The Induced Membrane Technique—The Filling Matters: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
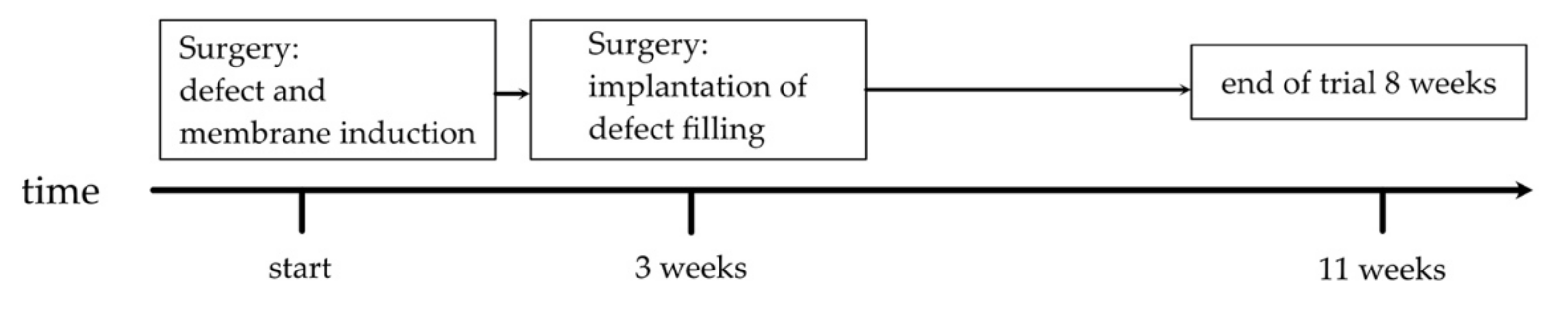
2.2. Animal Care and Group Setup
2.3. BMC Isolation and Seeding
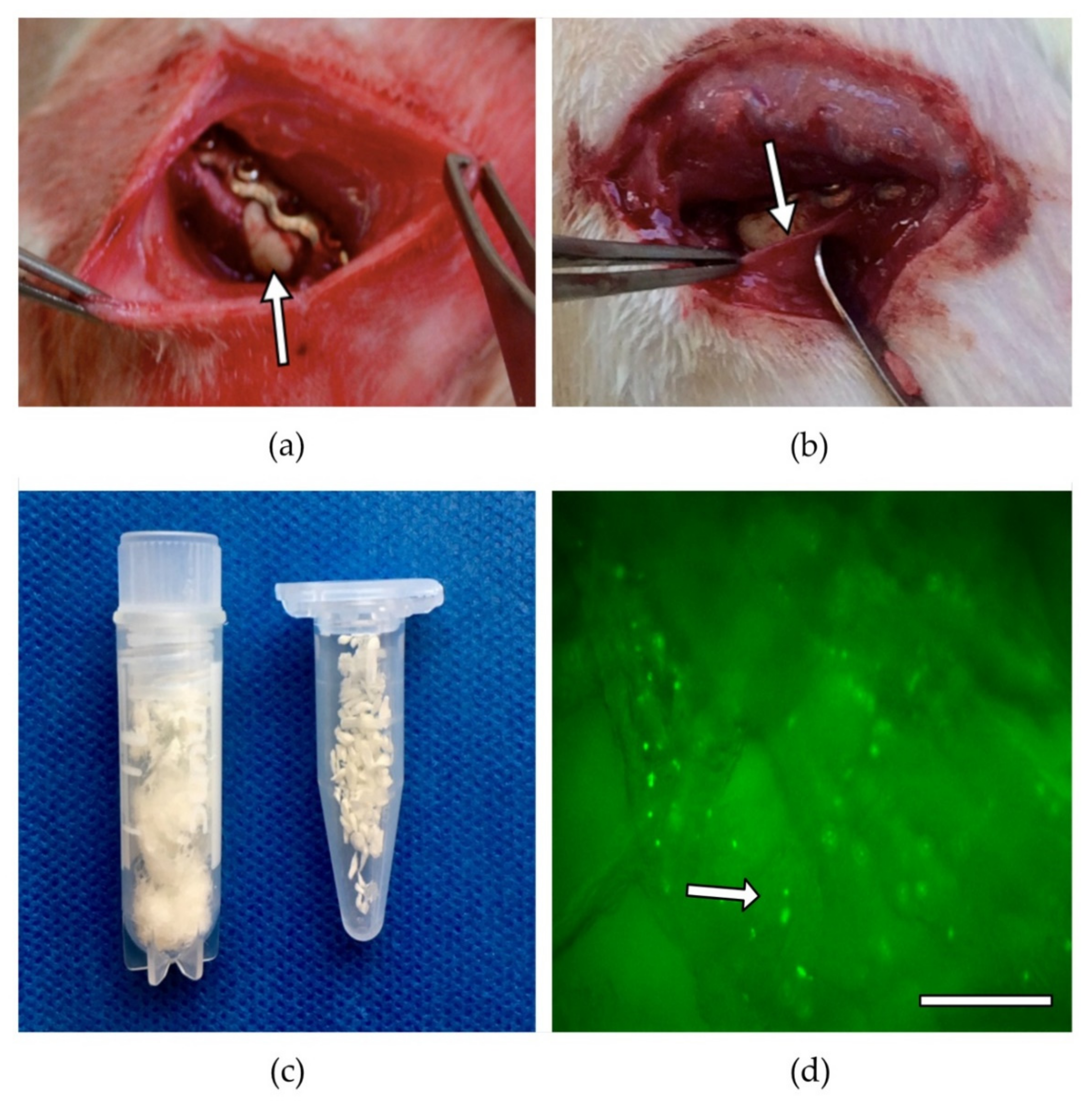
2.4. Surgical Procedure
2.5. Biomechanical Characterisation
2.6. Histological Assessment
2.7. μCT-Analysis
2.8. Statistics
3. Results
3.1. Animal Care/Complications
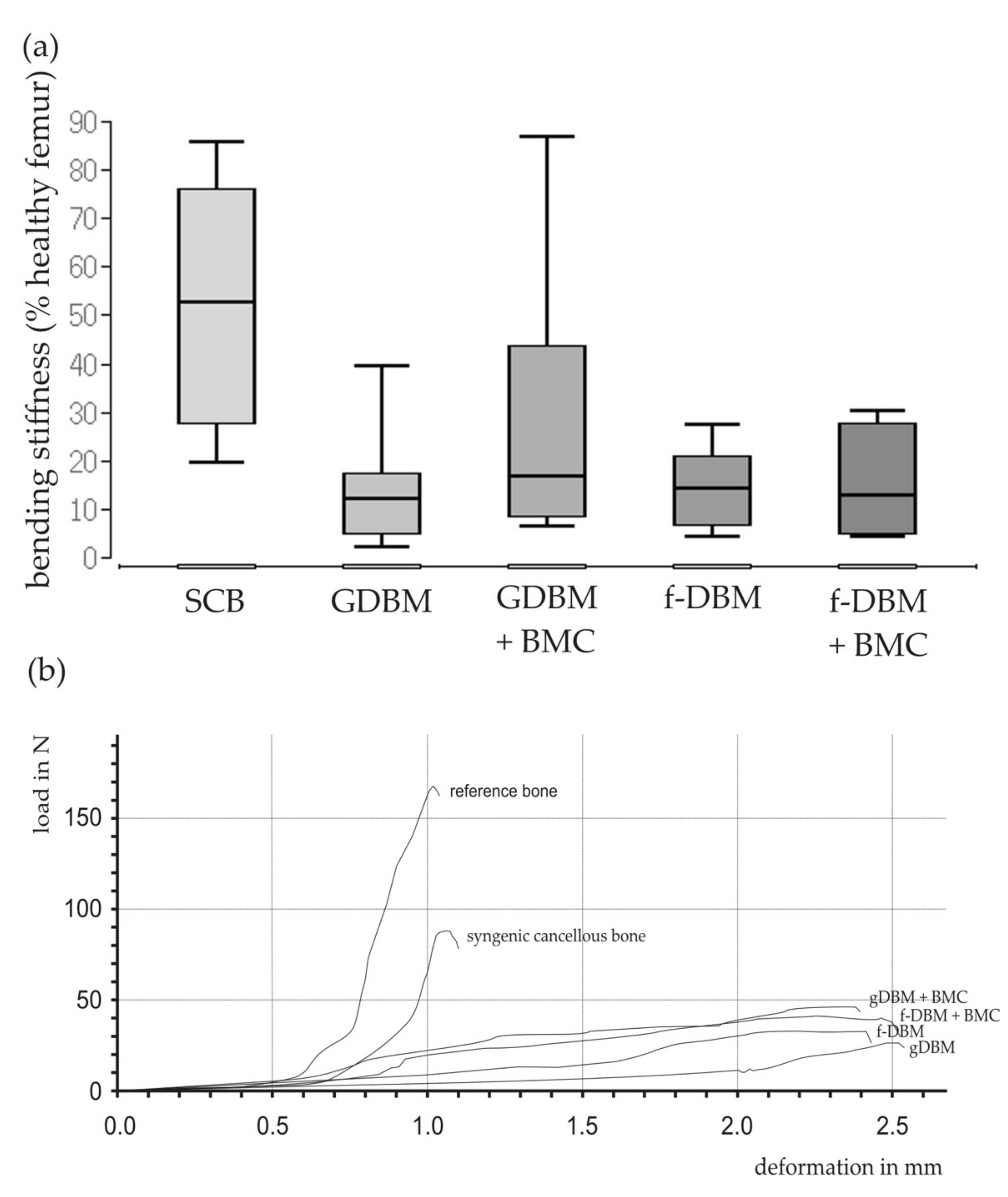
3.2. Similar Biomechanical Properties in All Induced Membrane Groups
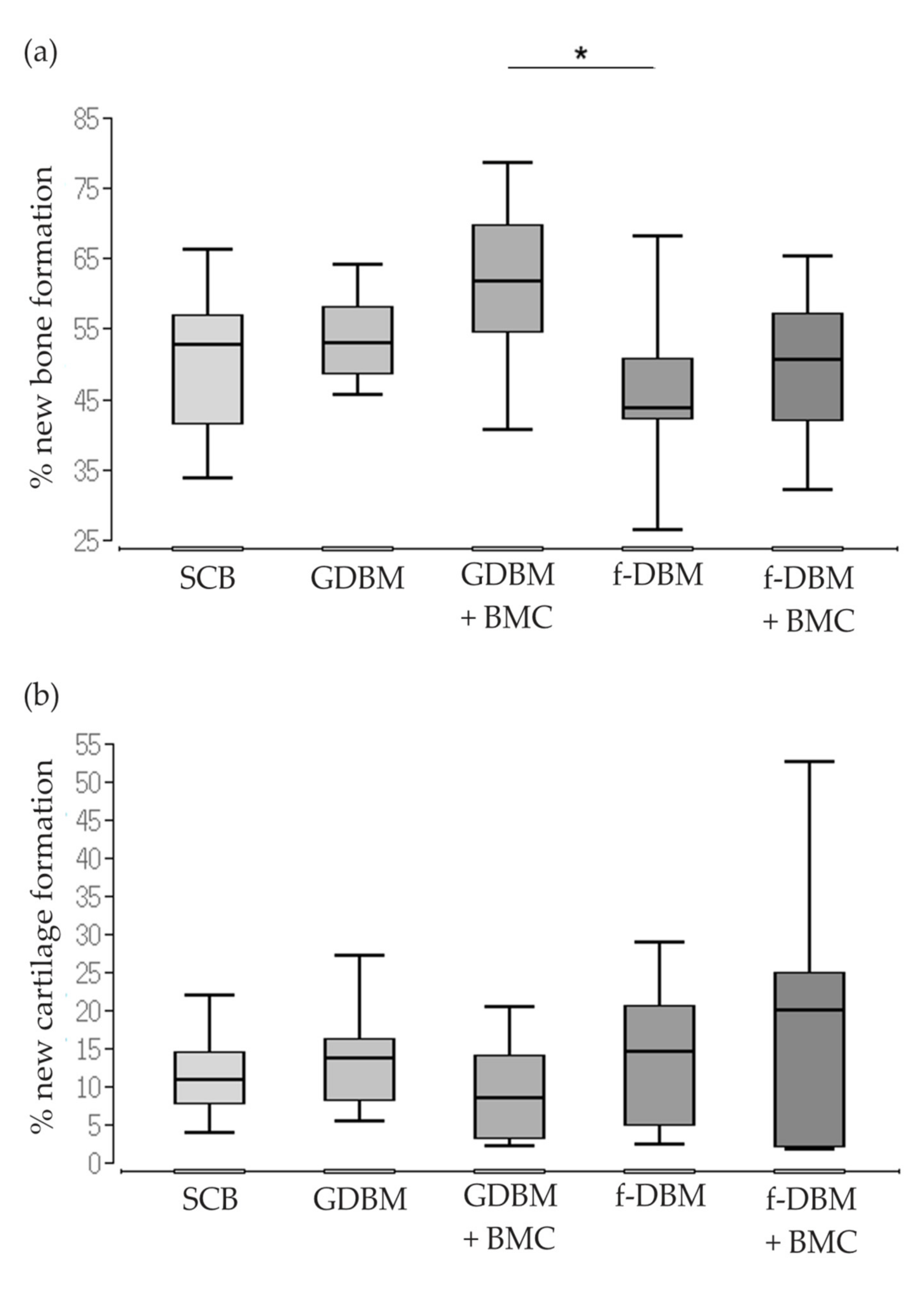
3.3. Significantly Better Bone Formation in GDBM + BMC vs. fDBM Group and Similar Cartilage Formation in All Groups
3.4. Highest Bone Healing Scores in Syngenic Cancellous Bone and f-DBM Groups
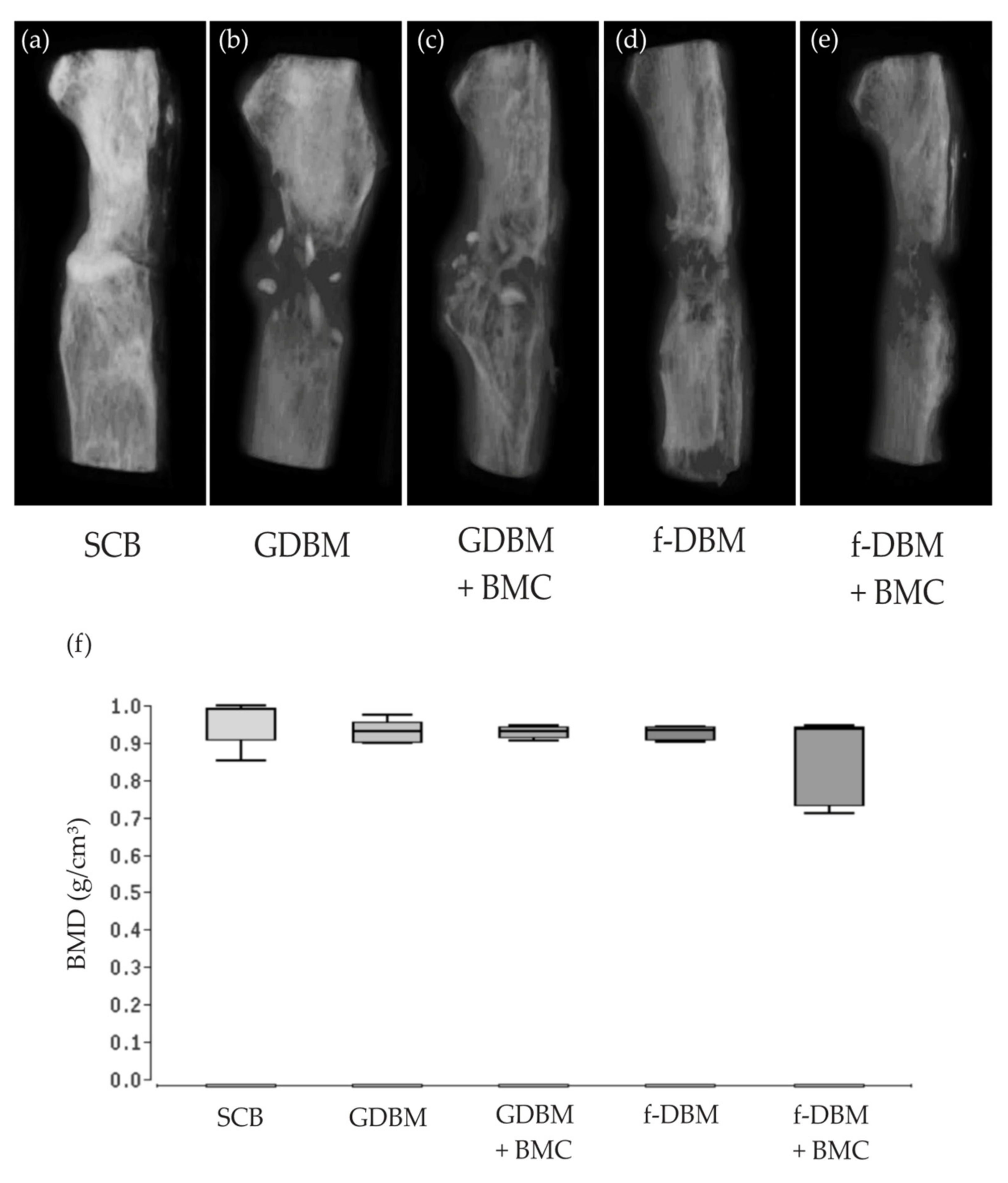
3.5. No Significant Differences Bone Mineral Density
3.6. Significantly Improved Bone Maturation in f-DBM + BMC vs. SCB, vs. GDBM, and vs. GDBM + BMC—No Differences in Vascularization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiese, A.; Pape, H.C. Bone defects caused by high-energy injuries, bone loss, infected nonunions, and nonunions. Orthop. Clin. N. Am. 2010, 41, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31 (Suppl. 5), S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38 (Suppl. 4), S3–S6. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42 (Suppl. 2), S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Nau, C.; Henrich, D.; Seebach, C.; Schroder, K.; Fitzsimmons, S.J.; Hankel, S.; Barker, J.H.; Marzi, I.; Frank, J. Treatment of Large Bone Defects with a Vascularized Periosteal Flap in Combination with Biodegradable Scaffold Seeded with Bone Marrow-Derived Mononuclear Cells: An Experimental Study in Rats. Tissue Eng. Part A 2016, 22, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Masquelet, A.C.; Fitoussi, F.; Begue, T.; Muller, G.P. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann. Chir. Plast. Esthet. 2000, 45, 346–353. [Google Scholar]
- Masquelet, A.C.; Begue, T. The concept of induced membrane for reconstruction of long bone defects. Orthop. Clin. N. Am 2010, 41, 27–37. [Google Scholar] [CrossRef]
