Molecular Basis for Paradoxical Activities of Polymorphonuclear Neutrophils in Inflammation/Anti-Inflammation, Bactericide/Autoimmunity, Pro-Cancer/Anticancer, and Antiviral Infection/SARS-CoV-II-Induced Immunothrombotic Dysregulation
Abstract
:1. Introduction
2. Regulation of Granulopoiesis, Response to Environmental Factors, and Destinies of PMNs in the Body
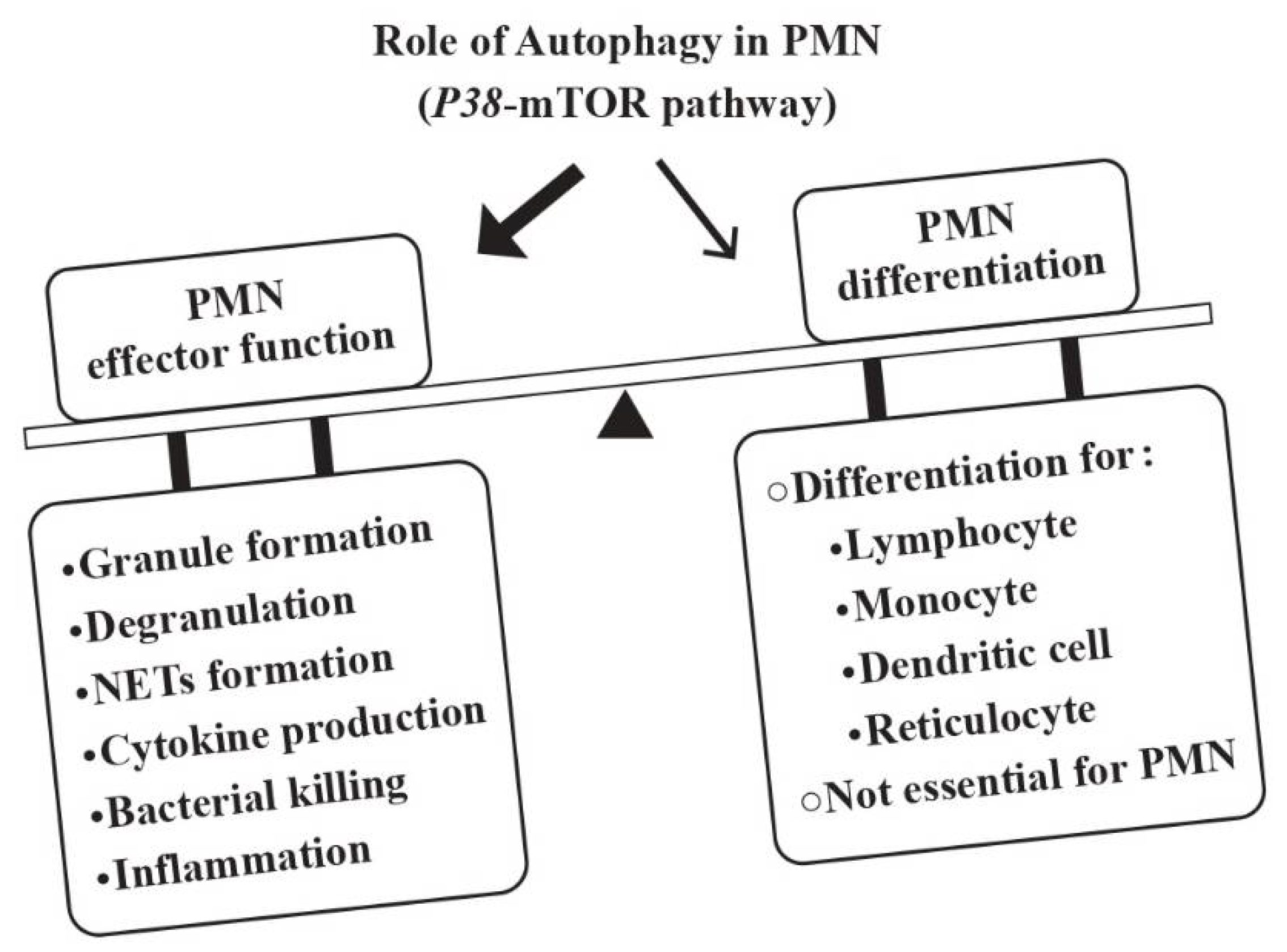
2.1. Regulatory Roles of Autophagy in Neutrophil Effector Functions
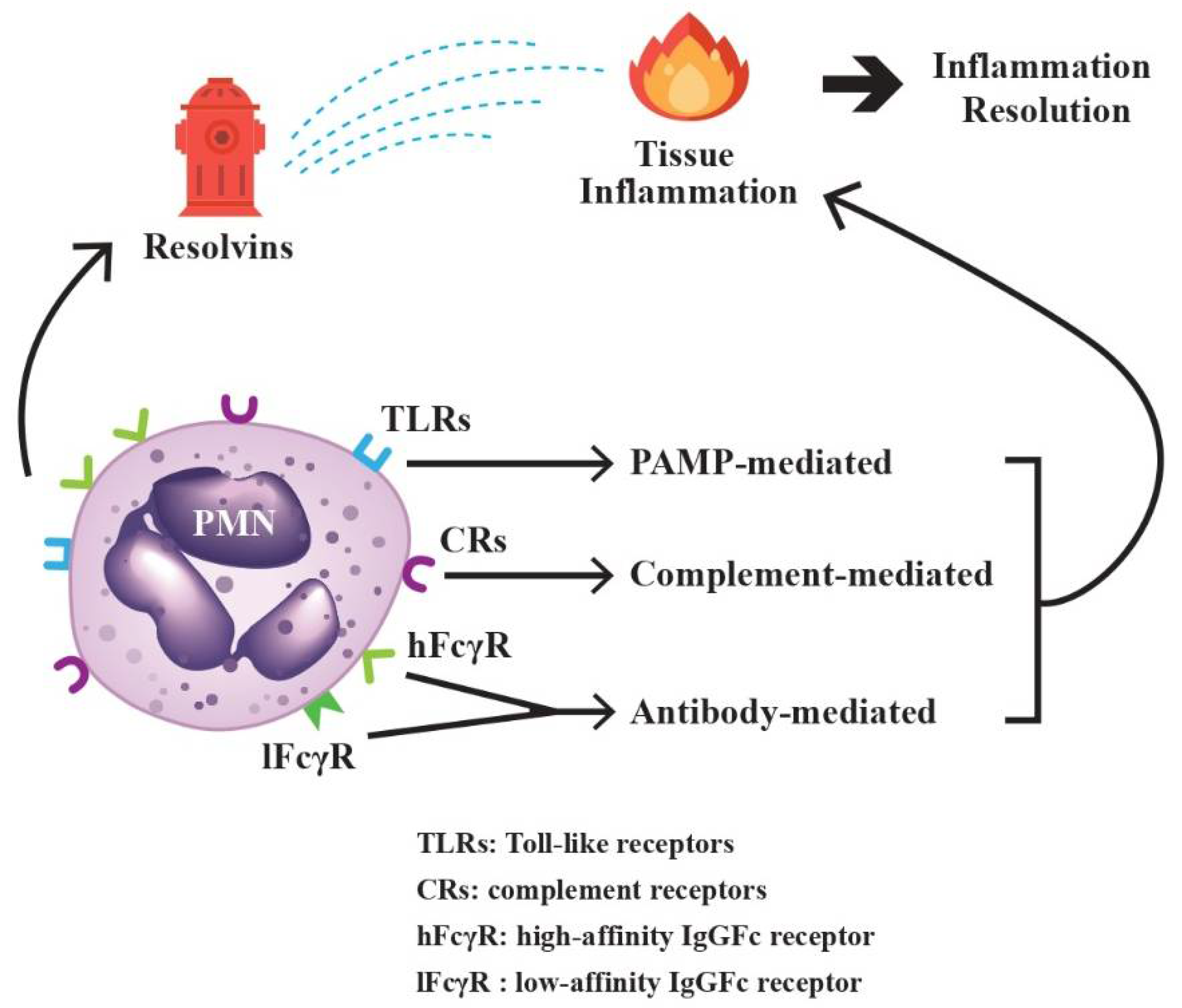
2.2. Rapid Sensing and Effective Response of PMNs to Environmental Factors
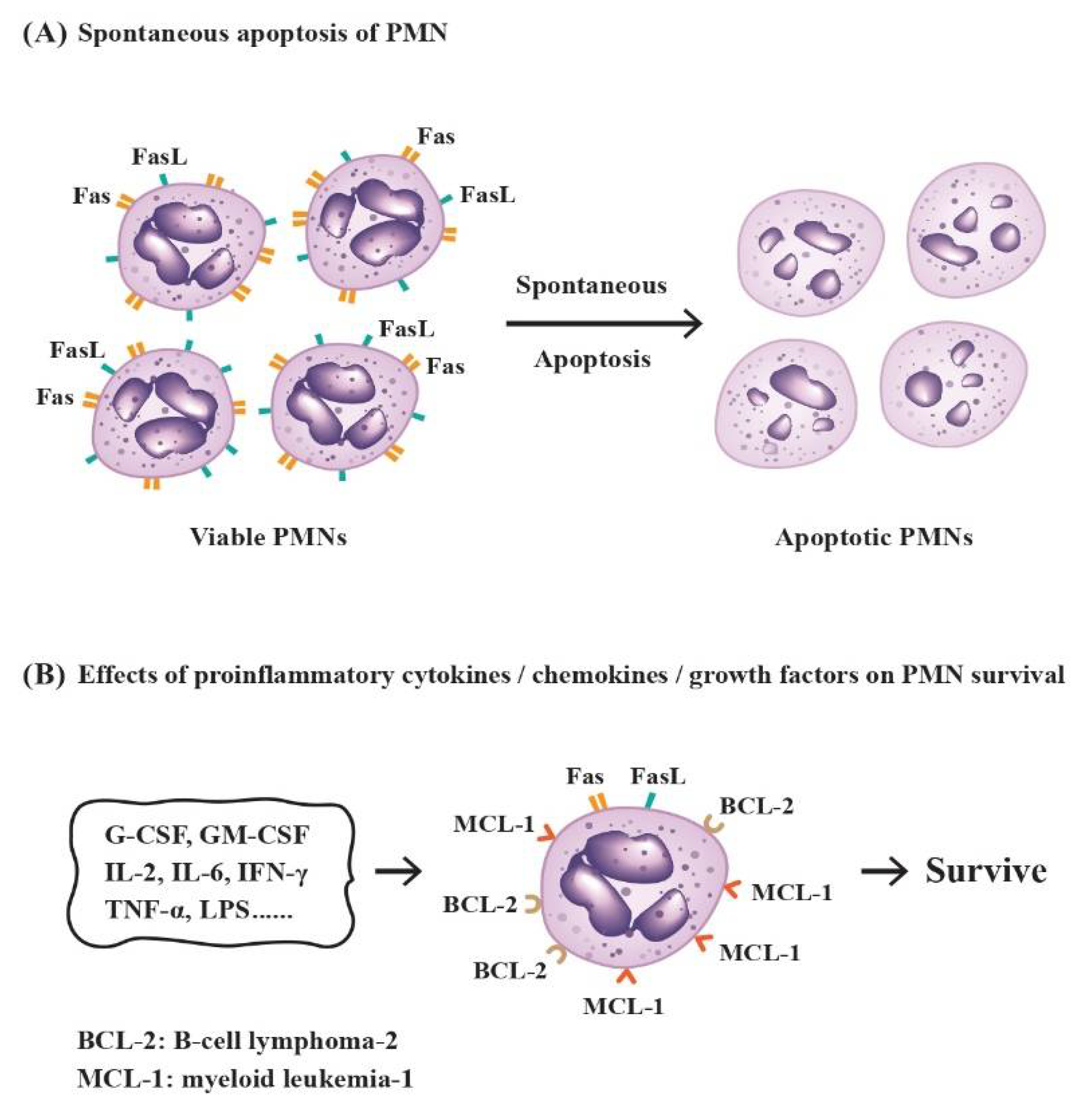
2.3. Factors Influencing the Destinies of PMNs
3. Novel Biological/Immunological Functions of PMNs
3.1. Biosynthesis and Secretion of Complement Component 3 (C3) and Factor B
3.2. Release of Granule Proteins, Cytokines, Chemokines, and Growth Factors from PMNs for Cell–Cell Communication and Immune Modulation
3.2.1. Degranulation to Liberate Azurophilic and Specific Granules
3.2.2. The Production of Cytokines/Chemokines/Growth Factors from PMNs for Immune Modulation
3.2.3. Liberation of Ectosomes and Exosomes from PMNs to Affect the Biological Functions of the Remote Cells or Tissues
Suppressive Effects and the Signaling Pathways of PMN-Derived Ectosomes (PMN-Ect) on Macrophage Maturation
The Modulatory Roles of PMN-Derived Exosomes (PMN-Exo) on the Immune Responses
