The Inflammatory Pattern of Chronic Limb-Threatening Ischemia in Muscles: The TNF-α Hypothesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Informed Consent and Recruitment
2.2. Medical Screening through Medical History and Physical Examination
2.3. Inclusion/Exclusion/Withdrawal Criteria
2.3.1. Inclusion Criteria:
- –
- Age > 18 years
- –
- Diagnosis of CLTI: presence of trophic lesions and/or rest pain plus ABI less than 0.4 and/or AP < 50 mmHg or plain or damped PPG curves or toe pressure (TP) < 30 mmHg [31].
- –
- Failure of a previous attempt of revascularization; patients considered at high risk of failure or at high risk of surgical complications during the procedure or in poor condition for surgery.
- –
- High risk of limb loss.
2.3.2. Exclusion Criteria
- –
- Pregnancy
- –
- Legally incapacitated.
- –
- Current cancer or during the last 5 years before the study.
- –
- Current pneumonia or sepsis or severe foot infection.
- –
- Untreated hypothyroidism and/or hypocortisolism.
2.3.3. Withdrawal Criteria
- –
- Patient’s own request.
- –
- Decision of the physician due to adverse reactions supposedly secondary to the drug.
- –
- Pneumonia/sepsis during the period of treatment.
- –
- Increase in levels of IGF-1 more than 2 standard deviations.
- –
- Increase in tumor markers.
2.4. Measurements
2.4.1. ABI and AP
2.4.2. Inflammatory and Vascular Circulating Biomarkers
2.4.3. Skeletal Muscle Samples
2.4.4. Real-Time PCR (RT-qPCR)
2.5. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Hemodynamic Parameters and Plasma Biomarkers
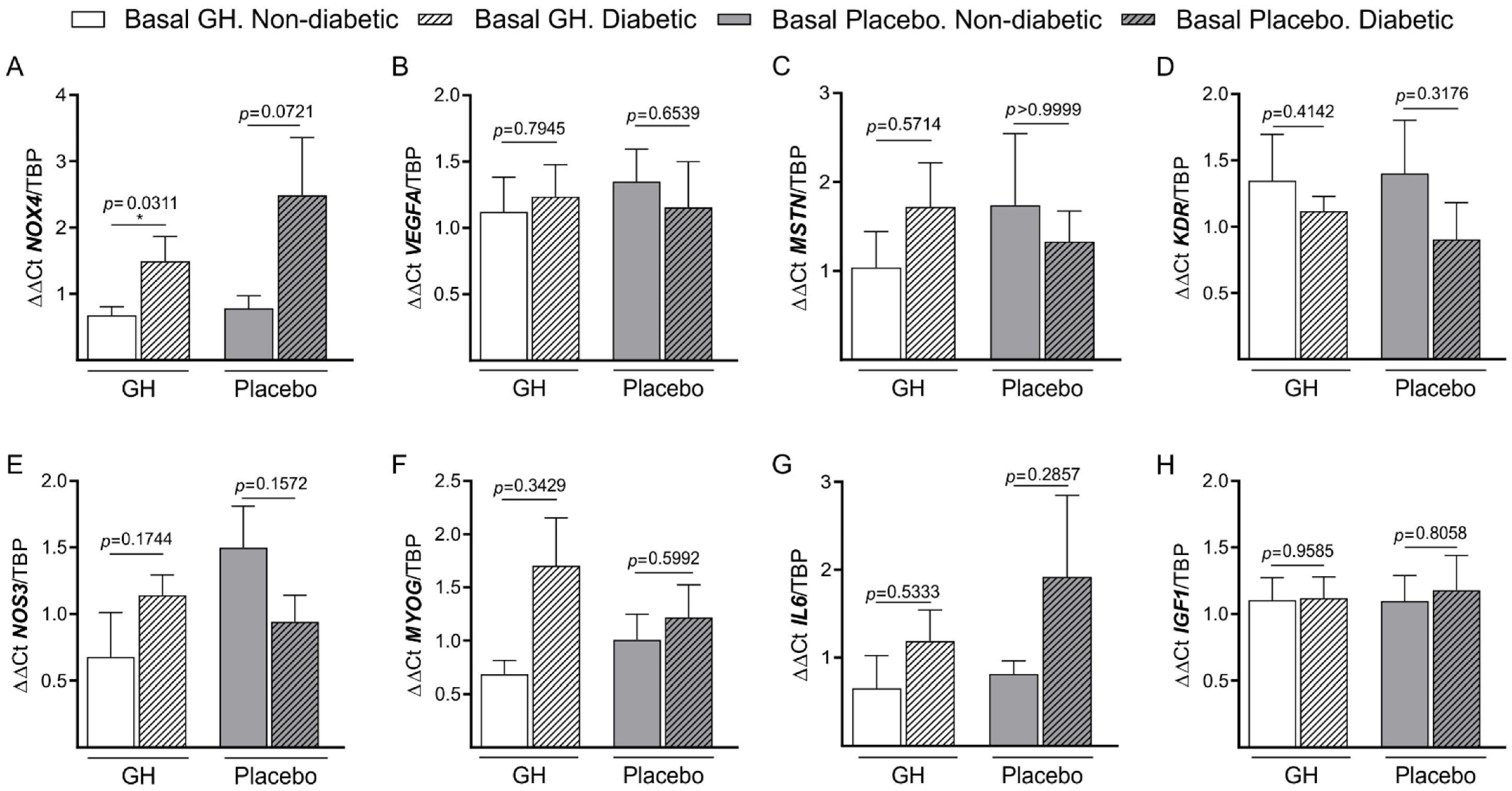
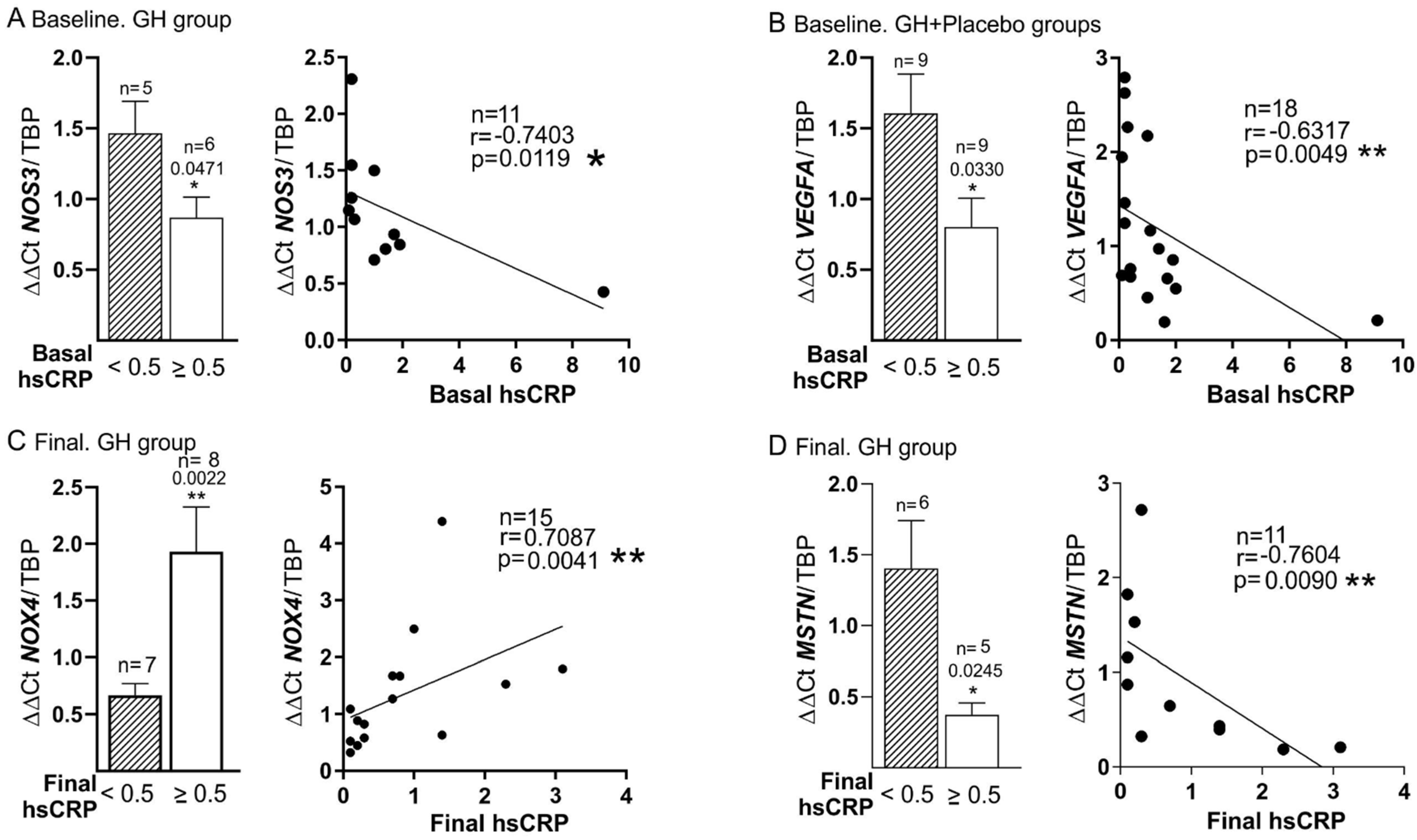
3.3. Basal mRNA Expression
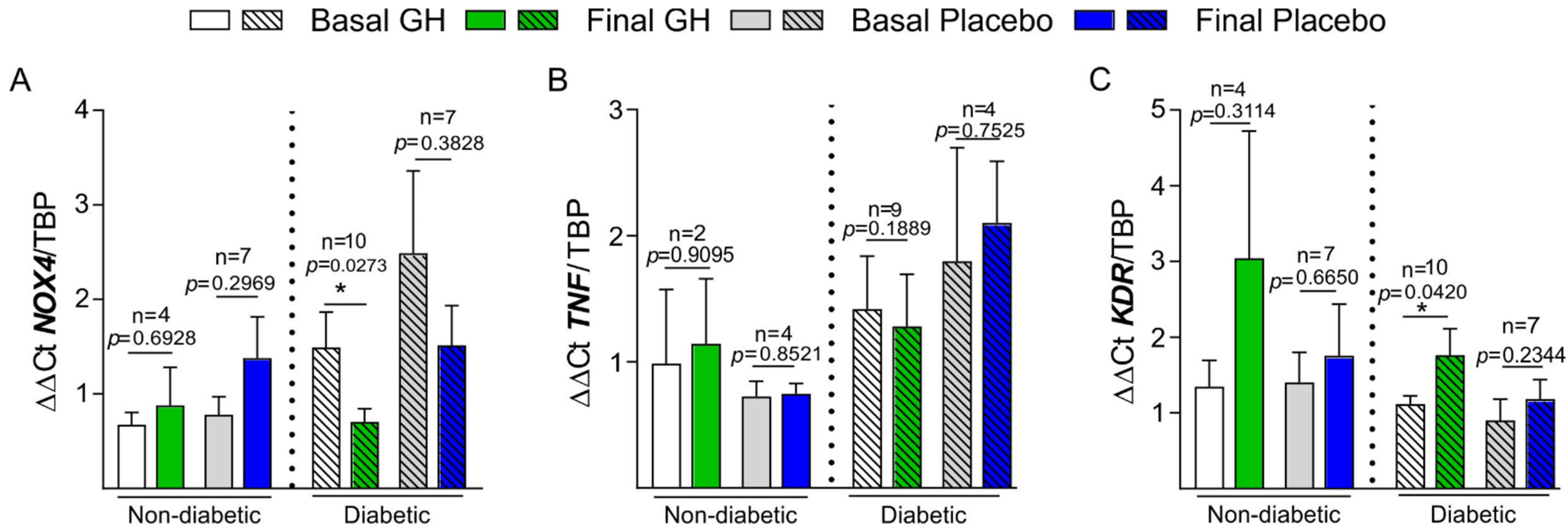
3.4. Basal and Final mRNA Expression
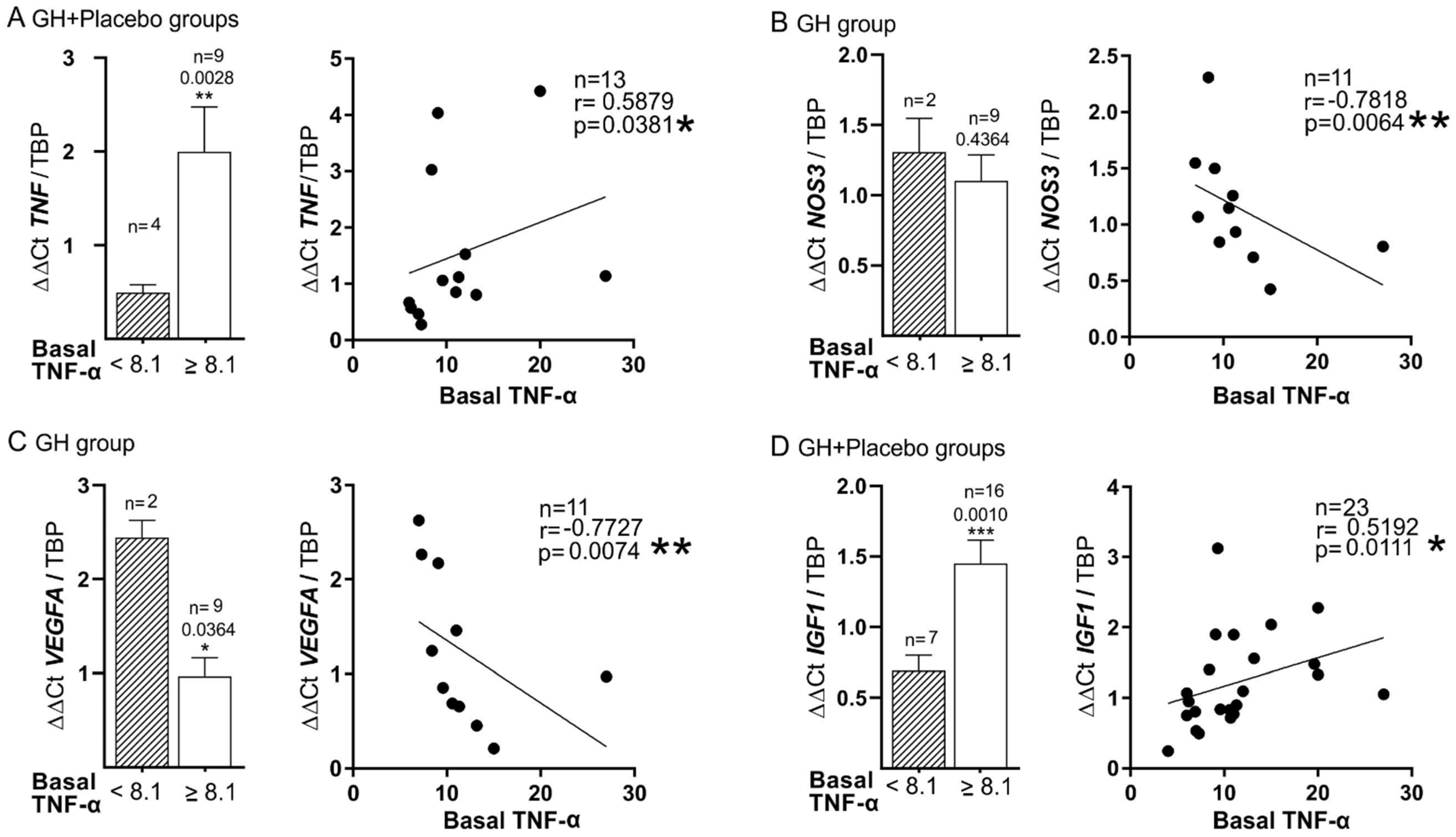
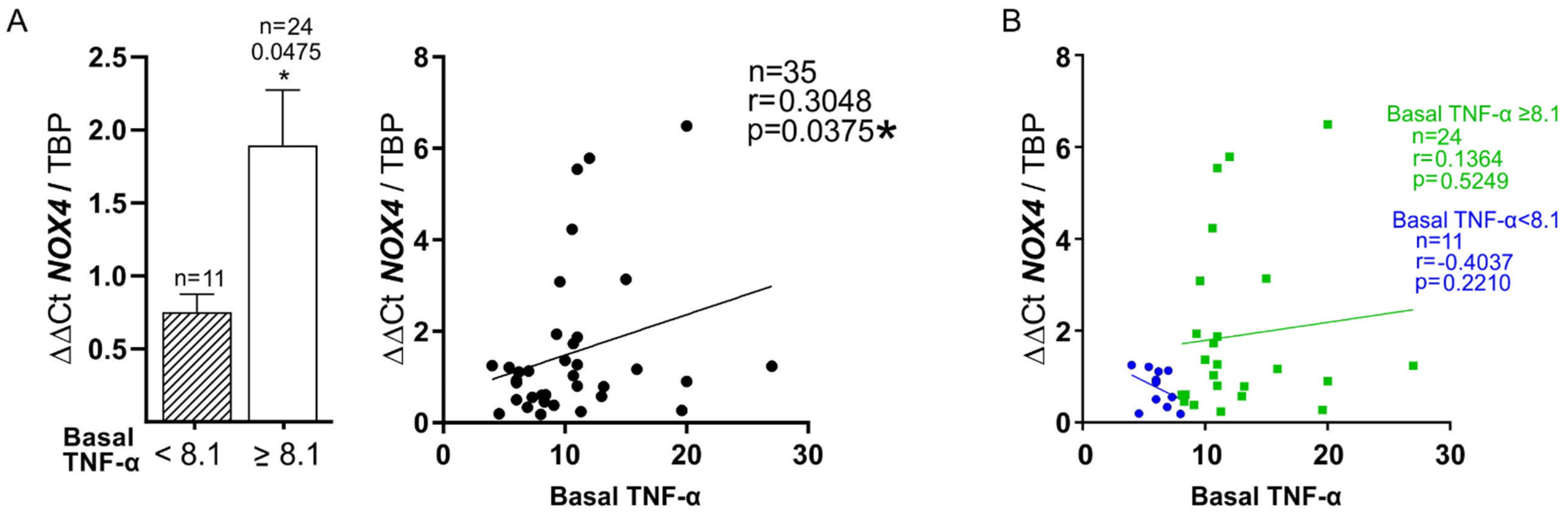
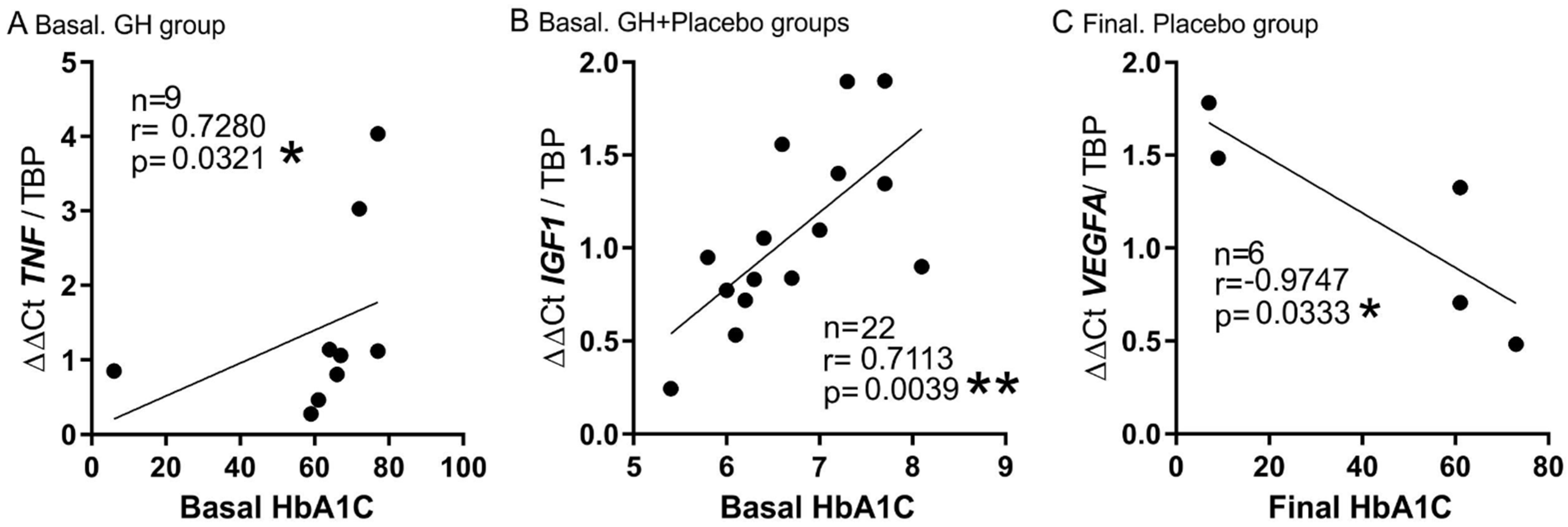
3.5. Plasma Biomarkers and mRNA Expression
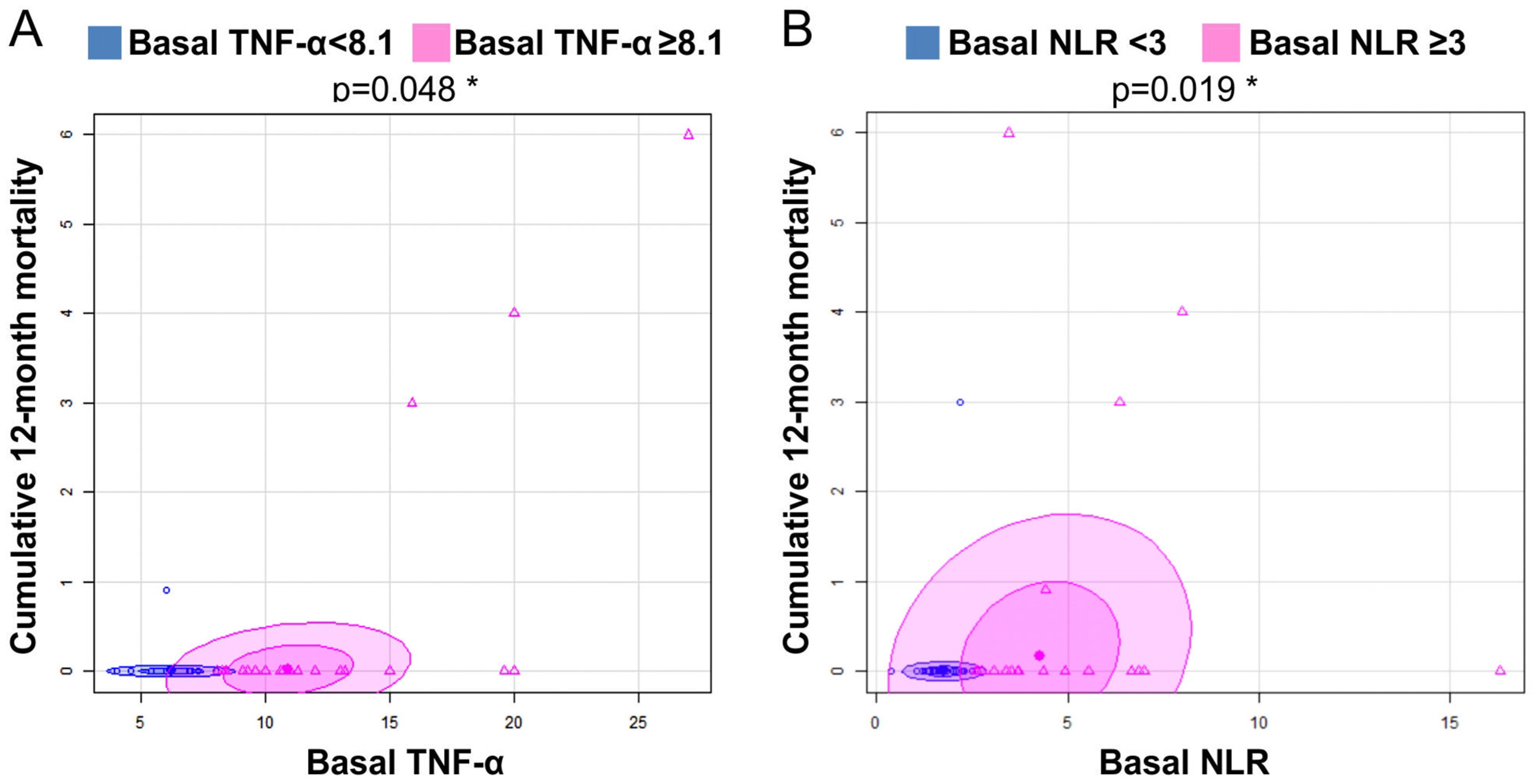
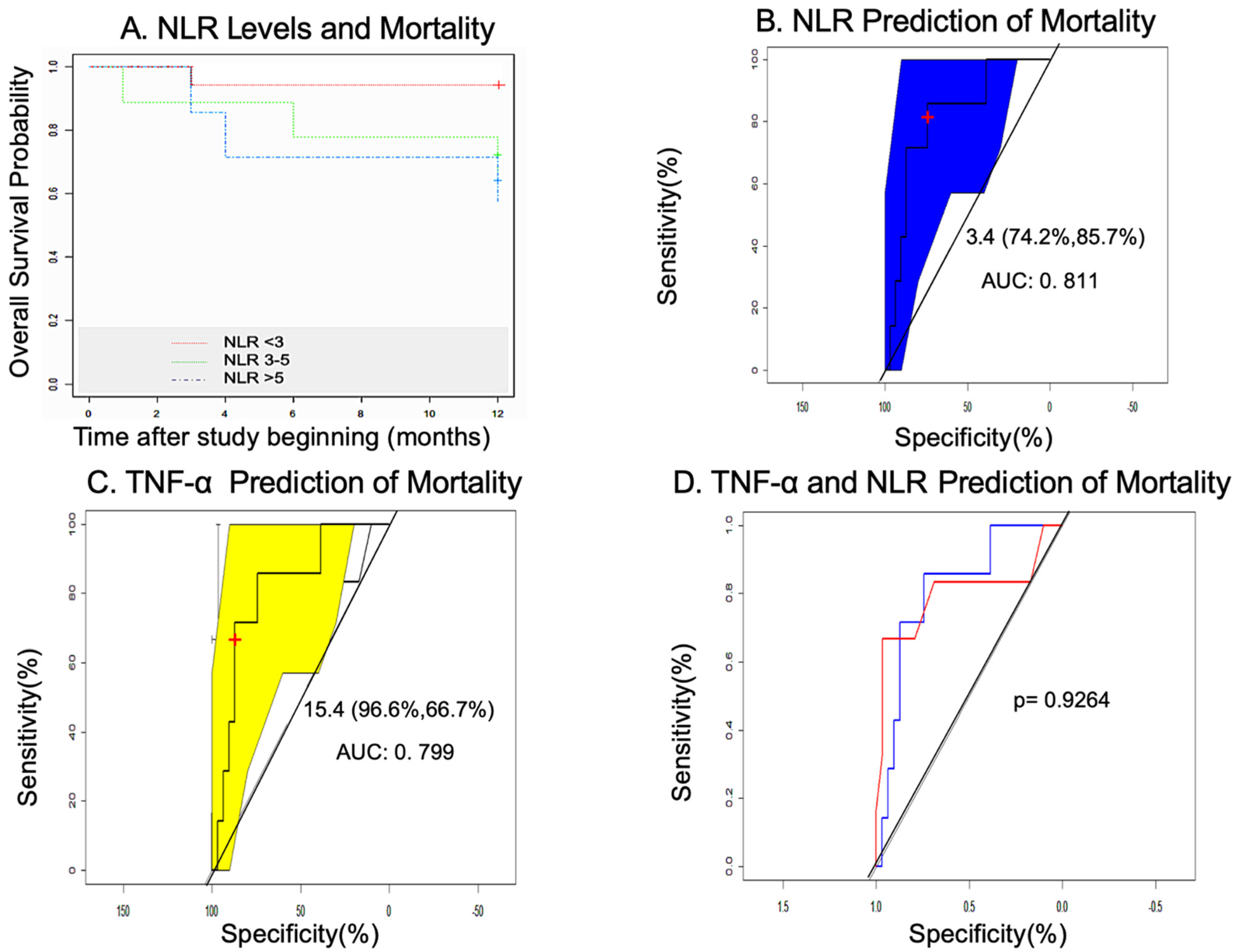
3.6. Mortality in the GHAS Trial
3.7. Statistical Study: Measures of Association of Variables
3.7.1. Univariate Analysis
3.7.2. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebocontrolled trial. Lancet 2002, 360, 7–22. [Google Scholar] [CrossRef]