- Klaue, K.; Knothe, U.; Anton, C.; Pfluger, D.H.; Stoddart, M.; Masquelet, A.C.; Perren, S.M. Bone regeneration in long-bone defects: Tissue compartmentalisation? In vivo study on bone defects in sheep. Injury 2009, 40 (Suppl. 4), S95–S102. [Google Scholar] [CrossRef]
- Pelissier, P.; Masquelet, A.C.; Bareille, R.; Pelissier, S.M.; Amedee, J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J. Orthop. Res. 2004, 22, 73–79. [Google Scholar] [CrossRef]
- Pelissier, P.; Martin, D.; Baudet, J.; Lepreux, S.; Masquelet, A.C. Behaviour of cancellous bone graft placed in induced membranes. Br. J. Plast. Surg. 2002, 55, 596–598. [Google Scholar] [CrossRef]
- Henrich, D.; Seebach, C.; Nau, C.; Basan, S.; Relja, B.; Wilhelm, K.; Schaible, A.; Frank, J.; Barker, J.; Marzi, I. Establishment and characterization of the Masquelet induced membrane technique in a rat femur critical-sized defect model. J. Tissue Eng. Regen. Med. 2016, 10, E382–E396. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S. Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects. Int. J. Mol. Sci. 2021, 22, 1538. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S. Concise Review: Adipose-Derived Stem Cells (ASCs) and Adipocyte-Secreted Exosomal microRNA (A-SE-miR) Modulate Cancer Growth and proMote Wound Repair. J. Clin. Med. 2019, 8, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentile, P.; Sterodimas, A.; Pizzicannella, J.; Dionisi, L.; De Fazio, D.; Calabrese, C.; Garcovich, S. Systematic Review: Allogenic Use of Stromal Vascular Fraction (SVF) and Decellularized Extracellular Matrices (ECM) as Advanced Therapy Medicinal Products (ATMP) in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 4982. [Google Scholar] [CrossRef]
- Gentile, P.; De Angelis, B.; Pasin, M.; Cervelli, G.; Curcio, C.B.; Floris, M.; Di Pasquali, C.; Bocchini, I.; Balzani, A.; Nicoli, F.; et al. Adipose-derived stromal vascular fraction cells and platelet-rich plasma: Basic and clinical evaluation for cell-based therapies in patients with scars on the face. J. Craniofac. Surg. 2014, 25, 267–272. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Concise Review: The Use of Adipose-Derived Stromal Vascular Fraction Cells and Platelet Rich Plasma in Regenerative Plastic Surgery. Stem Cells 2017, 35, 117–134. [Google Scholar] [CrossRef]