3.3. Induction of MHC-II Expression on PMNs by T-Cell-Derived Cytokines, Rendering PMN Mimicking Antigen-Presenting Cells (APC)
3.4. Trogocytosis (Plasma Membrane Transfer) among PMN, Non-Immune, and Immune-Related Cells
3.4.1. Elimination of the Intracellular Parasites or Unwanted Cells by PMN-Mediated Trogocytosis
3.4.2. Trogocytosis among PMNs and Other Immune-Related Cells for Immune Modulation
3.5. Biological and Pathobiological Roles of NET Formation from PMNs
4. Heterogeneity of PMN in Facilitating or Deterring Tumorigenesis
5. Impact of PMNs on Cardiovascular Disease (CVD)
6. The Role of PMNs in Antiviral Infection Processes
7. Overwhelming Immune Responses Relevant to PMNs in Pandemic Coronavirus Disease
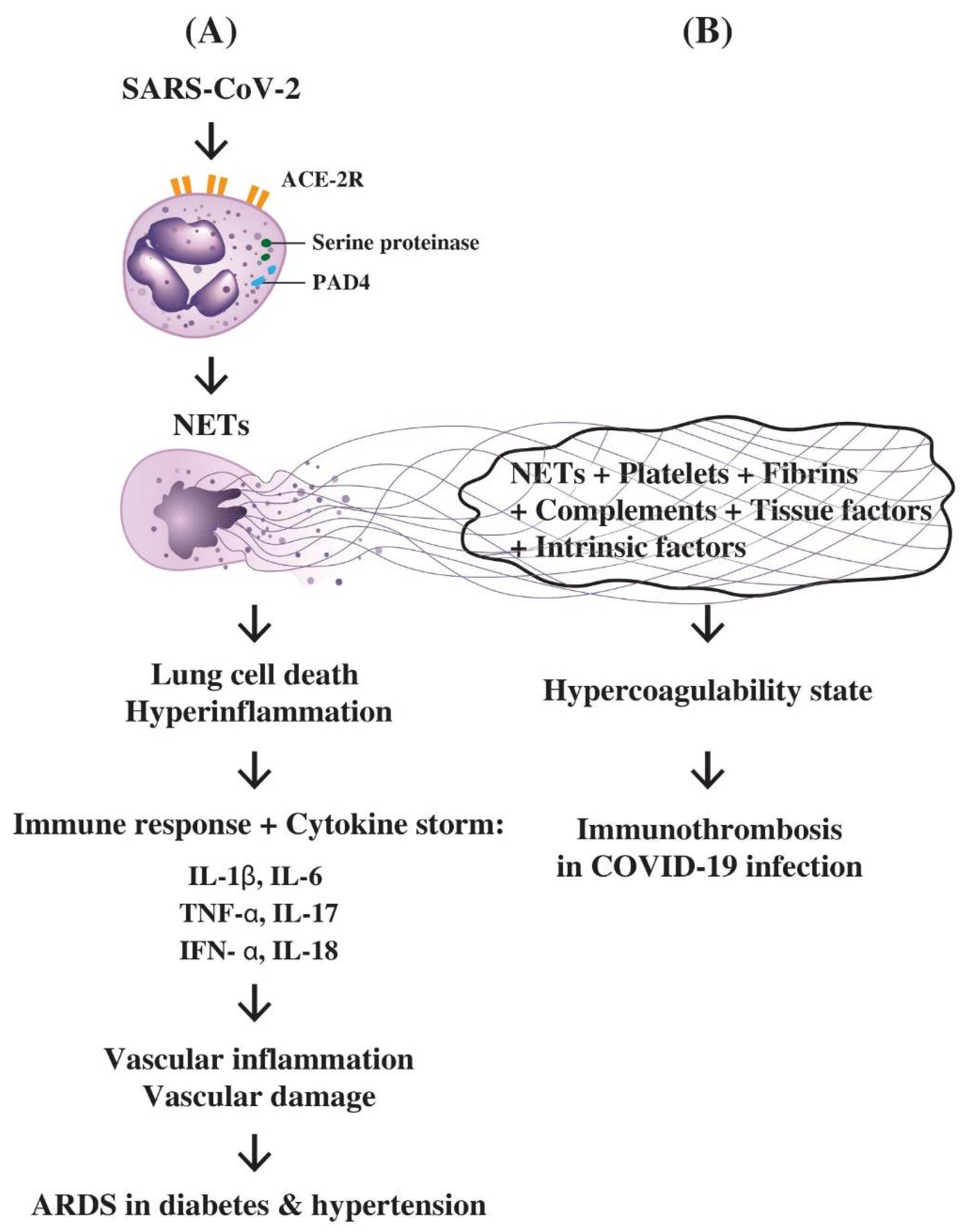
7.1. The role of PMN-Derived NETs in Inducing Hyperinflammation, Lung Cell Death, Cytokine Storm, ARDS, and Immunothrombotic Dysregulation in COVID-19 Disease
7.2. The Interactions of NETs, Complements, Coagulation Factors, and Platelets in the Immunothrombotic Dysregulation in COVID-19 Infection
8. Conclusions
- (1)
- Adequate use of the PMN-released defensins (HNP1, HD5) or antimicrobial peptide retrocyclin-101 (RC101) to block SARS-CoV-2 entry.
- (2)
- Facilitating NET release initially to trap and kill the virus followed by adding of complement C1q to rapidly clear the accumulated NETs.
- (3)
- Application of monoclonal anti-IL-17 antibody to suppress SARS-CoV-2 virus-induced hyperinflammation.