- Kumbhani, D.J.; Steg, G.; Cannon, C.P.; Eagle, K.A.; Smith, S.C.; Goto, S.; Magnus Ohman, E.; Elbez, Y.; Sritara, P.; Baumgartner, I.; et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: Insights from the REACH registry. Eur. Heart J. 2014, 35, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Ohman, E.M.; Bhatt, D.L.; Steg, P.G.; Goto, S.; Hirsch, A.T.; Liau, C.S.; Mas, J.L.; Richard, A.J.; Röther, J.; Wilson, P.W.F. The REduction of Atherothrombosis for Continued Health (REACH) Registry: An international, prospective, observational investigation in subjects at risk for atherothrombotic events-study design. Am. Heart J. 2006, 151, 786.e1–786.e10. [Google Scholar] [CrossRef] [PubMed]
- Haugen, S.; Casserly, I.P.; Regensteiner, J.G.; Hiatt, W.R. Risk assessment in the patient with established peripheral arterial disease. Vasc. Med. 2007, 12, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Diehm, C. Association of low ankle brachial index with high mortality in primary care. Eur. Heart J. 2006, 27, 1743–1749. [Google Scholar] [CrossRef]
- Resnick, H.E.; Lindsay, R.S.; McDermott, M.M.; Devereux, R.B.; Jones, K.L.; Fabsitz, R.R.; Howard, B.V. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: The Strong Heart Study. Circulation 2004, 109, 733–739. [Google Scholar] [CrossRef]
- Kistorp, C. Risk Stratification in Secondary Prevention. Circulation 2006, 114, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Brevetti, G.; Giugliano, G.; Brevetti, L.; Hiatt, W.R. Inflammation in peripheral artery disease. Circulation 2010, 122, 1862–1875. [Google Scholar] [CrossRef]
- Saenz-pipaon, G.; Martinez-aguilar, E.; Orbe, J.; Miqueo, A.G.; Fernandez-alonso, L.; Paramo, J.A.; Roncal, C. The role of circulating biomarkers in peripheral arterial disease. Int. J. Mol. Sci. 2021, 22, 3601. [Google Scholar] [CrossRef]
- Aboyans, V.; Criqui, M.H. Can we improve cardiovascular risk prediction beyond risk equations in the physician’s office? J. Clin. Epidemiol. 2006, 59, 547–558. [Google Scholar] [CrossRef]
- Steering Committee of the Physicians’ Health Study Research Group. Physicians’ Health Study: Aspirin and Primary Prevention of Coronary Heart Disease. N. Engl. J. Med. 1989, 321, 1825–1828. [Google Scholar] [CrossRef] [PubMed]
- Steering Committee of the Physicians’ Health Study Research Group. Final Report on the Aspirin Component of the Ongoing Physicians’ Health Study. N. Engl. J. Med. 1989, 321, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, R.; Zadelaar, S.; Kooistra, T. Cytokines and atherosclerosis: A comprehensive review of studies in mice. Cardiovasc. Res. 2008, 79, 360–376. [Google Scholar] [CrossRef]
- Ridker, P.M. Anticytokine Agents Targeting Interleukin Signaling Pathways for the Treatment of Atherothrombosis. Circ. Res. 2019, 124, 437–450. [Google Scholar] [CrossRef]
