Comparative Study of the Aftereffect of CO2 Inhalation or Tiletamine–Zolazepam–Xylazine Anesthesia on Laboratory Outbred Rats and Mice
Abstract
:1. Introduction
2. Materials and Methods
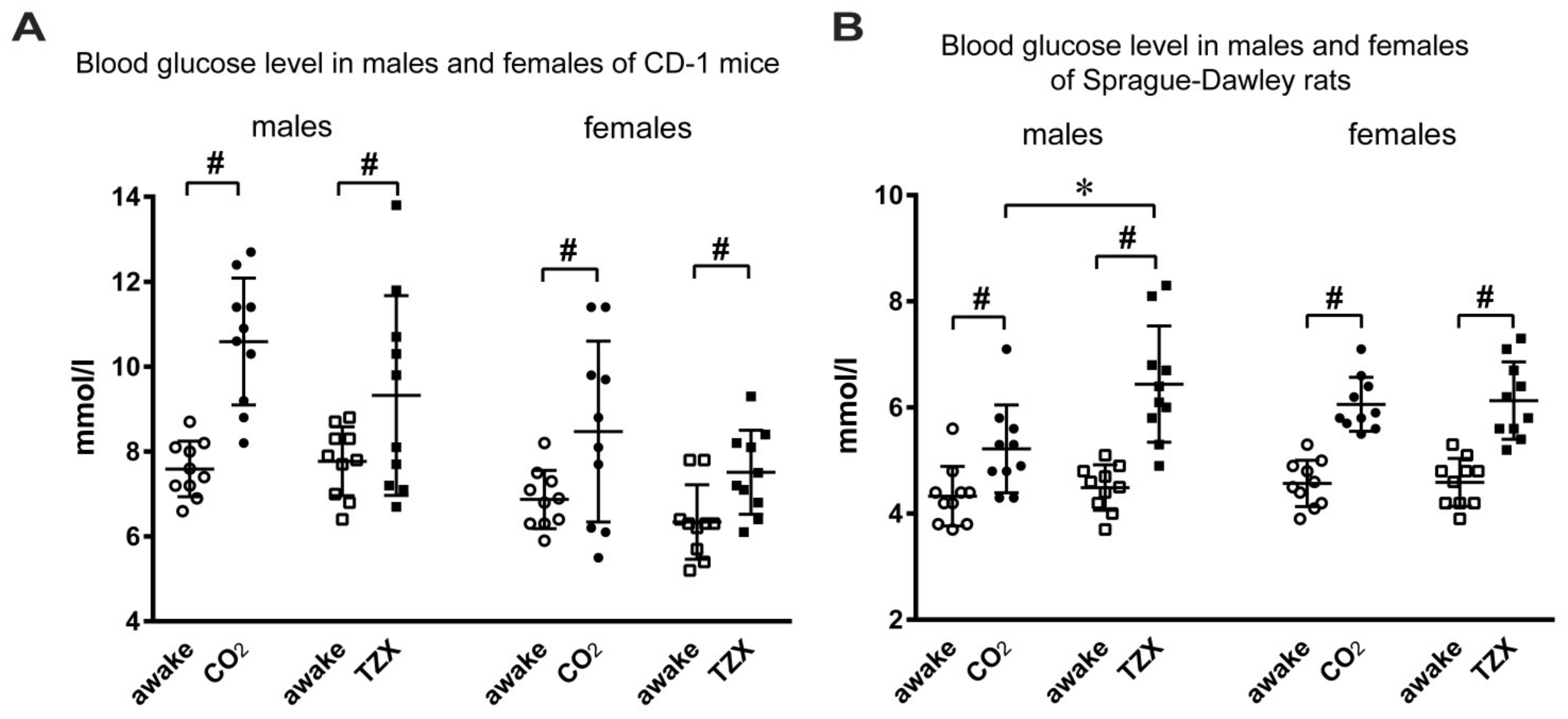
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| MCH | mean corpuscular hemoglobin |
| MCHC | mean corpuscular hemoglobin concentration |
| TZX | tiletamine-zolazepam-xylazine |
References
- Aldinger, K.A.; Sokoloff, G.; Rosenberg, D.M.; Palmer, A.A.; Millen, K.J. Genetic Variation and Population Substructure in Outbred CD-1 Mice: Implications for Genome-Wide Association Studies. PLoS ONE 2009, 4, e4729. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.-J.; Cho, Y.M.; Shin, H.J.; Kim, H.D.; Choi, K.M.; Kim, M.G.; Shin, H.D.; Chung, M.-W. Comparison of commonly used ICR stocks and the characterization of Korl:ICR. Lab. Anim. Res. 2017, 33, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hostetler, K.A.; Fisher, L.C.; Burruss, B.L. Prenatal developmental toxicity of alkyl dimethyl benzyl ammonium chloride and didecyl dimethyl ammonium chloride in CD rats and New Zealand White rabbits. Birth Defects Res. 2021, 113, 925–944. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-C.; Dai, Y.-Z.; Tzeng, Y.-M.; Liao, J.-W. Genotoxicity and 28-day repeated dose oral toxicity study of ovatodiolide in rats. Toxicol. Rep. 2021, 8, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Festing, M.F.W. Evidence Should Trump Intuition by Preferring Inbred Strains to Outbred Stocks in Preclinical Research. ILAR J. 2014, 55, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Schnell, M.A.; Hardy, C.; Hawley, M.; Propert, K.J.; Wilson, J.M. Effect of Blood Collection Technique in Mice on Clinical Pathology Parameters. Hum. Gene Ther. 2002, 13, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Nagate, T.; Chino, T.; Nishiyama, C.; Okuhara, D.; Tahara, T.; Maruyama, Y.; Kasahara, H.; Takashima, K.; Kobayashi, S.; Motokawa, Y.; et al. Diluted Isoflurane as a Suitable Alternative for Diethyl ether for Rat Anaesthesia in Regular Toxicology Studies. J. Vet. Med. Sci. 2007, 69, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- Fernández, I.; Peña, A.; Del Teso, N.; Pérez, V.; Rodríguez-Cuesta, J. Clinical biochemistry parameters in C57BL/6J mice after blood collection from the submandibular vein and retroorbital plexus. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 202–206. [Google Scholar]
- Harikrishnan, V.S.; Hansen, A.K.; Abelson, K.S.; Sørensen, D.B. A comparison of various methods of blood sampling in mice and rats: Effects on animal welfare. Lab. Anim. 2017, 52, 253–264. [Google Scholar] [CrossRef]