- (4)
- Induction of granulocytic MDSCs by TGF-β for anti-inflammation effect and immunosuppression of the cytokine storm.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| ADCC | antibody-dependent cellular cytotoxicity |
| Bak | Bcl-2 homologous antagonist killer |
| Bim | Bcl-2-like protein 11, commonly called BIM |
| Bcl-2 | B cell lymphoma 2 protein family (for regulation of apoptosis) |
| C | complement component |
| c-myc | human homologue of virus Myelocytomatosis, cytoplasmic domain |
| CVD | cardiovascular disease |
| C1q | q fragment of complement C1 |
| DAMP | Damage-associated molecular pattern |
| DNA | deoxyribonucleic acid |
| Ect | ectosome |
| ERK | extracellular signal-regulated kinase |
| EV | extracellular vesicle |
| Exo | exosome |
| FADD | Fas-associated protein with death domain |
| Fas | apoptosis antigen 1 (cluster of differentiation 95) |
| FasL | Fas ligand |
| FcγR | receptor for fragment C of immunoglobulin G |
| fMLP | formyl-methionyl-leucyl-phenylalanine |
| FXIa | coagulation factor 11, ‘a’ component |
| G-CSF | granulocyte colony stimulating factor |
| GM-CSF | granulocyte-macrophage colony stimulating factor |
| ICAM | intercellular adhesion molecule |
| IFN | interferon |
| IgG-Fc | Fragment C of immunoglobulin G |
| IL | interleukin |
| LDG | Low-density granulocyte |
| LPS | lipopolysaccharide |
| MCL-1 | Induced myeloid leukemia cell differentiation protein 1 |
| Mer-TK | proto-oncogene receptor tyrosine kinase |
| MDSC | myeloid-derived suppressor cell |
| MHC-II | class 2 major histocompatibility complex |
| MICC | mitogen-induced cellular cytotoxicity |
| mi-RNA | micro-ribonucleic acid |
| MPO | myeloperoxidase |
| N1 | type 1 neutrophil |
| N2 | type 2 neutrophil |
| NE | neutrophil elastase |
| NET | neutrophil extracellular trap |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| PAD-4 | protein arginine deiminase 4 |
| PAMP | pathogen-associated molecular pattern |
| PKC | protein kinase C |
| PMN | polymorphonuclear neutrophil |
| Pr3 | proteinase 3 |
| PS | phosphotidylserine |
| p53 | cellular tumor phosphoprotein 53 |
| ROS | reactive oxygen species |
| SARS-CoV-II | Severe acute respiratory syndrome-related coronavirus type II or coronavirus found in 2019 (COVID-19) |
| sC5b-9 | soluble complement fragments 5b to 9 complex |
| SIRPA | Signal regulatory protein alpha |
| SLE | systemic lupus erythematosus |
| SSc | systemic sclerosis |
| Syk | spleen tyrosine kinase |
| TAN | tumor-associated neutrophil |
| TF | tissue factor |
| TME | tumor microenvironment |
| TNF | tumor necrosis factor |
| TGF | transforming growth factor |
| TRADD | tumor necrosis factor receptor type 1-associated death domain |
| TRAIL-R | TNF-related apoptosis-inducing ligand receptor |
| TLR | Toll-like receptor |
References
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Cassatella, M.A. Neutrophil-derived proteins: Selling cytokines by the pound. Adv. Immunol. 1999, 73, 369–509. [Google Scholar] [CrossRef]
- Yang, P.; Li, Y.; Xie, Y.; Liu, Y. Different faces for different places: Heterogeneity of neutrophil phenotype and function. J. Immunol. Res. 2019, 2019, 8016254. [Google Scholar] [CrossRef] [Green Version]
- Fine, N.; Tasevski, N.; McCulloch, C.A.; Tenenbaum, H.C.; Glogauer, M. The neutrophil: Constant defender and first responder. Front. Immunol. 2020, 11, 571085. [Google Scholar] [CrossRef]
- Hacbarth, E.; Kajdacsy-Balla, A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986, 29, 1334–1342. [Google Scholar] [CrossRef]
- Bennett, L.; Palucka, A.K.; Arce, E.; Cantrell, V.; Borvak, J.; Banchereau, J.; Pascual, V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 2003, 197, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Carmona-Rivera, C.; Kaplan, M.J. Low-density granulocytes: A distinct class of neutrophils in systemic autoimmunity. Sem. Immunopathol. 2013, 35, 455–463. [Google Scholar] [CrossRef]
- Fu, J.; Tobin, M.C.; Thomas, L.L. Neutrophil-like low-density granulocytes are elevated in patients with moderate to severe persistent asthma. Ann. Allergy Asthma Immunol. 2014, 113, 635–640.e2. [Google Scholar] [CrossRef]
- Grayson, P.C.; Carmona-Rivera, C.; Xu, L.; Lim, N.; Gao, Z.; Asare, A.L.; Specks, U.; Stone, J.H.; Seo, P.; Spiera, R.F.; et al. Neutrophil-related gene expression and low-density granulocytes associated with disease activity and response to treatment in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2015, 57, 1922–1932. [Google Scholar] [CrossRef]
- Waight, J.D.; Hu, Q.; Miller, A.; Liu, S.; Abrams, S.I. Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS ONE 2011, 6, e27690. [Google Scholar] [CrossRef]
- Sagiv, J.Y.; Michaeli, J.; Assi, S.; Mishalian, I.; Kisos, H.; Levy, L.; Damti, P.; Lumbroso, D.; Polyansky, L.; Sionov, R.V.; et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015, 10, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Granot, Z.; Fridlender, Z.G. Plasticity beyond cancer cells and the “immunosuppressive switch”. Cancer Res. 2015, 75, 4441–4445. [Google Scholar] [CrossRef] [Green Version]
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2020, 108, 377–396. [Google Scholar] [CrossRef]
- Mitroulis, I.; Kourtzelis, I.; Kambas, K.; Rafail, S.; Chrysanthopoulou, A.; Speletas, M.; Ritis, K. Regulation of the autophagic machinery in human neutrophils. Eur. J. Immunol. 2010, 40, 1461–1472. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Stephenson, L.M.; Miller, B.C.; Ng, A.; Eisenberg, J.; Zhao, Z.; Cadwell, K.; Graham, D.B.; Mizushima, N.N.; Xavier, R.; Virgin, H.W.; et al. Identification of Atg5-dependent transcriptional changes and increases in mitochondrial mass in Atg5-deficient T lymphocyte. Autophagy 2009, 5, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Pua, H.H.; Dzhagalov, I.; Chuck, M.; Mizushima, N.; He, Y.W. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J. Exp. Med. 2007, 204, 25–31. [Google Scholar] [CrossRef]
- Miller, B.C.; Zhao, Z.; Stephenson, L.M.; Cadwell, K.; Pua, H.H.; Lee, H.K.; Mizushima, N.N.; Iwasaki, A.; He, Y.-W.; Swat, W.; et al. The autophagy gene ATG5 plays an essential role in B lymphocyte development. Autophagy 2008, 4, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Morgan, M.J.; Chen, K.; Choksi, S.; Liu, Z.G. Induction of autophagy is essential for monocyte-macrophage differentiation. Blood 2012, 119, 2895–2905. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.K.; Mattei, L.M.; Steinberg, B.E.; Alberts, P.; Lee, Y.H.; Chervonsky, A.; Mizushima, N.; Grinstein, S.; Iwasaki, A. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity 2010, 32, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Kundu, M.; Lindsten, T.; Yang, C.-Y.; Wu, J.; Zhao, F.; Zhang, J.; Selak, M.A.; Ney, P.A.; Thompson, C.B. Ulk 1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 2008, 112, 1493–1502. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, M.; Ferguson, D.J.P.; Edelmann, M.; Kessler, B.; Morten, K.J.; Komatsu, M.; Simon, A.K. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 832–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozman, S.; Yousefi, S.; Oberson, K.; Kaufmann, T.; Benarafa, C.; Simon, H.U. The generation of neutrophils in the bone marrow is controlled by autophagy. Cell Death Diff. 2015, 22, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.; Wei, Q.; Shin, J.N.; Fattah, E.A.; Bonilla, D.L.; Xiang, Q.; Eissa, N.T. Autophagy is required for neutrophil-mediated inflammation. Cell Rep. 2015, 12, 1731–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, I.; Ritchie, N.D.; Evans, T.J. The interrelationship between phagocytosis, autophagy and formation of neutrophil extracellular traps following infection of human neutrophils by Streptococcus pneumoniae. Innate Immun. 2017, 23, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Zhang, C.; Zou, Z.; Fan, E.K.Y.; Chen, L.; Li, Y.; Billiar, T.R.; Wilson, M.A.; Shi, X.; Fan, J. Aging-related Atg5 defect impairs neutrophil extracellular traps formation. Immunology 2017, 151, 417–432. [Google Scholar] [CrossRef] [Green Version]
- Skendros, P.; Mitroulis, I.; Ritis, K. Autophagy in neutrophils: From granulopoiesis to neutrophil extracellular traps. Front. Cell Dev. Biol. 2018, 6, 109. [Google Scholar] [CrossRef]
- Shrestha, S.; Lee, J.M.; Hong, C.-W. Autophagy in neutrophils. Korean J. Physiol. Pharmacol. 2020, 24, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.J.; Schroder, K. Pattern recognition receptor function in neutrophils. Trends Immunol. 2013, 34, 317–328. [Google Scholar] [CrossRef]
- Cumpelik, A.; Ankli, B.; Zecher, D.; Schifferli, J.A. Neutrophil microvesicles resolve gout by inhibiting C5a-mediated priming of the inflammasome. Ann. Rheum. Dis. 2016, 75, 1236–1245. [Google Scholar] [CrossRef] [Green Version]
