Influence of Fetomaternal Microchimerism on Maternal NK Cell Reactivity against the Child’s Leukemic Blasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cell Isolation and Treatment
2.3. DNA Isolation and Genotyping
2.4. Microchimerism Determination
2.5. Degranulation, KIR Phenotyping and Cytotoxicity Assay
2.6. Statistical and Flow Cytometry Data Analysis
3. Results
3.1. Study Cohort
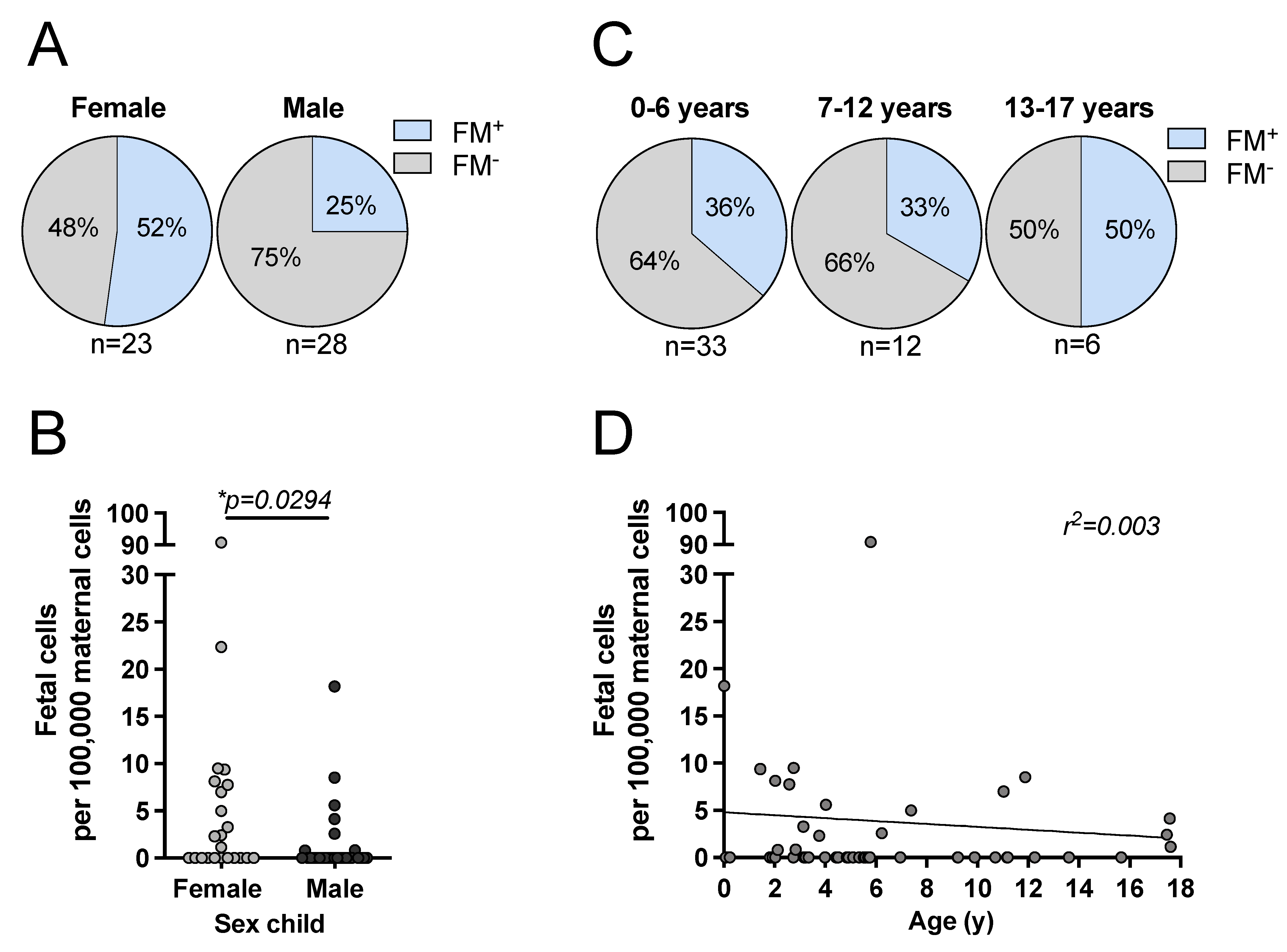
3.2. Effect of Filial Age and Sex on FM
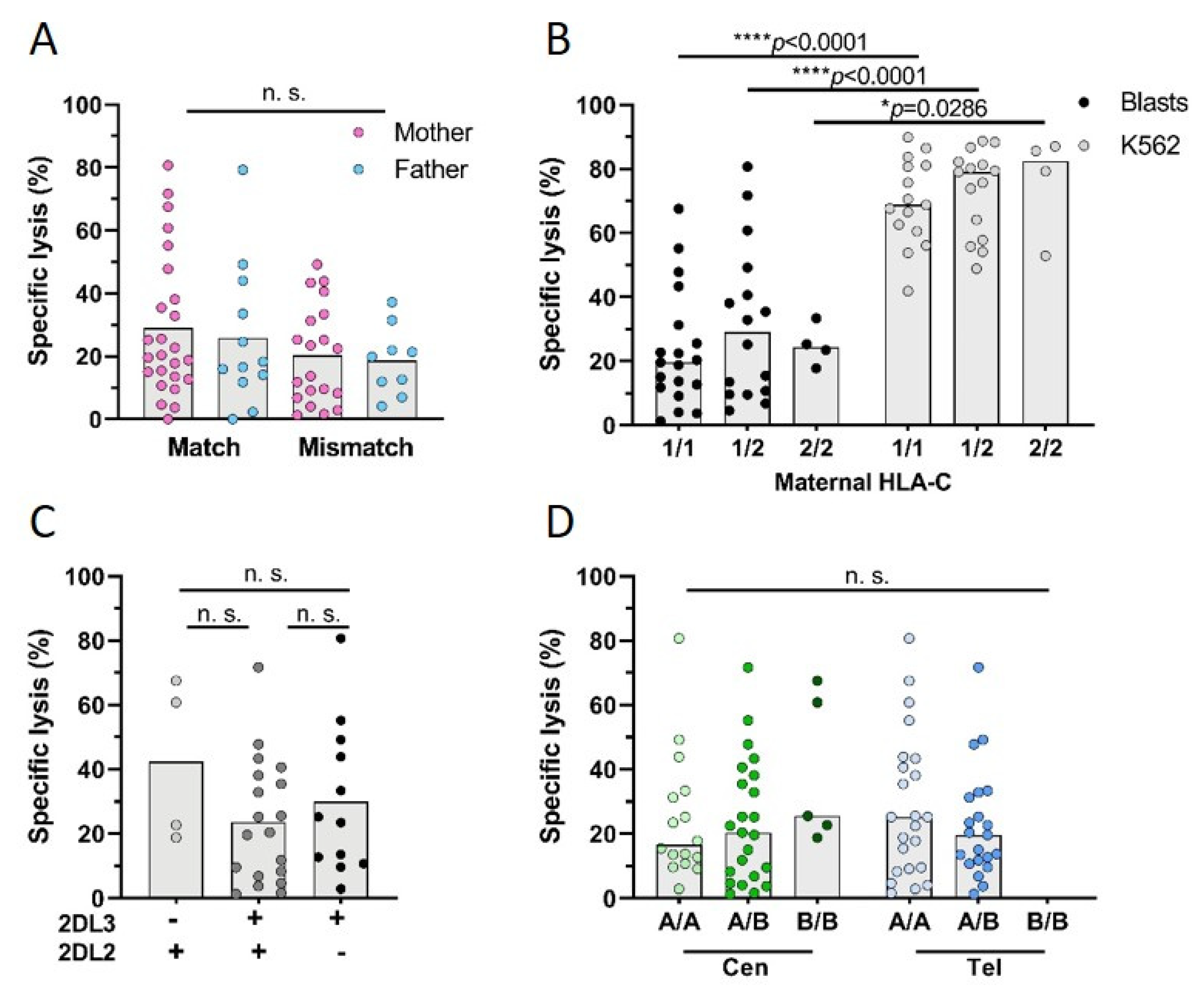
3.3. Influence of HLA-C on FM
3.4. Influence of KIR Genotype on FM
3.5. Influence of FM on Parental NK Cell KIR Phenotype
3.6. Influence of FM on Parental NK Cell Reactivity
4. Discussion
4.1. NK Cells in Establishment and Persistence of FM
4.2. NK Cell Alloreactivity, KIR and HSCT
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Reisner, Y.; Hagin, D.; Martelli, M.F. Haploidentical hematopoietic transplantation: Current status and future perspectives. Blood 2011, 118, 6006–6017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marks, D.I.; Khattry, N.; Cummins, M.; Goulden, N.; Green, A.; Harvey, J.; Hunt, L.P.; Keen, L.; Robinson, S.P.; Steward, C.G.; et al. Haploidentical stem cell transplantation for children with acute leukaemia. Br. J. Haematol. 2006, 134, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, Y.J.; Xu, L.P.; Liu, K.Y.; Liu, D.H.; Zhang, X.H.; Chen, H.; Han, W.; Chen, Y.H.; Wang, F.R.; et al. Who is the best donor for a related HLA haplotype-mismatched transplant? Blood 2014, 124, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, M.; Ruggeri, L.; Mancusi, A.; Bernardo, M.E.; de Angelis, C.; Bucher, C.; Locatelli, F.; Aversa, F.; Velardi, A. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood 2008, 112, 2990–2995. [Google Scholar] [CrossRef] [Green Version]
- Ruggeri, L.; Eikema, D.J.; Bondanza, A.; Noviello, M.; van Biezen, A.; de Wreede, L.C.; Crucitti, L.; Vago, L.; Ciardelli, S.; Bader, P.; et al. Mother donors improve outcomes after HLA haploidentical transplantation: A Study by the Cellular Therapy and Immunobiology Working Party of the EBMT. Transplant. Cell. Ther. 2022, in press. [Google Scholar] [CrossRef]
- Lo, Y.M.; Lau, T.K.; Chan, L.Y.; Leung, T.N.; Chang, A.M. Quantitative analysis of the bidirectional fetomaternal transfer of nucleated cells and plasma DNA. Clin. Chem. 2000, 46, 1301–1309. [Google Scholar] [CrossRef] [Green Version]
