Small Dense LDL: Scientific Background, Clinical Relevance, and Recent Evidence Still a Risk Even with ‘Normal’ LDL-C Levels
Abstract
:1. Introduction
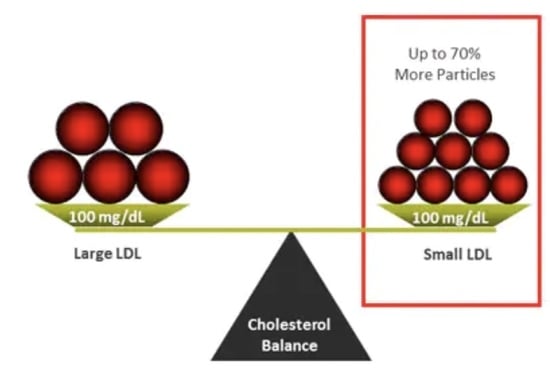
2. Clinical Relevance of LDL Heterogeneity
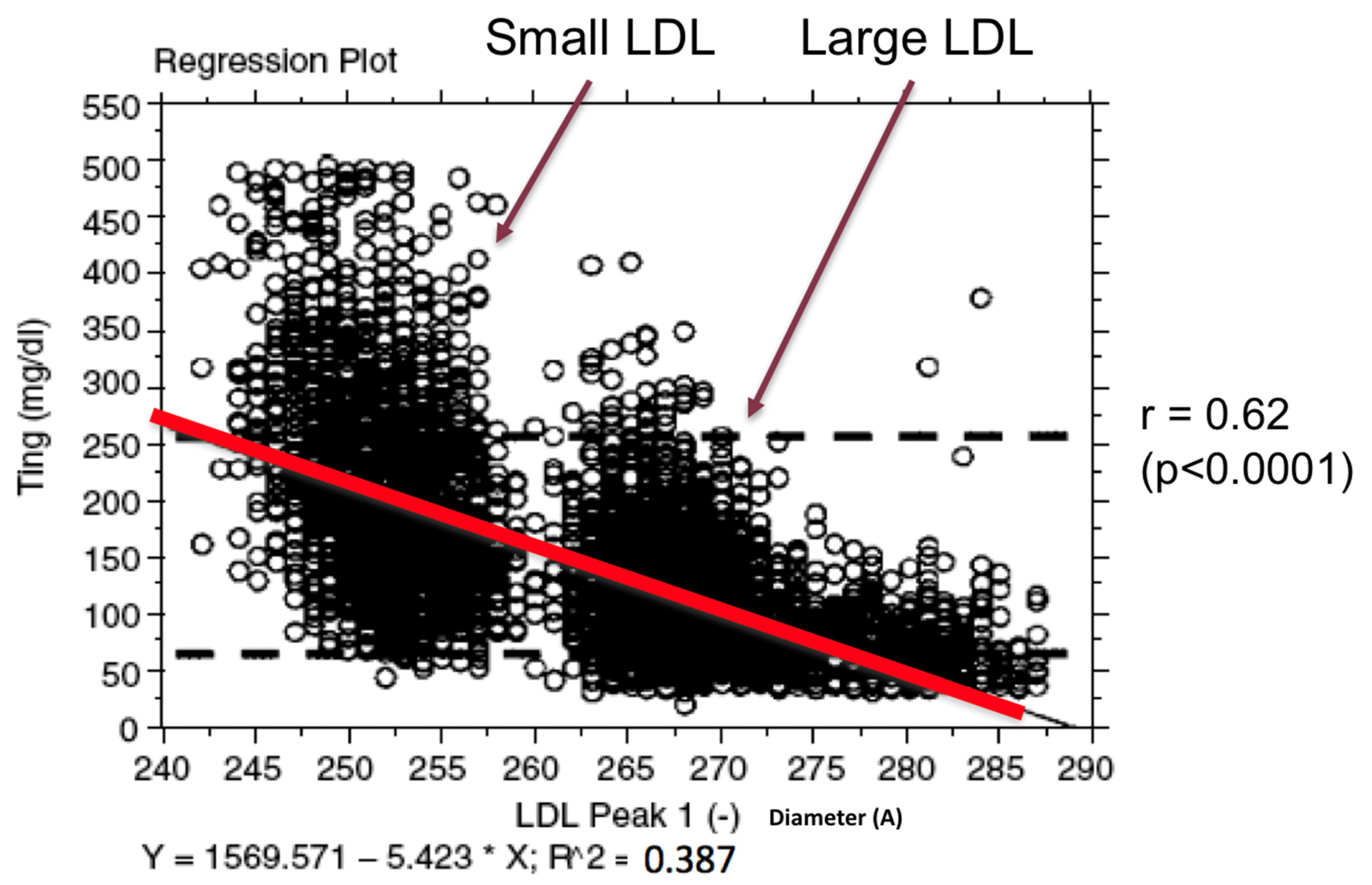
3. History of Lipoprotein Heterogeneity and Laboratory Methods
Indirect Approximation of LDL Heterogeneity
4. Evidence for LDL Heterogeneity Association with CHD Risk
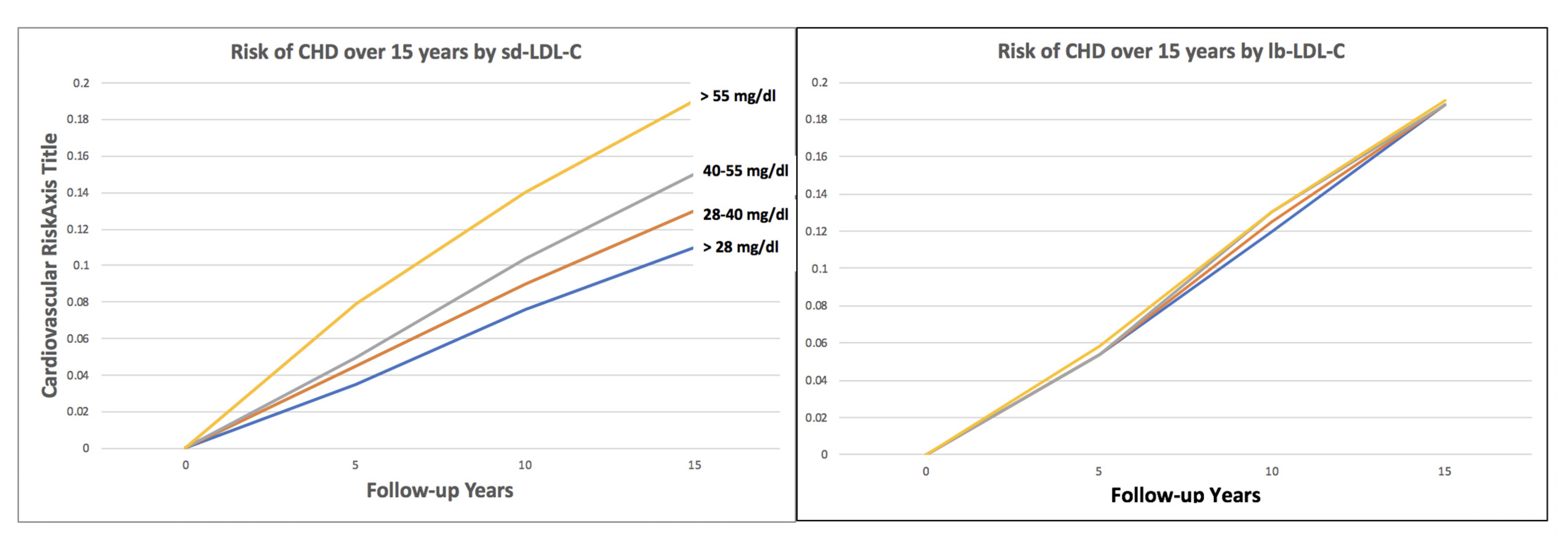
4.1. Small, Dense LDL-C Quantitative Level and CHD Risk
4.2. Independence of Small, Dense LDL-C as a Risk Predictor
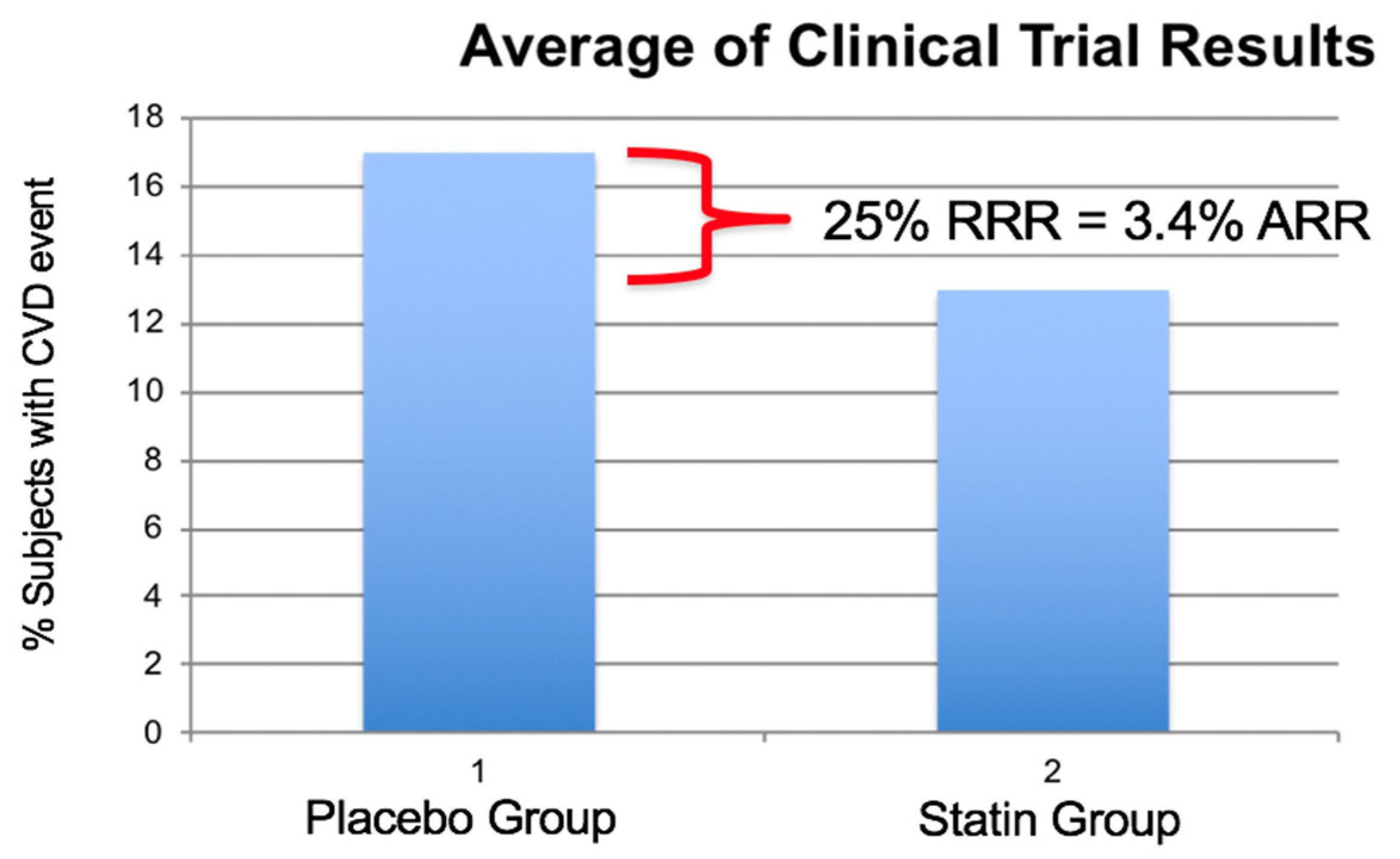
4.3. Statin Therapy and sdLDL-C CHD Risk
4.4. Small Dense LDL, Not Always a Significant Predictor of CVD Events
4.5. Non-Invasive Imaging and Small, Dense LDL
5. Evidence for Treatment Response
Lipid Medications and LDL Heterogeneity
6. Evidence for Clinical Outcome Response
Small Dense LDL and Interventional CV Outcomes
7. Guidelines and Small, Dense LDL
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Superko, H.R.; King, S., III. Lipid management to reduce cardiovascular risk: A new strategy is required. Circulation 2008, 117, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabatine, M.S.; Leiter, L.A.; Wiviott, S.D.; Giugliano, R.P.; Deedwania, P.; De Ferrari, G.M.; Murphy, S.A.; Kuder, J.F.; Gouni-Berthold, I.; Lewis, B.S.; et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: A prespecified analysis of the FOURIER randomized controlled trial. Lancet Diabetes Endocrinol. 2017, 12, 941–950. [Google Scholar] [CrossRef]
- Sachdeva, A.; Cannon, C.P.; Deedwania, P.C.; Labresh, K.A.; Smith, S.C., Jr.; Dai, D.; Hernandez, A.; Fonarow, G.C. Lipid levels in patients hospitalized with coronary artery disease: An analysis of 136,905 hospitalizations in Get with The Guidelines. Am. Heart J. 2009, 157, 111–117. [Google Scholar] [CrossRef]
- Berneis, K.K.; Krauss, R.M. Metabolic origins and clinical significance of LDL heterogeneity. J. Lipid Res. 2002, 43, 1363–1379. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.T.; Feldman, D.E. Prospective study of coronary heart disease vs. HDL2, HDL3, and other lipoproteins in Gofman’s Livermore cohort. Atherosclerosis 2011, 214, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, F.T.; Elliott, H.A.; Gofman, J.W. The ultracentrifugal characterization and isolation of human blood lipids and lipoproteins, with applications to the study of atherosclerosis. J. Phys. Colloid. Chem. 1951, 55, 80–93. [Google Scholar] [CrossRef]
- Krauss, R.M. All low-density lipoprotein particles are not created equal. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 959–961. [Google Scholar] [CrossRef] [Green Version]