- Miyabe, Y.; Miyabe, C.; Mani, V.; Mempel, T.R.; Luster, A.D. Atypical complement receptor C5aR2 transports C5a to initiate neutrophil adhesion and inflammation. Sci. Immunol. 2019, 4, eaav5951. [Google Scholar] [CrossRef] [PubMed]
- Van Rees, D.J.; Szilagyi, K.; Kuijpers, T.W.; Matlung, H.L.; van den Berg, T.K. Immunoreceptors on neutrophils. Sem. Immunol. 2016, 28, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dancey, J.T.; Deubelbeiss, K.A.; Harker, L.A.; Finch, C.A. Neutrophil kinetics in man. J. Clin. Investig. 1976, 58, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Hodgson, G.; Katz, M.; Dunn, A.R. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood 2002, 100, 854–861. [Google Scholar] [CrossRef] [Green Version]
- Pillay, J.; den Braber, I.; Vrisekoop, N.; Kwast, L.M.; de Boer, R.J.; Borghans, J.A.M.; Tesselaar, K.; Koenderman, L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 2010, 116, 625–627. [Google Scholar] [CrossRef]
- Simon, H.-U. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol. Rev. 2003, 193, 101–110. [Google Scholar] [CrossRef]
- Lopez, A.F.; Williamson, D.J.; Gamble, J.R.; Begley, C.G.; Harlan, J.M.; Klebanoff, S.J.; Waltersdorph, A.; Wong, G.; Clark, S.C.; Vadas, M.A. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J. Clin. Investig. 1986, 78, 1220–1228. [Google Scholar] [CrossRef]
- Begley, C.G.; Lopez, A.F.; Nicola, N.A.; Warren, D.J.; Vadas, M.A.; Sanderson, C.J.; Metcalf, D. Purified colony-stimulating factors enhance the survival of human neutrophils and eosinophils in vitro: A rapid and sensitive microassay for colony-stimulating factors. Blood 1986, 68, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Brach, M.A.; de Vos, S.; Gruss, H.J.; Herrmann, F. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood 1992, 80, 2920–2924. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, C.; Yoshida, S.; Taniguchi, H.; Qin, M.H.; Miyamoto, H.; Mizuguchi, Y. Lipopolysaccharide and granulocyte colony-stimulating factor delay neutrophil apoptosis and ingestion by guinea pig macrophages. Infect. Immun. 1993, 61, 1972–1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pericle, F.; Liu, J.H.; Diaz, J.I.; Blanchard, D.K.; Wei, S.; Forni, G.; Djeu, J.Y. Interleukin-2 prevention of apoptosis in human neutrophils. Eur. J. Immunol. 1994, 24, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.C.; Huang, M.-H.; Tsai, C.-Y.; Tsai, Y.-Y.; Tsai, S.-T.; Sun, K.-H.; Yu, H.-S.; Han, S.-H.; Yu, C.-L. The expression of genes modulating programmed cell death in normal human polymorphonuclear neutrophils. Biochem. Biophys. Res. Commun. 1997, 233, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Savill, J.; Dransfield, I.; Gregory, C.; Haslett, C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002, 2, 965–975. [Google Scholar] [CrossRef]
- Luo, H.R.; Loison, F. Constitutive neutrophil apoptosis: Mechanisms and regulation. Am. J. Hematol. 2008, 83, 288–295. [Google Scholar] [CrossRef]
- Edwards, S.W.; Derouet, M.; Howse, M.; Moots, R.J. Regulation of neutrophil apoptosis by Mcl-1. Biochem. Soc. Transac. 2004, 32, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Dzhagalov, I.; St. John, A.; He, Y.-W. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood 2007, 109, 1620–1626. [Google Scholar] [CrossRef] [Green Version]
- Reed, J.C. Proapoptotic multidomain Bcl-2/Bax-family proteins: Mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006, 13, 1378–1386. [Google Scholar] [CrossRef] [Green Version]
- Brenner, D.; Mak, T.W. Mitochondrial cell death effectors. Curr. Opin. Cell Biol. 2009, 21, 871–877. [Google Scholar] [CrossRef]
- Milot, E.; Filep, J.G. Regulation of neutrophil survival/apoptosis by Mcl-1. Sci. World J. 2011, 11, 1948–1962. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, A.D.; DeLeo, F.R. Neutrophil apoptosis and the resolution of infection. Immunol. Res. 2009, 43, 25–61. [Google Scholar] [CrossRef] [PubMed]
- Noseykina, E.M.; Schepetkin, I.A.; Atochin, D.N. Molecular mechanisms for regulation of neutrophil apoptosis under normal and pathological conditions. J. Evol. Biochem. Physiol. 2021, 57, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Influence of microbes on neutrophil life and death. Front. Cell. Inf. Microbiol. 2017, 7, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, C.; Yamashita, T.; Araki, A.; Terashita, M.; Watanabe, T.; Atsumi, M.; Tamura, M.; Sendo, F. Modulation of in vivo immune response by selective depletion of neutrophils using a monoclonal antibody, RP-3, I. Inhibition by RP-3 treatment of the priming and effector phases of delayed-type hypersensitivity to sheep red cells in rats. J. Immunol. 1993, 150, 3728–3738. [Google Scholar]
- Tamura, M.; Sekiya, S.; Terashita, M.; Sendo, F. Modulation of the in vivo immune response by selective depletion of neutrophils using a monoclonal antibody, RP-3, III. Enhancement by RP-3 treatment of the anti-sheep red blood cell plaque-forming cell response in rats. J. Immunol. 1994, 153, 1301–1308. [Google Scholar]
- Matsuzaki, J.; Tsuji, T.; Chamoto, K.; Takeshima, T.; Sendo, F.; Nishimura, T. Successful elimination of memory-type CD8+ T cell subsets by the administration of anti-Gr-1 monoclonal antibody in vivo. Cell. Immunol. 2003, 224, 98–105. [Google Scholar] [CrossRef]
- Yue, C.L.; Tanimoto, K.; Horiuchi, Y. Characterization and possible mechanisms of mitogen-induced cell-mediated cytotoxicity. Scand. J. Immunol. 1981, 14, 397–408. [Google Scholar] [CrossRef]
- Dallegri, F.; Frumento, G.; Maggi, A.; Patrone, F. PHA-induced neutrophil-mediated cytotoxicity. J. Clin. Lab. Immunol. 1983, 11, 203–206. [Google Scholar]
- Stiehm, E.R.; Roberts, R.L.; Ank, B.J.; Plaeger-Marshall, S.; Salman, N.; Shen, L.; Fanger, M.W. Comparison of cytotoxic properties of neonatal and adult neutrophils and monocytes and enhancement by cytokines. Clin. Diagn. Lab. Immunol. 1994, 1, 342–347. [Google Scholar] [CrossRef]
- Okuda, T. Murine polymorphonuclear leukocytes synthesize and secrete the third component and factor B of complement. Int. Immunol. 1991, 3, 293–296. [Google Scholar] [CrossRef]
- Botto, M.; Lissandrini, D.; Sorio, C.; Walport, M.J. Biosynthesis and secretion of complement component (C3) by activated human polymorphonuclear leukocytes. J. Immunol. 1992, 149, 1348–1355. [Google Scholar] [PubMed]
- Yu, C.L.; Tsai, C.Y.; Hsieh, S.C.; Tsai, Y.Y.; Tsai, S.T.; Sun, K.H.; Yu, H.S.; Han, S.H. Production of the third component of complement (C3) by peripheral polymorphonuclear neutrophils of the patients with rheumatoid arthritis. Proc. Natl. Sci. Counc. China B 1995, 19, 225–232. [Google Scholar]
- Bedouhène, S.; Dang, P.M.-C.; Hurtado-Nedelec, M.; El-Benna, J. Neutrophil degranulation of azurophil and specific granules. Methods Mol. Biol. 2020, 2087, 215–222. [Google Scholar] [PubMed]
- Gigon, L.; Yousefi, S.; Karaulov, A.; Simon, H.-U. Mechanisms of toxicity mediated by neutrophil and eosinophil granule proteins. Allergol. Int. 2021, 70, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-J.; Lu, M.-C.; Hsieh, S.-C.; Wu, C.-H.; Yu, H.-S.; Tsai, C.-Y.; Yu, C.-L. Release of surface-expressed lactoferrin from polymorphonuclear neutrophils after contact with CD4+ T cells and its modulation on Th1/Th2 cytokine production. J. Leukoc. Biol. 2006, 80, 350–358. [Google Scholar] [CrossRef]
- Bazzoni, F.; Cassatella, M.A.; Laudanna, C.; Rossi, F. Phagocytosis of opsonized yeast induces tumor necrosis factor-alpha mRNA accumulation and protein release by human polymorphonuclear leukocytes. J. Leukoc. Biol. 1991, 50, 223–228. [Google Scholar] [CrossRef]
- Cassatella, M.A. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today 1995, 16, 21–26. [Google Scholar] [CrossRef]
- Yoshimura, A.; Hara, Y.; Kaneko, T.; Kato, I. Secretion of IL-1β, TNF-α, IL-8, and IL-1ra by human polymorphonuclear leukocytes in response to lipopolysaccharides from periodontopathic bacteria. J. Periodont. Res. 1997, 32, 279–286. [Google Scholar] [CrossRef]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-derived cytokines: Facts beyond expression. Front. Immunol. 2014, 5, 508. [Google Scholar] [CrossRef] [Green Version]
- Tecchio, C.; Cassatella, M.A. Neutrophil-derived chemokines on the road to immunity. Semin. Immunol. 2016, 28, 119–128. [Google Scholar] [CrossRef]
- Tamassia, N.; Bianchetto-Aguilera, F.; Arruda-Silva, F.; Gardiman, E.; Gasperini, S.; Calzetti, F.; Cassatella, M.A. Cytokine production by human neutrophils: Revisiting the “dark side of the moon”. Eur. J. Clin. Investig. 2018, 48 (Suppl. S2), e12952. [Google Scholar] [CrossRef] [PubMed]