- AVMA Guidelines for the Euthanasia of Animals. Available online: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf (accessed on 25 February 2017).
- Valentine, H.; Williams, W.O.; Maurer, K.J. Sedation or inhalant anesthesia before euthanasia with CO2 does not reduce behavioral or physiologic signs of pain and stress in mice. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 50–57. [Google Scholar]
- Danneman, P.J.; Stein, S.; Walshaw, S.O. Humane and practical implications of using carbon dioxide mixed with oxygen for anesthesia or euthanasia of rats. Lab. Anim. Sci. 1997, 47, 376–385. [Google Scholar]
- Traslavina, R.P.; King, E.J.; Loar, A.S.; Riedel, E.R.; Garvey, M.S.; Ricart-Arbona, R.; Wolf, F.R.; Couto, S.S. Euthanasia by CO₂ inhalation affects potassium levels in mice. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 316–322. [Google Scholar] [PubMed]
- Valentim, A.M.; Guedes, S.; Pereira, A.M.; Antunes, L.M. Euthanasia using gaseous agents in laboratory rodents. Lab. Anim. 2016, 50, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Vahl, T.P.; Ulrich-Lai, Y.; Ostrander, M.M.; Dolgas, C.M.; Elfers, E.E.; Seeley, R.; D’Alessio, D.A.; Herman, J. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am. J. Physiol. Metab. 2005, 289, E823–E828. [Google Scholar] [CrossRef] [Green Version]
- Reed, B.; Varon, J.; Chait, B.T.; Kreek, M.J. Carbon Dioxide-Induced Anesthesia Results in a Rapid Increase in Plasma Levels of Vasopressin. Endocrinology 2009, 150, 2934–2939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zardooz, H.; Rostamkhani, F.; Zaringhalam, J.; Shahrivar, F.F. Plasma corticosterone, insulin and glucose changes induced by brief exposure to isoflurane, diethyl ether and CO2 in male rats. Physiol. Res. 2010, 59, 973–978. [Google Scholar] [CrossRef]
- Saha, D.C.; Saha, A.C.; Malik, G.; Astiz, M.E.; Rackow, E.C. Comparison of cardiovascular effects of tiletamine-zolazepam, pentobarbital, and ketamine-xylazine in male rats. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 74–80. [Google Scholar]
- Erhardt, W.; Hebestedt, A.; Aschenbrenner, G.; Pichotka, B.; Blümel, G. A comparative study with various anesthetics in mice (pentobarbi-tone, ketamine-xylazine, carfentanyl-etomidate). Res. Exp. Med. 1984, 184, 159–169. [Google Scholar] [CrossRef]
- Machado, E.; Normand, A.; Nunes, L.; Brenzikofer, R.; Macedo, D. Effects of different general anesthetics on serum hemolysis and hepatic and muscular glycogenolysis in rats. Braz. J. Med. Biol. Res. 2009, 42, 1035–1038. [Google Scholar] [CrossRef] [Green Version]