- Kruchen, A.; Stahl, T.; Gieseke, F.; Binder, T.M.; Ozcan, Z.; Meisel, R.; Kreyenberg, H.; Bader, P.; Gruhn, B.; Greil, J.; et al. Donor choice in haploidentical stem cell transplantation: Fetal microchimerism is associated with better outcome in pediatric leukemia patients. Bone Marrow Transpl. 2015, 50, 1367–1370. [Google Scholar] [CrossRef] [Green Version]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef]
- Kennedy, P.R.; Chazara, O.; Gardner, L.; Ivarsson, M.A.; Farrell, L.E.; Xiong, S.; Hiby, S.E.; Colucci, F.; Sharkey, A.M.; Moffett, A. Activating KIR2DS4 Is Expressed by Uterine NK Cells and Contributes to Successful Pregnancy. J. Immunol. 2016, 197, 4292–4300. [Google Scholar] [CrossRef] [Green Version]
- Moffett, A.; Chazara, O.; Colucci, F.; Johnson, M.H. Variation of maternal KIR and fetal HLA-C genes in reproductive failure: Too early for clinical intervention. Reprod. Biomed. Online 2016, 33, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Moffett-King, A. Natural killer cells and pregnancy. Nat. Rev. Immunol. 2002, 2, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; King, A.; Loke, Y.W. Expression of killer cell inhibitory receptors on human uterine natural killer cells. Eur. J. Immunol. 1997, 27, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Apps, R.; Gardner, L.; Traherne, J.; Male, V.; Moffett, A. Natural-killer cell ligands at the maternal-fetal interface: UL-16 binding proteins, MHC class-I chain related molecules, HLA-F and CD48. Hum. Reprod. 2008, 23, 2535–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiby, S.E.; Apps, R.; Chazara, O.; Farrell, L.E.; Magnus, P.; Trogstad, L.; Gjessing, H.K.; Carrington, M.; Moffett, A. Maternal KIR in combination with paternal HLA-C2 regulate human birth weight. J. Immunol. 2014, 192, 5069–5073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiby, S.E.; Apps, R.; Sharkey, A.M.; Farrell, L.E.; Gardner, L.; Mulder, A.; Claas, F.H.; Walker, J.J.; Redman, C.W.; Morgan, L.; et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J. Clin. Investig. 2010, 120, 4102–4110. [Google Scholar] [CrossRef]
- Herberman, R.B.; Nunn, M.E.; Lavrin, D.H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int. J. Cancer 1975, 16, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975, 5, 112–117. [Google Scholar] [CrossRef]
