Potential and Therapeutic Roles of Diosmin in Human Diseases
Abstract
:1. Introduction
2. Pharmacology of Diosmin
3. Critical Properties of Diosmin
3.1. Anti-Oxidant Property
3.2. Anti-Inflammatory Property
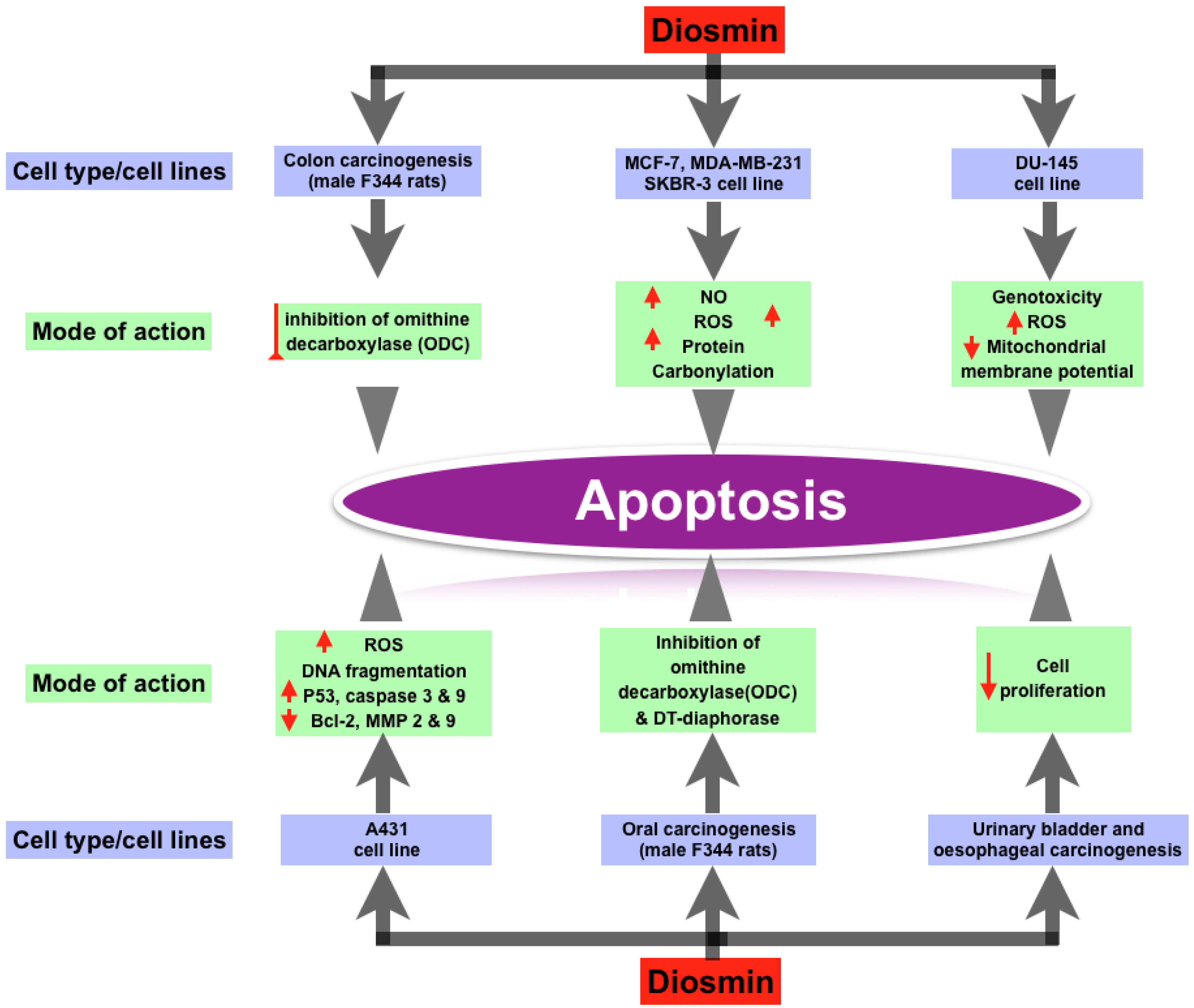
3.3. Anti-Cancer Property
3.4. Anti-Diabetic Property
3.5. Anti-Bacterial Property
3.6. Cardiovascular Protection
3.7. Liver Protection
3.8. Neuroprotection
3.9. Additional Roles
4. Potential Diosmin Target Proteins and the Pathways
5. Combinational Therapy, Side Effects, and the Administrative Route
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogucka-Kocka, A.; Woźniak, M.; Feldo, M.; Kockic, J.; Szewczyk, K. Diosmin—Isolation techniques, determination in plant material and pharmaceutical formulations, and clinical use. Nat. Prod. Commun. 2013, 8, 545–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerges, S.H.; Wahdan, S.A.; Elsherbiny, D.A.; El-Demerdash, E. Pharmacology of Diosmin, a Citrus Flavone Glycoside: An Updated Review. Eur. J. Drug Metab. Pharmacokinet. 2021, 47, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Liou, S.-S.; Hong, T.-Y.; Liu, I.-M. The Benefits of the Citrus Flavonoid Diosmin on Human Retinal Pigment Epithelial Cells under High-Glucose Conditions. Molecules 2017, 22, 2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldo, M.; Wójciak-Kosior, M.; Sowa, I.; Kocki, J.; Bogucki, J.; Zubilewicz, T.; Kęsik, J.; Bogucka-Kocka, A. Effect of Diosmin Administration in Patients with Chronic Venous Disorders on Selected Factors Affecting Angiogenesis. Molecules 2019, 24, 3316. [Google Scholar] [CrossRef] [Green Version]
- Fattori, V.; Rasquel-Oliveira, F.S.; Artero, N.A.; Ferraz, C.R.; Borghi, S.M.; Casagrande, R.; Verri, W.A. Diosmin Treats Lipopolysaccharide-Induced Inflammatory Pain and Peritonitis by Blocking NF-κB Activation in Mice. J. Nat. Prod. 2020, 83, 1018–1026. [Google Scholar] [CrossRef]
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Zoheir, K.M.A.; Iqbal, M.; Anwer, M.K.; Hoshani, A.A.R.; Attia, S.M.; Ahmad, S.F. Pharmacological Research. Pharmacol. Res. 2015, 102, 1–11. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Q.; Yang, J.; Yao, H.; Fan, R.; Cao, C.; Liu, C.; Zhang, S.; Lei, X.; Xu, S. The proteomic profiling of multiple tissue damage in chickens for a selenium deficiency biomarker discovery. Food Funct. 2020, 11, 1312–1321. [Google Scholar] [CrossRef]
- Ahmed, S.; Mundhe, N.; Borgohain, M.; Chowdhury, L.; Kwatra, M.; Bolshette, N.; Ahmed, A.; Lahkar, M. Diosmin Modulates the NF-kB Signal Transduction Pathways and Downregulation of Various Oxidative Stress Markers in Alloxan-Induced Diabetic Nephropathy. Inflammation 2016, 39, 1783–1797. [Google Scholar] [CrossRef]
- Crespo, M.E.; Gálvez, J.; Cruz, T.; Ocete, M.A.; Zarzuelo, A. Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Med. 1999, 65, 651–653. [Google Scholar] [CrossRef]
- Shalkami, A.S.; Hassan, M.; Bakr, A.G. Anti-inflammatory, antioxidant and anti-apoptotic activity of diosmin in acetic acid-induced ulcerative colitis. Hum. Exp. Toxicol. 2017, 37, 78–86. [Google Scholar] [CrossRef]
- Abdel-Reheim, M.A.; Messiha, B.A.S.; Abo-Saif, A.A. Hepatoprotective Effect of Diosmin on Iron-induced Liver Damage. Int. J. Pharmacol. 2017, 13, 529–540. [Google Scholar] [CrossRef]
- Carballo-Villalobos, A.I.; González-Trujano, M.-E.; Pellicer, F.; López-Muñoz, F.J. Antihyperalgesic Effect of Hesperidin Improves with Diosmin in Experimental Neuropathic Pain. BioMed Res. Int. 2016, 2016, 8263463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilit, A.C.; Kose, E.O.; Imir, N.G.; Aydemir, E. Anticancer and antimicrobial activities of diosmin. Genet. Mol. Res. 2021, 20, GMR18752. [Google Scholar] [CrossRef]
- Pushkaran, A.C.; Vinod, V.; Vanuopadath, M.; Nair, S.S.; Nair, S.V.; Vasudevan, A.K.; Biswas, R.; Mohan, C.G. Combination of Repurposed Drug Diosmin with Amoxicillin-Clavulanic acid Causes Synergistic Inhibition of Mycobacterial Growth. Sci. Rep. 2019, 9, 6800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, N.; AlAsmari, A.F.; Imam, F.; Ahmed, M.Z.; Alqahtani, F.; Alharbi, M.; AlSwayyed, M.; AlAsmari, F.; Alasmari, M.; Alshammari, A.; et al. Protective effect of diosmin against doxorubicin-induced nephrotoxicity. Saudi J. Biol. Sci. 2021, 28, 4375–4383. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Wnuk, M. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol. Lett. 2017, 265, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Gadewar, M.; Tahilyani, V.; Patel, D.K. A review on pharmacological and analytical aspects of diosmetin: A concise report. Chin. J. Integr. Med. 2013, 19, 792–800. [Google Scholar] [CrossRef]