- Saha, J.K.; Xia, J.; Grondin, J.M.; Engle, S.K.; Jakubowski, J.A. Acute Hyperglycemia Induced by Ketamine/Xylazine Anesthesia in Rats: Mechanisms and Implications for Preclinical Models. Exp. Biol. Med. 2005, 230, 777–784. [Google Scholar] [CrossRef]
- Manell, E.; Jensen-Waern, M.; Hedenqvist, P. Anaesthesia and changes in parameters that reflect glucose metabolism in pig—A pilot study. Lab. Anim. 2016, 51, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, Å.; Pringle, J.; Arnemo, J.M.; Swenson, J.E.; Brunberg, S.; Nyman, G. Treatment of Hypoxemia During Anesthesia of Brown Bears (Ursus arctos). J. Zoo Wildl. Med. 2010, 41, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Yaralioglu-Gurgoze, S.; Sindak, N.; Şahin, T.; Cen, O.; Şındak, N. Levels of glutathione peroxidase, lipoperoxidase and some biochemical and haematological parameters in gazelles anaesthetised with a tiletamin–zolazepam–xylazine combination. Vet. J. 2005, 169, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Thurmon, J.C.; Benson, G.J.; Tranquilli, W.J. Telazol—A review of its pharmacology and use in veterinary medicine. J. Vet. Pharmacol. Ther. 1993, 16, 383–418. [Google Scholar] [CrossRef]
- Seo, J.; Park, H.-P.; Jeon, Y.; Lim, Y.; Nam, K.; Hwang, J. Combined treatment with celecoxib and sevoflurane after global cerebral ischaemia has no additive neuroprotective effects in rats. Br. J. Anaesth. 2013, 110, 988–995. [Google Scholar] [CrossRef] [Green Version]
- An, S.; Jeon, Y.J.; Jo, A.; Lim, H.J.; Han, Y.E.; Cho, S.W.; Kim, H.Y.; Kim, H.J. Initial Influenza Virus Replication Can Be Limited in Allergic Asthma Through Rapid Induction of Type III Interferons in Respiratory Epithelium. Front. Immunol. 2018, 9, 986. [Google Scholar] [CrossRef] [Green Version]
- Wixon, S.K.; Smiler, K.L. Anesthesia and Analgesia in Laboratory Animals; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Tabata, H.; Kitamura, T.; Nagamatsu, N. Comparison of effects of restraint, cage transportation, anaesthesia and repeated bleeding on plasma glucose levels between mice and rats. Lab. Anim. 1998, 32, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Winder, W.W.; Fuller, E.O.; Conlee, R.K. Adrenal hormones and liver cAMP in exercising rats—Different modes of anesthesia. J. Appl. Physiol. 1983, 55, 1634–1636. [Google Scholar] [CrossRef]
- Toso, C.F.R.; Linares, L.M.; Rodríguez, R.R. Blood sugar concentrations during ketamine or pentobarbitone anesthesia in rats with or without alpha and beta adrenergic blockade. Medicina 1995, 55, 311–316. [Google Scholar]
