Effects of Reversal of Hypotension on Cerebral Microcirculation and Metabolism in Experimental Sepsis
Abstract
:1. Introduction
2. Methods
2.1. Experimental Animals
2.2. Surgical Procedures
2.3. Monitoring and Measurements
2.4. Cerebral Microcirculation
2.5. Cerebral Oxygenation and Metabolism
2.6. Experimental Protocol
2.7. Statistical Analysis
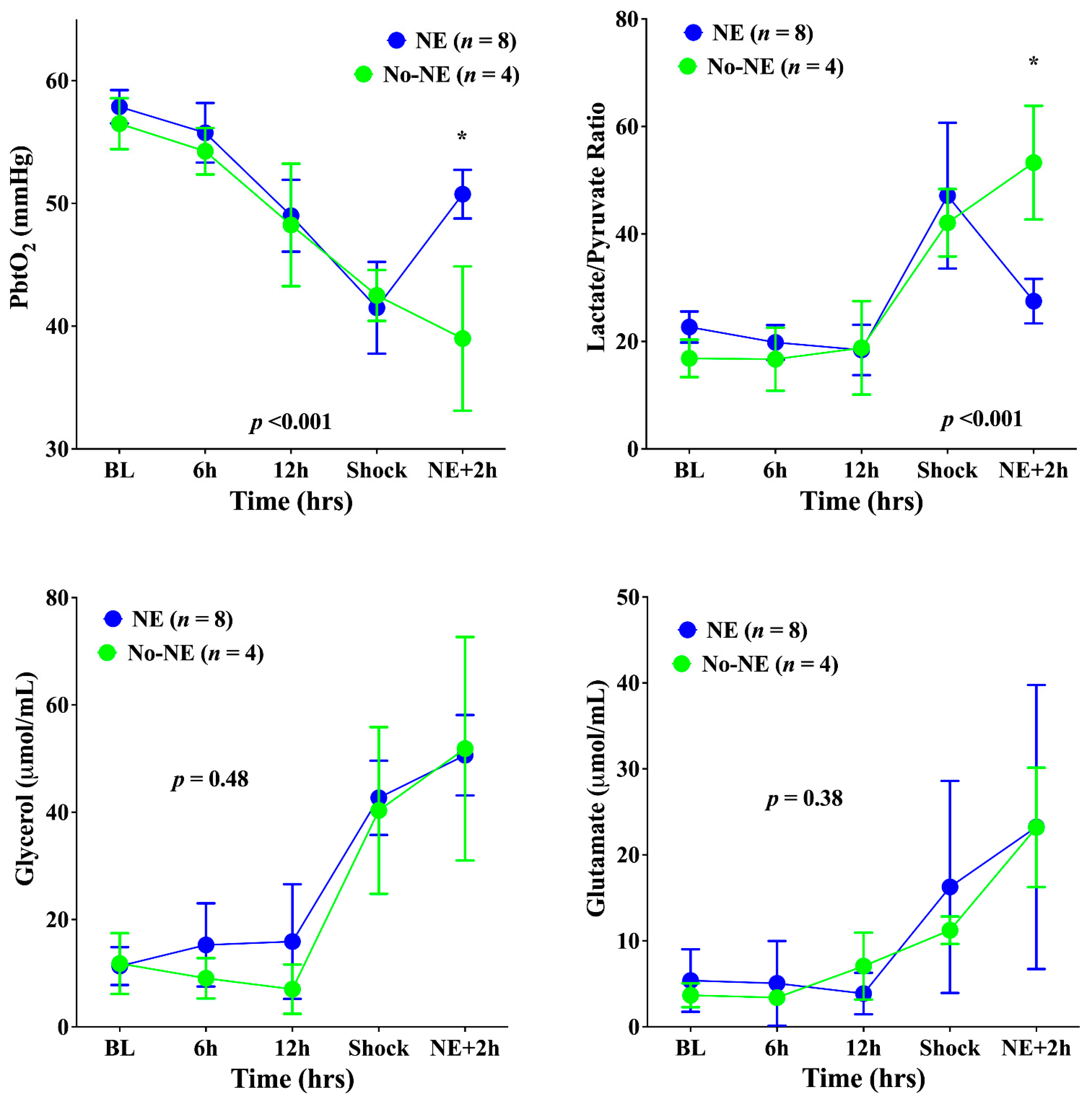
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farrah, K.; McIntyre, L.; Doig, C.J.; Talarico, R.; Taljaard, M.; Krahn, M.; Fergusson, D.; Forster, A.J.; Coyle, D.; Thavorn, K. Sepsis-associated mortality, resource use, and healthcare costs: A propensity-matched cohort study. Crit. Care Med. 2021, 49, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Guirgis, F.W.; Brakenridge, S.; Sutchu, S.; Khadpe, J.D.; Robinson, T.; Westenbarger, R.; Topp, S.T.; Kalynych, C.J.; Reynolds, J.; Dodani, S.; et al. The long-term burden of severe sepsis and septic shock: Sepsis recidivism and organ dysfunction. J. Trauma Acute Care Surg. 2016, 81, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J.; Cooke, C.R.; Wunsch, H.; Kahn, J.M. Population Burden of Long-Term Survivorship After Severe Sepsis in Older Americans. J. Am. Geriatr. Soc. 2012, 60, 1070–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sair, M.; Etherington, P.J.; Winlove, C.P.; Evans, T.W. Tissue oxygenation and perfusion in patients with systemic sepsis. Crit. Care Med. 2001, 29, 1343–1349. [Google Scholar] [CrossRef]
- Miranda, M.; Balarini, M.; Caixeta, D.; Bouskela, E. Microcirculatory dysfunction in sepsis: Pathophysiology, clinical monitoring, and potential therapies. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H24–H35. [Google Scholar] [CrossRef] [Green Version]
- De Backer, D.; Ospina-Tascon, G.; Salgado, D.; Favory, R.; Creteur, J.; Vincent, J.L. Monitoring the microcirculation in the critically ill patient: Current methods and future approaches. Intensive Care Med. 2010, 36, 1813–1825. [Google Scholar] [CrossRef]
- Crippa, I.A.; Taccone, F.S.; Wittebole, X.; Martin-Loeches, I.; Schroeder, M.E.; François, B.; Kotfis, K.; Ñamendys-Silva, S.A.; Forceville, X.; Solé-Violán, J.; et al. The prognostic value of brain dys-function in critically ill patients with and without sepsis: A post hoc analysis of the ICON Audit. Brain Sci. 2021, 11, 530. [Google Scholar] [CrossRef]