- Yao, X.; Gu, X.; Jin, S.; Shi, K.; Gao, X.; Wang, Q.; Zhao, J.; Zhang, H.; Lai, X. Anticancer and Anti-inflammatory Effect of Diosmin against Dalton Ascitic Lymphoma Induced Leukemia. J. Oleo Sci. 2021, 70, 665–673. [Google Scholar] [CrossRef]
- Tanaka, T.; Makita, H.; Kawabata, K.; Mori, H.; Kakumoto, M.; Satoh, K.; Hara, A.; Sumida, T.; Fukutani, K.; Ogawa, H. Modulation of N-methyl-N-amylnitrosamine-induced rat oesophageal tumourigenesis by dietary feeding of diosmin and hesperidin, both alone and in combination. Carcinogenesis 1997, 18, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Corsale, I.; Carrieri, P.; Martellucci, J.; Piccolomini, A.; Verre, L.; Rigutini, M.; Panicucci, S. Flavonoid mixture (diosmin, troxerutin, rutin, hesperidin, quercetin) in the treatment of I–III degree hemorroidal disease: A double-blind multicenter prospective comparative study. Int. J. Colorectal Dis. 2018, 33, 1595–1600. [Google Scholar] [CrossRef]
- Perumal, S. Effect of diosmin on apoptotic signaling molecules in N- nitrosodiethylamine-induced hepatocellular carcinoma in experimental rats. Mol. Cell. Biochem. 2018, 449, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Pari, L. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem.-Biol. Interact. 2012, 195, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.M.; Faria, B.M.D.; Ascari, L.M.; Souza, J.M.D.; Soares, A.G.; Cordeiro, Y.; Romão, L.F. Diosmin induces caspase-dependent apoptosis in human glioblastoma cells. An. Acad. Bras. Ciências 2019, 91, e20191031. [Google Scholar] [CrossRef] [PubMed]
- Dung, T.; Lin, C.-H.; Việt Bình, T.; Hsu, H.-H.; Su, C.-C.; Lin, Y.-M.; Tsai, C.-H.; Tsai, F.-J.; Kuo, W.-W.; Chen, L.-M.; et al. Diosmin induces cell apoptosis through protein phosphatase 2A activation in HA22T human hepatocellular carcinoma cells and blocks tumour growth in xenografted nude mice. Food Chem. 2012, 132, 2065–2073. [Google Scholar] [CrossRef]
- Dubey, K.; Dubey, R.; Gupta, R.; Gupta, A. Exploration of Diosmin to Control Diabetes and Its Complications-an In Vitro and In Silico Approach. Curr. Comput. Aided-Drug Des. 2021, 17, 307–313. [Google Scholar] [CrossRef]
- Li, T.; Zhu, W.; Liu, G.; Fang, C.; Quan, S. Diosmin for the prevention of ovarian hyperstimulation syndrome. Int. J. Gynecol. Obstet. 2020, 149, 166–170. [Google Scholar] [CrossRef]
- Eraslan, G.; Sarıca, Z.S.; Bayram, L.Ç.; Tekeli, M.Y.; Kanbur, M.; Karabacak, M. The effects of diosmin on aflatoxin-induced liver and kidney damage. Environ. Sci. Pollut. Res. Int. 2017, 24, 27931–27941. [Google Scholar] [CrossRef]
- Caltagirone, S.; Rossi, C.; Poggi, A.; Ranelletti, F.O.; Natali, P.G.; Brunetti, M.; Aiello, F.B.; Piantelli, M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int. J. Cancer 2000, 87, 595–600. [Google Scholar] [CrossRef]
- Kuntz, S.; Wenzel, U.; Daniel, H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999, 38, 133–142. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Bozdağ, M.; Eraslan, G. The effect of diosmin against lead exposure in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Waring, M.J.; Arrowsmith, J.; Leach, A.R.; Leeson, P.D.; Mandrell, S.; Owen, R.M.; Pairaudeau, G.; Pennie, W.D.; Pickett, S.D.; Wang, J.; et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Publ. Group 2015, 14, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, P.E.; Lu, H.; Mann, E.H.; Chen, Y.-H.; Ho, T.-R.; Cousins, D.J.; Corrigan, C.; Kelly, F.J.; Mudway, I.S.; Hawrylowicz, C.M. Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter. PLoS ONE 2018, 13, e0200040. [Google Scholar] [CrossRef] [Green Version]
- Zraika, S.; Hull, R.L.; Udayasankar, J.; Aston-Mourney, K.; Subramanian, S.L.; Kisilevsky, R.; Szarek, W.A.; Kahn, S.E. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia 2009, 52, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Khosravi, M.; Poursaleh, A.; Ghasempour, G.; Farhad, S.; Najafi, M. The effects of oxidative stress on the development of atherosclerosis. Biol. Chem. 2019, 400, 711–732. [Google Scholar] [CrossRef]
- Sepidarkish, M.; Farsi, F.; Akbari-Fakhrabadi, M.; Namazi, N.; Almasi-Hashiani, A.; Maleki Hagiagha, A.; Heshmati, J. The effect of vitamin D supplementation on oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 139, 141–152. [Google Scholar] [CrossRef]
- Hallak, M.; Vazana, L.; Shpilberg, O.; Levy, I.; Mazar, J.; Nathan, I. A molecular mechanism for mimosine-induced apoptosis involving oxidative stress and mitochondrial activation. Apoptosis 2008, 13, 147–155. [Google Scholar] [CrossRef]
- De Las Heras, N.; Martín Giménez, V.M.; Ferder, L.; Manucha, W.; Lahera, V. Implications of Oxidative Stress and Potential Role of Mitochondrial Dysfunction in COVID-19: Therapeutic Effects of Vitamin D. Antioxidants 2020, 9, 897. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [Green Version]