- Scioli, M.G.; Bielli, A.; Gentile, P.; Cervelli, V.; Orlandi, A. Combined treatment with platelet-rich plasma and insulin favours chondrogenic and osteogenic differentiation of human adipose-derived stem cells in three-dimensional collagen scaffolds. J Tissue Eng. Regen. Med. 2017, 11, 2398–2410. [Google Scholar] [CrossRef]
- De Angelis, B.; Orlandi, F.; Fernandes Lopes Morais D’Autilio, M.; Scioli, M.G.; Orlandi, A.; Cervelli, V.; Gentile, P. Long-term follow-up comparison of two different bi-layer dermal substitutes in tissue regeneration: Clinical outcomes and histological findings. Int. Wound J. 2018, 15, 695–706. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, B.; Orlandi, F.; Morais D’Autilio, M.F.L.; Di Segni, C.; Scioli, M.G.; Orlandi, A.; Cervelli, V.; Gentile, P. Vasculogenic Chronic Ulcer: Tissue Regeneration with an Innovative Dermal Substitute. J. Clin. Med. 2019, 8, 525. [Google Scholar] [CrossRef] [Green Version]
- Gentile, P.; Colicchia, G.M.; Nicoli, F.; Cervelli, G.; Curcio, C.B.; Brinci, L.; Cervelli, V. Complex abdominal wall repair using a porcine dermal matrix. Surg. Innov. 2013, 20, NP12–NP15. [Google Scholar] [CrossRef]
- De Angelis, B.; Gentile, P.; Tati, E.; Bottini, D.J.; Bocchini, I.; Orlandi, F.; Pepe, G.; Di Segni, C.; Cervelli, G.; Cervelli, V. One-Stage Reconstruction of Scalp after Full-Thickness Oncologic Defects Using a Dermal Regeneration Template (Integra). Biomed. Res. Int. 2015, 2015, 698385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentile, P.; Garcovich, S. Systematic Review-The Potential Implications of Different Platelet-Rich Plasma (PRP) Concentrations in Regenerative Medicine for Tissue Repair. Int. J. Mol. Sci. 2020, 21, 5702. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Bottini, D.J.; Spallone, D.; Curcio, B.C.; Cervelli, V. Application of platelet-rich plasma in maxillofacial surgery: Clinical evaluation. J. Craniofac. Surg. 2010, 21, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Gessmann, J.; Rosteius, T.; Baecker, H.; Sivalingam, K.; Peter, E.; Schildhauer, T.A.; Koller, M. Is the bioactivity of induced membranes time dependent? Eur. J. Trauma Emerg. Surg. 2021, 1–11. [Google Scholar] [CrossRef]
- Gindraux, F.; Loisel, F.; Bourgeois, M.; Oudina, K.; Melin, M.; de Billy, B.; Sergent, P.; Leclerc, G.; Petite, H.; Auber, F.; et al. Induced membrane maintains its osteogenic properties even when the second stage of Masquelet’s technique is performed later. Eur. J. Trauma Emerg. Surg. 2020, 46, 301–312. [Google Scholar] [CrossRef]