- Herberman, R.R.; Ortaldo, J.R.; Bonnard, G.D. Augmentation by interferon of human natural and antibody-dependent cell-mediated cytotoxicity. Nature 1979, 277, 221–223. [Google Scholar] [CrossRef]
- Bosch, M.; Khan, F.M.; Storek, J. Immune reconstitution after hematopoietic cell transplantation. Curr. Opin. Hematol. 2012, 19, 324–335. [Google Scholar] [CrossRef]
- Ruggeri, L.; Capanni, M.; Casucci, M.; Volpi, I.; Tosti, A.; Perruccio, K.; Urbani, E.; Negrin, R.S.; Martelli, M.F.; Velardi, A. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood 1999, 94, 333–339. [Google Scholar] [CrossRef]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pende, D.; Falco, M.; Vitale, M.; Cantoni, C.; Vitale, C.; Munari, E.; Bertaina, A.; Moretta, F.; Del Zotto, G.; Pietra, G.; et al. Killer Ig-Like Receptors (KIRs): Their Role in NK Cell Modulation and Developments Leading to Their Clinical Exploitation. Front. Immunol. 2019, 10, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhrberg, M.; Valiante, N.M.; Shum, B.P.; Shilling, H.G.; Lienert-Weidenbach, K.; Corliss, B.; Tyan, D.; Lanier, L.L.; Parham, P. Human diversity in killer cell inhibitory receptor genes. Immunity 1997, 7, 753–763. [Google Scholar] [CrossRef] [Green Version]
- Uhrberg, M.; Parham, P.; Wernet, P. Definition of gene content for nine common group B haplotypes of the Caucasoid population: KIR haplotypes contain between seven and eleven KIR genes. Immunogenetics 2002, 54, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Kulski, J.K.; Gaudieri, S.; Witt, C.S.; Freitas, E.M.; Trowsdale, J.; Christiansen, F.T. Comparative genomic analysis, diversity and evolution of two KIR haplotypes A and B. Gene 2004, 335, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Pyo, C.W.; Guethlein, L.A.; Vu, Q.; Wang, R.; Abi-Rached, L.; Norman, P.J.; Marsh, S.G.; Miller, J.S.; Parham, P.; Geraghty, D.E. Different patterns of evolution in the centromeric and telomeric regions of group A and B haplotypes of the human killer cell Ig-like receptor locus. PLoS ONE 2010, 5, e15115. [Google Scholar] [CrossRef] [Green Version]
- Raulet, D.H.; Vance, R.E.; McMahon, C.W. Regulation of the natural killer cell receptor repertoire. Annu. Rev. Immunol. 2001, 19, 291–330. [Google Scholar] [CrossRef]
- Parham, P.; Moffett, A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat. Rev. Immunol. 2013, 13, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Karre, K.; Ljunggren, H.G.; Piontek, G.; Kiessling, R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 1986, 319, 675–678. [Google Scholar] [CrossRef]