- Windeløv, J.A.; Pedersen, J.; Holst, J.J. Use of anesthesia dramatically alters the oral glucose tolerance and insulin secretion in C57Bl/6 mice. Physiol. Rep. 2016, 4, e12824. [Google Scholar] [CrossRef] [Green Version]
- Allen, B.W.; Stamler, J.S.; Piantadosi, C.A. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol. Med. 2009, 15, 452–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulandavelu, S.; Balkan, W.; Hare, J.M. Regulation of oxygen delivery to the body via hypoxic vasodilation. Proc. Natl. Acad. Sci. USA 2015, 112, 6254–6255. [Google Scholar] [CrossRef] [Green Version]
- Heuser, D.; Guggenberger, H. Ionic changes in brain ischemia and alterations produced by drugs. Br. J. Anesth. 1985, 57, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, O.; Zur, B.; Koch, A.; Tran, N.; Freyenhagen, R.; Hartmann, M.; Zacharowski, K. Clinical chemistry reference database for Wistar rats and C57/BL6 mice. Biol. Chem. 2007, 388, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Montford, J.R.; Linas, S. How Dangerous Is Hyperkalemia? J. Am. Soc. Nephrol. 2017, 28, 3155–3165. [Google Scholar] [CrossRef] [Green Version]
- Iyer, S.P.N.; Lee, L.; Li, L. Pharmacodynamic effects of the K + binder patiromer in a novel chronic hyperkalemia model in spontaneously hypertensive rats. Physiol. Rep. 2020, 8, e14572. [Google Scholar] [CrossRef]
- Conlee, K.M.; Stephens, M.L.; Rowan, A.N.; King, L.A. Carbon dioxide for euthanasia: Concerns regarding pain and distress, with special reference to mice and rats. Lab. Anim. 2005, 39, 137–161. [Google Scholar] [CrossRef]
- Améndola, L.; Weary, D.M. Understanding rat emotional responses to CO2. Transl. Psychiatry 2020, 10, 253. [Google Scholar] [CrossRef]
- Hodgkinson, A.; Hambleton, J. Elevation of serum calcium concentration and changes in other blood parameters after death. J. Surg. Res. 1969, 9, 567–574. [Google Scholar] [CrossRef]
- Mimura, Y. Phosphate and cyclic AMP excretion decreases during less than 12 hours of hypoxia in conscious rats. Acta Physiol. Scand. 1996, 158, 317–323. [Google Scholar] [CrossRef]
- Nwokocha, C.; Nwokocha, M.I.; Mounmbegna, P.P.E.; Owu, D.U.; Onyezuligbo, O.; Olu-Osifo, E.H.; Okojie, E.; Asuquo, E.; Thaxter, K.; Ogunsalu, C. Serum lipids, proteins and electrolyte profiles in rats following total body irradiation. West Indian Med. J. 2012, 61, 117–121. [Google Scholar] [PubMed]
- Popilskis, S.J.; Oz, M.C.; Gorman, P.; Florestal, A.; Kohn, D.F. Comparison of xylazine with tiletamine-zolazepam (Telazol) and xylazine-ketamine anesthesia in rabbits. Lab. Anim. Sci. 1991, 41, 51–53. [Google Scholar] [PubMed]