- Robba, C.; Crippa, I.A.; Taccone, F.S. Septic Encephalopathy. Curr. Neurol. Neurosci. Rep. 2018, 18, 82. [Google Scholar] [CrossRef]
- Bowton, D.L.; Bertels, N.H.; Prough, D.S.; Stump, D.A. Cerebral blood flow is reduced in patients with sepsis syndrome. Crit. Care Med. 1989, 17, 399–403. [Google Scholar] [CrossRef]
- Crippa, I.A.; Subirà, C.; Vincent, J.L.; Fernandez, R.F.; Hernandez, S.C.; Cavicchi, F.Z.; Creteur, J.; Taccone, F.S. Impaired cerebral autoregulation is asso-ciated with brain dysfunction in patients with sepsis. Crit. Care 2018, 22, 327. [Google Scholar] [CrossRef] [Green Version]
- Al Tayar, A.; Abouelela, A.; Mohiuddeen, K. Can the cerebral regional oxygen saturation be a perfusion parameter in shock? J. Crit. Care 2017, 38, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Su, F.; Pierrakos, C.; He, X.; James, S.; Dewitte, O.; Vincent, J.-L.; de Backer, D. Cerebral microcirculation is impaired during sepsis: An experimental study. Crit. Care 2010, 14, R140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosengarten, B.; Hecht, M.; Auch, D.; Ghofrani, H.A.; Schermuly, R.T.; Grimminger, F.; Kaps, M. Microcirculatory Dysfunction in the Brain Precedes Changes in Evoked Potentials in Endotoxin-Induced Sepsis Syndrome in Rats. Cerebrovasc. Dis. 2007, 23, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Comim, C.M.; Vilela, M.C.; Constantino, L.S.; Petronilho, F.; Vuolo, F.; Lacerda-Queiroz, N.; Rodrigues, D.H.; Da Rocha, J.L.; Teixeira, A.L.; de Quevedo, J.L.; et al. Traffic of leukocytes and cytokine up-regulation in the central nervous system in sepsis. Intensive Care Med. 2011, 37, 711–718. [Google Scholar] [CrossRef]
- Taccone, F.S.; Su, F.; de Deyne, C.; Abdellhai, A.; Pierrakos, C.; He, X.; Donadello, K.; Dewitte, O.; Vincent, J.-L.; de Backer, D. Sepsis Is Associated With Altered Cerebral Microcirculation and Tissue Hypoxia in Experimental Peritonitis. Crit. Care Med. 2014, 42, e114–e122. [Google Scholar] [CrossRef]
- Ince, C.; Boerma, E.C.; Cecconi, M.; de Backer, D.; Shapiro, N.I.; Duranteau, J.; Pinsky, M.R.; Artigas, A.; Teboul, J.-L.; Reiss, I.K.M.; et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: Results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2018, 44, 281–299. [Google Scholar] [CrossRef] [Green Version]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Johnston, A.J.; Steiner, L.A.; Chatfield, D.A.; Coles, J.P.; Hutchinson, P.J.; Al-Rawi, P.G.; Menon, D.K.; Gupta, A.K. Effect of cerebral perfusion pressure augmentation with dopamine and norepinephrine on global and focal brain oxygenation after traumatic brain injury. Intensive Care Med. 2004, 30, 791–797. [Google Scholar] [CrossRef]
