Extremely Low-Frequency Electromagnetic Fields Increase Cytokines in Human Hair Follicles through Wnt/β-Catenin Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primary Culture of DP and ORS Cells
2.2. EMF Exposure
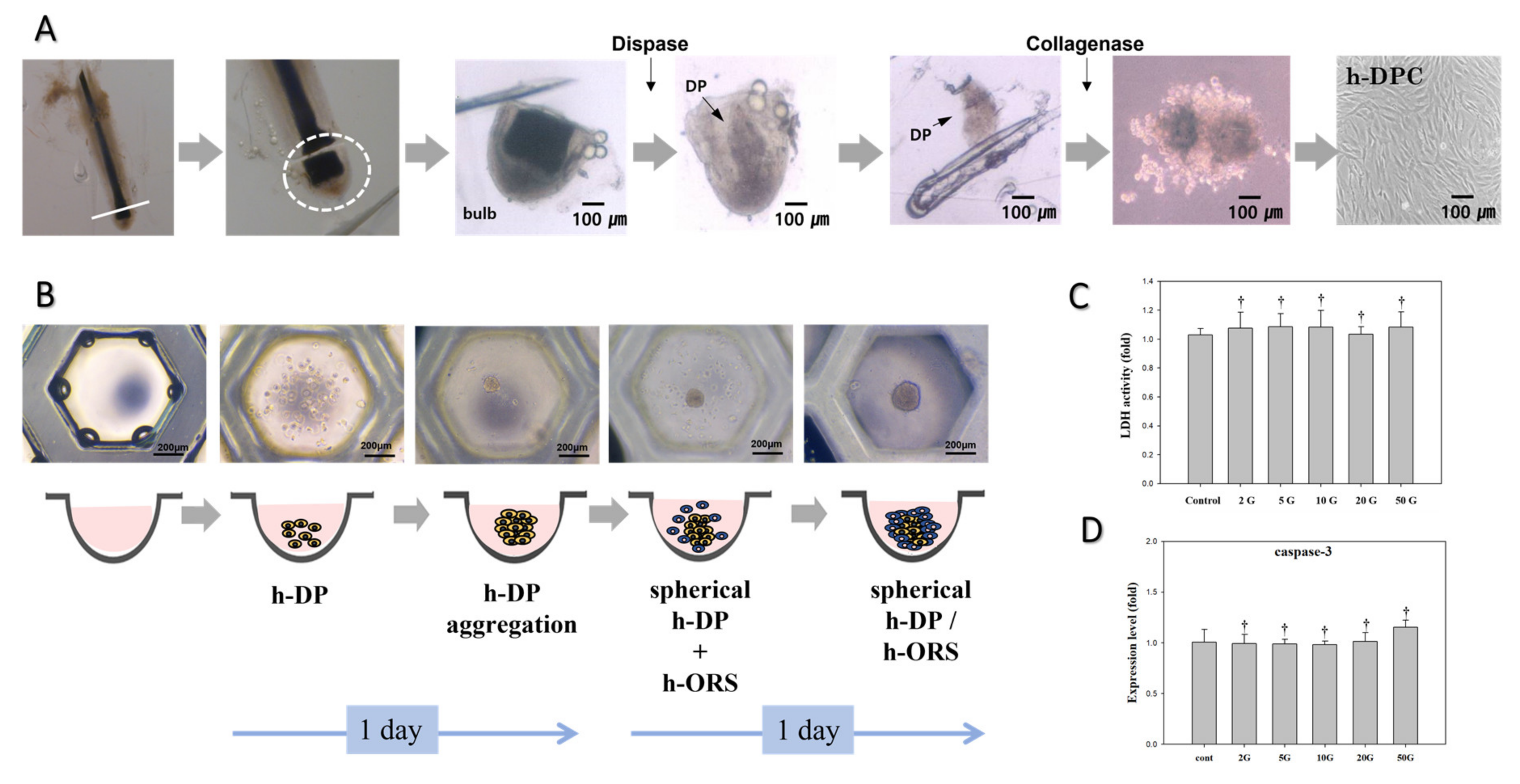
2.3. Hair Bulb Spheroid Culture
2.4. Human Hair Follicle Organ Cultures
2.5. Lactate Dehydrogenase Activity Assay
2.6. Quantitative Real-Time PCR
2.7. Western Blotting
2.8. Immunohistochemistry and Imaging Analysis
2.9. Statistical Analysis
3. Results
3.1. Evaluation of EMF Cytotoxicity
3.2. Analysis of HBS Activity after EMF Treatment
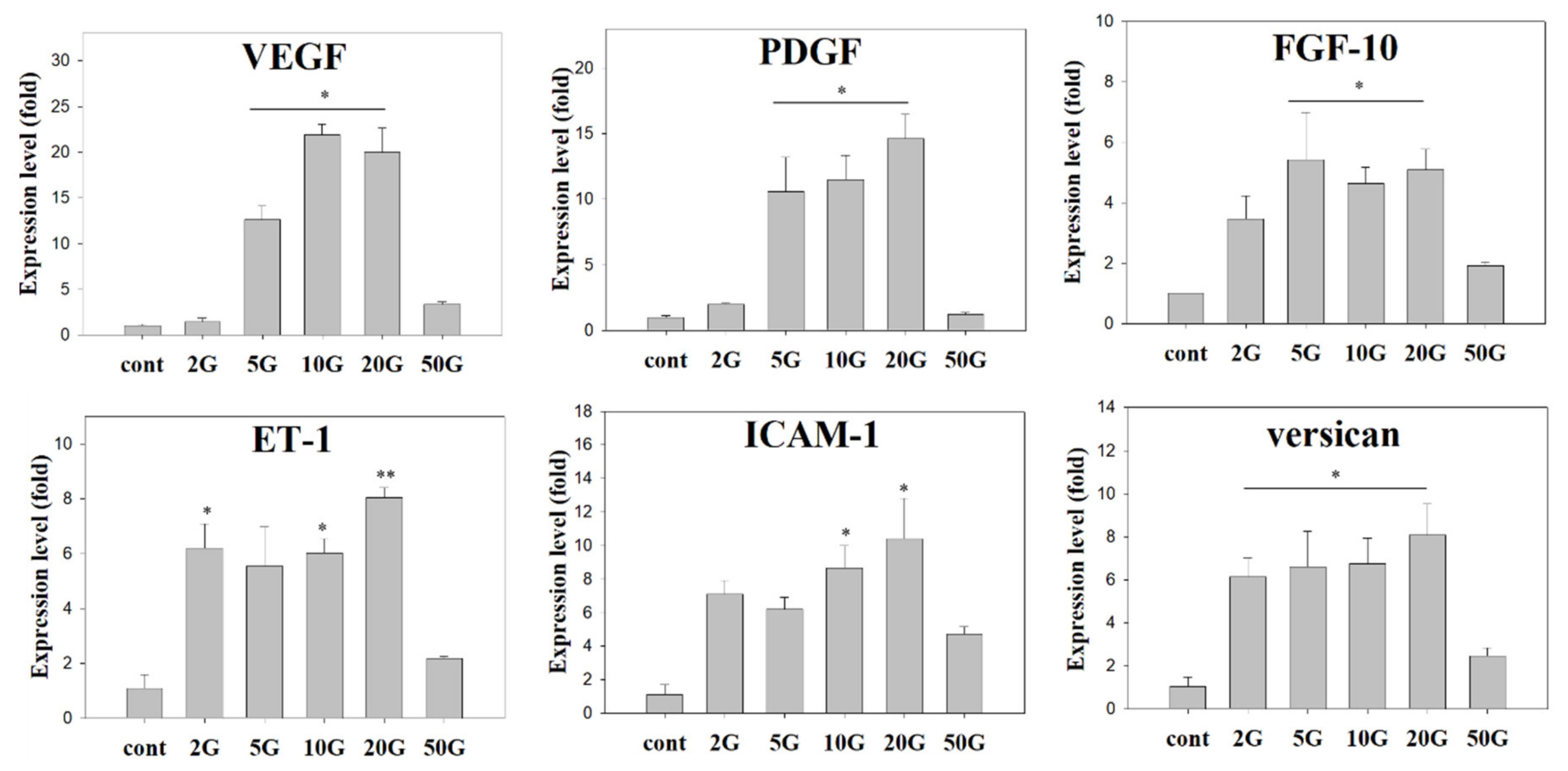
3.3. Evaluation of EMF Efficiency for Hair Growth and Activity
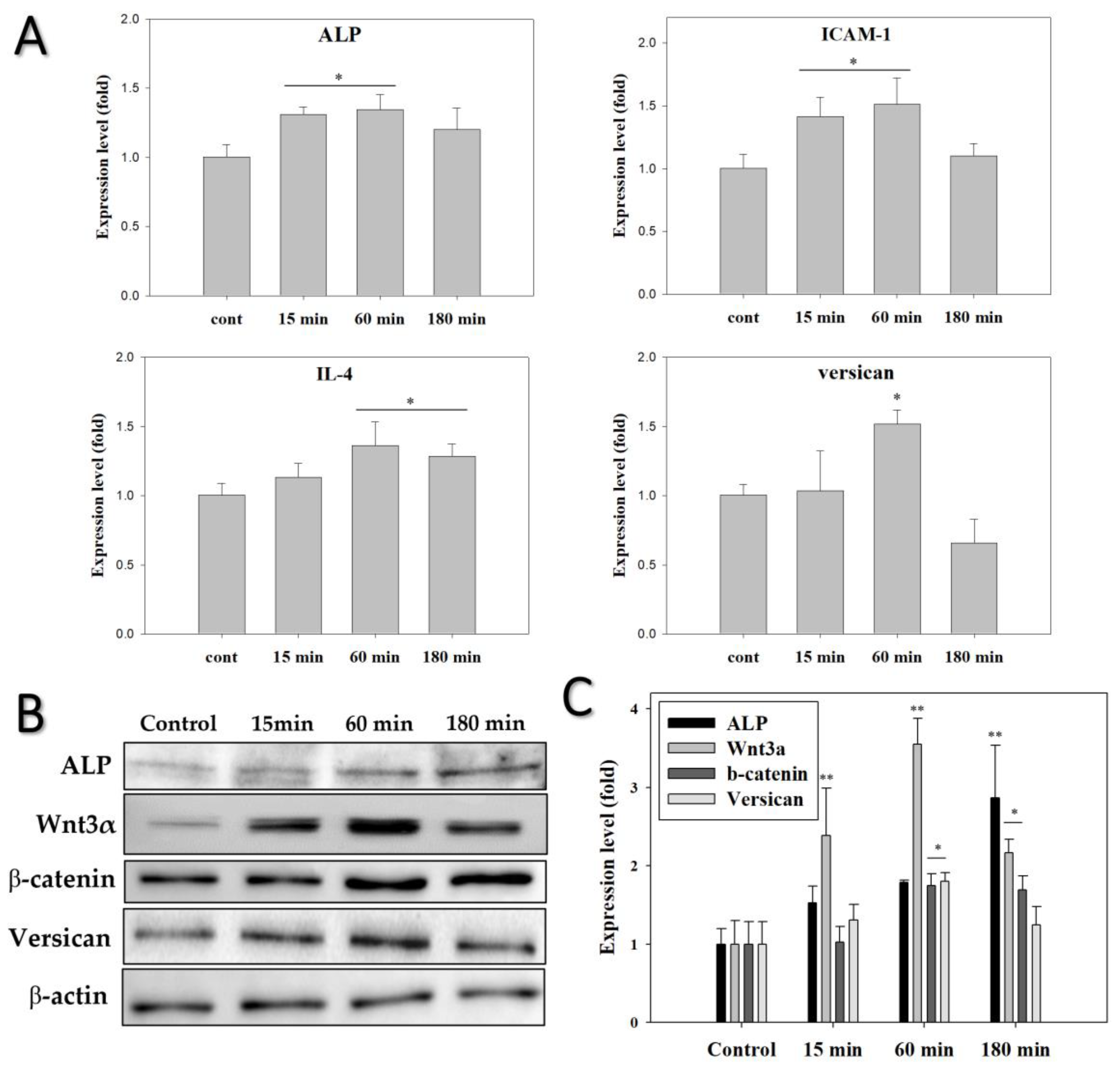
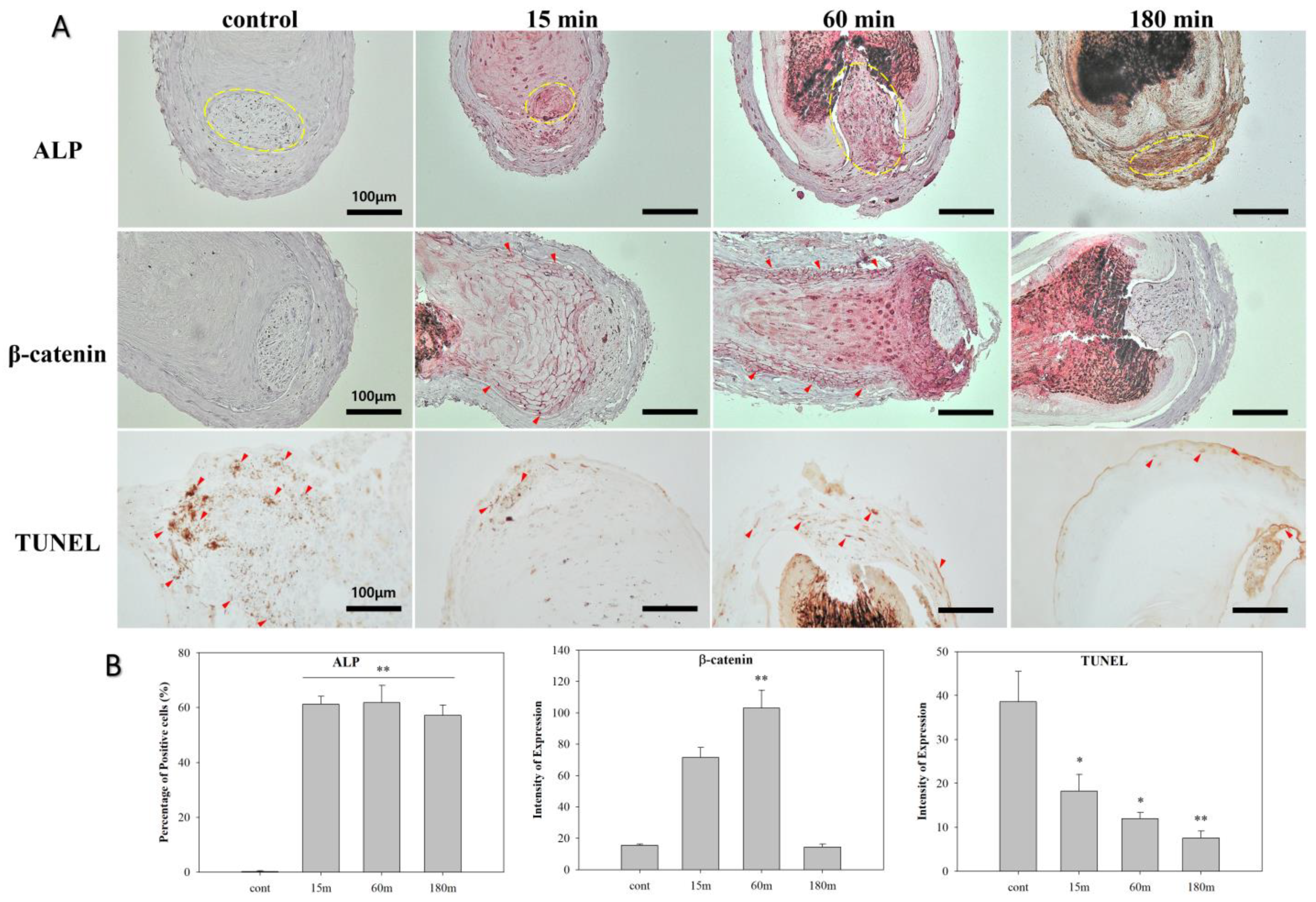
3.4. EMF Regulates Anagen-Related Molecules in HFs in an Exposure Time-Dependent Manner
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwack, M.H.; Kang, B.M.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Minoxidil activates β-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.J.; Choi, S.-J.; Yoon, S.-Y.; Lee, J.Y.; Park, W.-S.; Park, P.-J.; Kim, K.H.; Eun, H.C.; Kwon, O. Valproic acid promotes human hair growth in in vitro culture model. J. Dermatol. Sci. 2013, 72, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Herczeg-Lisztes, E.; Funk, W.; Zillikens, D.; Bíró, T.; Paus, R. Differential effects of caffeine on hair shaft elongation, matrix and outer root sheath keratinocyte proliferation, and transforming growth factor-β2/insulin-like growth factor-1-mediated regulation of the hair cycle in male and female human hair follicles in vitro. Br. J. Dermatol. 2014, 171, 1031–1043. [Google Scholar] [PubMed]