- Kaptoge, S.; Seshasai, S.R.K.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.O.; et al. Inflammatory cytokines and risk of coronary heart disease: New prospective study and updated meta-analysis. Eur. Heart J. 2014, 35, 578–589. [Google Scholar] [CrossRef]
- Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: A mendelian randomisation analysis. Lancet 2012, 379, 1205–1213. [Google Scholar] [CrossRef]
- Swerdlow, D.I.; Holmes, M.V.; Kuchenbaecker, K.B.; Engmann, J.; Shah, T.; Sofat, R. The interleukin-6 receptor as a potential target for coronary heart disease prevention: Evaluation using Mendelian randomization. Lancet 2012, 379, 1214–1224. [Google Scholar] [CrossRef]
- Qasim, A.N.; Reilly, M.P. Genetics of Atherosclerotic Cardiovascular Disease. In Emery and Rimoin’s Principles and Practice of Medical Genetics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–37. [Google Scholar]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Anzaldi, M.; Libra, M.; Navolanic, P.M.; Malaponte, G.; Mangano, K.; Quattrocchi, C.; Di Marco, R.; Fiore, V.; Neri, S. Plasma Levels of Inflammatory Biomarkers in Peripheral Arterial Disease. Angiology 2016, 67, 870–874. [Google Scholar] [CrossRef]
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S.; Sosnowska, D.; Casanegra, A.I.; Esponda, O.L.; Ungvari, Z.; Csiszar, A.; Sonntag, W.E. Impaired Vascular Endothelial Growth Factor A and Inflammation in Patients With Peripheral Artery Disease. Angiology 2014, 165, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.; Pedersen, B.K. The Role of Exercise-Induced Myokines in Muscle Homeostasis and the Defense against Chronic Diseases. J. Biomed. Biotechnol. 2010, 2010, 520258. [Google Scholar] [CrossRef] [PubMed]
- Barnabe, C.; Martin, B.-J.; Ghali, W.A. Systematic review and meta-analysis: Anti-tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res. 2011, 63, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Spanò, F.; Cagnati, P.; Puppo, F. Free radicals and endothelial dysfunction: Potential positive effects of TNF-α inhibitors. Redox Rep. 2013, 18, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Paquissi, F. The role of inflammation in cardiovascular diseases: The predictive value of neutrophil–lymphocyte ratio as a marker in peripheral arterial disease. Ther. Clin. Risk Manag. 2016, 12, 851. [Google Scholar] [CrossRef]
- Bir, S.C.; Kolluru, G.K.; Fang, K.; Kevil, C.G. Redox balance dynamically regulates vascular growth and remodeling. Semin. Cell Dev. Biol. 2012, 23, 745–757. [Google Scholar] [CrossRef]
