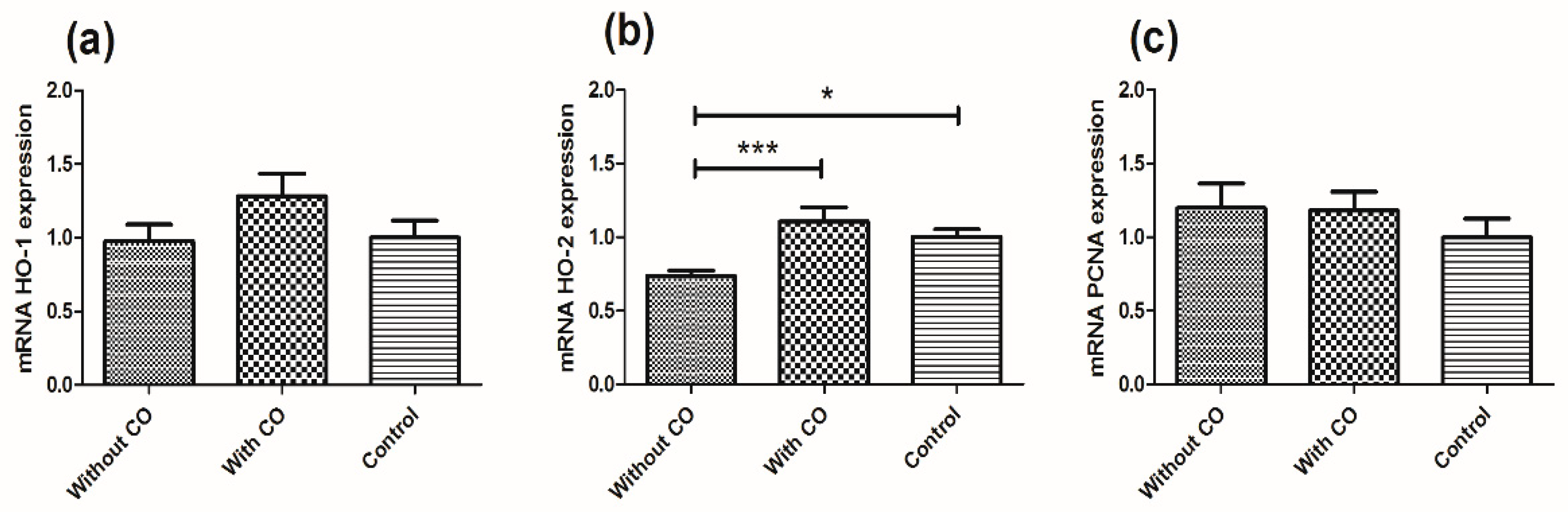
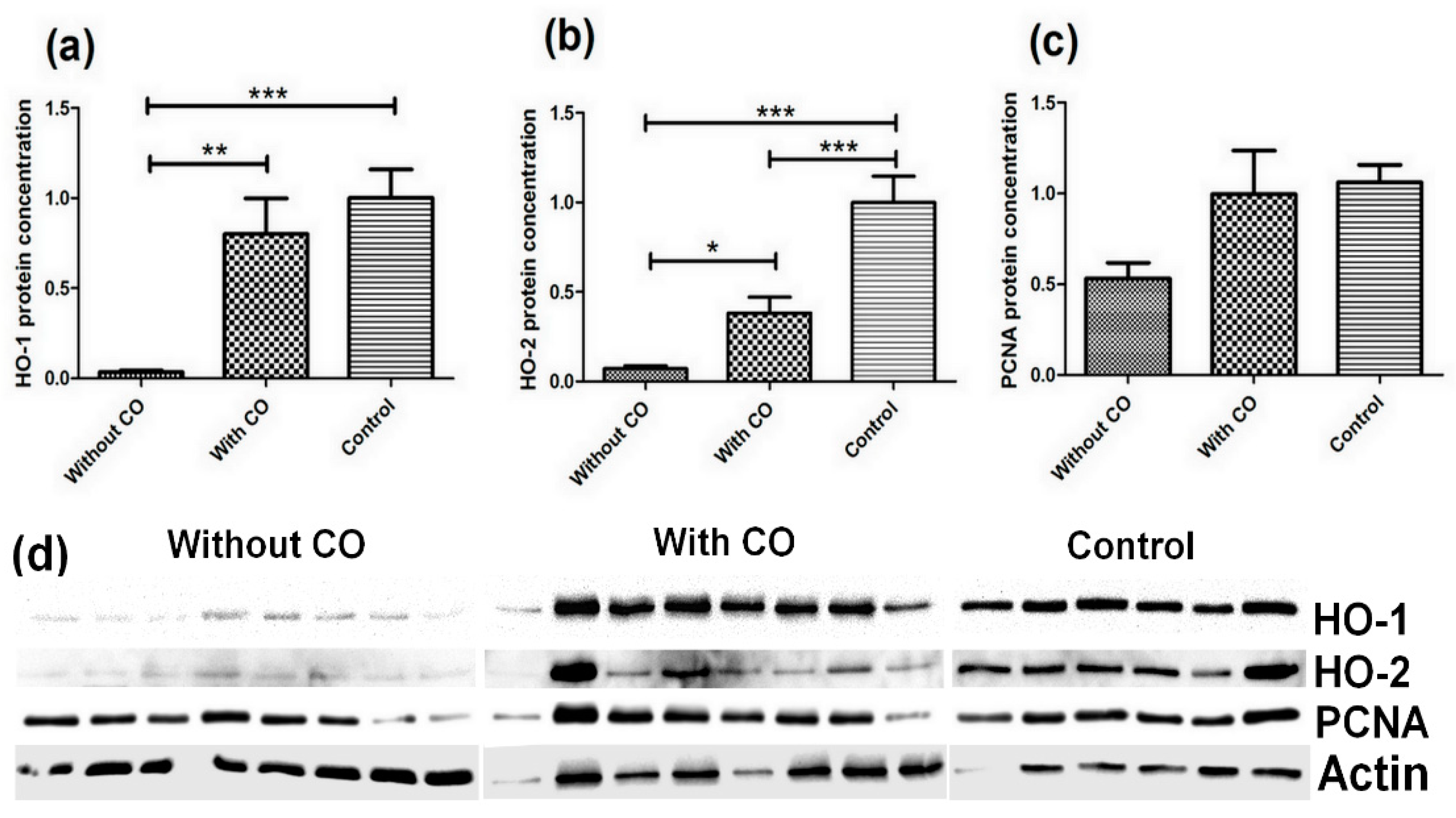
3. Results
4. Discussion
- -
- Carbon monoxide in small quantities is not toxic to organisms but has a stimulating function;
- -
- Carbon monoxide modulates the expression of studied factors (HO-1, HO-2 and PCNA), e.g., humoral light signal;
- -
- CO synthesis in natural conditions upregulates itself and depends on the amount of particles which are consistent with light intensity during the different seasons;
- -
- The amount of HO-1 and HO-2 proteins in the retina is dependent on the CO concentration in the blood flowing to the eye;
- -
- Many of the changes we have observed are on a translational level, and we conclude that CO particles regulate cofactor-triggered translation.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, Y.; Liao, H.-W.; Do, M.; Yau, K.-W. Non-image-forming ocular photoreception in vertebrates. Curr. Opin. Neurobiol. 2010, 15, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Was, H.; Dulak, J.; Jozkowicz, A. Heme oxygenase-1 in tumor biology and therapy. Curr. Drug Targets 2010, 11, 1551–1570. [Google Scholar] [CrossRef] [PubMed]
- Resch, H.; Zawinka, C.; Weigert, G.; Schmetterer, L.; Garhofer, G. Inhaled Carbon Monoxide Increases Retinal and Choroidal Blood Flow in Healthy Humans. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4275–7280. [Google Scholar] [CrossRef] [PubMed]
- Dolinay, T.; Szilasi, M.; Liu, M.; Choi, A.M.K. Inhaled Carbon Monoxide Confers Antiinflammatory Effects against Ventilator-induced Lung Injury. Am. J. Respir. Crit. Care Med. 2004, 170, 613–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motterlini, R.; Clark, J.E.; Foresti, R.; Sarathchandra, P.; Mann, B.E.; Green, C.J. Carbon monoxide-releasing molecules: Characterization of biochemical and vascular activities. Circ. Res. 2002, 90, E17–E24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szade, K.; Zukowska, M.; Szade, A.; Nowak, W.; Skulimowska, I.; Ciesla, M.; Bukowska-Strakova, K.; Gulati, G.S.; Kachamakova-Trojanowska, N.; Kusienicka, A.; et al. Heme oxygenase-1 deficiency triggers exhaustion of hematopoietic stem cells. EMBO Rep. 2019, 29, e47895. [Google Scholar] [CrossRef]
- Bucolo, C.; Drago, F. Focus on molecules: Heme oxygenase-1. Exp. Eye Res. 2009, 89, 822–823. [Google Scholar] [CrossRef]