- Butin-Israeli, V.; Houser, M.C.; Feng, M.; Thorp, E.B.; Nusrat, A.; Parkos, C.A.; Sumagin, R. Deposition of microparticles by neutrophils onto inflamed epithelium: A new mechanism to disrupt epithelial intercellular adhesions and promote transepithelial migration. FASEB J. 2016, 30, 4007–4020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasser, O.; Schifferli, J.A. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 2004, 104, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Finkielsztein, A.; Mascarenhas, L.; Butin-Israeli, V.; Sumagin, R. Isolation and characterization of neutrophil-derived microparticles for functional studies. J. Vis. Exp. 2018, 133, e56949. [Google Scholar] [CrossRef]
- Takashima, A.; Yao, Y. Neutrophil plasticity: Acquisition of phenotype and functionality of antigen-presenting cell. J. Leukoc. Biol. 2015, 98, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.; Loré, K. Granulocytes: New members of the antigen presenting cell family. Front. Immunol. 2017, 8, 1781. [Google Scholar] [CrossRef] [Green Version]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, R.A.; Liang, F.; Loré, K. Neutrophils acquire the capacity for antigen presentation to memory CD4+ T cells in vitro and ex vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef] [Green Version]
- Meinderts, S.M.; Baker, G.; van Wijk, S.; Beuger, B.M.; Geissler, J.; Jansen, M.H.; Saris, A.; Brinke, A.T.; Kuijpers, T.W.; van den Berg, T.K.; et al. Neutrophils acquire antigen-presenting cell features after phagocytosis of IgG-opsonized erythrocytes. Blood Adv. 2019, 3, 1761–1773. [Google Scholar] [CrossRef]
- Polak, D.; Hafner, C.; Briza, P.; Kitzmüller, C.; Elbe-Bürger, A.; Samadi, N.; Gschwandtner, M.; Pfützner, W.; Zlabinger, G.J.; Jahn-Schmid, B.; et al. A novel role for neutrophils in IgE-mediated allergy: Evidence for antigen presentation in late-phase reactions. J. Allergy Clin. Immunol. 2019, 143, 1143–1152.e4. [Google Scholar] [CrossRef] [Green Version]
- Samadi, N.; Polak, D.; Kitzmüller, C.; Steinberger, P.; Zlabinger, G.J.; Jahn-Schmid, B.; Bohle, B. T-cell-derived cytokines enhance the antigen-presenting capacity of human neutrophils. Eur. J. Immunol. 2019, 49, 1441–1443. [Google Scholar] [CrossRef]
- Li, K.-J.; Wu, C.-H.; Lu, C.-H.; Shen, C.-Y.; Kuo, Y.-M.; Tsai, C.-Y.; Hsieh, S.-C.; Yu, C.-L. Trogocytosis between non-immune cells for cell clearance, and among immune-related cells for modulating immune responses and autoimmunity. Int. J. Mol. Sci. 2021, 22, 2236. [Google Scholar] [CrossRef] [PubMed]
- Dance, A. Core concept: Cells nibble one another via the under-appreciated process of trogocytosis. Proc. Natl. Acad. Sci. USA 2019, 116, 17608–17610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, R.P.; Lindorfer, M.A. Fcγ-receptor-mediated trogocytosis impacts mAb-based therapies: Historical precedence and recent developments. Blood 2015, 125, 762–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valgardsdottir, R.; Cattaneo, I.; Klein, C.; Introna, M.; Figliuzzi, M.; Golay, J. Human neutrophils mediate trogocytosis rather than phagocytosis of CLL B cells opsonized with anti-CD20 antibodies. Blood 2017, 129, 2636–2644. [Google Scholar] [CrossRef] [Green Version]
- Poupot, M.; Fournié, J.-J. Spontaneous membrane transfer through homotypic synapses between lymphoma cells. J. Immunol. 2003, 171, 2517–2523. [Google Scholar] [CrossRef] [Green Version]
- Honer, H.; Frank, C.; Dechant, C.; Repp, R.; Glennie, M.; Herrmann, M. Intimate cell conjugate formation and exchange of membrane lipids procede apoptosis induction in target cells during antibody-dependent, granulocyte-mediated cytotoxicity. J. Immunol. 2007, 179, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Li, K.-J.; Wu, C.-H.; Shen, C.-Y.; Kuo, Y.-M.; Yu, C.-L.; Hsieh, S.-C. Membrane transfer from mononuclear cells to polymorphonuclear neutrophils transduces cell survival and activation signals in the recipient cells via anti-extrinsic apoptotic and MAP kinase signaling pathways. PLoS ONE 2016, 11, e0156262. [Google Scholar] [CrossRef] [Green Version]
- Hudrisier, D.; Riond, J.; Mazarguil, H.; Gairin, J.E.; Joly, E. Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J. Immunol. 2001, 166, 3645–3649. [Google Scholar] [CrossRef] [Green Version]
- Batista, F.D.; Iber, D.; Neuberger, M.S. B cells acquire antigen from target cells after synapse formation. Nature 2001, 411, 489–494. [Google Scholar] [CrossRef]
- Dömer, D.; Walther, T.; Möller, S.; Behnen, M.; Laskay, T. Neutrophil extracellular traps activate proinflammatory functions of human neutrophils. Front. Immunol. 2021, 12, 636954. [Google Scholar] [CrossRef]
- Douglas, R.G.; Alford, R.H.; Cate, T.R.; Couch, R.B. The leukocyte response during viral respiratory illness in man. Ann. Intern. Med. 1966, 64, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Faden, H.; Hong, J.J.; Ogra, P.L. Interaction of polymorphonuclear leukocytes and viruses in humans: Adherence of polymorphonuclear leukocytes to respiratory syncytial virus-infected cells. J. Virol. 1984, 52, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacGregor, R.R.; Friedman, H.M.; Macarak, E.J.; Kefalides, N.A. Virus infection of endothelial cells increases granulocyte adherence. J. Clin. Investig. 1980, 65, 1469–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratcliffe, D.R.; Nolin, S.L.; Cramer, E.B. Neutrophil interaction with influenza-infected epithelial cells. Blood 1988, 72, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouse, B.T.; Babiuk, L.A.; Henson, P.M. Neutrophils in antiviral immunity: Inhibition of virus replication by a mediator produced by bovine neutrophils. J. Infect. Dis. 1980, 141, 223–232. [Google Scholar] [CrossRef]
- Tumpey, T.M.; Chen, S.-H.; Oakes, J.E.; Lausch, R.N. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J. Virol. 1996, 70, 898–904. [Google Scholar] [CrossRef] [Green Version]
- Tumpey, T.M.; García-Sastre, A.; Taubenberger, J.K.; Palese, P.; Swayne, D.E.; Pantin-Jackwood, M.J.; Schultz-Cherry, S.; Solórzano, A.; Van Rooijen, N.; Katz, J.M.; et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: Functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J. Virol. 2005, 79, 14933–14944. [Google Scholar] [CrossRef] [Green Version]
- Tate, M.D.; Brooks, A.G.; Reading, P.C. The role of neutrophils in the upper and lower respiratory tract during influenza virus infection of mice. Respir. Res. 2008, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Wojtasiak, M.; Pickett, D.L.; Tate, M.D.; Londrigan, S.L.; Bedoui, S.; Brooks, A.G.; Reading, P.C. Depletion of Gr-1+, but not Ly6G+, immune cells exacerbates virus replication and disease in an intranasal model of herpes simplex virus type 1 infection. J. Gen. Virol. 2010, 91, 2158–2166. [Google Scholar] [CrossRef]
- Tate, M.D.; Brooks, A.G.; Reading, P.C.; Mintern, J.D. Neutrophils sustain effective CD8+ T-cell responses in the respiratory tract following influenza infection. Immunol. Cell Biol. 2012, 90, 197–205. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Zhu, L. Role of neutrophils in acute viral infection. Immun. Inflamm. Dis. 2021, 9, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-hussaniy, H.A.; Al-Harcan, N.A.H.; Alexiou, A.; Batiha, G.E.-S. Neutrophil extracellular traps (NETs) and COVID-19: A new frontiers for therapeutic modality. Int. Immunopharmacol. 2022, 104, 108516. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Means, T.K.; Luster, A.D. Toll-like receptors stimulate human neutrophil function. Blood 2003, 102, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000, 80, 617–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, S.C.; Tsai, C.Y.; Sun, K.H.; Yu, H.S.; Tsai, S.T.; Wang, J.C.; Tsai, Y.Y.; Han, S.H.; Yu, C.L. Decreased spontaneous and lipopolysaccharide stimulated production of interleukin 8 by polymorphonuclear neutrophils of patients with active systemic lupus erythematosus. Clin. Exp. Rheumatol. 1994, 12, 627–633. [Google Scholar] [PubMed]
- Hsieh, S.C.; Wu, T.-H.; Tsai, C.-Y.; Li, K.-J.; Lu, M.-C.; Wu, C.-H.; Yu, C.-L. Abnormal in vitro CXCR2 modulation and defective cationic ion transporter expression on polymorphonuclear neutrophils responsible for hyporesponsiveness to IL-8 stimulation in patients with active systemic lupus erythematosus. Rheumatology 2008, 47, 150–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, S.C.; Tsai, C.Y.; Sun, K.H.; Tsai, Y.Y.; Tsai, S.T.; Han, S.H.; Yu, H.S.; Yu, C.L. Defective spontaneous and bacterial lipopolysaccharide-stimulated production of interleukin-1 receptor antagonist by polymorphonuclear neutrophils of patients with active systemic lupus erythematosus. Br. J. Rheumatol. 1995, 34, 107–112. [Google Scholar] [CrossRef]