- Ljunggren, H.G.; Karre, K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- He, Y.; Tian, Z. NK cell education via nonclassical MHC and non-MHC ligands. Cell. Mol. Immunol. 2017, 14, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooley, S.; Trachtenberg, E.; Bergemann, T.L.; Saeteurn, K.; Klein, J.; Le, C.T.; Marsh, S.G.; Guethlein, L.A.; Parham, P.; Miller, J.S.; et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood 2009, 113, 726–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooley, S.; Weisdorf, D.J.; Guethlein, L.A.; Klein, J.P.; Wang, T.; Le, C.T.; Marsh, S.G.; Geraghty, D.; Spellman, S.; Haagenson, M.D.; et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 2010, 116, 2411–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Bao, X.; Wu, X.; Tang, X.; Wang, M.; Wu, D.; He, J. Donor selection for killer immunoglobulin-like receptors B haplotype of the centromeric motifs can improve the outcome after HLA-identical sibling hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2014, 20, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Babor, F.; Peters, C.; Manser, A.R.; Glogova, E.; Sauer, M.; Potschger, U.; Ahlmann, M.; Cario, G.; Feuchtinger, T.; Gruhn, B.; et al. Presence of centromeric but absence of telomeric group B KIR haplotypes in stem cell donors improve leukaemia control after HSCT for childhood ALL. Bone Marrow Transpl. 2019, 54, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Oevermann, L.; Michaelis, S.U.; Mezger, M.; Lang, P.; Toporski, J.; Bertaina, A.; Zecca, M.; Moretta, L.; Locatelli, F.; Handgretinger, R. KIR B haplotype donors confer a reduced risk for relapse after haploidentical transplantation in children with ALL. Blood 2014, 124, 2744–2747. [Google Scholar] [CrossRef]
- Frohn, C.; Schlenke, P.; Ebel, B.; Dannenberg, C.; Bein, G.; Kirchner, H. DNA typing for natural killer cell inhibiting HLA-Cw groups NK1 and NK2 by PCR-SSP. J. Immunol. Methods 1998, 218, 155–160. [Google Scholar] [CrossRef]
- Alves, L.G.; Rajalingam, R.; Canavez, F. A novel real-time PCR method for KIR genotyping. Tissue Antigens 2009, 73, 188–191. [Google Scholar] [CrossRef]
- Stahl, T.; Bohme, M.U.; Kroger, N.; Fehse, B. Digital PCR to assess hematopoietic chimerism after allogeneic stem cell transplantation. Exp. Hematol. 2015, 43, 462–468. [Google Scholar] [CrossRef]