- Gofman, J.W.; Young, W.; Tandy, R. Ischemic heart disease, atherosclerosis and longevity. Circulation 1966, 34, 679–697. [Google Scholar] [CrossRef] [Green Version]
- Nichols, A.V.; Krauss, R.M.; Musliner, T.A. Nondenaturing polyacrylamide gradient gel electrophoresis. Methods Enzymol. 1986, 128, 417–431. [Google Scholar]
- Chapman, M.J.; Goldstein, S.; Lagrange, D.; Laplaud, P.M. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J. Lipid Res. 1981, 22, 339–358. [Google Scholar] [CrossRef]
- Hirano, T.; Ito, Y.; Saegusa, H.; Yoshino, G. A novel and simple method for quantification of small, dense LDL. J. Lipid Res. 2003, 44, 2193–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otvos, J.D.; Jeyarajah, E.J.; Bennett, D.W. Quantification of plasma lipoproteins by proton nuclear magnetic resonance spectroscopy. Clin. Chem. 1991, 37, 377–386. [Google Scholar] [CrossRef]
- Williams, P.T.; Zhao, X.Q.; Marcovina, S.M.; Otvos, J.D.; Brown, B.G.; Krauss, R.M. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis 2014, 233, 713–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Superko, H.R. Advanced lipoprotein testing and subfractionation research Tool or Clinical Utility? Circulation 2009, 119, 2383–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virani, S.S.; Morris, P.B.; Agarwala, A.; Ballantyne, C.M.; Birtcher, K.K.; Kris-Etherton, P.M.; Ladden-Stirling, A.B.; Miller, M.; Orringer, C.E.; Stone, N.J. ACC Exert consensus decision pathway on the management of ASCVD risk reduction in patients with persistent hypertriglyceridemia. JACC 2021, 9, 960–993. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.; Wolska, A.; Warnick, R.; Lucero, D.; Remaley, A.T. A New Equation Based on the Standard lipid panel for calculating small dense low-density lipoprotein cholesterol and its use as a risk-enhancer test. Clin. Chem. 2021, 67, 987–997. [Google Scholar] [CrossRef]
- Austin, M.A.; Breslow, J.L.; Hennekens, C.H.; Buring, J.E.; Willett, W.C.; Krauss, R.M. Low density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 1988, 260, 1917–1921. [Google Scholar] [CrossRef]
- Lamarche, B.; Tchernof, A.; Moorjani, S.; Cantin, B.; Dagenais, G.R.; Lupien, P.J.; Despres, J.P. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men: Prospective results from the Quebec Cardiovascular Study. Circulation 1997, 95, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Gardner, C.D.; Fortmann, S.P.; Krauss, R.M. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA 1996, 276, 875–881. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Krauss, R.M.; Blanche, P.J.; Holl, L.G.; Sacks, F.M.; Hennekens, C.H. A prospective study of triglyceride level, low density lipoprotein particle diameter, and risk of myocardial infarction. JAMA 1996, 276, 882–888. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, A.C.; Cantin, B.; Dagenais, G.R.; Mauriège, P.; Bernard, P.M.; Després, J.P.; Lamarche, B. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Québec Cardiovascular Study. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 553–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, S.; Otvos, J.D.; Rifai, N.; Rosenson, R.S.; Buring, J.E.; Ridker, P.M. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009, 119, 931–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musunuru, K.; Orho-Melander, M.; Caulfield, M.P.; Li, S.; Salameh, W.A.; Reitz, R.E.; Berglund, G.; Hedblad, B.; Engström, G.; Williams, P.T.; et al. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler. Thromb. Vasc. Bio. L. 2009, 29, 1975–1980. [Google Scholar] [CrossRef] [Green Version]
- Hoogeveen, R.C.; Gaubatz, J.W.; Sun, W.; Dodge, R.C.; Crosby, J.R.; Jiang, J.; Couper, D.; Virani, S.S.; Kathiresan, S.; Boerwinkle, E.; et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.Y.; Steffen, B.T.; Guan, W.; McClelland, R.L.; Warnick, R.; McConnell, J.; Hoefner, D.M.; Remaley, A.T. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: The Multi-ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Superko, H.R. Nontraditional Markers of Cardiovascular Disease Risk Can Improve the 2013 American College of Cardiology/American Heart Association Prevention Guidelines: Insights from the Multi-Ethnic Study of Atherosclerosis Investigation. Circulation 2015, 132, 904–906. [Google Scholar] [CrossRef]
- Krauss, R.; Williams, P.; Brensike, J.; Detre, K.; Lindgren, F.; Kelsey, S.; Levy, R. Intermediate-density lipoproteins and progression of coronary artery disease in hypercholesterolaemic men. Lancet 1987, 2, 62–65. [Google Scholar] [CrossRef]
- Watts, G.F.; Lewis, B.; Lewis, E.S.; Coltart, D.J.; Smith, L.D.R.; Swan, A.V.; Mann, J.I. Effects on Coronary artery disease of lipid-lowering diet, or diet pluscholestyramine, in the St Thomas’ Atherosclerosis Regression Study (STARS). Lancet 1992, 339, 563–569. [Google Scholar] [CrossRef]
- Mack, W.J.; Krauss, R.M.; Hodis, N.H. Lipoprotein subclasses in the monitored atherosclerosis regression study (MARS). Arterioscler. Thromb. Vasc. Biol. 1996, 16, 697–704. [Google Scholar] [CrossRef]