- Thorup, L.; Koch, K.U.; Upton, R.N.; Østergaard, L.; Rasmussen, M. Effects of Vasopressors on Cerebral Circulation and Oxygenation: A Narrative Review of Pharmacodynamics in Health and Traumatic Brain Injury. J. Neurosurg. Anesthesiol. 2020, 32, 18–28. [Google Scholar] [CrossRef]
- Ferlini, L.; Su, F.; Creteur, J.; Taccone, F.S.; Gaspard, N. Cerebral autoregulation and neurovascular coupling are progressively impaired during septic shock: An experimental study. Intensive Care Med. Exp. 2020, 8, 44. [Google Scholar] [CrossRef]
- De Santis, P.; de Fazio, C.; Franchi, F.; Bond, O.; Vincent, J.-L.; Creteur, J.; Taccone, F.S.; Scolletta, S. Incoherence between Systemic Hemodynamic and Microcirculatory Response to Fluid Challenge in Critically Ill Patients. J. Clin. Med. 2021, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Rosengarten, B.; Wolff, S.; Klatt, S.; Schermuly, R.T. Effects of inducible nitric oxide synthase inhibition or norepinephrine on the neurovascular coupling in an endotoxic rat shock model. Crit. Care 2009, 13, R139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okazaki, N.; Lankadeva, Y.R.; Peiris, R.M.; Birchall, I.E.; May, C.N. Rapid and persistent decrease in brain tissue oxygenation in ovine gram-negative sepsis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R990–R996. [Google Scholar] [CrossRef] [PubMed]
- Tasdemiroglu, E.; Macfarlane, R.; Wei, E.P.; Kontos, H.A.; Moskowitz, M.A. Pial vessel caliber and cerebral blood flow become dissociated during ischemia-reperfusion in cats. Am. J. Physiol. 1992, 263 Pt 2, H533–H536. [Google Scholar] [CrossRef]
- Kurtz, P.; D’Avila, J.C.; Prado, D.; Madeira, C.; Vargas-Lopes, C.; Panizzutti, R.; Azevedo, L.C.P.; Bozza, F.A. Cerebral Multimodal Monitoring in Sepsis: An Experimental Study. Shock 2019, 51, 228–234. [Google Scholar] [CrossRef]
- Nyberg, A.; Gremo, E.; Blixt, J.; Sperber, J.; Larsson, A.; Lipcsey, M.; Pikwer, A.; Castegren, M. Lung-protective ventilation increases cerebral metabolism and non-inflammatory brain injury in porcine experimental sepsis. BMC Neurosci. 2021, 22, 31. [Google Scholar] [CrossRef]