- Fehse, B.; Chukhlovin, A.; Kuhlcke, K.; Marinetz, O.; Vorwig, O.; Renges, H.; Kruger, W.; Zabelina, T.; Dudina, O.; Finckenstein, F.G.; et al. Real-time quantitative Y chromosome-specific PCR (QYCS-PCR) for monitoring hematopoietic chimerism after sex-mismatched allogeneic stem cell transplantation. J. Hematother. Stem Cell Res. 2001, 10, 419–425. [Google Scholar] [CrossRef]
- Alizadeh, M.; Bernard, M.; Danic, B.; Dauriac, C.; Birebent, B.; Lapart, C.; Lamy, T.; Le Prise, P.Y.; Beauplet, A.; Bories, D.; et al. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood 2002, 99, 4618–4625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paximadis, M.; Mathebula, T.Y.; Gentle, N.L.; Vardas, E.; Colvin, M.; Gray, C.M.; Tiemessen, C.T.; Puren, A. Human leukocyte antigen class I (A, B, C) and II (DRB1) diversity in the black and Caucasian South African population. Hum. Immunol. 2012, 73, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Kolialexi, A.; Tsangaris, G.T.; Antsaklis, A.; Mavroua, A. Rapid clearance of fetal cells from maternal circulation after delivery. Ann. N. Y. Acad. Sci. 2004, 1022, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, J.; Mottonen, M.; Alanen, A.; Lassila, O. Phenotypic characterization of regulatory T cells in the human decidua. Clin. Exp. Immunol. 2004, 136, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.T.; Chaturvedi, V.; Ertelt, J.M.; Kinder, J.M.; Clark, D.R.; Valent, A.M.; Xin, L.; Way, S.S. Regulatory T cells: New keys for further unlocking the enigma of fetal tolerance and pregnancy complications. J. Immunol. 2014, 192, 4949–4956. [Google Scholar] [CrossRef]
- Erlebacher, A.; Vencato, D.; Price, K.A.; Zhang, D.; Glimcher, L.H. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J. Clin. Investig. 2007, 117, 1399–1411. [Google Scholar] [CrossRef] [Green Version]
- Hiby, S.E.; Regan, L.; Lo, W.; Farrell, L.; Carrington, M.; Moffett, A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum. Reprod. 2008, 23, 972–976. [Google Scholar] [CrossRef]
- Hiby, S.E.; Walker, J.J.; O’Shaughnessy, K.M.; Redman, C.W.; Carrington, M.; Trowsdale, J.; Moffett, A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 2004, 200, 957–965. [Google Scholar] [CrossRef]