- Henrich, D.; Verboket, R.; Schaible, A.; Kontradowitz, K.; Oppermann, E.; Brune, J.C.; Nau, C.; Meier, S.; Bonig, H.; Marzi, I.; et al. Characterization of bone marrow mononuclear cells on biomaterials for bone tissue engineering in vitro. Biomed. Res. Int. 2015, 2015, 762407. [Google Scholar] [CrossRef] [Green Version]
- Janko, M.; Sahm, J.; Schaible, A.; Brune, J.C.; Bellen, M.; Schroder, K.; Seebach, C.; Marzi, I.; Henrich, D. Comparison of three different types of scaffolds preseeded with human bone marrow mononuclear cells on the bone healing in a femoral critical size defect model of the athymic rat. J. Tissue Eng. Regen. Med. 2018, 12, 653–666. [Google Scholar] [CrossRef]
- Nau, C.; Simon, S.; Schaible, A.; Seebach, C.; Schroder, K.; Marzi, I.; Henrich, D. Influence of the induced membrane filled with syngeneic bone and regenerative cells on bone healing in a critical size defect model of the rat’s femur. Injury 2018, 49, 1721–1731. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Kahling, C.; Wilhelm, K.; Tami, A.E.; Alini, M.; Marzi, I. Endothelial progenitor cells and mesenchymal stem cells seeded onto beta-TCP granules enhance early vascularization and bone healing in a critical-sized bone defect in rats. Tissue Eng. Part A 2010, 16, 1961–1970. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Moghaddam, A. Long Bone Nonunion. Z. Orthop. Unfall. 2015, 153, 659–674. [Google Scholar] [CrossRef]
- Donegan, D.J.; Scolaro, J.; Matuszewski, P.E.; Mehta, S. Staged bone grafting following placement of an antibiotic spacer block for the management of segmental long bone defects. Orthopedics 2011, 34, e730–e735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosemark, P.; Perdikouri, C.; Pelkonen, M.; Isaksson, H.; Tagil, M. The masquelet induced membrane technique with BMP and a synthetic scaffold can heal a rat femoral critical size defect. J. Orthop. Res. 2015, 33, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Chan, Y.H.; Hsieh, S.C.; Lew, W.Z.; Feng, S.W. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int. J. Mol. Sci. 2019, 20, 5015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.S.; Tsuang, Y.H.; Liao, C.J.; Liu, H.C.; Hang, Y.S.; Lin, F.H. The effect of sintered beta-dicalcium pyrophosphate particle size on newborn Wistar rat osteoblasts. Artif. Organs 1999, 23, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Shapoff, C.A.; Bowers, G.M.; Levy, B.; Mellonig, J.T.; Yukna, R.A. The effect of particle size on the osteogenic activity of composite grafts of allogeneic freeze-dried bone and autogenous marrow. J. Periodontol. 1980, 51, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Aaboe, M.; Janner, S.F.; Saulacic, N.; Bornstein, M.M.; Bosshardt, D.D.; Buser, D. Influence of particle size of deproteinized bovine bone mineral on new bone formation and implant stability after simultaneous sinus floor elevation: A histomorphometric study in minipigs. Clin. Implant. Dent. Relat. Res. 2015, 17, 274–285. [Google Scholar] [CrossRef] [Green Version]
- Ghanaati, S.; Barbeck, M.; Orth, C.; Willershausen, I.; Thimm, B.W.; Hoffmann, C.; Rasic, A.; Sader, R.A.; Unger, R.E.; Peters, F.; et al. Influence of beta-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 2010, 6, 4476–4487. [Google Scholar] [CrossRef]
- Coathup, M.J.; Cai, Q.; Campion, C.; Buckland, T.; Blunn, G.W. The effect of particle size on the osteointegration of injectable silicate-substituted calcium phosphate bone substitute materials. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 902–910. [Google Scholar] [CrossRef] [Green Version]
- Sohling, N.; Leiblein, M.; Schaible, A.; Janko, M.; Schwable, J.; Seidl, C.; Brune, J.C.; Nau, C.; Marzi, I.; Henrich, D.; et al. First Human Leucocyte Antigen (HLA) Response and Safety Evaluation of Fibrous Demineralized Bone Matrix in a Critical Size Femoral Defect Model of the Sprague-Dawley Rat. Materials 2020, 13, 3120. [Google Scholar] [CrossRef]
- Verboket, R.D.; Irrle, T.; Busche, Y.; Schaible, A.; Schroder, K.; Brune, J.C.; Marzi, I.; Nau, C.; Henrich, D. Fibrous Demineralized Bone Matrix (DBM) Improves Bone Marrow Mononuclear Cell (BMC)-Supported Bone Healing in Large Femoral Bone Defects in Rats. Cells 2021, 10, 1249. [Google Scholar] [CrossRef]
- Pruss, A.; Gobel, U.B.; Pauli, G.; Kao, M.; Seibold, M.; Monig, H.J.; Hansen, A.; von Versen, R. Peracetic acid-ethanol treatment of allogeneic avital bone tissue transplants—A reliable sterilization method. Ann. Transplant. 2003, 8, 34–42. [Google Scholar] [PubMed]