- Dupras, J.; Vachon, P.; Cuvelliez, S.; Blais, D. Anesthesie du lapin de Nouvelle-Zelandeutilisant les combinaisons tileta-mine-zolazepamet ketamine-midazolam avec ou sans xylazine. Can. Vet. J. 2001, 42, 455–460. (In French) [Google Scholar]
- Lefkov, S.H.; Müssig, D. Tiletamine-zolazepam and xylazine is a potent cardiodepressive combination: A case report. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 63–64. [Google Scholar] [PubMed]
- Noble, B.J.; Drinkhill, M.J.; Myers, D.S.; Hainsworth, R. Blood mobilization from the liver of the anaesthetized dog. Exp. Physiol. 1998, 83, 513–522. [Google Scholar] [CrossRef]
- Schaffner, A.; Augustiny, N.; Otto, R.C.; Fehr, J. The hypersplenic spleen: A contractile reservoir of granulocytes and platelets. Arch. Intern. Med. 1985, 145, 651–654. [Google Scholar] [CrossRef]
- Khokhlova, O.; Tukhovskaya, E.A.; Kravchenko, I.N.; Sadovnikova, E.S.; Pakhomova, I.A.; Kalabina, E.A.; Lobanov, A.; Shaykhutdinova, E.R.; Ismailova, A.M.; Murashev, A.N. Using Tiletamine-Zolazepam-Xylazine Anesthesia Compared to CO2-inhalation for Terminal Clinical Chemistry, Hematology, and Coagulation Analysis in Mice. J. Pharmacol. Toxicol. Methods 2017, 84, 11–19. [Google Scholar] [CrossRef]
- Jadaho, S.B.; Yang, R.-Z.; Lin, Q.; Hu, H.; Anania, F.A.; Shuldiner, A.R.; Gong, D.-W. Murine alanine aminotransferase: cDNA cloning, functional expression, and differential gene regulation in mouse fatty liver. Hepatology 2004, 39, 1297–1302. [Google Scholar] [CrossRef]
- Yang, R.-Z.; Park, S.; Reagan, W.J.; Goldstein, R.; Zhong, S.; Lawton, M.; Rajamohan, F.; Qian, K.; Liu, L.; Gong, D.-W. Alanine aminotransferase isoenzymes: Molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology 2009, 49, 598–607. [Google Scholar] [CrossRef] [Green Version]
- Ennulat, D.; Walker, D.; Clemo, F.; Magid-Slav, M.; Ledieu, D.; Graham, M.; Botts, S.; Boone, L. Effects of Hepatic Drug-metabolizing Enzyme Induction on Clinical Pathology Parameters in Animals and Man. Toxicol. Pathol. 2010, 38, 810–828. [Google Scholar] [CrossRef]
- Reagan, W.J.; Yang, R.Z.; Park, S.; Goldstein, R.; Brees, D.; Gong, D.W. Metabolic adaptive ALT isoenzyme response in livers of C57/BL6 mice treated with dexamethasone. Toxicol. Pathol. 2012, 40, 1117–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellington, D.; Mikaelian, I.; Singer, L. Comparison of ketamine-xylazine and ketamine-dexmedetomidine anesthesia and intraperitoneal tolerance in rats. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 481–487. [Google Scholar] [PubMed]