- Joo, H.J.; Jeong, K.H.; Kim, J.E.; Kang, H. Various wavelengths of light-emitting diode light regulate the proliferation of human dermal papilla cells and hair follicles via Wnt/β-catenin and the extracellular signal-regulated kinase pathways. Ann. Dermatol. 2017, 29, 747–754. [Google Scholar] [CrossRef]
- Jampa-Ngern, S.; Viravaidya-Pasuwat, K.; Suvanasuthi, S.; Khantachawana, A. Wnt signaling maintains the hair-inducing activity of the dermal papilla. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Korea, 11–15 July 2017; pp. 3592–3595. [Google Scholar]
- Yoon, S.-Y.; Kim, K.-T.; Jo, S.J.; Cho, A.-R.; Jeon, S.-I.; Choi, H.-D.; Kim, K.H.; Park, G.-S.; Pack, J.-K.; Kwon, O.S. Induction of hair growth by insulin-like growth factor-1 in 1,763 MHz radiofrequency-irradiated hair follicle cells. PLoS ONE 2011, 6, e28474. [Google Scholar] [CrossRef] [Green Version]
- Sohn, K.M.; Jeong, K.H.; Kim, J.E.; Park, Y.M.; Kang, H. Hair growth-promotion effects of different alternating current parameter settings are mediated by the activation of Wnt/β-catenin and MAPK pathway. Exp. Dermatol. 2015, 24, 958–963. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of Hair Follicle Dermal Papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef] [Green Version]
- Bureau, J.; Ginouves, P.; Guilbaud, J.; Roux, M.J. Essential oils and low-intensity electromagnetic pulses in the treatment of androgen-dependent alopecia. Adv. Ther. 2003, 20, 220–229. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Bai, L.; Zhao, P.; Zhang, M. Exposure to 50 Hz electromagnetic fields enhances hair follicle regrowth in C57BL/6 mice. Exp. Biol. Med. 2019, 244, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ye, Y.; Liu, X.; Bai, L.; Zhao, P.; Bai, W.; Zhang, M. Low-frequency electromagnetic fields promote hair follicles regeneration by injection a mixture of epidermal stem cells and dermal papilla cells. Electromagn. Biol. Med. 2020, 39, 251–256. [Google Scholar] [CrossRef]
- Ki, G.-E.; Kim, Y.-M.; Lim, H.-M.; Lee, E.-C.; Choi, Y.-K.; Seo, Y.-K. Extremely Low-Frequency Electromagnetic Fields Increase the Expression of Anagen-Related Molecules in Human Dermal Papilla Cells via GSK-3β/ERK/Akt Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 784. [Google Scholar] [CrossRef] [Green Version]
- Philpott, M. In vitro maintenance of isolated hair follicles: Current status and future development. Exp. Dermatol. 1999, 8, 317. [Google Scholar]
- Higgins, C.A.; Richardson, G.D.; Ferdinando, D.; Westgate, G.E.; Jahoda, C.A. Modelling the hair follicle dermal papilla using spheroid cell cultures. Exp. Dermatol. 2010, 19, 546–548. [Google Scholar] [CrossRef]
- Higgins, C.A.; Chen, J.C.; Cerise, J.E.; Jahoda, C.A.; Christiano, A.M. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc. Natl. Acad. Sci. USA 2013, 110, 19679–19688. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Miao, Y.; Wang, J.; Fan, Z.; Du, L.; Su, Y.; Liu, B.; Hu, Z.; Xing, M. Surface tension guided hanging-drop: Producing controllable 3D spheroid of high-passaged human dermal papilla cells and forming inductive microtissues for hair-follicle regeneration. ACS Appl. Mater. Interfaces 2016, 8, 5906–5916. [Google Scholar] [CrossRef]