- Caicedo, D.; Devesa, P.; Alvarez, C.V.; Devesa, J. Why Should Growth Hormone (GH) Be Considered a Promising Therapeutic Agent for Arteriogenesis? Insights from the GHAS Trial. Cells 2020, 9, 807. [Google Scholar] [CrossRef]
- Caicedo, D.; Díaz, O.; Devesa, P.; Devesa, J. Growth Hormone (GH) and Cardiovascular System. Int. J. Mol. Sci. 2018, 19, 290. [Google Scholar] [CrossRef]
- Caicedo, D.; Devesa, J. Growth Hormone (GH) and Wound Healing. In Wound Healing-Current Perspectives; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur. J. Vasc. Endovasc. Surg. 2019, 58, S1–S109.e33. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 305–368. [Google Scholar] [CrossRef]
- Suárez, C.; Lozano, F. Guía Española de Consenso Multidisciplinar en Enfermedad Arterial Periférica de Extremidades Inferiores, 1st ed.; Luzán, S.A., Ed.; FESEMI: Madrid, Spain, 2012; ISBN 978-84-7989-716-1. [Google Scholar]
- Garcia-Rendueles, A.R.; Rodrigues, J.S.; Garcia-Rendueles, M.E.R.; Suarez-Fariña, M.; Perez-Romero, S.; Barreiro, F.; Bernabeu, I.; Rodriguez-Garcia, J.; Fugazzola, L.; Sakai, T.; et al. Rewiring of the apoptotic TGF-β-SMAD/NFκB pathway through an oncogenic function of p27 in human papillary thyroid cancer. Oncogene 2017, 36, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Chenlo, M.; Rodriguez-Gomez, I.A.; Serramito, R.; Garcia-Rendueles, A.R.; Villar-Taibo, R.; Fernandez-Rodriguez, E.; Perez-Romero, S.; Suarez-Fariña, M.; Garcia-Allut, A.; Cabezas-Agricola, J.M.; et al. Unmasking a new prognostic marker and therapeutic target from the GDNF-RET/PIT1/p14ARF/p53 pathway in acromegaly. EBioMedicine 2019, 43, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.M. Part.II. Developmento of the body systems. Cardiovascular System. In Human Embryology and Developmental Biology; Carlson, B., Ed.; Elsevier: St Louis, MO, USA, 2019; p. 496. ISBN 9780323523752. [Google Scholar]
- Byrd, N.; Grabel, L. Hedgehog signaling in murine vasculogenesis and angiogenesis. Trends Cardiovasc. Med. 2004, 14, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; McClelland, R.L.; McDermott, M.M.; Allison, M.A.; Blumenthal, R.S.; Aboyans, V.; Ix, J.H.; Burke, G.L.; Liu, K.; Shea, S. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2010, 56, 1506–1512. [Google Scholar] [CrossRef]
- Fragoso, J.; Sierra, M.; Vargas, G.; Barrios, A.; Ramírez, J. El factor de necrosis tumoral α (TNF-α) en las enfermedades cardiovasculares: Biología molecular y genética. Gac. Med. Mex. 2013, 149, 521–530. [Google Scholar]