- Hatayama, K.; Ichikawa, Y.; Nishihara, Y.; Goto, K.; Nakamura, D.; Wakita, A.; Kobayashi, J. Serum alkaline phosphatase isoenzymes in SD rats detected by poly-acrylamide-gel disk electrophoresis. Toxicol. Mech. Methods 2012, 22, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Hatayama, K.; Nishihara, Y.; Kimura, S.; Goto, K.; Nakamura, D.; Wakita, A.; Urasoko, Y. Alkaline phosphatase isoenzymes in mouse plasma detected by poly-acrylamide-gel disk electrophoresis. J. Toxicol. Sci. 2011, 36, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krones, E.; Erwa, W.; Trauner, M.; Fickert, P. Serum alkaline phosphatase levels accurately reflect cholestasis in mice. Hepatology 2015, 62, 981–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overmyer, K.A.; Thonusin, C.; Qi, N.R.; Burant, C.F.; Evans, C.R. Impact of Anesthesia and Euthanasia on Metabolomics of Mammalian Tissues: Studies in a C57BL/6J Mouse Model. PLoS ONE 2015, 10, e0117232. [Google Scholar] [CrossRef]
| Males | Females | |||
|---|---|---|---|---|
| CO2 (n = 10) | TZX (n = 10) | CO2 (n = 10) | TZX (n = 10) | |
| Total protein (g/L) | 54.5 ± 0.8 | 42.4 ± 0.8 *** | 56.3 ± 1.1 | 42.3 ± 1.3 *** |
| Albumin (g/L) | 33.5 ± 0.5 | 26.6 ± 0.4 *** | 40.0 ± 1.8 | 31.7 ± 1.9 ** |
| Globulins (g/L) | 21.1 ± 0.4 | 15.8 ± 0.5 *** | 16.2 ± 1.1 | 10.6 ± 0.8 ** |
| Albumin:Globulin Ratio | 1.6 ± 0.1 | 1.7 ± 0.1 * | 2.7 ± 0.5 | 3.3 ± 0.4 |
| Urea (mmol/L) | 9.5 ± 0.4 | 9.1 ± 0.4 | 7.8 ± 0.2 | 9.0 ± 0.5 |
| Creatinine (µmol/L) | 38.7 ± 0.8 | 41.3 ± 1.1 | 48.7 ± 2.5 | 42.1 ± 1.8 * |
| Cholesterol (mmol/L) | 3.29 ± 0.20 | 2.72 ± 0.19 | 2.73 ± 0.25 | 1.93 ± 0.11 * |
| Triglycerides (mmol/L) | 1.50 ± 0.13 | 1.21 ± 0.08 | 2.16 ± 0.43 | 1.18 ± 0.12 |
| Total bilirubin (µmol/L) | 7.55 ± 0.73 | 5.50 ± 0.43 * | 6.94 ± 0.82 | 6.07 ± 0.32 * |
| ALT (U/L) | 58.9 ± 7.2 | 44.3 ± 6.4 * | 44.1 ± 4.5 | 30.6 ± 1.6 * |
| AST (U/L) | 77.6 ± 5.3 | 49.2 ± 3.0 *** | 66.5 ± 2.5 | 53.3 ± 2.0 ** |
| ALP (U/L) | 76.9 ± 9.1 | 70.0 ± 8.8 | 143.6 ± 9.3 | 128.3 ± 11.7 |
| Calcium (mmol/L) | 2.81 ± 0.03 | 2.30 ± 0.02 *** | 3.15 ± 0.09 | 2.44 ± 0.07 *** |
| Inorganic phosphorus (mmol/L) | 3.87 ± 0.08 | 3.04 ± 0.13 *** | 4.14 ± 0.25 | 3.23 ± 0.18 ** |
| Sodium (mmol/L) | 142.3 ± 1.6 | 152.2 ± 0.6 *** | 144.5 ± 0.9 | 150.6 ± 0.6 *** |
| Potassium (mmol/L) | 16.2 ± 0.4 | 5.2 ± 0.1 *** | 13.5 ± 0.3 | 4.4 ± 0.1 *** |
| Chloride (mmol/L) | 112.0 ± 1.3 | 110.4 ± 0.6 * | 112.6 ± 0.6 | 110.2 ± 0.4 ** |
| Males | Females | |||
|---|---|---|---|---|
| CO2 (n = 10) | TZX (n = 10) | CO2 (n = 10) | TZX (n = 10) | |
| Total protein (g/L) | 70.9 ± 5.1 | 59.2 ± 4.6 *** | 67.0 ± 3.7 | 57.6 ± 4.0 *** |
| Albumin (g/L) | 41.0 ± 1.5 | 34.8 ± 2.2 *** | 39.8 ± 1.5 | 35.1 ± 1.9 *** |
| Globulins (g/L) | 29.8 ± 3.6 | 24.3 ± 3.0 ** | 27.1 ± 2.4 | 22.5 ± 2.4 *** |