- Korzeniewski, C.; Callewaert, D.M. An enzyme-release assay for natural cytotoxicity. J. Immunol. Methods 1983, 64, 313–320. [Google Scholar] [CrossRef]
- Iida, M.; Ihara, S.; Matsuzaki, T. Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Dev. Growth Differ. 2007, 49, 185–195. [Google Scholar] [CrossRef]
- Liu, B.; Chen, X.; Yi, H.; Han, L.; Ji, B.; Chen, H.; Deng, W.; Wan, M. β-Catenin is involved in oleanolic acid-dependent promotion of proliferation in human hair matrix cells in an in vitro organ culture model. Fitoterapia 2017, 121, 136–140. [Google Scholar] [CrossRef]
- Lindner, G.; Botchkarev, V.A.; Botchkarev, N.V.; Ling, G.; Van Der Veen, C.; Paus, R. Analysis of apoptosis during hair follicle regression (catagen). Am. J. Pathol. 1997, 151, 1601. [Google Scholar]
- Li, W.; Lu, Z.-F.; Man, X.-Y.; Li, C.-M.; Zhou, J.; Chen, J.-Q.; Yang, X.-H.; Wu, X.-J.; Cai, S.-Q.; Zheng, M. VEGF upregulates stimulates proliferation through ERK pathway. Mol. Biol. Rep. 2012, 39, 8687–8694. [Google Scholar] [CrossRef]
- Kozlowska, U.; Blume-Peytavi, U.; Kodelja, V.; Sommer, C.; Goerdt, S.; Majewski, S.; Jablonska, S.; Orfanos, C.E. Expression of vascular endothelial growth factor (VEGF) in various compartments of the human hair follicle. Arch. Dermatol. Res. 1998, 290, 661–668. [Google Scholar] [CrossRef]
- Lachgar, S.; Moukadiri, H.; Jonca, F.; Charveron, M.; Bouhaddioui, N.; Gall, Y.; Bonafe, J.L.; Plouët, J. Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J. Investig. Dermatol. 1996, 106, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Lachgar, S.; Charveron, M.; Gall, Y.; Bonafe, J. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br. J. Dermatol. 1998, 138, 407–411. [Google Scholar] [CrossRef]