- Miller, B.D.; Alderman, E.L.; Haskell, W.L.; Fair, J.M.; Krauss, R.M. Predominance of dense low-density lipoprotein particles predicts angiographic benefit of therapy in the Stanford Coronary Risk Intervention Project (SCRIP). Circulation 1996, 94, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Campos, H.; Moye, L.A.; Glasser, S.P.; Stampfer, M.J.; Sacks, F.M. Low-density lipoprotein size, pravastatin treatment and coronary events. JAMA 2001, 286, 1468–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackey, R.H.; Kuller, L.H.; Sutton-Tyrrell, K.; Evans, R.W.; Holubkov, R.; Matthews, K.A. Lipoprotein subclasses and coronary artery calcium in postmenopausal women from the healthy women study. Am. J. Cardiol. 2002, 90, 71i–76i. [Google Scholar] [CrossRef]
- Williams, P.T.; Superko, H.R.; Haskell, W.L.; Alderman, E.L.; Blanche, P.J.; Holl, L.G.; Krauss, R.M. Smallest LDL particles are most strongly related to coronary disease progression in men. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 314–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.Q.; Kosinski, A.J.S.; Barnhart, H.X.; Superko, H.R.; King, S.B. Prediction of native coronary artery disease progression following PTCA or CABG in the emory angioplasty verus surgery trial. Med. Sci. Monit. 2003, 9, 48–54. [Google Scholar]
- Miyazaki, T.; Shimada, K.; Miyauchi, K.; Kume, A.; Tanimoto, K.; Kiyanagi, T.; Sumiyoshi, K.; Hiki, M.; Mokuno, H.; Okazaki, S.; et al. Effects of fenofibrate on lipid profiles, cholesterol ester transfer activity, and in-stent intimal hyperplasia in patients after elective coronary stenting. Lipids Health Dis. 2010, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Mora, S.; Caulfield, M.P.; Wohlgemuth, J.; Chen, Z.; Superko, H.R.; Rowland, C.M.; Glynn, R.J.; Ridker, P.M.; Krauss, R.M. Atherogenic Lipoprotein Subfractions Determined by Ion Mobility and First Cardiovascular Events After Random Allocation to High-Intensity Statin or Placebo: The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial. Circulation 2015, 132, 2220–2229. [Google Scholar]
- Albers, J.J.; Slee, A.; Fleg, J.L.; O’Brien, K.D.; Marcovina, S.M. Relationship of baseline HDL subclasses, small dense LDL and LDL triglyceride to cardiovascular events in the AIM-HIGH clinical trial. Atherosclerosis 2016, 251, 454–459. [Google Scholar] [CrossRef] [Green Version]
- Shiffman, D.; Louiea, J.Z.; Michael, P.; Caulfield, M.P.; Nilssonb, P.M.; Devlin, J.J.; Olle Melander, O. LDL subfractions are associated with incident cardiovascular disease in the Malmo Prevention Project Study. Atherosclerosis 2017, 263, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Sakai, K.; Koba, S.; Nakamura, Y.; Yokota, Y.; Tsunoda, F.; Shoji, M.; Itoh, Y.; Hamazaki, Y.; Kobayashi, Y. Small dense low-density lipoprotein cholesterol is a promising biomarker for secondary prevention in older men with stable coronary artery disease. Geriatr. Gerontol. Int. 2018, 18, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Nishikura, T.; Koba, S.; Yokota, Y.; Hirano, T.; Tsunoda, F.; Shoji, M.; Hamazaki, Y.; Suzuki, H.; Itoh, Y.; Katagiri, T.; et al. Elevated small dense low-density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J. Atheroscler. Thromb. 