| Albumin:Globulin Ratio | 1.4 ± 0.1 | 1.4 ± 0.2 | 1.5 ± 0.1 | 1.6 ± 0.1 |
| Urea (mmol/L) | 9.5 ± 1.1 | 9.0 ± 1.5 | 7.9 ± 0.9 | 7.5 ± 1.1 |
| Creatinine (µmol/L) | 72.3 ± 4.5 | 52.2 ± 3.3 *** | 70.0 ± 3.4 | 59.2 ± 4.3 *** |
| Cholesterol (mmol/L) | 2.79 ± 0.45 | 1.98 ± 0.45 ** | 2.78 ± 0.38 | 2.23 ± 0.31 ** |
| Triglycerides (mmol/L) | 1.04 ± 0.26 | 0.62 ± 0.12 *** | 0.94 ± 0.19 | 0.57 ± 0.12 *** |
| Total bilirubin (µmol/L) | 5.23 ± 1.29 | 3.33 ± 0.80 ** | 3.93 ± 1.11 | 3.21 ± 0.82 |
| ALT (U/L) | 68.2 ± 10.1 | 52.1 ± 8.0 ** | 60.1 ± 11.9 | 37.8 ± 7.3 *** |
| AST (U/L) | 83.8 ± 6.7 | 72.4 ± 8.3 * | 83.7 ± 8.5 | 74.7 ± 6.7 ** |
| ALP (U/L) | 160.0 ± 27.0 | 138.1 ± 26.0 | 100.1 ± 26.3 | 72.3 ± 25.3 * |
| Calcium (mmol/L) | 3.28 ± 0.27 | 2.51 ± 0.20 *** | 2.94 ± 0.31 | 2.56 ± 0.15 ** |
| Inorganic phosphorus (mmol/L) | 4.41 ± 0.68 | 2.17 ± 0.33 *** | 3.65 ± 0.92 | 1.75 ± 0.27 *** |
| Sodium (mmol/L) | 141.9 ± 7.2 | 144.1 ± 6.0 | 132.0 ± 3.3 | 138.1 ± 2.3 *** |
| Potassium (mmol/L) | 11.0 ± 1.5 | 4.6 ± 0.3 *** | 12.5 ± 1.8 | 4.2 ± 0.3 *** |
| Chloride (mmol/L) | 119.9 ± 7.8 | 117.2 ± 5.5 | 114.1 ± 2.1 | 112.8 ± 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khokhlova, O.N.; Borozdina, N.A.; Sadovnikova, E.S.; Pakhomova, I.A.; Rudenko, P.A.; Korolkova, Y.V.; Kozlov, S.A.; Dyachenko, I.A. Comparative Study of the Aftereffect of CO2 Inhalation or Tiletamine–Zolazepam–Xylazine Anesthesia on Laboratory Outbred Rats and Mice. Biomedicines 2022, 10, 512. https://doi.org/10.3390/biomedicines10020512
Khokhlova ON, Borozdina NA, Sadovnikova ES, Pakhomova IA, Rudenko PA, Korolkova YV, Kozlov SA, Dyachenko IA. Comparative Study of the Aftereffect of CO2 Inhalation or Tiletamine–Zolazepam–Xylazine Anesthesia on Laboratory Outbred Rats and Mice. Biomedicines. 2022; 10(2):512. https://doi.org/10.3390/biomedicines10020512
Chicago/Turabian StyleKhokhlova, Oksana N., Natalya A. Borozdina, Elena S. Sadovnikova, Irina A. Pakhomova, Pavel A. Rudenko, Yuliya V. Korolkova, Sergey A. Kozlov, and Igor A. Dyachenko. 2022. "Comparative Study of the Aftereffect of CO2 Inhalation or Tiletamine–Zolazepam–Xylazine Anesthesia on Laboratory Outbred Rats and Mice" Biomedicines 10, no. 2: 512. https://doi.org/10.3390/biomedicines10020512
APA StyleKhokhlova, O. N., Borozdina, N. A., Sadovnikova, E. S., Pakhomova, I. A., Rudenko, P. A., Korolkova, Y. V., Kozlov, S. A., & Dyachenko, I. A. (2022). Comparative Study of the Aftereffect of CO2 Inhalation or Tiletamine–Zolazepam–Xylazine Anesthesia on Laboratory Outbred Rats and Mice. Biomedicines, 10(2), 512. https://doi.org/10.3390/biomedicines10020512