- Karlsson, L.; Bondjers, C.; Betsholtz, C. Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development 1999, 126, 2611–2621. [Google Scholar] [CrossRef] [PubMed]
- Kamp, H.; Geilen, C.; Sommer, C.; Blume-Peytavi, U. Regulation of PDGF and PDGF receptor in cultured dermal papilla cells and follicular keratinocytes of the human hair follicle. Exp. Dermatol. 2003, 12, 662–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiso, M.; Hamazaki, T.S.; Itoh, M.; Kikuchi, S.; Nakagawa, H.; Okochi, H. Synergistic effect of PDGF and FGF2 for cell proliferation and hair inductive activity in murine vibrissal dermal papilla in vitro. J. Dermatol. Sci. 2015, 79, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H. Stimulation of human hair growth by the recombinant human keratinocyte growth factor-2 (KGF-2). Biotechnol. Lett. 2005, 27, 749–752. [Google Scholar] [CrossRef]
- Kawano, M.; Komi-Kuramochi, A.; Asada, M.; Suzuki, M.; Oki, J.; Jiang, J.; Imamura, T. Comprehensive analysis of FGF and FGFR expression in skin: FGF18 is highly expressed in hair follicles and capable of inducing anagen from telogen stage hair follicles. J. Investig. Dermatol. 2005, 124, 877–885. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.-H.; Xiang, L.-J.; Shi, H.-X.; Zhang, J.; Jiang, L.-P.; Cai, P.-T.; Lin, Z.-L.; Lin, B.-B.; Huang, Y.; Zhang, H.-L. Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. BioMed Res. Int. 2015, 2015, 730139. [Google Scholar]
- Lü, Z.-F.; Cai, S.-Q.; Wu, J.-J.; Zheng, M. Biological characterization of cultured dermal papilla cells and hair follicle regenerationin vitroandin vivo. Chin. Med. J. 2006, 119, 275–281. [Google Scholar] [CrossRef]