2014, 21, 755–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, P.T.; Zhao, X.Q.; Marcovina, S.M.; Brown, B.G.; Krauss, R.M. Levels of cholesterol in small LDL particles predict atherosclerosis progression and incident CHD in the HDL-Atherosclerosis treatment study (HATS). PLoS ONE 2013, 8, e56782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, T.; Yagi, H.; Sumino, H.; Tsunekawa, K.; Araki, O.; Kimura, T.; Nara, M.; Ogiwara, T.; Nakajima, K.; Murakami, M. Relationship between carotid artery intima-media thickness and small dense low-density lipoprotein cholesterol concentrations measured by homogenous assay in Japanese subjects. Clin. Chim. Acta. 2015, 442, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Jug, B.; Papazian, J.; Lee, R.; Budoff, M.J. Association of lipoprotein subfractions and coronary artery calcium in patient at intermediate cardiovascular risk. Am. J. Cardiol. 2013, 111, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Wood, P.D.; Haskell, W.; Klein, H.; Lewis, S.; Stern, M.P.; Farquhar, J.W. The distribution of plasma lipoproteins in middle-aged male runners. Metabolism 1976, 25, 1249–1257. [Google Scholar] [CrossRef]
- Williams, P.T.; Krauss, R.M.; Stefanick, M.L.; Vranizan, K.M.; Wood, P.D. Effects of low-fat diet, calorie restriction, and running on lipoprotein subfraction concentrations in moderately overweight men. Metabolism 1994, 43, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Siri-Tarino, P.W.; Chiu, S.; Bergeron, N.; Krauss, R.M. Saturated fats versus polyunsaturated fats versus carbohydrates for cardiovascular disease prevention and treatment. Annu. Rev. Nutr. 2015, 35, 517–543. [Google Scholar] [CrossRef] [Green Version]
- Rundblad, A.; Holven, K.B.; Ottestad, I.; Myhrstad, M.C.; Ulven, S.M. High-quality fish oil has a more favorable effect than oxidized fish oil on intermediate-density lipoprotein and LDL subclasses: A randomized controlled trial. Br. J. Nutr. 2017, 117, 1291–1298. [Google Scholar] [CrossRef] [Green Version]
- Masuda, D.; Miyata, Y.; Matsui, S.; Yamashita, S. Omega-3 fatty acid ethyl esters improve low-density lipoprotein subclasses without increasing low-density lipoprotein-cholesterol levels: A phase 4, randomized study. Atherosclerosis 2020, 292, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Superko, H.R.; Krauss, R.M.; Haskell, W.L. Stanford Coronary Risk Intervention Project Investigators. Association of lipoprotein subclass distribution with use of selective and non-selective beta-blocker medications in patients with coronary heart disease. Atherosclerosis 1993, 101, 1–8. [Google Scholar] [CrossRef]