- Anderson, H.D.I.; Rahmutula, D.; Gardner, D.G. Tumor Necrosis Factor-α Inhibits Endothelial Nitric-oxide Synthase Gene Promoter Activity in Bovine Aortic Endothelial Cells. J. Biol. Chem. 2004, 279, 963–969. [Google Scholar] [CrossRef]
- Liu, S.-J.; Zhong, Y.; You, X.-Y.; Liu, W.-H.; Li, A.-Q.; Liu, S.-M. Insulin-like growth factor 1 opposes the effects of C-reactive protein on endothelial cell activation. Mol. Cell. Biochem. 2014, 385, 199–205. [Google Scholar] [CrossRef]
- Åberg, N.D.; Wall, A.; Anger, O.; Jood, K.; Andreasson, U.; Blennow, K.; Zetterberg, H.; Isgaard, J.; Jern, C.; Svensson, J. Circulating levels of vascular endothelial growth factor and post-stroke long-term functional outcome. Acta Neurol. Scand. 2020, 141, 405–414. [Google Scholar] [CrossRef]
- Giles, J.T.; Sattar, N.; Gabriel, S.; Ridker, P.M.; Gay, S.; Warne, C.; Musselman, D.; Brockwell, L.; Shittu, E.; Klearman, M.; et al. Cardiovascular Safety of Tocilizumab Versus Etanercept in Rheumatoid Arthritis: A Randomized Controlled Trial. Arthritis Rheumatol. 2020, 72, 31–40. [Google Scholar] [CrossRef]
- Murdaca, G.; Spanò, F.; Miglino, M.; Puppo, F. Effects of TNF-α inhibitors upon the mechanisms of action of VEGF. Immunotherapy 2013, 5, 113–115. [Google Scholar] [CrossRef]
- De Simone, C.; Farina, M.; Maiorino, A.; Fanali, C.; Perino, F.; Flamini, A.; Caldarola, G.; Sgambato, A. TNF-alpha gene polymorphisms can help to predict response to etanercept in psoriatic patients. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1786–1790. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; You, B.; Chen, S.-P.; Liao, J.K.; Sun, J. Tumor Necrosis Factor-α Downregulates Endothelial Nitric Oxide Synthase mRNA Stability via Translation Elongation Factor 1-α 1. Circ. Res. 2008, 103, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Kaptoge, S.; Di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J.; Tipping, R.W.; Ford, C.E.; Pressel, S.L.; et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Guralnik, J.M.; Corsi, A.; Albay, M.; Macchi, C.; Bandinelli, S.; Ferrucci, L. Patterns of inflammation associated with peripheral arterial disease: The InCHIANTI study. Am. Heart J. 2005, 150, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Basuroy, S.; Tcheranova, D.; Bhattacharya, S.; Leffler, C.W.; Parfenova, H. Nox4 NADPH oxidase-derived reactive oxygen species, via endogenous carbon monoxide, promote survival of brain endothelial cells during TNF-α-induced apoptosis. Am. J. Physiol. Cell Physiol. 2011, 300, C256–C265. [Google Scholar] [CrossRef]