- Foresti, R.; Bains, S.K.; Pitchumony, T.S.; de Castro Brás, L.E.; Drago, F.; Dubois-Randé, J.; Bucolo, C.; Motterlini, R. Small molecule activators of the Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol. Res. 2013, 76, 132–148. [Google Scholar] [CrossRef] [Green Version]
- Pittalà, V.; Fidilio, A.; Lazzara, F.; Platania, C.B.M.; Salerno, D.; Foresti, R.; Drago, F.; Bucolo, C. Effects of Novel Nitric Oxide-Releasing Molecules against Oxidative Stress on Retinal Pigmented Epithelial Cells. Oxid. Med. Cell. Longev. 2017, 2017, 1420892. [Google Scholar] [CrossRef]
- Kim, Y.M.; Pae, H.O.; Park, J.E.; Lee, Y.C.; Woo, J.M.; Kim, N.H.; Choi, Y.K.; Lee, B.S.; Kim, S.R.; Chung, H.T. Heme oxygenase in the regulation of vascular biology: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 14, 137–167. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, S.; Omata, Y.; Sakamoto, H.; Higashimoto, Y.; Hara, T.; Sagara, Y.; Noguchi, M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene 2004, 336, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, M.L. Chapter 12—Carbon monoxide intoxication. In Handbook of Clinical Neurology; 3rd Series; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 131, pp. 191–203. [Google Scholar]
- Oren, D.A.; Duda, M.; Kozioł, K.; Romerowicz-Misielak, M.; Koziorowska, A.; Sołek, P.; Nowak, S.; Kulpa, M.; Koziorowski, M. Retinal venous blood carbon monoxide response to bright light in male pigs: A Preliminary Study. J. Photochem. Photobiol. B Biol. 2017, 168, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Gilun, P.; Stefanczyk-Krzymowska, S.; Romerowicz-Misielak, M.; Tabecka-Lonczynska, A.; Przekop, F.; Koziorowski, M. Carbon monoxide-mediated humoral pathway for the transmission of light signal to the hypothalamus. J. Physiol. Pharmacol. 2013, 64, 761–777. [Google Scholar] [PubMed]
- Romerowicz-Misielak, M.; Tabecka-Lonczynska, A.; Koziol, K.; Gilun, P.; Stefanczyk-Krzymowska, S.; Och, W.; Koziorowski, M. Changes in gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor gene expression after an increase in carbon monoxide concentration in the cavernous sinus of male wild boar and pig crossbred. J. Physiol. Pharmacol. 2016, 67, 431–442. [Google Scholar] [PubMed]