- Pari, L.; Srinivasan, S. Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2010, 64, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Adouani, I.; Qureshi, A.S.; Hang, T.-J. Preparation, evaluation and pharmacokinetics of diosmin herbosomein beagle dogs. Pak. J. Pharm. Sci. 2019, 33, 033–040. [Google Scholar]
- Senthamizhselvan, O.; Manivannan, J.; Silambarasan, T.; Raja, B. Diosmin pretreatment improves cardiac function and suppresses oxidative stress in rat heart after ischemia/reperfusion. Eur. J. Pharmacol. 2014, 736, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Tong, N.; Zhang, Z.; Zhang, W.; Qiu, Y.; Gong, Y.; Yin, L.; Qiu, Q.; Wu, X. Diosmin Alleviates Retinal Edema by Protecting the Blood-Retinal Barrier and Reducing Retinal Vascular Permeability during Ischemia/Reperfusion Injury. PLoS ONE 2013, 8, e61794. [Google Scholar] [CrossRef] [Green Version]
- Calvert, J.W. Chapter 5—Ischemic Heart Disease and its Consequences. In Cellular and Molecular Pathobiology of Cardiovascular Disease; Willis, M.S., Homeister, J.W., Stone, J.R., Eds.; Cellular and Molecular Pathobiology of Cardiovascular Disease; Academic Press: San Diego, CA, USA, 2014; pp. 79–100. [Google Scholar]
- Queenthy, S.S.; John, B. Diosmin exhibits anti-hyperlipidemic effects in isoproterenol induced myocardial infarcted rats. Eur. J. Pharmacol. 2013, 718, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Makita, H.; Kawabata, K.; Mori, H.; Kakumoto, M.; Satoh, K.; Hara, A.; Sumida, T.; Tanaka, T.; Ogawa, H. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis 1997, 18, 957–965. [Google Scholar] [CrossRef] [Green Version]
- Pendeville, H.; Carpino, N.; Marine, J.C.; Takahashi, Y.; Muller, M.; Martial, J.A.; Cleveland, J.L. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol. Cell. Biol. 2001, 21, 6549–6558. [Google Scholar] [CrossRef] [Green Version]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.-W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [Green Version]
- Browning, A.M.; Walle, U.K.; Walle, T. Flavonoid glycosides inhibit oral cancer cell proliferation—role of cellular uptake and hydrolysis to the aglycones. J. Pharm. Pharmacol. 2005, 57, 1037–1041. [Google Scholar] [CrossRef]
- Sahu, N.; Soni, D.; Chandrashekhar, B.; Satpute, D.B.; Saravanadevi, S.; Sarangi, B.K.; Pandey, R.A. Synthesis of silver nanoparticles using flavonoids: Hesperidin, naringin and diosmin, and their antibacterial effects and cytotoxicity. Int. Nano Lett. 2016, 6, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Martínez, C.; Vicente, V.; Yáñez, J.; Alcaraz, M.; Castells, M.T.; Canteras, M.; Benavente-García, O.; Castillo, J. The effect of the flavonoid diosmin, grape seed extract and red wine on the pulmonary metastatic B16F10 melanoma. Histol. Histopathol. 2005, 20, 1121–1129. [Google Scholar] [PubMed]
- Álvarez, N.; Vicente, V.; Martínez, C. Synergistic Effect of Diosmin and Interferon-α on Metastatic Pulmonary Melanoma. Cancer Biother. Radiopharm. 2009, 24, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Martínez Conesa, C.; Vicente Ortega, V.; Yáñez Gascón, M.J.; Alcaraz Baños, M.; Canteras Jordana, M.; Benavente-García, O.; Castillo, J. Treatment of Metastatic Melanoma B16F10 by the Flavonoids Tangeretin, Rutin, and Diosmin. J. Agric. Food Chem. 2005, 53, 6791–6797. [Google Scholar] [CrossRef]
- Dung, T.D.; Day, C.H.; Binh, T.V.; Lin, C.-H.; Hsu, H.-H.; Su, C.-C.; Lin, Y.-M.; Tsai, F.-J.; Kuo, W.-W.; Chen, L.-M.; et al. PP2A mediates diosmin p53 activation to block HA22T cell proliferation and tumor growth in xenografted nude mice through PI3K–Akt–MDM2 signaling suppression. Food Chem. Toxicol. 2012, 50, 1802–1810. [Google Scholar] [CrossRef]
- Farmer, J.A. Diabetic dyslipidemia and atherosclerosis: Evidence from clinical trials. Curr. Diabetes Rep. 2008, 8, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J. Does statin monotherapy address the multiple lipid abnormalities in type 2 diabetes? Atheroscler. Suppl. 2005, 6, 15–19. [Google Scholar] [CrossRef]
- Jain, D.; Bansal, M.K.; Dalvi, R.; Upganlawar, A.; Somani, R. Protective effect of diosmin against diabetic neuropathy in experimental rats. J. Integr. Med. 2014, 12, 35–41. [Google Scholar] [CrossRef]
- Zielinska, D.F.; Gnad, F.; Schropp, K.; Wiśniewski, J.R.; Mann, M. Short Article. MOLCEL 2012, 46, 542–548. [Google Scholar]
- Luepker, R.V. Cardiovascular disease: Rise, fall, and future prospects. Annu. Rev. Public Health 2011, 32, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Nabel, E.G. Cardiovascular disease. N. Engl. J. Med. 2003, 349, 60–72. [Google Scholar] [CrossRef]