- Moe, K.T.; Yin, N.O.; Naylynn, T.M.; Khairunnisa, K.; Wutyi, M.A.; Gu, Y.; Atan, M.S.M.; Wong, M.C.; Koh, T.H.; Wong, P. Nox2 and Nox4 mediate tumour necrosis factor-α-induced ventricular remodelling in mice. J. Cell. Mol. Med. 2011, 15, 2601–2613. [Google Scholar] [CrossRef]
- Moe, K.T.; Aulia, S.; Jiang, F.; Chua, Y.L.; Koh, T.H.; Wong, M.C.; Dusting, G.J. Differential upregulation of Nox homologues of NADPH oxidase by tumor necrosis factor-alpha in human aortic smooth muscle and embryonic kidney cells. J. Cell. Mol. Med. 2006, 10, 231–239. [Google Scholar] [CrossRef]
- Xia, C.; Meng, Q.; Liu, L.-Z.; Rojanasakul, Y.; Wang, X.-R.; Jiang, B.-H. Reactive Oxygen Species Regulate Angiogenesis and Tumor Growth through Vascular Endothelial Growth Factor. Cancer Res. 2007, 67, 10823–10830. [Google Scholar] [CrossRef]
- Maekawa, Y.; Ishikawa, K.; Yasuda, O.; Oguro, R.; Hanasaki, H.; Kida, I.; Takemura, Y.; Ohishi, M.; Katsuya, T.; Rakugi, H. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine 2009, 35, 341–346. [Google Scholar] [CrossRef]
- Signorelli, S.; Marino, E.; Scuto, S. Inflammation and Peripheral Arterial Disease. J 2019, 2, 142–151. [Google Scholar] [CrossRef]
- Brevetti, G.; Colao, A.; Schiano, V.; Pivonello, R.; Laurenzano, E.; Di Somma, C.; Lombardi, G.; Chiariello, M. IGF system and peripheral arterial disease: Relationship with disease severity and inflammatory status of the affected limb. Clin. Endocrinol. 2008, 69, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Coelho, N.H.; Coelho, A.; Augusto, R.; Semião, C.; Peixoto, J.; Fernandes, L.; Martins, V.; Canedo, A.; Gregório, T. Pre-operative Neutrophil to Lymphocyte Ratio is Associated With 30 Day Death or Amputation After Revascularisation for Acute Limb Ischaemia. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Brizuela Sanz, J.A.; González Fajardo, J.A.; Taylor, J.H.; Río Solá, L.; Muñoz Moreno, M.F.; Vaquero Puerta, C. Design of a New Risk Score in Critical Limb Ischaemia: The ERICVA Model. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 90–99. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequence | Amplification Size | Annealing Tª |
|---|---|---|---|
| TBP | Fw: 5′-GCCCGAAACGCCGAATAT-3′ | 67 bp | 60 °C |
| Rv: 5′-TTCGTGGCTCTCTTATCCTCATG-3′ | |||
| Pb: 5′-TCCCAAGCGGTTTGCTGCGGTA-3′ | |||
| VEGFA | Applied Biosystems: Hs00900055_m1 | 67 bp | 60 °C |
| IGF1 | Applied Biosystems: Hs01547656_m1 | 68 bp | 60 °C |
| NOS3 | Applied Biosystems: Hs01574665_m1 | 86 bp | 60 °C |
| MSTN | Applied Biosystems: Hs00976237_m1 | 69 bp | 60 °C |
| NOX4 | Applied Biosystems: Hs01379108_m1 | 64 bp | 60 °C |