- Li, J.; Tzu, J.; Chen, Y.; Zhang, Y.P.; Nguyen, N.T.; Gao, J.; Bradley, M.; Keene, D.R.; Oro, A.E.; Miner, J.H. Laminin-10 is crucial for hair morphogenesis. EMBO J. 2003, 22, 2400–2410. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; DeRouen, M.C.; Chen, C.-H.; Nguyen, M.; Nguyen, N.T.; Ido, H.; Harada, K.; Sekiguchi, K.; Morgan, B.A.; Miner, J.H. Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev. 2008, 22, 2111–2124. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, J.; Ehama, R.; Wu, L.; Jiang, S.; Jiang, N.; Burgeson, R.E. Selective activation of the versican promoter by epithelial–mesenchymal interactions during hair follicle development. Proc. Natl. Acad. Sci. USA 1999, 96, 7336–7341. [Google Scholar] [CrossRef] [Green Version]
- Soma, T.; Tajima, M.; Kishimoto, J. Hair cycle-specific expression of versican in human hair follicles. J. Dermatol. Sci. 2005, 39, 147–154. [Google Scholar] [CrossRef]
- Jo, S.J.; Kim, J.Y.; Jang, S.; Choi, S.J.; Kim, K.H.; Kwon, O. Decrease of versican levels in the follicular dermal papilla is a remarkable aging-associated change of human hair follicles. J. Dermatol. Sci. 2016, 84, 354. [Google Scholar] [CrossRef]
- Handjiski, B.; Eichmüller, S.; Hofmann, U.; Czarnetzki, B.; Paus, R. Alkaline phosphatase activity and localization during the murine hair cycle. Br. J. Dermatol. 1994, 131, 303–310. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yoon, J.; Shin, S.H.; Zahoor, M.; Kim, H.J.; Park, P.J.; Park, W.-S.; Kim, H.-Y.; Choi, K.-Y. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS ONE 2012, 7, e34152. [Google Scholar] [CrossRef] [Green Version]