- Ditz, C.; Klaus, S.; Bahlmann, L.; Onken, N.; Keck, A.; Gliemroth, J. Microdialysis as a Part of Invasive Cerebral Monitoring During Porcine Septic Shock. J. Neurosurg. Anesthesiol. 2016, 28, 323–330. [Google Scholar] [CrossRef]
- He, X.; Su, F.; Xie, K.; Taccone, F.S.; Donadello, K.; Vincent, J.-L. Should Hyperoxia Be Avoided During Sepsis? An Experimental Study in Ovine Peritonitis. Crit. Care Med. 2017, 45, e1060–e1067. [Google Scholar] [CrossRef]
- Bernini, A.; Masoodi, M.; Solari, D.; Miroz, J.-P.; Carteron, L.; Christinat, N.; Morelli, P.; Beaumont, M.; Abed-Maillard, S.; Hartweg, M.; et al. Modulation of cerebral ketone metabolism following traumatic brain injury in humans. J. Cereb. Blood Flow Metab. 2020, 40, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Carteron, L.; Solari, D.; Patet, C.; Quintard, H.; Miroz, J.-P.; Bloch, J.; Daniel, R.T.; Hirt, L.; Eckert, P.; Magistretti, P.J.; et al. Hypertonic Lactate to Improve Cerebral Perfusion and Glucose Availability After Acute Brain Injury. Crit. Care Med. 2018, 46, 1649–1655. [Google Scholar] [CrossRef]
- O’Donnell, J.; Zeppenfeld, D.; McConnell, E.; Pena, S.; Nedergaard, M. Norepinephrine: A Neuromodulator That Boosts the Function of Multiple Cell Types to Optimize CNS Performance. Neurochem. Res. 2012, 37, 2496–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandi, M.; Jackson, S.K.; Macrae, D.; Shankar-Hari, M.; Tremoleda, J.L.; Lilley, E. Rethinking animal models of sepsis—Working towards improved clinical translation whilst integrating the 3Rs. Clin. Sci. 2020, 134, 1715–1734. [Google Scholar] [CrossRef] [PubMed]
- Buras, J.A.; Holzmann, B.; Sitkovsky, M. Animal Models of sepsis: Setting the stage. Nat. Rev. Drug Discov. 2005, 4, 854–865. [Google Scholar] [CrossRef] [PubMed]


| Baseline | 6 h | 12 h | Shock | NE + 2 h | p Value | ||
|---|---|---|---|---|---|---|---|
| Temperature, °C | NE | 39.6 ± 0.5 | 40.1 ± 0.4 | 40.6 ± 0.6 | 41.3 ± 0.6 | 41.1 ± 0.6 | 0.75 |
| No-NE | 39.7 ± 0.3 | 40.2 ± 0.2 | 40.8 ± 0.6 | 41.8 ± 0.2 | 41.3 ± 0.7 | ||
| HR, beats/min | NE | 113 ± 14 | 144 ± 21 | 147 ± 19 | 145 ± 25 | 151 ± 21 | 0.47 |
| No-NE | 120 ± 8 | 156 ± 29 | 154 ± 19 | 141 ± 6 | 140 ± 11 | ||
| MPAP, mmHg | NE | 15 ± 2 | 13 ± 3 | 16 ± 3 | 18 ± 5 | 17 ± 6 | 0.39 |
| No-NE | 14 ± 3 | 15 ± 2 | 15 ± 6 | 20 ± 4 | 18 ± 4 | ||
| PAOP, mmHg | NE | 4 ± 1 | 3 ± 2 | 4 ± 1 | 5 ± 2 | 5 ± 1 | 0.82 |