| MYOG | Applied Biosystems: Hs01072232_m1 | 76 bp | 60 °C |
| KDR | Applied Biosystems: Hs00911700_m1 | 83 bp | 60 °C |
| IL6 | Applied Biosystems: Hs00174131_m1 | 95 bp | 60 °C |
| TNF | Applied Biosystems: Hs00174128_m1 | 80 bp | 60 °C |
| TG | HDLc | LDLc | ABI | AP | Age | IGF-I | IGFBP3 | TNF-α | hsCRP | B2-M | CyC | HbA1C | NLR | Fibrin. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 164.1 | 45.5 | 97.9 | 0.23 | 38.6 | 71.5 | 134.9 | 3.06 | 10.66 | 1.6 | 0.4 | 1.45 | 6.5 | 3.5 | 560.21 |
| Median | 132.5 | 41 | 100 | 0.2 | 31.5 | 72 | 125 | 2.9 | 10 | 0.95 | 0.3 | 1.2 | 6.3 | 2.6 | 505 |
| SD | 88.1 | 15.1 | 32.1 | 0.23 | 37.02 | 12.4 | 53.2 | 1.1 | 4.9 | 2.2 | 0.3 | 0.9 | 1.02 | 2.8 | 128.51 |
| Mín. | 46 | 29 | 36 | 0 | 0 | 49 | 38 | 0.5 | 4 | 0.1 | 0.14 | 0.55 | 5.1 | 0.4 | 403 |
| Max. | 412 | 97 | 161 | 0.93 | 140 | 93 | 275 | 5.2 | 27 | 9.1 | 1.1 | 3.8 | 8.9 | 16.3 | 853 |
| Plasma Marker | GH | Placebo | |||||
|---|---|---|---|---|---|---|---|
| Obs. | Mean | SD | Obs. | Mean | SD | p-Value | |
| TNF-α (Basal) | 16 | 12.35 | 5.2 | 16 | 8.78 | 3.9 | 0.0184 * |
| TNF-α (Final) | 15 | 10.93 | 5.12 | 14 | 8.04 | 3.6 | 0.0464 * |
| hsCRP (Basal) | 18 | 2.07 | 2.86 | 16 | 0.79 | 0.70 | 0.0454 * |
| hsCRP (Final) | 17 | 1.1 | 1.38 | 14 | 3.42 | 7.51 | 0.2188 |
| B2M (Basal) | 7 | 0.47 | 0.27 | 16 | 0.22 | 0.08 | 0.1269 |
| B2M (Final) | 8 | 0.56 | 0.51 | 14 | 0.21 | 0.12 | 0.3894 |
| CyC (Basal) | 7 | 1.75 | 0.93 | 4 | 0.76 | 0.17 | 0.035 * |
| CyC (Final) | 8 | 1.71 | 1.12 | 2 | 0.8 | 0.14 | 0.3054 |
| Plasma Marker/ Gene | NOS3 (Basal) | NOS3 (Final) | VEGFA (Basal) | VEGFA (Final) | TNF (Basal) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Both | Placebo | GH | GH | Both | GH | Placebo | Both | GH | ||
| TNF-α (Basal) | r = −0.49 p = 0.015 | r = −0.78 p = 0.0064 | r = −0.432 p = 0.039 | r = −0.773 p = 0.005 | r = 0.588 p = 0.035 * | |||||
| TNF-α (Final) | r = 0.866 p = 0.012 | |||||||||
| TNF-α > 8.1 (Basal) | r = 0.802, p = 0.001 * | |||||||||
| hsCRP (Basal) | r = 0.74 p = 0.009 | r = −0.632 p = 0.005 | r = −0.775 p = 0.041 | |||||||
| hsCRP > 0.5 (Basal) | r = −0.693 p = 0.018 | r = −0.546 p = 0.019 | r = −0.866 p = 0.012 | |||||||
| DM | hsCRP > 0.5 (Final) | r = −0.711 p = 0.021 * | r = −0.866, p = 0.012 | |||||||
| NLR > 3 (Basal) | r = −0.645 p = 0.032 | |||||||||
| NLR > 5 (Basal) | r = −0.69 p = 0.018 | |||||||||
| HbA1C (Basal) | r = 0.728 p = 0.026 | |||||||||
| HbA1C (Final) | r = −0.975 p = 0.005 | |||||||||
| Non-DM | hsCRP (Basal) | r = −0.9 p = 0.037 | ||||||||