- Yu, C.-L.; Sun, K.-H.; Tsai, C.Y.; Tsai, Y.-Y.; Tsai, S.-T.; Huang, D.-F.; Han, S.-H.; Yu, H.-S. Expression of Th1/Th2 cytokine mRNA in peritoneal exudative polymorphonuclear neutrophils and their effects on mononuclear Th1/Th2 cytokine production in MRL-lpr/lpr mice. Immunology 1998, 95, 480–487. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Wu, T.-H.; Yu, C.-L.; Tsai, Y.-Y.; Chou, C.-T. Decreased IL-12 production by polymorphonuclear leukocytes in patients with active systemic lupus erythematosus. Immunol. Investig. 2002, 31, 177–189. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Li, K.-J.; Hsieh, S.-C.; Liao, H.-T.; Yu, C.-L. What’s wrong with neutrophils in lupus? Clin. Exp. Rheumatol. 2019, 37, 684–693. [Google Scholar]
- Stein, J.M.; Luzio, J.P. Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem. J. 1991, 274 Pt 2, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.; Sadallah, S.; Hefti, A.; Landmann, R.; Schifferli, J.A. Ectosomes released by human neutrophils are specialized functional units. J. Immunol. 1999, 163, 4564–4573. [Google Scholar] [CrossRef]
- Johnstone, R.M. Exosomes biological significance: A concise review. Blood Cells Mol. Dis. 2006, 36, 315–321. [Google Scholar] [CrossRef]
- Sadallah, S.; Eken, C.; Schifferli, J.A. Ectosomes as modulators of inflammation and immunity. Clin. Exp. Immunol. 2011, 163, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Eken, C.; Gasser, O.; Zenhaeusern, G.; Oehri, I.; Hess, C.; Schifferli, J.A. Polymorphonuclear neutrophil-derived ectosomes interfere with the maturation of monocyte-derived dendritic cells. J. Immunol. 2008, 180, 817–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eken, C.; Martin, P.J.; Sadallah, S.; Treves, S.; Schaller, M.; Schifferli, J.A. Ectosomes released by polymorphonuclear neutrophils induce a MerTK-dependent anti-inflammatory pathway in macrophages. J. Biol. Chem. 2010, 285, 39914–39921. [Google Scholar] [CrossRef] [Green Version]
- Eken, C.; Sadallah, S.; Martin, P.J.; Treves, S.; Schifferli, J.A. Ectosomes of polymorphonuclear neutrophils activate multiple signaling pathways in macrophages. Immunobiology 2013, 218, 382–392. [Google Scholar] [CrossRef]
- Rawat, K.; Syeda, S.; Shrivastava, A. Neutrophil-derived granule cargoes: Paving the way for tumor growth and progression. Cancer Metastasis Rev. 2021, 40, 221–244. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, Y.; Jin, H.; Li, L. The role of exosome in autoimmune connective tissue disease. Ann. Med. 2019, 51, 101–108. [Google Scholar] [CrossRef]
- Mirzaei, R.; Zamani, F.; Hajibaba, M.; Rasouli-Saravani, A.; Noroozbeygi, M.; Gorgani, M.; Hosseini-Fard, S.R.; Jalalifar, S.; Ajdarkosh, H.; Abedi, S.H.; et al. The pathogenic therapeutic and diagnostic role of exosomal microRNA in the autoimmune diseases. J. Neuroimmunol. 2021, 358, 577640. [Google Scholar] [CrossRef]
- Miao, C.; Wang, X.; Zhou, W.; Huang, J. The emerging roles of exosomes in autoimmune diseases, with special emphasis on microRNAs in exosomes. Pharmacol. Res. 2021, 169, 105680. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-Y.; Hsieh, S.-C.; Lu, C.-S.; Wu, T.-H.; Liao, H.-T.; Wu, C.-H.; Li, K.-J.; Kuo, Y.-M.; Lee, H.-T.; Shen, C.-Y.; et al. Cross-talk between mitochondrial dysfunction-provoked oxidative stress and aberrant noncoding RNA expression in the pathogenesis and pathophysiology of SLE. Int. J. Mol. Sci. 2019, 20, 5183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.Y.; Shen, C.-Y.; Liu, C.-W.; Hsieh, S.-C.; Liao, H.-T.; Li, K.-J.; Lu, C.-S.; Lee, H.-T.; Lin, C.-S.; Wu, C.-H.; et al. Aberrant non-coding RNA expression in patients with systemic lupus erythematosus: Consequences for immune dysfunctions and tissue damage. Biomolecules 2020, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-Y.; Hsieh, S.-C.; Liu, C.-W.; Lu, C.-H.; Liao, H.-T.; Chen, M.-H.; Li, K.-J.; Wu, C.-H.; Shen, C.-Y.; Kuo, Y.-M.; et al. The expression of non-coding RNAs and their target molecules in rheumatoid arthritis: A molecular basis for rheumatoid pathogenesis and its potential clinical applications. Int. J. Mol. Sci. 2021, 22, 5689. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-Y.; Hsieh, S.-C.; Wu, T.-H.; Li, K.-J.; Shen, C.-Y.; Liao, H.-T.; Wu, C.H.; Kuo, Y.-M.; Lu, C.-S.; Yu, C.-L. Pathogenic roles of autoantibodies and aberrant epigenetic regulation of immune and connective tissue cells in the tissue fibroosis of patients with systemic sclerosis. Int. J. Mol. Sci. 2020, 21, 3069. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Hsieh, S.-C.; Liu, C.-W.; Lu, C.-S.; Wu, C.-H.; Liao, H.-T.; Chen, M.-H.; Li, K.-J.; Shen, C.-Y.; Kuo, Y.-M.; et al. Cross-talk among polymorphonuclear neutrophils, immune and non-immune cells via released cytokines, granule proteins, microvesicles, and neutrophil extracellular trap formation: A novel concept of biology and pathobiology for neutrophils. Int. J. Mol. Sci. 2021, 22, 3119. [Google Scholar] [CrossRef]
- Li, L.; Zuo, X.; Xiao, Y.; Liu, D.; Luo, H.; Zhu, H. Neutrophil-derived exosome from systemic sclerosis inhibits the proliferation and migration of endothelial cells. Biochem. Biophys. Res. Commun. 2020, 526, 334–340. [Google Scholar] [CrossRef]
- Li, L.; Zuo, X.; Liu, D.; Luo, H.; Zhu, H. The functional roles of RNAs cargoes released by neutrophil-derived exosomes in dermatomyositis. Front. Pharmacol. 2021, 12, 727901. [Google Scholar] [CrossRef]
- Oehler, L.; Majdic, O.; Pickl, W.F.; Stockl, J.; Riedl, E.; Drach, J.; Rappersberger, K.; Geissler, K.; Knapp, W. Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics. J. Exp. Med. 1998, 187, 1019–1028. [Google Scholar] [CrossRef] [Green Version]
- Sandilands, G.P.; McCrae, J.; Hill, K.; Perry, M.; Baxter, D. Major histocompatibility complex class II (DR) antigen and costimulatory molecules on in vitro and in vivo activated human polymorphonuclear neutrophils. Immunology 2006, 119, 562–571. [Google Scholar] [CrossRef]
- Mercer, F.; Ng, S.H.; Brown, T.M.; Boatman, G.; Johnson, P.J. Neutrophils kill the parasite Trichomonas vaginalis using trogocytosis. PLoS Biol. 2018, 16, e2003885. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Valle, I.; Latorre, M.C.; Calvo, M.; Gaspar, B.; Gómez-Oro, C.; Collazos, A.; Breton, A.; Caballero-Campo, P.; Ardoy, M.; Asensio, F.; et al. Vaginal neutrophils eliminate sperm by trogocytosis. Hum. Reprod. 2020, 35, 2567–2578. [Google Scholar] [CrossRef] [PubMed]
- Vanherberghen, B.; Andersson, K.; Carlin, L.M.; Nolte-‘t Hoen, E.N.M.; Williams, G.S.; Höglund, P.; Davis, D.M. Human and murine inhibitory natural killer cell receptors transfer from natural killer cells to target cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16873–16878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.; Mero, P.; Booth, J.W. Dynamics of macrophage trogocytosis of rituximab-coated B cells. PLoS ONE 2001, 6, e14498. [Google Scholar] [CrossRef] [Green Version]
- Miyake, K.; Shiozawa, N.; Nagao, T.; Yoshikawa, S.; Yamanishi, Y.; Karasuyama, H. Trogocytosis of peptide-MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proc. Natl. Acad. Sci. USA 2017, 114, 1111–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliphant, C.J.; Hwang, Y.Y.; Walker, J.A.; Salimi, M.; Wong, S.H.; Brewer, J.M.; Englezakis, A.; Barlow, J.L.; Hams, E.; Scanlon, S.T.; et al. MHC II-mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 2014, 41, 283–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, K.; Karasuyama, H. The role of trogocytosis in the modulation of immune cell functions. Cells 2021, 10, 1255. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. The multiple roles of trogocytosis in immunity, the nervous system and development. BioMed. Res. Int. 2021, 2021, 1601565. [Google Scholar] [CrossRef]
- Nakayama, M.; Hori, A.; Toyoura, S.; Yamaguchi, S.-I. Shaping of T cell functions by trogocytosis. Cells 2021, 10, 1155. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Ribon, M.; Seninet, S.; Mussard, J.; Sebbag, M.; Clavel, C.; Serre, G.; Boissier, M.-C.; Semerano, L.; Decker, P. Neutrophil extracellular traps exert both pro- and anti-inflammatory actions in rheumatoid arthritis that are modulated by C1q and LL-37. J. Autoimmun. 2019, 98, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Döring, Y.; Libby, P.; Soehnlein, O. Neutrophil extracellular traps participate in cardiovascular diseases: Recent experimental and clinical insights. Circ. Res. 2020, 126, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, W.; Shen, F.; Du, W.; Xu, Z.; Liu, Z. The emerging role of neutrophil extracellular traps in arterial, venous and cancer-associated thrombosis. Front. Cardiovasc. Med. 2021, 8, 786387. [Google Scholar] [CrossRef] [PubMed]
- Klopf, J.; Brostjan, C.; Eilenberg, W.; Neumayer, C. Neutrophil extracellular traps and their implications in cardiovascular and inflammatory disease. Int. J. Mol. Sci. 2021, 22, 559. [Google Scholar] [CrossRef]