- Vijayan, V.; Wagener, F.A.D.T.G.; Immenschuh, S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018, 153, 159–167. [Google Scholar] [CrossRef]
- Joe, Y.; Kim, S.; Kim, H.J.; Park, J.; Chen, Y.; Park, H.J.; Jekal, S.J.; Ryter, S.W.; Kim, U.H.; Chung, H.T. FGF21-induced by carbon monoxide mediates metabolic homeostasis via the PERK/ATF4 pathway. FASEB J. 2018, 32, 2630–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romerowicz-Misielak, M.; Oren, D.A.; Sowa-Kucma, M.; Tabecka-Lonczynska, A.; Gilun, P.; Stefanczyk-Krzymowska, S.; Koziorowski, M. Changes in melatonin synthesis parameters after carbon monoxide concentration increase in the cavernous sinus. J. Physiol. Pharmacol. 2015, 66, 505–514. [Google Scholar] [PubMed]
- Bucolo, C.; Drago, F. Carbon monoxide and the eye: Implications for glaucoma therapy. Pharmacol Ther. 2011, 130, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zhan, Y.; Wang, H.; Luo, Z.; Zheng, F.; Zhou, Y.; Wu, Y.; Wang, S.; Wu, Y.; Xiang, G.; et al. Carbon monoxide releasing molecule-3 alleviates neuron death after spinal injury via inflammasome regulation. EBioMedicine 2019, 40, 643–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilun, P.; Flisikowski, L.; Flisikowska, T.; Kwiatkowska, J.; Wąsowska, B.; Koziorowska-Gilun, M. Role of methylation in Period 2 (PER2) transcription in the context of the presence or absence of light signals: Natural and chemical—Studies on the pig model. Int. J. Mol. Sci. 2021, 22, 7769. [Google Scholar] [CrossRef]
- Koziorowski, M.; Stefańczyk-Krzymowska, S.; Tabecka-Lonczyńska, A.; Gilun, P.; Kamiński, M. The gaseous messenger carbon monoxide is released from the eye into the ophthalmic venous blood depending on the intensity of sunlight. J. Biol. Regul. Homeost. Agents 2012, 26, 111–118. [Google Scholar]
- Chomczynski, P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 1993, 15, 532–534. [Google Scholar] [PubMed]
- Tabecka-Lonczynska, A.; Mytych, J.; Solek, P.; Abrachamowicz, A.; Welz, M.; Koziorowski, M. Local regulators of seasonal reproduction processes in uterus masculinus of an adult male European bison (Bison bonasus, Linnaeus 1758). J. Physiol. Pharmacol. 2018, 69, 747–753. [Google Scholar]