- Blokhuis, J.H.; Hilton, H.G.; Guethlein, L.A.; Norman, P.J.; Nemat-Gorgani, N.; Nakimuli, A.; Chazara, O.; Moffett, A.; Parham, P. KIR2DS5 allotypes that recognize the C2 epitope of HLA-C are common among Africans and absent from Europeans. Immun. Inflamm. Dis. 2017, 5, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Nakimuli, A.; Chazara, O.; Hiby, S.E.; Farrell, L.; Tukwasibwe, S.; Jayaraman, J.; Traherne, J.A.; Trowsdale, J.; Colucci, F.; Lougee, E.; et al. A KIR B centromeric region present in Africans but not Europeans protects pregnant women from pre-eclampsia. Proc. Natl. Acad. Sci. USA 2015, 112, 845–850. [Google Scholar] [CrossRef] [Green Version]
- Rowe, J.H.; Ertelt, J.M.; Xin, L.; Way, S.S. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 2012, 490, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Li, D.K.; Wi, S. Changing paternity and the risk of preeclampsia/eclampsia in the subsequent pregnancy. Am. J. Epidemiol. 2000, 151, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granne, I.; Shen, M.; Rodriguez-Caro, H.; Chadha, G.; O’Donnell, E.; Brosens, J.J.; Quenby, S.; Child, T.; Southcombe, J.H. Characterisation of peri-implantation endometrial Treg and identification of an altered phenotype in recurrent pregnancy loss. Mucosal Immunol. 2022, 15, 120–129. [Google Scholar] [CrossRef]
- Ruggeri, L.; Mancusi, A.; Burchielli, E.; Capanni, M.; Carotti, A.; Aloisi, T.; Aversa, F.; Martelli, M.F.; Velardi, A. NK cell alloreactivity and allogeneic hematopoietic stem cell transplantation. Blood Cells Mol. Dis. 2008, 40, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Vago, L.; Eikema, D.J.; de Wreede, L.C.; Ciceri, F.; Diaz, M.A.; Locatelli, F.; Jindra, P.; Milone, G.; Diez-Martin, J.L.; et al. Natural killer cell alloreactivity in HLA-haploidentical hematopoietic transplantation: A study on behalf of the CTIWP of the EBMT. Bone Marrow Transpl. 2021, 56, 1900–1907. [Google Scholar] [CrossRef] [PubMed]
- Oevermann, L.; Schulte, J.H.; Hundsdorfer, P.; Hakimeh, D.; Kogel, F.; Lang, P.; Corbacioglu, S.; Eggert, A.; Sodani, P. HLA-haploidentical hematopoietic stem cell transplantation in pediatric patients with hemoglobinopathies: Current practice and new approaches. Bone Marrow Transpl. 2019, 54, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Meazza, R.; Falco, M.; Loiacono, F.; Canevali, P.; Della Chiesa, M.; Bertaina, A.; Pagliara, D.; Merli, P.; Indio, V.; Galaverna, F.; et al. Phenotypic and Functional Characterization of NK Cells in alphabetaT-Cell and B-Cell Depleted Haplo-HSCT to Cure Pediatric Patients with Acute Leukemia. Cancers 2020, 12, 2187. [Google Scholar] [CrossRef]