- Leffler, J.; Martin, M.; Gullstrand, B.; Tydén, H.; Lood, C.; Truedsson, L.; Bengtsson, A.A.; Blom, A.M. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J. Immunol. 2012, 188, 3522–3531. [Google Scholar] [CrossRef] [Green Version]
- Söderberg, D.; Segelmark, M. Neutrophil extracellular traps in ANCA-associated vasculitis. Front. Immunol. 2016, 7, 256. [Google Scholar] [CrossRef] [Green Version]
- Masucci, M.T.; Minopoli, M.; Del Vecchio, S.; Carriero, M.V. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front. Immunol. 2020, 11, 1749. [Google Scholar] [CrossRef]
- Hazeldine, J.; Harris, P.; Chapple, I.L.; Grant, M.; Greenwood, H.; Livesey, A.; Sapey, E.; Lord, J.M. Impaired neutrophil extracellular trap formation: A novel defect in the innate immune system of aged individuals. Aging Cell 2014, 13, 690–698. [Google Scholar] [CrossRef]
- Lu, C.-H.; Li, K.-J.; Wu, C.-H.; Shen, C.-Y.; Kuo, Y.-M.; Hsieh, S.-C.; Yu, C.-L. The FcγRIII engagement augments PMA-stimulated neutrophil extracellular traps (NETs) formation by granulocytes partially via cross-talk between Syk-ERK-NF-κB and PKC-ROS signaling pathways. Biomedicines 2021, 9, 1127. [Google Scholar] [CrossRef]
- Farrera, C.; Fadeel, B. Macrophage clearance of neutrophil extracellular traps is a silent process. J. Immunol. 2013, 191, 2647–2656. [Google Scholar] [CrossRef] [Green Version]
- Eruslanov, E.B.; Bhojnagarwala, P.S.; Quatromoni, J.G.; Stephen, T.L.; Ranganathan, A.; Deshpande, C.; Akimova, T.; Vachani, A.; Litzky, L.; Hancock, W.W.; et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J. Clin. Investig. 2014, 124, 5466–5480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Duckworth, C.A.; Zhao, Q.; Pritchard, D.M.; Rhodes, J.M.; Yu, L.-G. Increased circulation of galectin-3 in cancer induces secretion of metastasis-promoting cytokines from blood vascular endothelium. Clin. Cancer Res. 2013, 19, 1693–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Aparicio, M.; Alfaro, C. Significance of the IL-8 pathway for immunotherapy. Hum. Vaccines Immunother. 2020, 16, 2312–2317. [Google Scholar] [CrossRef] [PubMed]
- Furumaya, C.; Martinez-Sanz, P.; Bouti, P.; Kuijpers, T.W.; Matlung, H.L. Plasticity in pro- and anti-tumor activity of neutrophils: Shifting the balance. Front. Immunol. 2020, 11, 2100. [Google Scholar] [CrossRef] [PubMed]
- Avtenyuk, N.U.; Visser, N.; Bremer, E.; Wiersma, V.R. The neutrophil: The underdog that packs a punch in the fight against cancer. Int. J. Mol. Sci. 2020, 21, 7820. [Google Scholar] [CrossRef]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.-F.; Gereke, M.; von Köckritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Shaul, M.E.; Levy, L.; Sun, J.; Mishalian, I.; Singhal, S.; Kapoor, V.; Horng, W.; Fridlender, G.; Albelda, S.M.; Fridlender, Z.G. Tumor-associated neutrophils display a distinct N1 profile following TGFβ modulation: A transcriptomics analysis of pro- vs. antitumor TANs. Oncoimmunology 2016, 5, e1232221. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.Y.; Tai, J.A.; Li, S.; Nishikawa, T.; Kaneda, Y. Virus-stimulated neutrophils in the tumor microenvironment enhance T cell-mediated anti-tumor immunity. Oncotarget 2016, 7, 42195–42207. [Google Scholar] [CrossRef]
- Heemskerk, N.; van Egmond, M. Monoclonal antibody-mediated killing of tumour cells by neutrophils. Eur. J. Clin. Investig. 2018, 48 (Suppl. S2), e12962. [Google Scholar] [CrossRef] [Green Version]
- Matlung, H.L.; Babes, L.; Zhao, X.W.; van Houdt, M.; Treffers, L.W.; van Rees, D.J.; Franke, K.; Schornagel, K.; Verkuijlen, P.; Janssen, H.; et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 2018, 23, 3946–3959.e6. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, A.M.; Bondza, S.; Evers, M.; Koutstaal, R.; Nederend, M.; Marco Jansen, J.H.; Rösner, T.; Valerius, T.; Leusen, J.H.W.; Ten Broeke, T. Potent Fc receptor signaling by IgA leads to superior killing of cancer cells by neutrophils compared to IgG. Front. Immunol. 2019, 10, 704. [Google Scholar] [CrossRef] [Green Version]
- Treffers, L.W.; van Houdt, M.; Bruggeman, C.W.; Heineke, M.H.; Zhao, X.W.; van der Heijden, J.; Nagelkerke, S.Q.; Verkuijlen, P.J.J.H.; Geissler, J.; Lissenberg-Thunnissen, S.; et al. FcγRIIIb restricts antibody-dependent destruction of cancer cells by human neutrophils. Front. Immunol. 2019, 9, 3124. [Google Scholar] [CrossRef] [PubMed]
- Albrengues, J.; Shields, M.A.; Ng, D.; Park, C.G.; Ambrico, A.; Poindexter, M.E.; Upadhyay, P.; Uyeminami, D.L.; Pommier, A.; Küttner, V.; et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018, 361, eaao4227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thålin, C.; Hisada, Y.; Lundström, S.; Mackman, N.; Wallén, H. Neutrophil extracellular traps: Villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1724–1738. [Google Scholar] [CrossRef]
- Jung, H.S.; Gu, J.; Kim, J.-E.; Nam, Y.; Song, J.W.; Kim, H.K. Cancer cell-induced neutrophil extracellular traps promote both hypercoagulability and cancer progression. PLoS ONE 2019, 14, e0216055. [Google Scholar] [CrossRef] [Green Version]
- Shao, B.-Z.; Yao, Y.; Li, J.-P.; Chai, N.-L.; Linghu, E.-Q. The role of neutrophil extracellular traps in cancer. Front. Oncol. 2021, 11, 714357. [Google Scholar] [CrossRef]
- Kramer, E.D.; Abrams, S.I. Granulocytic myeloid-derived suppressor cells as negative regulators of anticancer immunity. Front. Immuunol. 2020, 11, 1963. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Gerhardt, L.; Vareki, S.M. Immunosuppressive effects of myeloid-derived suppressor cells in cancer and immunotherapy. Cells 2021, 10, 1170. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997, 336, 973–979. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Hattori, S.; Katsuda, S.; Nakanishi, I.; Nagai, Y. Human neutrophil elastase: Degradation of basement membrane components and immunolocalization in the tissue. J. Biochem. 1990, 108, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, G.; Nakano, M.; Prati, F.; Niccoli, G.; Mallus, M.T.; Ramazzotti, V.; Montone, R.A.; Kolodgie, F.D.; Virmani, R.; Crea, F. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: A clinicopathological study. Circulation 2010, 122, 2505–2513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naruko, T.; Ueda, M.; Haze, K.; van der Wal, A.C.; van der Loos, C.M.; Itoh, A.; Komatsu, R.; Ikura, Y.; Ogami, M.; Shimada, Y.; et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation 2002, 106, 2894–2900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.J.; Kettritz, R.; Falk, R.J.; Jennette, J.C.; Gaido, M.L. Apoptosis of endothelial cells induced by the neutrophil serine proteases proteinase 3 and elastase. Am. J. Pathol. 1996, 149, 1617–1626. [Google Scholar]
- Soehnlein, O. Multiple roles for neutrophils in atherosclerosis. Circ. Res. 2012, 110, 875–888. [Google Scholar] [CrossRef] [Green Version]
- Sreejit, G.; Latif, A.A.; Murphy, A.J.; Nagareddy, P.R. Emerging roles of neutrophil-borne S100A8/A9 in cardiovascular inflammation. Pharmacol. Res. 2020, 161, 105212. [Google Scholar] [CrossRef]
- Bonaventura, A.; Montecucco, F.; Dallegri, F.; Carbone, F.; Lüscher, T.F.; Camici, G.G.; Liberale, L. Novel findings in neutrophil biology and their impact on cardiovascular disease. Cardiovasc. Res. 2019, 115, 1266–1285. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Braster, Q.; Ortega-Gomez, A.; Soehnlein, O. Neutrophils as regulators of cardiovascular inflammation. Nat. Rev. Cardiol. 2020, 17, 327–340. [Google Scholar] [CrossRef]
- Gan, T.; Yang, Y.; Hu, F.; Chen, X.; Zhou, J.; Li, Y.; Xu, Y.; Wang, H.; Chen, Y.; Zhang, M. TLR3 regulated poly I:C-induced neutrophil extracellular traps and acute lung injury partly through p38 MAP kinase. Front. Microbiol. 2018, 9, 3174. [Google Scholar] [CrossRef]
- Stegelmeier, A.A.; Darzianiazizi, M.; Hanada, K.; Sharif, S.; Wootton, S.K.; Bridle, B.W.; Karimi, K. Type I interferon-mediated regulation of antiviral capabilities of neutrophils. Int. J. Mol. Sci. 2021, 22, 4726. [Google Scholar] [CrossRef] [PubMed]
- Khosroshahi, L.M.; Rezaei, N. Dysregulation of the immune response in coronavirus disease 2019. Cell Biol. Int. 2021, 45, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Schönrich, G.; Raftery, M.J.; Samstag, Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regul. 2020, 77, 100741. [Google Scholar] [CrossRef] [PubMed]
- Chau, A.S.; Weber, A.G.; Maria, N.I.; Narain, S.; Liu, A.; Hajizadeh, N.; Malhotra, P.; Bloom, O.; Marder, G.; Kaplan, B. The longitudinal immune response to coronavirus disease 2019: Chasing the cytokine storm. Arthritis Rheumatol. 2021, 73, 23–35. [Google Scholar] [CrossRef]
- Matthay, M.A.; Leligdowicz, A.; Liu, K.D. Biological mechanisms of COVID-19 acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020, 202, 1489–1491. [Google Scholar] [CrossRef]