- Iafisco, M.; Alogna, A.; Miragoli, M.; Catalucci, D. Cardiovascular nanomedicine: The route ahead. Nanomedicine 2019, 14, 2391–2394. [Google Scholar] [CrossRef] [PubMed]
- Martín Giménez, V.M.; Kassuha, D.E.; Manucha, W. Nanomedicine applied to cardiovascular diseases: Latest developments. Ther. Adv. Cardiovasc. Dis. 2017, 11, 133–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashour, N.H.; Lin, G.I.; Frishman, W.H. Herbal medicine for the treatment of cardiovascular disease: Clinical considerations. Arch. Intern. Med. 1998, 158, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Stansfield, W.E.; Ranek, M.; Pendse, A.; Schisler, J.C.; Wang, S.; Pulinilkunnil, T.; Willis, M.S. Chapter 4—The Pathophysiology of Cardiac Hypertrophy and Heart Failure. In Cellular and Molecular Pathobiology of Cardiovascular Disease; Willis, M.S., Homeister, J.W., Stone, J.R., Eds.; Cellular and Molecular Pathobiology of Cardiovascular Disease; Academic Press: San Diego, CA, USA, 2014; pp. 51–78. [Google Scholar]
- Katsenis, K. Micronized purified flavonoid fraction (MPFF): A review of its pharmacological effects, therapeutic efficacy and benefits in the management of chronic venous insufficiency. Curr. Vasc. Pharmacol. 2005, 3, 1–9. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [Green Version]
- Munjal, A.; Khandia, R. Atherosclerosis: Orchestrating Cells and Biomolecules Involved in Its Activation and Inhibition; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 1–38. [Google Scholar]
- Renehan, A.G.; Zwahlen, M.; Egger, M. Adiposity and cancer risk:new mechanistic insightsfrom epidemiology. Nat. Rev. Cancer 2015, 15, 484–498. [Google Scholar] [CrossRef]
- Lee, J.; Song, K.-M.; Jung, C.H. Diosmin restores the skin barrier by targeting the aryl hydrocarbon receptor in atopic dermatitis. Phytomedicine 2021, 81, 153418. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, B.; Kanwar, S.S.; Kumar, A. Lead Phytochemicals for Anticancer Drug Development. Front. Plant Sci. 2016, 7, 1667. [Google Scholar] [CrossRef] [Green Version]
- Warsi, M.K.; Kamal, M.A.; Baeshen, M.N.; Izhari, M.A.; Mobashir, A.F.A.M. Comparative Study of Gene Expression Profiling Unravels Functions associated with Pathogenesis of Dengue Infection. Curr. Pharm. Des. 2020, 26, 5293–5299. [Google Scholar] [CrossRef]
- Kamal, M.A.; Warsi, M.K.; Alnajeebi, A.; Ali, H.A.; Helmi, N.; Izhari, M.A.; Mustafa, S.; Mobashir, M. Gene expression profiling and clinical relevance unravel the role hypoxia and immune signaling genes and pathways in breast cancer: Role of hypoxia and immune signaling genes in breast cancer. J. Intern. Med. Sci. Art 2020, 1, 2–10. [Google Scholar] [CrossRef]
- Bajrai, L.; Sohrab, S.S.; Alandijany, T.A.; Mobashir, M.; Parveen, S.; Kamal, M.A.; Azhar, E.I. Gene expression profiling of early acute febrile stage of dengue infection and its comparative analysis with Streptococcus pneumoniae infection. Front. Cell. Infect. Microbiol. 2021, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- Alexeyenko, A.; Sonnhammer, E.L.L. Global networks of functional coupling in eukaryotes from comprehensive data integration. Genome Res. 2009, 19, 1107–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobashir, M.; Schraven, B.; Beyer, T. Simulated evolution of signal transduction networks. PLoS ONE 2012, 7, e50905. [Google Scholar] [CrossRef] [PubMed]
- Mobashir, M.; Madhusudhan, T.; Isermann, B.; Beyer, T.; Schraven, B. Negative Interactions and Feedback Regulations Are Required for Transient Cellular Response. Sci. Rep. 2014, 4, 3718. [Google Scholar] [CrossRef] [Green Version]
- Eldakhakhny, B.M.; Sadoun, A.H.; Choudhry, H.; Mobashir, M. In-Silico Study of Immune System Associated Genes in Case of Type-2 Diabetes with Insulin Action and Resistance, and/or Obesity. Front. Endocrinol. 2021, 12, 281. [Google Scholar] [CrossRef]
- Krishnamoorthy, P.K.P.; Kamal, M.A.; Warsi, M.K.; Alnajeebi, A.; Ali, H.A.; Helmi, N.; Izhari, M.A.; Mustafa, S.; Firoz, A.; Mobashir, M. In-silico study reveals immunological signaling pathways, their genes, and potential herbal drug targets in ovarian cancer. Inform. Med. Unlocked 2020, 20, 100422. [Google Scholar] [CrossRef]
- Christopoulos, D.; Nicolaides, A.N.; Szendro, G. Venous reflux: Quantification and correlation with the clinical severity of chronic venous disease. Br. J. Surg. 1988, 75, 352–356. [Google Scholar] [CrossRef]
- De Smet, F.; Christopoulos, A.; Carmeliet, P. Allosteric targeting of receptor tyrosine kinases. Nat. Biotechnol. 2014, 32, 1113–1120. [Google Scholar] [CrossRef]