| N | % | ||
|---|---|---|---|
| Patients, N | 70 | ||
| Sex | Female | 35 | 50 |
| Male | 35 | 50 | |
| Age (years) | Median (range) | 5.72 (0.02–17.60) | |
| Disease | c-ALL | 35 | 50 |
| T-ALL | 8 | 11 | |
| B-ALL | 13 | 19 | |
| AML | 2 | 3 | |
| Others | 12 | 17 | |
| FM determination possible | 51 | 73 | |
| FM+ | 19 | 37 | |
| FM− | 32 | 63 | |
| N | Value | p-Value | Cramer’s V | ||
|---|---|---|---|---|---|
| Child | Age | 51 | 45.975 | 1 | 1 |
| Sex | 51 | 3.989 | 0.080 | 0.280 | |
| Disease | 51 | 9.223 | 0.088 | 0.457 | |
| Centromeric KIR | 51 | 0.716 | 0.143 | 0.104 | |
| Telomeric KIR | 51 | 4.127 | 0.105 | 0.289 | |
| KIR content score | 51 | 4.660 | 0.148 | 0.308 | |
| HLA-C | 51 | 2.215 | 0.298 | 0.212 | |
| Mother | Centromeric KIR | 51 | 8.551 | 0.011 | 0.422 |
| Telomeric KIR | 51 | 0.944 | 0.702 | 0.112 | |
| KIR content score | 51 | 6.595 | 0.072 | 0.372 | |
| HLA-C | 51 | 6.979 | 0.031 | 0.387 | |
| HLA-C match/mismatch | 51 | 9.188 | 0.009 | 0.434 | |
| Father | Centromeric KIR | 17 | 0.032 | 1 | 0.044 |
| Telomeric KIR | 18 | 0.692 | 1 | 0.177 | |
| KIR content score | 18 | 1.313 | 1 | 0.226 | |
| HLA-C | 20 | 0.672 | 1 | 0.169 | |
| HLA-C match/mismatch | 20 | 0.020 | 1 | 0.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, L.-M.; Kruchen, A.; Fehse, B.; Müller, I. Influence of Fetomaternal Microchimerism on Maternal NK Cell Reactivity against the Child’s Leukemic Blasts. Biomedicines 2022, 10, 603. https://doi.org/10.3390/biomedicines10030603
Martin L-M, Kruchen A, Fehse B, Müller I. Influence of Fetomaternal Microchimerism on Maternal NK Cell Reactivity against the Child’s Leukemic Blasts. Biomedicines. 2022; 10(3):603. https://doi.org/10.3390/biomedicines10030603
Chicago/Turabian StyleMartin, Lena-Marie, Anne Kruchen, Boris Fehse, and Ingo Müller. 2022. "Influence of Fetomaternal Microchimerism on Maternal NK Cell Reactivity against the Child’s Leukemic Blasts" Biomedicines 10, no. 3: 603. https://doi.org/10.3390/biomedicines10030603
APA StyleMartin, L.-M., Kruchen, A., Fehse, B., & Müller, I. (2022). Influence of Fetomaternal Microchimerism on Maternal NK Cell Reactivity against the Child’s Leukemic Blasts. Biomedicines, 10(3), 603. https://doi.org/10.3390/biomedicines10030603