- Hue, S.; Beldi-Ferchiou, A.; Bendib, I.; Surenaud, M.; Fourati, S.; Frapard, T.; Rivoal, S.; Razazi, K.; Carteaux, G.; Delfau-Larue, M.-H.; et al. Uncontrolled innate and impaired adaptive immune responses in patients with COVID-19 acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020, 202, 1509–1519. [Google Scholar] [CrossRef]
- Yao, C.; Bora, S.A.; Parimon, T.; Zaman, T.; Friedman, O.A.; Palatinus, J.A.; Surapaneni, N.S.; Matusov, Y.P.; Chiang, G.C.; Kassar, A.G.; et al. Cell-type-specific immune dysregulation in severely ill COVID-19 patients. Cell Rep. 2021, 34, 108590. [Google Scholar] [CrossRef]
- De Candia, P.; Prattichizzo, F.; Garavelli, S.; Matarese, G. T cells: Warriors of SARS-CoV-2 infection. Trends Immunol. 2021, 42, 18–30. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. for the China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Veras, F.P.; Pontelli, M.C.; Silva, C.M.; Toller-Kawahisa, J.E.; de Lima, M.; Nascimento, D.C.; Schneider, A.H.; Caetité, D.; Tavares, L.A.; Paiva, I.M.; et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020, 217, e20201129. [Google Scholar] [CrossRef]
- Borges, L.; Pithon-Curi, T.C.; Curi, R.; Hatanaka, E. COVID-19 and neutrophils: The relationship between hyperinflammation and neutrophil extracellular traps. Mediat. Inflamm. 2020, 2020, 8829674. [Google Scholar] [CrossRef] [PubMed]
- Yaqinuddin, A.; Kvietys, P.; Kashir, J. COVID-19: Role of neutrophil extracellular traps in acute lung injury. Respir. Investig. 2020, 58, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Szturmowicz, M.; Demkow, U. Neutrophil extracellular traps (NETs) in severe SARS-CoV-2 lung disease. Int. J. Mol. Sci. 2021, 22, 8854. [Google Scholar] [CrossRef] [PubMed]
- Tomar, B.; Anders, H.-J.; Desai, J.; Mulay, S.R. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells 2020, 9, 1383. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Chan, L.; Karimi, N.; Morovati, S.; Alizadeh, K.; Kakish, J.E.; Vanderkamp, S.; Fazel, F.; Napoleoni, C.; Alizadeh, K.; Mehrani, Y.; et al. The roles of neutrophil in cytokine storms. Viruses 2021, 13, 2318. [Google Scholar] [CrossRef]
- Nicolai, L.; Leunig, A.; Brambs, S.; Kaiser, R.; Weinberger, T.; Weigand, M.; Muenchhoff, M.; Hellmuth, J.C.; Ledderose, S.; Schulz, H.; et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation 2020, 142, 1176–1189. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Radermecker, C.; Detrembleur, N.; Guiot, J.; Cavalier, E.; Henket, M.; d’Emal, C.; Vanwinge, C.; Cataldo, D.; Oury, C.; Delvenne, P.; et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J. Exp. Med. 2020, 217, e20201012. [Google Scholar] [CrossRef]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Busch, M.H.; Timmermans, S.A.M.E.G.; Nagy, M.; Visser, M.; Huckriede, J.; Aendekerk, J.P.; de Vries, F.; Potjewijd, J.; Jallah, B.; Ysermans, R.; et al. Neutrophils and contact activation of coagulation as potential drivers of COVID-19. Circulation 2020, 142, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Ercan, H.; Schrottmaier, W.C.; Pirabe, A.; Schmuckenschlager, A.; Pereyra, D.; Santol, J.; Pawelka, E.; Traugott, M.T.; Schörgenhofer, C.; Seitz, T.; et al. Platelet phenotype analysis of COVID-19 patients reveals progressive changes in the activation of integrin αIIbβ3, F13A1, the SARS-CoV-2 target EIF4A1 and annexin A5. Front. Cardiovasc. Med. 2021, 8, 779073. [Google Scholar] [CrossRef] [PubMed]
- Iliadi, V.; Konstantinidou, I.; Aftzoglou, K.; Iliadis, S.; Konstantinidis, T.G.; Tsigalou, C. The emerging role of neutrophils in the pathogenesis of thrombosis in COVID-19. Int. J. Mol. Sci. 2021, 22, 5368. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Becerril, B.; Campi-Caballero, R.; Sevilla-Fuentes, S.; Hernández-Regino, L.M.; Hanono, A.; Flores-Bustamante, A.; González-Flores, J.; García-Ávila, C.A.; Aquino-Gálvez, A.; Castillejos-López, M.; et al. Immunothrombosis in COVID-19: Implications of neutrophil extracellular traps. Biomolecules 2021, 11, 694. [Google Scholar] [CrossRef] [PubMed]

| Functions | References |
|---|---|
| Mitogen-induced cell-mediated cytotoxicity (MICC) | [57,58] |
| Antibody-dependent cell-mediated cytotoxicity (ADCC) | [59] |
| Biosynthesis & secretion of complement components C3 and factor B | [60,61,62] |
| Degranulation of azurophilic and specific granule proteins | [63,64,65] |
| Production of cytokines/chemokines/growth factors | [66,67,68,69,70,71] |
| Exocytosis of ectosomes and exosomes | [72,73,74] |
| Expression of MHC-II antigens for antigen-presenting activity | [75,76,77,78,79,80,81,82] |
| Trogocytosis (plasma membrane exchange) by PMN | [83,84,85,86,87,88,89] |
| Neutrophil extracellular traps (NET) formation by PMN | [90] |
| Antiviral activity by PMNs | [91,92,93,94,95,96,97,98,99,100,101,102,103] |
| In Vitro | In Vivo |
|---|---|
| IL-1α/IL-1β | IL-1α |
| IL-1ra | IL-1β |
| IL-8 | IL-1ra |
| IL-12 | IL-6 |
| TNF-α | IL-8 |
| IFN-α | IL-10 |
| CD30L | IL-12 |
| GROα, GROβ | MIP2 |
| CINC-1, 2a, 3 | KC/GROα |
| IP-10 | CINC |
| MIG | MIP-1α |
| MIP-1α/-1β | MIP-β |
| TGF-α, TGF-β1 | MCP-1 |
| IL-3, G-CSF, M-CSF | TNF-α |
| GM-CSF (?) | TGF-β1 |
| IL-6 (?) | |
| MCP-1 (?) | |
| SCF (?) |
| Neutrophil Type 1 (N1) | Neutrophil Type 2 (N2) | Granulocytic MDSC | |
|---|---|---|---|
| Function | Anti-tumor | Pro-tumor | Pro-tumor |
| Stimulated by | IFN-β [156] | TGF-β [157] | Interferon regulatory factor-8 deficiency [168] |
| Expression of | TNF-α, Fas, ICAM | FcγRIIIb [163] | CD11b+ Ly6CloLy6G+ |
| ROS, NET | |||
| Proteolytic enzymes | |||
| Arginase [158] | |||
| Immunity | Promote CD8+ T activation & cell-mediated cytotoxicity [160,161,162] | CD8+ T cells | Suppressive effect on T cell immunity [167,168] |
| IL-4 and IL-13 secretion [157] | Suppression of NK activity | ||
| ADCC [159] | |||
| FcγT-mediated trogocytosis [161] | |||
| FcαR-mediated ADCC [162,163] |
| Untoward Effects | Pathology & References |
|---|---|
| PMN-derived ectosomes | Tumor growth and tumor progression [119] |
| PMN-derived exosomes | Systemic sclerosis [127,128] Dermatomyositis [129] |
| Excessive NETs formation or insufficient NETs clearance | Rheumatoid arthritis [90] Vascular thrombosis/atherosclerosis [143,144,145] Autoimmune diseases [146,147] |
| Impaired NETs formation by PMN | Tumor progression/metastasisInfection [144,148] |
| Excessive N2 in tumor-associated neutrophils oversecreting TGF-β and arginase | Tumorigenesis [158] |
| FcγRIIb on PMN | ADCC-mediated tumor cell killing [164] |
| Abnormal NET formation | Pro-tumor effects by inflammatory microenvironment Interaction with inflammasomes and autophagy |
| Release of proteinase 3, neutrophil elastase, and myeloperoxidase | Endothelial cell apoptosis Acute myocardial infarction [171,172,173,174] |
| Release of Alamins (S100A8/A9) | Inflammation and cardiac injury [171,172,173,174,175,176,177,178] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-H.; Hsieh, S.-C.; Li, T.-H.; Lu, C.-H.; Liao, H.-T.; Shen, C.-Y.; Li, K.-J.; Wu, C.-H.; Kuo, Y.-M.; Tsai, C.-Y.; et al. Molecular Basis for Paradoxical Activities of Polymorphonuclear Neutrophils in Inflammation/Anti-Inflammation, Bactericide/Autoimmunity, Pro-Cancer/Anticancer, and Antiviral Infection/SARS-CoV-II-Induced Immunothrombotic Dysregulation. Biomedicines 2022, 10, 773. https://doi.org/10.3390/biomedicines10040773
Wu T-H, Hsieh S-C, Li T-H, Lu C-H, Liao H-T, Shen C-Y, Li K-J, Wu C-H, Kuo Y-M, Tsai C-Y, et al. Molecular Basis for Paradoxical Activities of Polymorphonuclear Neutrophils in Inflammation/Anti-Inflammation, Bactericide/Autoimmunity, Pro-Cancer/Anticancer, and Antiviral Infection/SARS-CoV-II-Induced Immunothrombotic Dysregulation. Biomedicines. 2022; 10(4):773. https://doi.org/10.3390/biomedicines10040773
Chicago/Turabian StyleWu, Tsai-Hung, Song-Chou Hsieh, Tsu-Hao Li, Cheng-Hsun Lu, Hsien-Tzung Liao, Chieh-Yu Shen, Ko-Jen Li, Cheng-Han Wu, Yu-Min Kuo, Chang-Youh Tsai, and et al. 2022. "Molecular Basis for Paradoxical Activities of Polymorphonuclear Neutrophils in Inflammation/Anti-Inflammation, Bactericide/Autoimmunity, Pro-Cancer/Anticancer, and Antiviral Infection/SARS-CoV-II-Induced Immunothrombotic Dysregulation" Biomedicines 10, no. 4: 773. https://doi.org/10.3390/biomedicines10040773
APA StyleWu, T.-H., Hsieh, S.-C., Li, T.-H., Lu, C.-H., Liao, H.-T., Shen, C.-Y., Li, K.-J., Wu, C.-H., Kuo, Y.-M., Tsai, C.-Y., & Yu, C.-L. (2022). Molecular Basis for Paradoxical Activities of Polymorphonuclear Neutrophils in Inflammation/Anti-Inflammation, Bactericide/Autoimmunity, Pro-Cancer/Anticancer, and Antiviral Infection/SARS-CoV-II-Induced Immunothrombotic Dysregulation. Biomedicines, 10(4), 773. https://doi.org/10.3390/biomedicines10040773