- Ramelet, A.A.; Boisseau, M.R.; Allegra, C.; Nicolaides, A.; Jaeger, K.; Carpentier, P.; Cappelli, R.; Forconi, S. Veno-active drugs in the management of chronic venous disease. An international consensus statement: Current medical position, prospective views and final resolution. Clin. Hemorheol. Microcirc. 2005, 33, 309–319. [Google Scholar]
- Cazaubon, M.; Benigni, J.-P.; Steinbruch, M.; Jabbour, V.; Gouhier-Kodas, C. Is There a Difference in the Clinical Efficacy of Diosmin and Micronized Purified Flavonoid Fractio.on for the Treatment of Chronic Venous Disorders? Review of Available Evidence. Vasc. Health Risk Manag. 2021, 17, 591–600. [Google Scholar] [CrossRef]
- Serra, R.; Ielapi, N.; Bitonti, A.; Candido, S.; Fregola, S.; Gallo, A.; Loria, A.; Muraca, L.; Raimondo, L.; Velcean, L.; et al. Efficacy of a Low-Dose Diosmin Therapy on Improving Symptoms and Quality of Life in Patients with Chronic Venous Disease: Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 999. [Google Scholar] [CrossRef]
- Meshikhes, A.W.N. Daflon for haemorrhoids: A prospective, multi-centre observational study. Surgeon 2004, 2, 335–338+361. [Google Scholar] [CrossRef]
- Cypriani, B.; Limasset, B.; Carrié, M.L.; Le Doucen, C.; Roussie, M.; de Paulet, A.C.; Damon, M. Antioxidant activity of micronized diosmin on oxygen species from stimulated human neutrophils. Biochem. Pharmacol. 1993, 45, 1531–1535. [Google Scholar] [CrossRef]
- Oh-hora, M.; Komatsu, N.; Pishyareh, M.; Feske, S.; Hori, S.; Taniguchi, M.; Rao, A.; Takayanagi, H. Agonist-Selected T Cell Development Requires Strong T Cell Receptor Signaling and Store-Operated Calcium Entry. Immunity 2013, 38, 881–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castle, A.R.; Gill, A.C. Physiological Functions of the Cellular Prion Protein. Front. Mol. Biosci. 2017, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borisov, N.; Aksamitiene, E.; Kiyatkin, A.; Legewie, S.; Berkhout, J.; Maiwald, T.; Kaimachnikov, N.P.; Timmer, J.; Hoek, J.B.; Kholodenko, B.N. Systems-level interactions between insulin–EGF networks amplify mitogenic signaling. Mol. Syst. Biol. 2009, 5, 256. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Umar, T.; Kausar, T.; Mobashir, M.; Nayeem, S.M.; Hoda, N. Identification of lead BAY60-7550 analogues as potential inhibitors that utilize the hydrophobic groove in PDE2A: A molecular dynamics simulation study. J. Mol. Modeling 2017, 23, 7. [Google Scholar] [CrossRef]
- Pinu, F.R.; Beale, D.J.; Paten, A.M.; Kouremenos, K.; Swarup, S.; Schirra, H.J.; Wishart, D. Systems Biology and Multi-Omics Integration: Viewpoints from the Metabolomics Research Community. Metabolites 2019, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Rekhi, R.; Qutub, A.A. Systems approaches for synthetic biology: A pathway toward mammalian design. Front. Physiol. 2013, 4, 285. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, S.; Mobashir, M. LC–MS and docking profiling reveals potential difference between the pure and crude fucoidan metabolites. Int. J. Biol. Macromol. 2020, 143, 11–29. [Google Scholar] [CrossRef]
- Mobashir, M. Mathematical Modeling and Evolution of Signal Transduction Pathways and Networks. Ph.D. Thesis, Magdeburg University, Magdeburg, Germany, 2013. [Google Scholar]
- Singh, A.; Maqbool, M.; Mobashir, M.; Hoda, N. Dihydroorotate dehydrogenase: A drug target for the development of antimalarials. Eur. J. Med. Chem. 2017, 125, 640–651. [Google Scholar] [CrossRef]
- Maqbool, M.; Mobashir, M.; Hoda, N. Pivotal role of glycogen synthase kinase-3: A therapeutic target for Alzheimer’s. Eur. J. Med. Chem. 2015, 107, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Helmi, N.; Alammari, D.; Mobashir, M. Role of Potential COVID-19 Immune System Associated Genes and the Potential Pathwayslinkage with Type-2 Diabetes. Comb. Chem. High. Throughput Screen. 2021, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Meena, P.; Singh, A.; Jameel, E.; Maqbool, M.; Mobashir, M.; Shandilya, A.; Tiwari, M.; Hoda, N.; Jayaram, B. Synthesis and screening of triazolopyrimidine scaffold as multi-functional agents for Alzheimer’s disease therapies. Eur. J. Med. Chem. 2016, 119, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, P.K.P.; Subasree, S.; Arthi, U.; Mobashir, M.; Gowda, C.; Revanasiddappa, P.D. T-cell Epitope-based Vaccine Design for Nipah Virus by Reverse Vaccinology Approach. Comb. Chem. High. Throughput Screen. 2020, 23, 788–796. [Google Scholar] [CrossRef]
- Bajrai, L.H.; Sohrab, S.S.; Mobashir, M.; Kamal, M.A.; Rizvi, M.A.; Azhar, E.I. Understanding the role of potential pathways and its components including hypoxia and immune system in case of oral cancer. Sci. Rep. 2021, 11, 19576. [Google Scholar] [CrossRef]

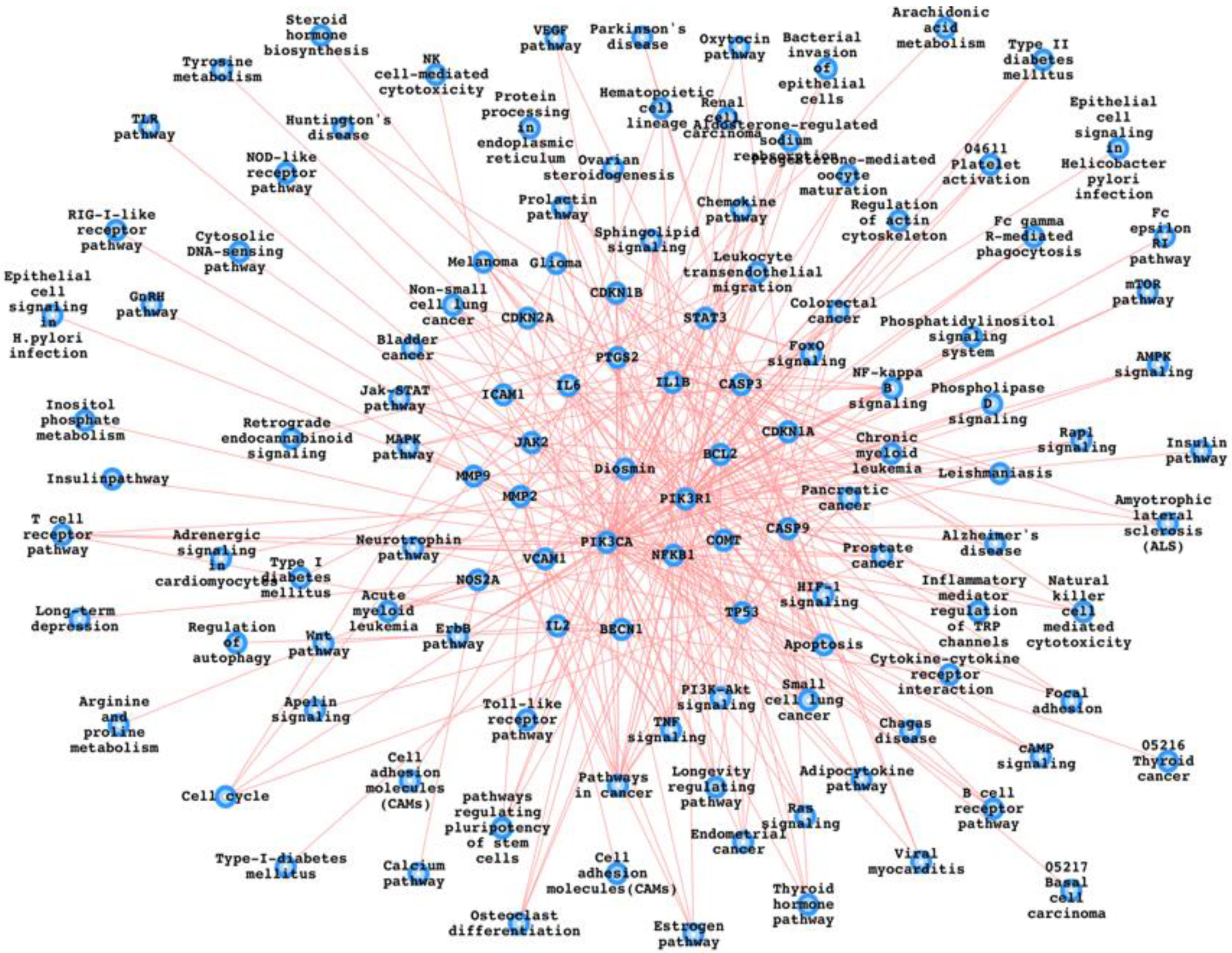
| Diosmin | NOS2A | Arginine_and_proline_metabolism |