- Tokura, Y.; Sugita, K.; Kabashima, K.; Ito, T.; Yagi, H. Alopecia universalis associated with impaired interleukin-4 production and low serum IgE level. J. Am. Acad. Dermatol. 2007, 57, S22–S25. [Google Scholar] [CrossRef]
- Choi, N.; Sung, J.-H. Udenafil induces the hair growth effect of adipose-derived stem cells. Biomol. Ther. 2019, 27, 404. [Google Scholar] [CrossRef]
- Mandt, N.; Geilen, C.C.; Wrobel, A.; Gelber, A.; Hartwig, K.; Orfanos, C.E.; Blume-Peytavi, U. Interleukin-4 induces apoptosis in cultured human follicular keratinocytes, but not in dermal papilla cells. Eur. J. Dermatol. 2002, 12, 432–438. [Google Scholar]
- Müller-Röver, S.; Bulfone-Paus, S.; Handjiski, B.; Welker, P.; Sundberg, J.P.; McKay, I.A.; Botchkarev, V.A.; Paus, R. Intercellular adhesion molecule-1 and hair follicle regression. J. Histochem. Cytochem. 2000, 48, 557–568. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Moon, S.-O.; Kim, S.H.; Kim, H.J.; Koh, Y.S.; Koh, G.Y. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-κB activation in endothelial cells. J. Biol. Chem. 2001, 276, 7614–7620. [Google Scholar] [CrossRef] [Green Version]
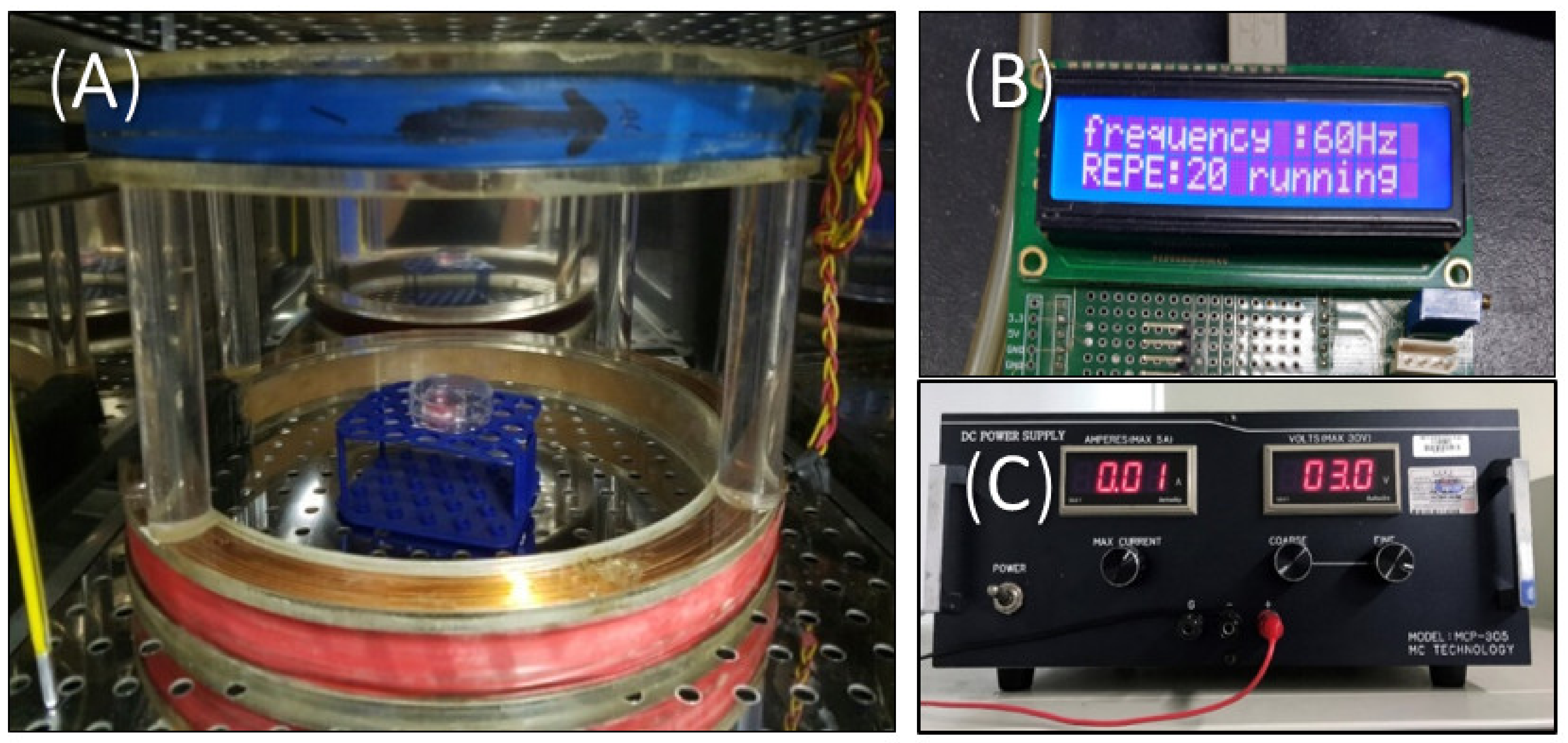


| Model Tissue | ELF-EMF Irradiation Conditions | Irradiation Period |
|---|---|---|
| Hair bulb spheroid | Intensity: 2, 5, 10, 20, 50 G Frequency: 60 Hz Exposure time: 15 min/day | 7 days |
| Hair follicle | Intensity: 10 G Frequency: 60 Hz Exposure time: 15, 60, 180 min/day | 14 days |
| Gene | Primer Sequences | Amplicon Length (bp) |
|---|---|---|
| β-actin | 5′-GAAAGCCTGCCGGTGACTAA-3′ 5′-TTCCCGTTCTCAGCCTTGAC-3′ | 301 |