- Seebach, C.; Henrich, D.; Schaible, A.; Relja, B.; Jugold, M.; Bonig, H.; Marzi, I. Cell-based therapy by implanted human bone marrow-derived mononuclear cells improved bone healing of large bone defects in rats. Tissue Eng. Part A 2015, 21, 1565–1578. [Google Scholar] [CrossRef] [PubMed]
- Drosse, I.; Volkmer, E.; Seitz, S.; Seitz, H.; Penzkofer, R.; Zahn, K.; Matis, U.; Mutschler, W.; Augat, P.; Schieker, M. Validation of a femoral critical size defect model for orthotopic evaluation of bone healing: A biomechanical, veterinary and trauma surgical perspective. Tissue Eng. Part C Methods 2008, 14, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.S.; Mao, Z.; Spengler, D.M. Young’s modulus, bending strength, and tissue physical properties of human compact bone. J. Orthop. Res. 1990, 8, 592–603. [Google Scholar] [CrossRef]
- Garvey, W.; Fathi, A.; Bigelow, F.; Carpenter, B.; Jimenez, C. Improved Movat pentachrome stain. Stain Technol 1986, 61, 60–62. [Google Scholar] [CrossRef]
- Han, Z.; Bhavsar, M.; Leppik, L.; Oliveira, K.M.C.; Barker, J.H. Histological Scoring Method to Assess Bone Healing in Critical Size Bone Defect Models. Tissue Eng. Part C Methods 2018, 24, 272–279. [Google Scholar] [CrossRef]
- Verboket, R.D.; Leiblein, M.; Janko, M.; Schaible, A.; Brune, J.C.; Schroder, K.; Heilani, M.; Fremdling, C.; Busche, Y.; Irrle, T.; et al. From two stages to one: Acceleration of the induced membrane (Masquelet) technique using human acellular dermis for the treatment of non-infectious large bone defects. Eur. J. Trauma Emerg. Surg. 2020, 46, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Verboket, R.; Leiblein, M.; Seebach, C.; Nau, C.; Janko, M.; Bellen, M.; Bonig, H.; Henrich, D.; Marzi, I. Autologous cell-based therapy for treatment of large bone defects: From bench to bedside. Eur. J. Trauma Emerg. Surg. 2018, 44, 649–665. [Google Scholar] [CrossRef] [Green Version]
- Seebach, C.; Schultheiss, J.; Wilhelm, K.; Frank, J.; Henrich, D. Comparison of six bone-graft substitutes regarding to cell seeding efficiency, metabolism and growth behaviour of human mesenchymal stem cells (MSC) in vitro. Injury 2010, 41, 731–738. [Google Scholar] [CrossRef]