| Diosmin | NOS2A | Calcium pathway |
| Diosmin | NOS2A | Long-term_depression |
| Diosmin | NOS2A | Small_cell_lung_cancer |
| Diosmin | PTGS2 | Arachidonic_acid_metabolism |
| Diosmin | PTGS2 | VEGF pathway |
| Diosmin | PTGS2 | Leishmaniasis |
| Diosmin | PTGS2 | Pathways_in_cancer |
| Diosmin | PTGS2 | Small_cell_lung_cancer |
| Diosmin | MMP2 | Leukocyte_transendothelial_migration |
| Diosmin | MMP2 | GnRH pathway |
| Diosmin | MMP2 | Pathways_in_cancer |
| Diosmin | MMP2 | Bladder_cancer |
| Diosmin | ICAM1 | Cell_adhesion_molecules_(CAMs) |
| Diosmin | ICAM1 | Natural_killer_cell_mediated_cytotoxicity |
| Diosmin | ICAM1 | Leukocyte_transendothelial_migration |
| Diosmin | ICAM1 | Viral_myocarditis |
| Diosmin | COMT | Steroid_hormone_biosynthesis |
| Diosmin | COMT | Tyrosine_metabolism |
| Diosmin | JAK2 | Chemokine pathway |
| Diosmin | JAK2 | Jak-STAT pathway |
| Diosmin | JAK2 | Adipocytokine pathway |
| Diosmin | JAK2 | Leishmaniasis |
| Diosmin | MMP9 | Leukocyte_transendothelial_migration |
| Diosmin | MMP9 | Pathways_in_cancer |
| Diosmin | MMP9 | Bladder_cancer |
| Diosmin | NFKB1 | MAPK pathway |
| Diosmin | NFKB1 | Chemokine pathway |
| Diosmin | NFKB1 | Apoptosis |
| Diosmin | NFKB1 | Toll-like_receptor pathway |
| Diosmin | NFKB1 | NOD-like_receptor pathway |
| Diosmin | NFKB1 | RIG-I-like_receptor pathway |
| Diosmin | NFKB1 | Cytosolic_DNA-sensing_pathway |
| Diosmin | NFKB1 | T_cell_receptorpathway |
| Diosmin | NFKB1 | B_cell_receptorpathway |
| Diosmin | NFKB1 | Neurotrophin pathway |
| Diosmin | NFKB1 | Adipocytokine pathway |
| Diosmin | NFKB1 | Epithelial_cell_signaling_in_Helicobacter_pylori_infection |
| Diosmin | NFKB1 | Leishmaniasis |
| Diosmin | NFKB1 | Chagas_disease |
| Diosmin | NFKB1 | Pathways_in_cancer |
| Diosmin | NFKB1 | Pancreatic_cancer |
| Diosmin | NFKB1 | Prostate_cancer |
| Diosmin | NFKB1 | Chronic_myeloid_leukemia |
| Diosmin | NFKB1 | Acute_myeloid_leukemia |
| Diosmin | NFKB1 | Small_cell_lung_cancer |
| Diosmin | IL2 | Cytokine-cytokine_receptor_interaction |
| Diosmin | IL2 | T_cell_receptor pathway |
| Diosmin | IL2 | Type_I_diabetes_mellitus |
| Diosmin | CDKN1B | ErbB pathway |
| Diosmin | CDKN1B | Cell_cycle |
| Diosmin | CDKN1B | Pathways_in_cancer |
| Diosmin | CDKN1B | Prostate_cancer |
| Diosmin | CDKN1B | Chronic_myeloid_leukemia |
| Diosmin | CDKN1B | Small_cell_lung_cancer |
| Diosmin | PIK3CA | Inositol_phosphate_metabolism |
| Diosmin | PIK3CA | ErbB pathway |
| Diosmin | PIK3CA | Chemokine pathway |
| Diosmin | PIK3CA | Phosphatidylinositol_signaling_system |
| Diosmin | PIK3CA | mTOR pathway |
| Diosmin | PIK3CA | Apoptosis |
| Diosmin | PIK3CA | Focal_adhesion |
| Diosmin | PIK3CA | Toll-like_receptor pathway |
| Diosmin | PIK3CA | Jak-STAT pathway |
| Diosmin | PIK3CA | Natural_killer_cell_mediated_cytotoxicity |
| Diosmin | PIK3CA | T_cell_receptor pathway |
| Diosmin | PIK3CA | B_cell_receptor pathway |
| Diosmin | PIK3CA | Fc_epsilon_RI pathway |
| Diosmin | PIK3CA | Fc_gamma_R-mediated_phagocytosis |
| Diosmin | PIK3CA | Leukocyte_transendothelial_migration |
| Diosmin | PIK3CA | Neurotrophin pathway |
| Diosmin | PIK3CA | Regulation_of_actin_cytoskeleton |
| Diosmin | PIK3CA | Insulinpathway |
| Diosmin | PIK3CA | Progesterone-mediated_oocyte_maturation |
| Diosmin | PIK3CA | Type_II_diabetes_mellitus |
| Diosmin | PIK3CA | Aldosterone-regulated_sodium_reabsorption |
| Diosmin | PIK3CA | Bacterial_invasion_of_epithelial_cells |
| Diosmin | PIK3CA | Chagas_disease |
| Diosmin | PIK3CA | Pathways_in_cancer |
| Diosmin | PIK3CA | Colorectal_cancer |
| Diosmin | PIK3CA | Renal_cell_carcinoma |
| Diosmin | PIK3CA | Pancreatic_cancer |
| Diosmin | PIK3CA | Endometrial_cancer |
| Diosmin | PIK3CA | Glioma |
| Diosmin | PIK3CA | Prostate_cancer |
| Diosmin | PIK3CA | Melanoma |
| Diosmin | PIK3CA | Chronic_myeloid_leukemia |
| Diosmin | PIK3CA | Acute_myeloid_leukemia |
| Diosmin | PIK3CA | Small_cell_lung_cancer |
| Diosmin | PIK3CA | Non-small_cell_lung_cancer |
| Diosmin | CDKN1A | ErbB pathway |
| Diosmin | CDKN1A | Cell_cycle |
| Diosmin | CDKN1A | Pathways_in_cancer |
| Diosmin | CDKN1A | Glioma |
| Diosmin | CDKN1A | Prostate_cancer |
| Diosmin | CDKN1A | Melanoma |
| Diosmin | CDKN1A | Bladder_cancer |
| Diosmin | CDKN1A | Chronic_myeloid_leukemia |
| Diosmin | IL1B | MAPK pathway |
| Diosmin | IL1B | Cytokine-cytokine_receptor_interaction |
| Diosmin | IL1B | Apoptosis |
| Diosmin | IL1B | Toll-like_receptor pathway |
| Diosmin | IL1B | Hematopoietic_cell_lineage |
| Diosmin | IL1B | Type_I_diabetes_mellitus |
| Diosmin | IL1B | Alzheimer’s_disease |
| Diosmin | BECN1 | Regulation_of_autophagy |
| Diosmin | CASP9 | Apoptosis |