- Harada, E.; Sugishima, M.; Harada, J.; Noguchi, M.; Fukuyama, K.; Sugase, K. Backbone assignments of the apo and Zn(II) protoporphyrin IX-bound states of the soluble form of rat heme oxygenase-1. Biomol. NMR Assign. 2015, 9, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Nowak, S.; Romerowicz-Misielak, M.; Kozioł, K.; Koziorowski, M. The Seasonal Changes of the Heme Oxygenase in the Retina Pig. Exp. Eye Res. 2020, 190, 107870. [Google Scholar] [CrossRef] [PubMed]
- Krzymowski, T.; Stefanczyk-Krzymowska, S. Local retrograde and destination transfer of physiological regulators as an important regulatory system and its role. Facts and hypothesis. J. Physiol. Pharmacol. 2012, 63, 3–16. [Google Scholar] [PubMed]
- Kutty, R.K.; Kutty, G.; Wiggert, B.; Chader, G.J.; Darrow, R.M.; Organisciak, D.T. Induction of heme oxygenase 1 in the retina by intense visible light: Suppression by the antioxidant dimetylthiourea. Proc. Natl. Acad. Sci. USA 1995, 92, 1177–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonneaux, V.; Ribelayga, C. Generation of the Melatonin Endocrine Message in Mammals: A Review of the Complex Regulation of Melatonin Synthesis by Norepinephrine, Peptides, and Other Pineal Transmitters. Pharmacol. Rev. 2003, 55, 325–395. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Koh, S.-H.; Yoo, M.; Choi, Y.K. Regenerative potential of carbon monoxide in adult neural circuits of the central nervous system. Int. J. Mol. Sci. 2020, 21, 2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, S.; Gilun, P.; Kozioł, K.; Romerowicz-Misielak, M.; Koziorowska-Gilun, M.; Wąsowska, B. Carbon Monoxide (CO) as a Retinal Regulator of Heme Oxygenases -1, and -2 (HO’s) Expression. Biomedicines 2022, 10, 358. https://doi.org/10.3390/biomedicines10020358
Nowak S, Gilun P, Kozioł K, Romerowicz-Misielak M, Koziorowska-Gilun M, Wąsowska B. Carbon Monoxide (CO) as a Retinal Regulator of Heme Oxygenases -1, and -2 (HO’s) Expression. Biomedicines. 2022; 10(2):358. https://doi.org/10.3390/biomedicines10020358
Chicago/Turabian StyleNowak, Sławomir, Przemysław Gilun, Katarzyna Kozioł, Maria Romerowicz-Misielak, Magdalena Koziorowska-Gilun, and Barbara Wąsowska. 2022. "Carbon Monoxide (CO) as a Retinal Regulator of Heme Oxygenases -1, and -2 (HO’s) Expression" Biomedicines 10, no. 2: 358. https://doi.org/10.3390/biomedicines10020358