- Superko, H.R.; Wood, P.D.; Krauss, R.M. Effect of alpha- and selective beta-blockade for hypertension control on plasma lipoproteins, apoproteins, lipoprotein subclasses, and postprandial lipemia. Am. J. Med. 1989, 86 (Suppl. 1B), 26–31. [Google Scholar] [CrossRef]
- Superko, H.R.; McGovern, M.E.; Raul, E.; Garrett, B. Nicotinic Acid has a Differential Effect on Low Density Lipoprotein Subclass Distribution in Patients Classified as LDL pattern A., B, or I. Am. J. Cardiol. 2004, 94, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Superko, H.R.; Zhao, X.Q.; Hodis, H.N.; Guyton, J.R. Niacin and Heart Disease Prevention: Engraving its Tombstone is a Mistake. J. Clin. Lipidol. 2017, 11, 1309–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Superko, H.R.; Garrett, B.C.; King, S.B., III; Momary, K.M.; Chronos, N.A.; Wood, P.D. Effect of combination nicotinic acid and gemfibrozil treatment on IDL and subclasses of LDL and HDL in patients with combined hyperlipidemia. Am. J. Cardiol. 2009, 103, 387–392. [Google Scholar] [CrossRef]
- Zambon, A.; Hokanson, J.E.; Brown, B.G.; Brunzell, J.D. Evidence for a new pathophysiological mechanism for coronary artery disease regression: Hepatic lipase-mediated changes in LDL density. Circulation 1999, 99, 1959–1964. [Google Scholar] [CrossRef] [Green Version]
- Watts, G.F.; Mandalia, S.; Brunt, J.N.H.; Slavin, B.M.; Coltart, D.J.; Lewis, B. Independent associations between plasma lipoprotein subfraction levels and the course of coronary artery disease in the St. Thomas’ Atherosclerosis Regression Study (STARS). Metabolism 1993, 42, 1461–1467. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Otvos, J.D.; Freedman, D.S. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am. J. Cardiol. 2002, 90, 89–94. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, M.H.; Lee, B.K.; Rim, S.J.; Min, P.K.; Yoon, S.J.; Kim, J.H.; Rhee, J.H.; Yoon, Y.W.; Hong, B.K.; et al. Effects of increasing particle size of low-density lipoprotein on restenosis after coronary stent implantation. Circ. J. 2008, 72, 1059–1064. [Google Scholar] [CrossRef] [Green Version]
- Tsujita, K.; Yamanaga, K.; Komura, N.; Sakamoto, K.; Sugiyama, S.; Sumida, H.; Shimomura, H.; Yamashita, T.; Oka, H.; Nakao, K.; et al. PRECISE-IVUS Investigators. Synergistic effect of ezetimibe addition on coronary atheroma regression in patients with prior statin therapy: Subanalysis of PRECISE-IVUS trial. Eur. J. Prev Cardiol. 2016, 23, 1524–1528. [Google Scholar] [CrossRef]