| Diosmin | CASP9 | VEGF pathway |
| Diosmin | CASP9 | Alzheimer’s_disease |
| Diosmin | CASP9 | Parkinson’s_disease |
| Diosmin | CASP9 | Amyotrophic_lateral_sclerosis_(ALS) |
| Diosmin | CASP9 | Huntington’s_disease |
| Diosmin | CASP9 | Pathways_in_cancer |
| Diosmin | CASP9 | Colorectal_cancer |
| Diosmin | CASP9 | Pancreatic_cancer |
| Diosmin | CASP9 | Endometrial_cancer |
| Diosmin | CASP9 | Prostate_cancer |
| Diosmin | CASP9 | Small_cell_lung_cancer |
| Diosmin | CASP9 | Non-small_cell_lung_cancer |
| Diosmin | CASP9 | Viral_myocarditis |
| Diosmin | IL6 | Cytokine-cytokine_receptor_interaction |
| Diosmin | IL6 | Toll-like_receptor pathway |
| Diosmin | IL6 | Hematopoietic_cell_lineage |
| Diosmin | IL6 | Pathways_in_cancer |
| Diosmin | TP53 | MAPK pathway |
| Diosmin | TP53 | Cell_cycle |
| Diosmin | TP53 | Apoptosis |
| Diosmin | TP53 | Wnt pathway |
| Diosmin | TP53 | Neurotrophin pathway |
| Diosmin | TP53 | Pathways_in_cancer |
| Diosmin | TP53 | Colorectal_cancer |
| Diosmin | TP53 | Pancreatic_cancer |
| Diosmin | TP53 | Endometrial_cancer |
| Diosmin | TP53 | Glioma |
| Diosmin | TP53 | Prostate_cancer |
| Diosmin | TP53 | 05216_Thyroid_cancer |
| Diosmin | TP53 | 05217_Basal_cell_carcinoma |
| Diosmin | TP53 | Melanoma |
| Diosmin | TP53 | Bladder_cancer |
| Diosmin | TP53 | Chronic_myeloid_leukemia |
| Diosmin | TP53 | Small_cell_lung_cancer |
| Diosmin | TP53 | Non-small_cell_lung_cancer |
| Diosmin | PIK3R1 | ErbB pathway |
| Diosmin | PIK3R1 | Chemokine pathway |
| Diosmin | PIK3R1 | Phosphatidylinositol_signaling_system |
| Diosmin | PIK3R1 | mTOR pathway |
| Diosmin | PIK3R1 | Apoptosis |
| Diosmin | PIK3R1 | Focal_adhesion |
| Diosmin | PIK3R1 | Toll-like_receptor pathway |
| Diosmin | PIK3R1 | Jak-STATpathway |
| Diosmin | PIK3R1 | Natural_killer_cell_mediated_cytotoxicity |
| Diosmin | PIK3R1 | T_cell_receptor pathway |
| Diosmin | PIK3R1 | B_cell_receptor pathway |
| Diosmin | PIK3R1 | Fc_epsilon_RI pathway |
| Diosmin | PIK3R1 | Fc_gamma_R-mediated_phagocytosis |
| Diosmin | PIK3R1 | Leukocyte_transendothelial_migration |
| Diosmin | PIK3R1 | Neurotrophinpathway |
| Diosmin | PIK3R1 | Regulation_of_actin_cytoskeleton |
| Diosmin | PIK3R1 | Insulinpathway |
| Diosmin | PIK3R1 | Progesterone-mediated_oocyte_maturation |
| Diosmin | PIK3R1 | Type_II_diabetes_mellitus |
| Diosmin | PIK3R1 | Aldosterone-regulated_sodium_reabsorption |
| Diosmin | PIK3R1 | Bacterial_invasion_of_epithelial_cells |
| Diosmin | PIK3R1 | Chagas_disease |
| Diosmin | PIK3R1 | Pathways_in_cancer |
| Diosmin | PIK3R1 | Colorectal_cancer |
| Diosmin | PIK3R1 | Renal_cell_carcinoma |
| Diosmin | PIK3R1 | Pancreatic_cancer |
| Diosmin | PIK3R1 | Endometrial_cancer |
| Diosmin | PIK3R1 | Glioma |
| Diosmin | PIK3R1 | Prostate_cancer |
| Diosmin | PIK3R1 | Melanoma |
| Diosmin | PIK3R1 | Chronic_myeloid_leukemia |
| Diosmin | PIK3R1 | Acute_myeloid_leukemia |
| Diosmin | PIK3R1 | Small_cell_lung_cancer |
| Diosmin | PIK3R1 | Non-small_cell_lung_cancer |
| Diosmin | CDKN2A | Cell_cycle |
| Diosmin | CDKN2A | Pathways_in_cancer |
| Diosmin | CDKN2A | Pancreatic_cancer |
| Diosmin | CDKN2A | Glioma |
| Diosmin | CDKN2A | Melanoma |
| Diosmin | CDKN2A | Bladder_cancer |
| Diosmin | CDKN2A | Chronic_myeloid_leukemia |
| Diosmin | CDKN2A | Non-small_cell_lung_cancer |
| Diosmin | VCAM1 | Cell_adhesion_molecules_(CAMs) |
| Diosmin | VCAM1 | Leukocyte_transendothelial_migration |
| Diosmin | CASP3 | MAPK pathway |
| Diosmin | CASP3 | Apoptosis |
| Diosmin | CASP3 | Natural_killer_cell_mediated_cytotoxicity |
| Diosmin | CASP3 | Alzheimer’s_disease |
| Diosmin | CASP3 | Parkinson’s_disease |
| Diosmin | CASP3 | Amyotrophic_lateral_sclerosis_(ALS) |
| Diosmin | CASP3 | Huntington’s_disease |
| Diosmin | CASP3 | Epithelial_cell_signaling_in_Helicobacter_pylori_infection |
| Diosmin | CASP3 | Pathways_in_cancer |
| Diosmin | CASP3 | Colorectal_cancer |
| Diosmin | CASP3 | Viral_myocarditis |
| Diosmin | STAT3 | Chemokine pathway |
| Diosmin | STAT3 | Jak-STAT pathway |
| Diosmin | STAT3 | Adipocytokine pathway |
| Diosmin | STAT3 | Pathways_in_cancer |
| Diosmin | STAT3 | Pancreatic_cancer |
| Diosmin | STAT3 | Acute_myeloid_leukemia |
| Diosmin | BCL2 | Apoptosis |
| Diosmin | BCL2 | Focal_adhesion |
| Diosmin | BCL2 | Amyotrophic_lateral_sclerosis_(ALS) |
| Diosmin | BCL2 | Pathways_in_cancer |
| Diosmin | BCL2 | Colorectal_cancer |
| Diosmin | BCL2 | Prostate_cancer |
| Diosmin | BCL2 | Small_cell_lung_cancer |
| Diosmin | BCL2 | Protein_processing_in_endoplasmic_reticulum |
| Diosmin | NFKB1 | Ras_signaling |
| Diosmin | PIK3CA | Ras_signaling |
| Diosmin | PIK3R1 | Ras_signaling |
| Diosmin | PIK3CA | Rap1_signaling |
| Diosmin | PIK3R1 | Rap1_signaling |