- Henrich, D.; Seebach, C.; Kaehling, C.; Scherzed, A.; Wilhelm, K.; Tewksbury, R.; Powerski, M.; Marzi, I. Simultaneous cultivation of human endothelial-like differentiated precursor cells and human marrow stromal cells on beta-tricalcium phosphate. Tissue Eng. Part C Methods 2009, 15, 551–560. [Google Scholar] [CrossRef]
- Janko, M.; Pollinger, S.; Schaible, A.; Bellen, M.; Schroder, K.; Heilani, M.; Fremdling, C.; Marzi, I.; Nau, C.; Henrich, D.; et al. Determination of the effective dose of bone marrow mononuclear cell therapy for bone healing in vivo. Eur. J. Trauma Emerg. Surg. 2020, 46, 265–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, T.; Mifune, Y.; Matsumoto, T.; Shoji, T.; Kawakami, Y.; Kawamoto, A.; Ii, M.; Akimaru, H.; Kuroda, T.; Horii, M.; et al. Superior Potential of CD34-Positive Cells Compared to Total Mononuclear Cells for Healing of Nonunion Following Bone Fracture. Cell Transplant. 2015, 24, 1379–1393. [Google Scholar] [CrossRef] [PubMed]
- Leiblein, M.; Kolb, T.; Christian, L.; Schroder, K.; Yaman, C.; Schaible, A.; Marzi, I.; Henrich, D.; Janko, M. Introduction of a New Surgical Method to Improve Bone Healing in a Large Bone Defect by Replacement of the Induced Membrane by a Human Decellularized Dermis Repopulated with Bone Marrow Mononuclear Cells in Rat. Materials 2020, 13, 2629. [Google Scholar] [CrossRef] [PubMed]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. 2020, 15, 493–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, H.; Shih, Y.V.; Varghese, S. Resolution of inflammation in bone regeneration: From understandings to therapeutic applications. Biomaterials 2021, 277, 121114. [Google Scholar] [CrossRef]
- Wildemann, B.; Kandziora, F.; Krummrey, G.; Palasdies, N.; Haas, N.P.; Raschke, M.; Schmidmaier, G. Local and controlled release of growth factors (combination of IGF-I and TGF-beta I, and BMP-2 alone) from a polylactide coating of titanium implants does not lead to ectopic bone formation in sheep muscle. J. Control Release 2004, 95, 249–256. [Google Scholar] [CrossRef]
- Wildemann, B.; Lange, K.; Strobel, C.; Fassbender, M.; Willie, B.; Schmidmaier, G. Local BMP-2 application can rescue the delayed osteotomy healing in a rat model. Injury 2011, 42, 746–752. [Google Scholar] [CrossRef]