- Davidson, M.H.; Ballantyne, C.M.; Jacobson, T.A.; Bittner, V.A.; Braun, L.T.; Brown, A.S.; Brown, W.V.; Cromwell, W.C.; Goldberg, R.B.; McKenney, J.M.; et al. Clinical utility of inflammatory markers and advanced lipoprotein testing: Advice from an expert panel of lipid specialists. J. Clin. Lipidol. 2011, 5, 338–367. [Google Scholar]
- Mikhailidis, D.P.; Elisaf, M.; Rizzo, M.; Berneis, K.; Griffin, B.; Zambon, A.; Liberopoulos, E. European panel on low density lipoprotein (LDL) subclasses: A statement on the pathophysiology, atherogenicity and clinical significance of LDL subclasses. Curr. Vasc. Pharmacol. 2011, 9, 531–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashioka, M.; Sakata, S.; Honda, T.; Hata, J.; Yoshida, D.; Hirakawa, Y.; Shibata, M.; Goto, K.; Kitazono, T.; Osawa, H.; et al. Small Dense Low-Density Lipoprotein Cholesterol and the Risk of Coronary Heart Disease in a Japanese Community. J. Atheroscler. Thromb. 2020, 27, 669–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balling, M.; Nordestgaard, B.G.; Langsted, A.; Varbo, A.; Kamstrup, P.R.; Afzal, S. Small dense low-density lipoprotein predicts atherosclerotic cardiovascular the Copenhagen General population. JACC 2020, 75, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
| Study | Year | Findings |
|---|---|---|
| Framingham [9] | 1966 | Trig rich Sf20-400 lipoproteins associated with CAD risk |
| Lawrence Livermore [6] | 1966 | Trig rich Sf20-100 lipoproteins associated with CAD risk |
| NHLBI-II [28] | 1987 | IDL linked to arteriographic progression of CAD |
| Boston Area Heart [18] | 1988 | LDL pattern B associated with 3-fold increased CAD risk |
| STARS [29] | 1992 | Dense (small) LDL best predictor of arteriographic outcome |
| Physician’s Health survey [21] | 1996 | LDL pattern B associated with 3.4-fold increased CAD risk independent of total and HDL cholesterol and apo B |
| Stanford 5 City Project [20] | 1996 | LDL size best predictor of CAD risk by conditional logistic regression |
| MARS [30] | 1996 | In statin treated subjects with LDL-C < 85 mg/dL, triglyceride-rich lipoproteins were correlated with disease progression |
| SCRIP [31] | 1996 | Dense LDL predicts arteriographic benefit in the Stanford Coronary Risk Intervention project. |
| Quebec CV Study [19] | 1997 | Small LDL related to CHD risk. |
| Statistical adjustment for LDL-C, triglycerides, HDL-C, and apoB had virtually no impact on the relationship of small LDL and CHD risk. | ||
| CARE [32] | 2001 | Large LDL size was an independent predictor of CHD events. Identifying patients on the basis of LDL size may not be useful clinically since pravastatin effectively treats risk associated with large LDL. |