| Diosmin | BECN1 | Apelin_signaling |
| Diosmin | ICAM1 | NF-kappa_B_signaling |
| Diosmin | IL1B | NF-kappa_B_signaling |
| Diosmin | NFKB1 | NF-kappa_B_signaling |
| Diosmin | PTGS2 | NF-kappa_B_signaling |
| Diosmin | BCL2 | NF-kappa_B_signaling |
| Diosmin | VCAM1 | NF-kappa_B_signaling |
| Diosmin | ICAM1 | TNF_signaling |
| Diosmin | IL1B | TNF_signaling |
| Diosmin | IL6 | TNF_signaling |
| Diosmin | MMP9 | TNF_signaling |
| Diosmin | NFKB1 | TNF_signaling |
| Diosmin | PIK3CA | TNF_signaling |
| Diosmin | PIK3R1 | TNF_signaling |
| Diosmin | PTGS2 | TNF_signaling |
| Diosmin | VCAM1 | TNF_signaling |
| Diosmin | CASP3 | TNF_signaling |
| Diosmin | CDKN1A | HIF-1_signaling |
| Diosmin | CDKN1B | HIF-1_signaling |
| Diosmin | IL6 | HIF-1_signaling |
| Diosmin | NFKB1 | HIF-1_signaling |
| Diosmin | PIK3CA | HIF-1_signaling |
| Diosmin | PIK3R1 | HIF-1_signaling |
| Diosmin | BCL2 | HIF-1_signaling |
| Diosmin | STAT3 | HIF-1_signaling |
| Diosmin | CDKN1A | FoxO_signaling |
| Diosmin | CDKN1B | FoxO_signaling |
| Diosmin | IL6 | FoxO_signaling |
| Diosmin | PIK3CA | FoxO_signaling |
| Diosmin | PIK3R1 | FoxO_signaling |
| Diosmin | STAT3 | FoxO_signaling |
| Diosmin | PIK3CA | Phospholipase_D_signaling |
| Diosmin | PIK3R1 | Phospholipase_D_signaling |
| Diosmin | NFKB1 | Sphingolipid_signaling |
| Diosmin | PIK3CA | Sphingolipid_signaling |
| Diosmin | PIK3R1 | Sphingolipid_signaling |
| Diosmin | BCL2 | Sphingolipid_signaling |
| Diosmin | TP53 | Sphingolipid_signaling |
| Diosmin | NFKB1 | cAMP_signaling |
| Diosmin | PIK3CA | cAMP_signaling |
| Diosmin | PIK3R1 | cAMP_signaling |
| Diosmin | CDKN1A | PI3K-Akt_signaling |
| Diosmin | CDKN1B | PI3K-Akt_signaling |
| Diosmin | IL2 | PI3K-Akt_signaling |
| Diosmin | IL6 | PI3K-Akt_signaling |
| Diosmin | JAK2 | PI3K-Akt_signaling |
| Diosmin | NFKB1 | PI3K-Akt_signaling |
| Diosmin | BCL2 | PI3K-Akt_signaling |
| Diosmin | TP53 | PI3K-Akt_signaling |
| Diosmin | CASP9 | PI3K-Akt_signaling |
| Diosmin | PIK3CA | AMPK_signaling |
| Diosmin | PIK3R1 | AMPK_signaling |
| Diosmin | JAK2 | pathways_regulating_pluripotency_of_stem_cells |
| Diosmin | PIK3CA | pathways_regulating_pluripotency_of_stem_cells |
| Diosmin | PIK3R1 | pathways_regulating_pluripotency_of_stem_cells |
| Diosmin | STAT3 | pathways_regulating_pluripotency_of_stem_cells |
| Diosmin | PTGS2 | Retrograde_endocannabinoid_signaling |
| Diosmin | IL1B | Inflammatory_mediator_regulation_of_TRP_channels |
| Diosmin | PIK3CA | Inflammatory_mediator_regulation_of_TRP_channels |
| Diosmin | PIK3R1 | Inflammatory_mediator_regulation_of_TRP_channels |
| Diosmin | IL1B | Osteoclast_differentiation |
| Diosmin | NFKB1 | Osteoclast_differentiation |
| Diosmin | PIK3CA | Osteoclast_differentiation |
| Diosmin | PIK3R1 | Osteoclast_differentiation |
| Diosmin | NFKB1 | Longevity_regulating_pathway |
| Diosmin | PIK3CA | Longevity_regulating_pathway |
| Diosmin | PIK3R1 | Longevity_regulating_pathway |
| Diosmin | TP53 | Longevity_regulating_pathway |
| Diosmin | IL1B | Hematopoietic_cell_lineage |
| Diosmin | IL6 | Hematopoietic_cell_lineage |
| Diosmin | PIK3CA | 04611_Platelet_activation |
| Diosmin | PIK3R1 | 04611_Platelet_activation |
| Diosmin | PTGS2 | Ovarian_steroidogenesis |
| Diosmin | MMP2 | Estrogen pathway |
| Diosmin | MMP9 | Estrogen pathway |
| Diosmin | PIK3CA | Estrogen pathway |
| Diosmin | PIK3R1 | Estrogen pathway |
| Diosmin | JAK2 | Prolactin pathway |
| Diosmin | NFKB1 | Prolactin pathway |
| Diosmin | PIK3CA | Prolactin pathway |
| Diosmin | PIK3R1 | Prolactin pathway |
| Diosmin | STAT3 | Prolactin pathway |
| Diosmin | CDKN1A | Oxytocin pathway |
| Diosmin | PTGS2 | Oxytocin pathway |
| Diosmin | PIK3CA | Thyroid_hormone pathway |
| Diosmin | PIK3R1 | Thyroid_hormone pathway |
| Diosmin | TP53 | Thyroid_hormone pathway |
| Diosmin | CASP9 | Thyroid_hormone pathway |
| Diosmin | BCL2 | Adrenergic_signaling_in_cardiomyocytes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huwait, E.; Mobashir, M. Potential and Therapeutic Roles of Diosmin in Human Diseases. Biomedicines 2022, 10, 1076. https://doi.org/10.3390/biomedicines10051076
Huwait E, Mobashir M. Potential and Therapeutic Roles of Diosmin in Human Diseases. Biomedicines. 2022; 10(5):1076. https://doi.org/10.3390/biomedicines10051076
Chicago/Turabian StyleHuwait, Etimad, and Mohammad Mobashir. 2022. "Potential and Therapeutic Roles of Diosmin in Human Diseases" Biomedicines 10, no. 5: 1076. https://doi.org/10.3390/biomedicines10051076
APA StyleHuwait, E., & Mobashir, M. (2022). Potential and Therapeutic Roles of Diosmin in Human Diseases. Biomedicines, 10(5), 1076. https://doi.org/10.3390/biomedicines10051076