| LDLc | r = −0.827 p = 0.002 | |||||||||
| Plasma Marker/ Gene | IGF-I (Basal) | IGF-I (Final) | MSTN (Basal) | MSTN (Final) | MYOG (Final) | KDR (Final) | NOX4 (Basal) | NOX4 (Final) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Both | Placebo | GH | Both | GH | GH | Placebo | Placebo | Both | GH | ||
| TNF-α (Basal) | r = 0.519 p = 0.011 | r = 0.821 p = 0.0341 | r = 0.8 p = 0.023 * | r = 0.305 p = 0.037 | |||||||
| TNF-α > 8.1 (Basal) | r = 0.598 p = 0.003 | r = 0.586 p = 0.011 | |||||||||
| hsCRP (Basal) | r = −0.648 p = 0.031 | ||||||||||
| hsCRP (Final) | r = −0.691 p = 0.027 | r = 0.709 p = 0.003 * | |||||||||
| DM | hsCRP > 0.5 (Final) | r = 0.773 p = 0.001 * | |||||||||
| NLR > 3 (Basal) | r = −0.662 p = 0.019 | ||||||||||
| NLR > 5 (Basal) | r = −0.857 p = 0.014 * | ||||||||||
| HbA1C (Basal) | r = 0.597 p = 0.019 | ||||||||||
| Non-DM | TNF-α (Basal) | r = 0.645 p = 0.032 | |||||||||
| TNF-α > 8.1 (Basal) | r = 0.717 p = 0.009 | ||||||||||
| hsCRP (Basal) | r = 0.9 p = 0.037 | r = −0.847 p = 0.016 | |||||||||
| 0–2 Months | Mortality | p-Value | 2–12 Months | Mortality | p-Value | |
|---|---|---|---|---|---|---|
| Placebo | 0/16 | 0% | 2/16 | 12.5% | ||
| GH | 1/18 | 5.5% | 0.42 | 5/17 | 29.4% | 0.23 |
| Cumulative | 5.5% | 47.4% |
| Predictors of Mortality in the GHAS Trial | |||
|---|---|---|---|
| p-Value | OR | CI (95%) | |
| NLR ≥ 3 (Basal) | 0.019 | 6.9 | 0.71–353.7 |
| TNF-α ≥ 8.1 (Basal) | 0.0487 | 2.5 | 0.23–136.6 |
| COPD | 0.042 | 5.8 | 0.84–40.7 |
| ASA4-ASA3 | 0.0119 | 15.7 | 0.87–284.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caicedo, D.; Alvarez, C.V.; Perez-Romero, S.; Devesa, J. The Inflammatory Pattern of Chronic Limb-Threatening Ischemia in Muscles: The TNF-α Hypothesis. Biomedicines 2022, 10, 489. https://doi.org/10.3390/biomedicines10020489
Caicedo D, Alvarez CV, Perez-Romero S, Devesa J. The Inflammatory Pattern of Chronic Limb-Threatening Ischemia in Muscles: The TNF-α Hypothesis. Biomedicines. 2022; 10(2):489. https://doi.org/10.3390/biomedicines10020489
Chicago/Turabian StyleCaicedo, Diego, Clara V. Alvarez, Sihara Perez-Romero, and Jesús Devesa. 2022. "The Inflammatory Pattern of Chronic Limb-Threatening Ischemia in Muscles: The TNF-α Hypothesis" Biomedicines 10, no. 2: 489. https://doi.org/10.3390/biomedicines10020489
APA StyleCaicedo, D., Alvarez, C. V., Perez-Romero, S., & Devesa, J. (2022). The Inflammatory Pattern of Chronic Limb-Threatening Ischemia in Muscles: The TNF-α Hypothesis. Biomedicines, 10(2), 489. https://doi.org/10.3390/biomedicines10020489