| Healthy Women Study [33] | 2002 | Small low-density lipoprotein (LDL) was positively associated with coronary artery calcium (p < 0.01), but medium and large LDL were not. |
| SCRIP [34] | 2003 | Small low-density lipoprotein III but not low-density lipoprotein cholesterol is related to arteriographic progression |
| EAST [35] | 2003 | Arteriographic CAD progression over three years was significantly and independently linked to small, dense LDL particles. |
| Healthy Women Study [23] | 2009 | CVD risk prediction associated with lipoprotein profiles evaluated by NMR was comparable but not superior to that of standard lipids or apolipoproteins |
| HATS [36] | 2014 | Four laboratory methodologies confirm the association of small, dense LDL with greater coronary atherosclerosis progression and the associations were independent of standard lipid measurements. |
| ARIC [25] | 2014 | sdLDL-C was associated with future CHD events even in individuals considered at low CVD risk based on their LDL-C level. |
| MESA [26] | 2014 | sdLDL-C significantly associated with CHD risk even in subjects with LDL-C < 100 mg/dL who were normoglycemic |
| JUPITERr [37] | 2015 | Baseline LDL-C was not associated with CVD events, in contrast with significant associations for non-HDL-C and atherogenic particles including select subfractions of LDL particles. |
| AIM-HIGH [38] | 2016 | Levels of HDL3-C, but not HDL-C, HDL2-C, sdLDL, or LDL-TG, predict CV events in patients with metabolic dyslipidemia. |
| Malmo Heart [39] | 2017 | Smaller LDL particles are associated with incident CVD independently of traditional risk-factors, including standard lipids |
| Sakai [40] | 2018 | sdLDL-C was the most effective predictor of residual risk of future CHD events in stable older male CAD patients using statins and was independent of LDL-C |
| Copenhagen Heart Study [30] | 2020 | Individuals with high sdLDL-C had higher MI and ASCVD risk in 38,322 subjects. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Superko, H.; Garrett, B. Small Dense LDL: Scientific Background, Clinical Relevance, and Recent Evidence Still a Risk Even with ‘Normal’ LDL-C Levels. Biomedicines 2022, 10, 829. https://doi.org/10.3390/biomedicines10040829
Superko H, Garrett B. Small Dense LDL: Scientific Background, Clinical Relevance, and Recent Evidence Still a Risk Even with ‘Normal’ LDL-C Levels. Biomedicines. 2022; 10(4):829. https://doi.org/10.3390/biomedicines10040829
Chicago/Turabian StyleSuperko, Harold, and Brenda Garrett. 2022. "Small Dense LDL: Scientific Background, Clinical Relevance, and Recent Evidence Still a Risk Even with ‘Normal’ LDL-C Levels" Biomedicines 10, no. 4: 829. https://doi.org/10.3390/biomedicines10040829