| Material | Histology | Radiology/Biomechanical Testing |
|---|---|---|
| syngenic cancellous bone (SCB) | n = 5 | n = 8 |
| DBM granules (GDBM) | n = 5 | n = 8 |
| DBM granules +BMC (GDBM + BMC) | n = 5 | n = 8 |
| fibrous demineralized bone matrix (f-DBM) | n = 5 | n = 8 |
| fibrous demineralized bone matrix + BMC (f-DBM + BMC) | n = 5 | n = 8 |
| Material | Bone Healing Score |
|---|---|
| syngenic cancellous bone (SCB) | 22 |
| DBM granules (GDBM) | 18 |
| DBM granules + BMC (GDBM + BMC) | 19 |
| fibrous demineralized bone matrix (f-DBM) | 20 |
| fibrous demineralized bone matrix + BMC (f-DBM + BMC) | 19 |
| Material | Bone Volume/Total Volume (BV/TV) |
|---|---|
| syngenic cancellous bone (SCB) | 0.78 (±4.4%) |
| DBM granules (GDBM) | 0.61 (±4.2%) |
| DBM granules + BMC (GDBM + BMC) | 0.73 (±5.1%) |
| fibrous demineralized bone matrix (f-DBM) | 0.64 (±6.1%) |
| fibrous demineralized bone matrix + BMC (f-DBM + BMC) | 0.62 (±4.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verboket, R.D.; Söhling, N.; Heilani, M.; Fremdling, C.; Schaible, A.; Schröder, K.; Brune, J.C.; Marzi, I.; Henrich, D. The Induced Membrane Technique—The Filling Matters: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats. Biomedicines 2022, 10, 642. https://doi.org/10.3390/biomedicines10030642
Verboket RD, Söhling N, Heilani M, Fremdling C, Schaible A, Schröder K, Brune JC, Marzi I, Henrich D. The Induced Membrane Technique—The Filling Matters: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats. Biomedicines. 2022; 10(3):642. https://doi.org/10.3390/biomedicines10030642
Chicago/Turabian StyleVerboket, René D., Nicolas Söhling, Myriam Heilani, Charlotte Fremdling, Alexander Schaible, Katrin Schröder, Jan C. Brune, Ingo Marzi, and Dirk Henrich. 2022. "The Induced Membrane Technique—The Filling Matters: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats" Biomedicines 10, no. 3: 642. https://doi.org/10.3390/biomedicines10030642
APA StyleVerboket, R. D., Söhling, N., Heilani, M., Fremdling, C., Schaible, A., Schröder, K., Brune, J. C., Marzi, I., & Henrich, D. (2022). The Induced Membrane Technique—The Filling Matters: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats. Biomedicines, 10(3), 642. https://doi.org/10.3390/biomedicines10030642