| Caspase-3 | 5′-TACCAGTGGAGGCCGACTTC-3′ 5′-GCGACTGGATGAACCAGGAG-3′ | 96 |
| ET-1 | 5′-GCTGCCTTTTCTCCCCGTTA-3′ 5′-GCTTCAGGTCCCTCAAAGCG-3′ | 89 |
| FGF-10 | 5′-AGCCCCAAACAACAACAACAG-3′ 5′-GCCATCCTCGTTTCCAATTCAT-3′ | 187 |
| ICAM-1 | 5′-ACCATCTACAGCTTTCCGGC-3′ 5′-CAATCCCTCTCGTCCAGTCG-3′ | 293 |
| PDGF | 5′-GATCCGCTCCTTTGATGATC-3′ 5′-GTCTCACACTTGCATGCCAG-3′ | 435 |
| VEGF | 5′-GCCATCCAATCGAGACCCTG-3′ 5′-ATTAGACAGCAGCGGGCAC-3′ | 367 |
| Versican | 5′-GTGTTCCACCTCACTGTCCC-3′ 5′-CCTGGAGTTCCCCCACTGTT-3′ | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-H.; Kim, Y.-M.; Park, H.-J.; Nam, M.-H.; Seo, Y.-K. Extremely Low-Frequency Electromagnetic Fields Increase Cytokines in Human Hair Follicles through Wnt/β-Catenin Signaling. Biomedicines 2022, 10, 924. https://doi.org/10.3390/biomedicines10040924
Choi J-H, Kim Y-M, Park H-J, Nam M-H, Seo Y-K. Extremely Low-Frequency Electromagnetic Fields Increase Cytokines in Human Hair Follicles through Wnt/β-Catenin Signaling. Biomedicines. 2022; 10(4):924. https://doi.org/10.3390/biomedicines10040924
Chicago/Turabian StyleChoi, Ju-Hye, Yu-Mi Kim, Hee-Jung Park, Myeong-Hyun Nam, and Young-Kwon Seo. 2022. "Extremely Low-Frequency Electromagnetic Fields Increase Cytokines in Human Hair Follicles through Wnt/β-Catenin Signaling" Biomedicines 10, no. 4: 924. https://doi.org/10.3390/biomedicines10040924
APA StyleChoi, J.-H., Kim, Y.-M., Park, H.-J., Nam, M.-H., & Seo, Y.-K. (2022). Extremely Low-Frequency Electromagnetic Fields Increase Cytokines in Human Hair Follicles through Wnt/β-Catenin Signaling. Biomedicines, 10(4), 924. https://doi.org/10.3390/biomedicines10040924