| No-NE | 4 ± 1 | 3 ± 1 | 4 ± 1 | 5 ± 2 | 5 ± 1 | ||
| PaCO2, mmHg | NE | 39 ± 3 | 38 ± 1 | 36 ± 3 | 42 ± 7 | 43 ± 8 | 0.66 |
| No-NE | 41 ± 5 | 36 ± 3 | 37 ± 1 | 40 ± 8 | 41 ± 11 | ||
| PaO2, mmHg | NE | 133 ± 9 | 129 ±9 | 119 ± 14 | 110 ± 6 | 106 ± 11 | 0.11 |
| No-NE | 117 ± 14 | 117 ± 5 | 112 ± 18 | 111 ± 4 | 109 ± 9 | ||
| pH | NE | 7.41 ± 0.04 | 7.38 ± 0.03 | 7.36 ± 0.03 | 7.25 ± 0.08 | 7.26 ± 0.08 | 0.52 |
| No-NE | 7.37 ± 0.06 | 7.37 ± 0.06 | 7.36 ± 0.04 | 7.25 ± 0.10 | 7.28 ± 0.13 | ||
| TPC, mL/mmHg | NE | 19 ± 4 | 17 ± 2 | 16 ± 4 | 12 ± 2 | 14 ± 2 | 0.69 |
| No-NE | 21 ± 4 | 17 ± 3 | 16 ± 5 | 13 ± 3 | 15 ± 5 | ||
| Hemoglobin, g/dL | NE | 9.8 ± 1.4 | 11.6 ± 1.1 | 12.5 ± 1.0 | 13.2 ± 1.8 | 12.7 ± 2.0 | 0.17 |
| No-NE | 10.5 ± 0.7 | 11.2 ± 1.2 | 11.1 ± 1.2 | 11.9 ± 2.7 | 12.1 ± 2.9 | ||
| Urine Output, mL | NE | 89 ± 66 | 319 ± 98 | 689 ± 89 | 798 ± 129 | 804 ± 128 | 0.49 |
| No-NE | 105 ± 98 | 389 ± 112 | 703 ± 109 | 811 ± 133 | 813 ± 135 | ||
| Fluid Amount, mL | NE | 317 ± 99 | 1423 ± 616 | 1840 ± 622 | 2310 ± 777 | 2490 ± 971 | 0.61 |
| No-NE | 299 ± 111 | 1530 ± 866 | 1980 ± 855 | 2198 ± 1012 | 2318 ± 999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taccone, F.S.; Su, F.; He, X.; Peluso, L.; Donadello, K.; Scolletta, S.; De Backer, D.; Vincent, J.-L. Effects of Reversal of Hypotension on Cerebral Microcirculation and Metabolism in Experimental Sepsis. Biomedicines 2022, 10, 923. https://doi.org/10.3390/biomedicines10040923
Taccone FS, Su F, He X, Peluso L, Donadello K, Scolletta S, De Backer D, Vincent J-L. Effects of Reversal of Hypotension on Cerebral Microcirculation and Metabolism in Experimental Sepsis. Biomedicines. 2022; 10(4):923. https://doi.org/10.3390/biomedicines10040923
Chicago/Turabian StyleTaccone, Fabio Silvio, Fuhong Su, Xinrong He, Lorenzo Peluso, Katia Donadello, Sabino Scolletta, Daniel De Backer, and Jean-Louis Vincent. 2022. "Effects of Reversal of Hypotension on Cerebral Microcirculation and Metabolism in Experimental Sepsis" Biomedicines 10, no. 4: 923. https://doi.org/10.3390/biomedicines10040923
APA StyleTaccone, F. S., Su, F., He, X., Peluso, L., Donadello, K., Scolletta, S., De Backer, D., & Vincent, J.-L. (2022). Effects of Reversal of Hypotension on Cerebral Microcirculation and Metabolism in Experimental Sepsis. Biomedicines, 10(4), 923. https://doi.org/10.3390/biomedicines10040923








