Enhancing the Bioactivity of Bicyclic Peptides Targeted to Grb7-SH2 by Restoring Cell Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptide Synthesis and Concentration Determination
2.2. Protein Expression and Purification
2.3. Surface Plasmon Resonance
2.4. Crystal Structure Determination
2.5. Cell Culture and Reagents
2.6. Wound Healing Assay
3. Results
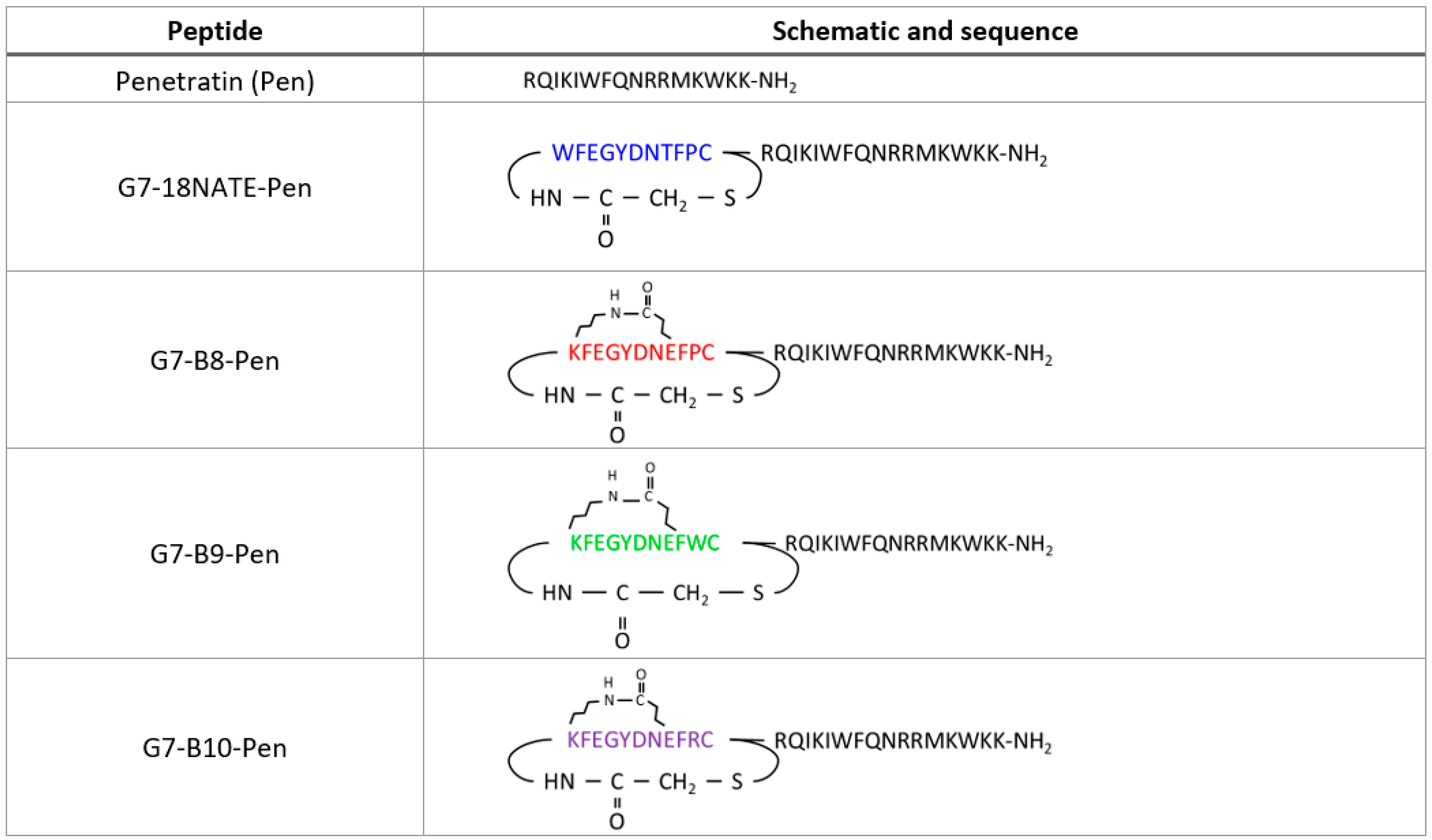
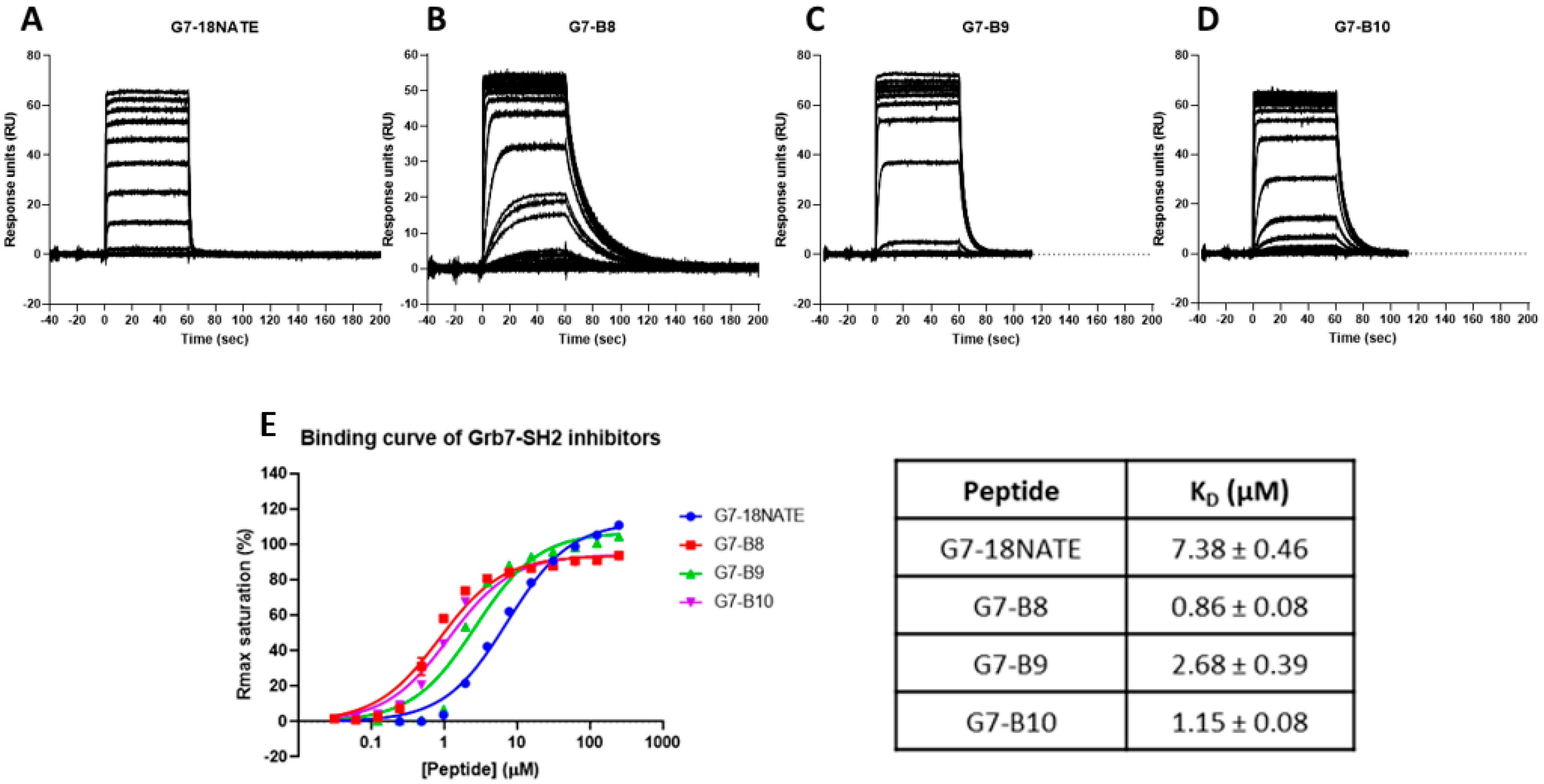
3.1. G7 Peptide Binding to Grb7-SH2
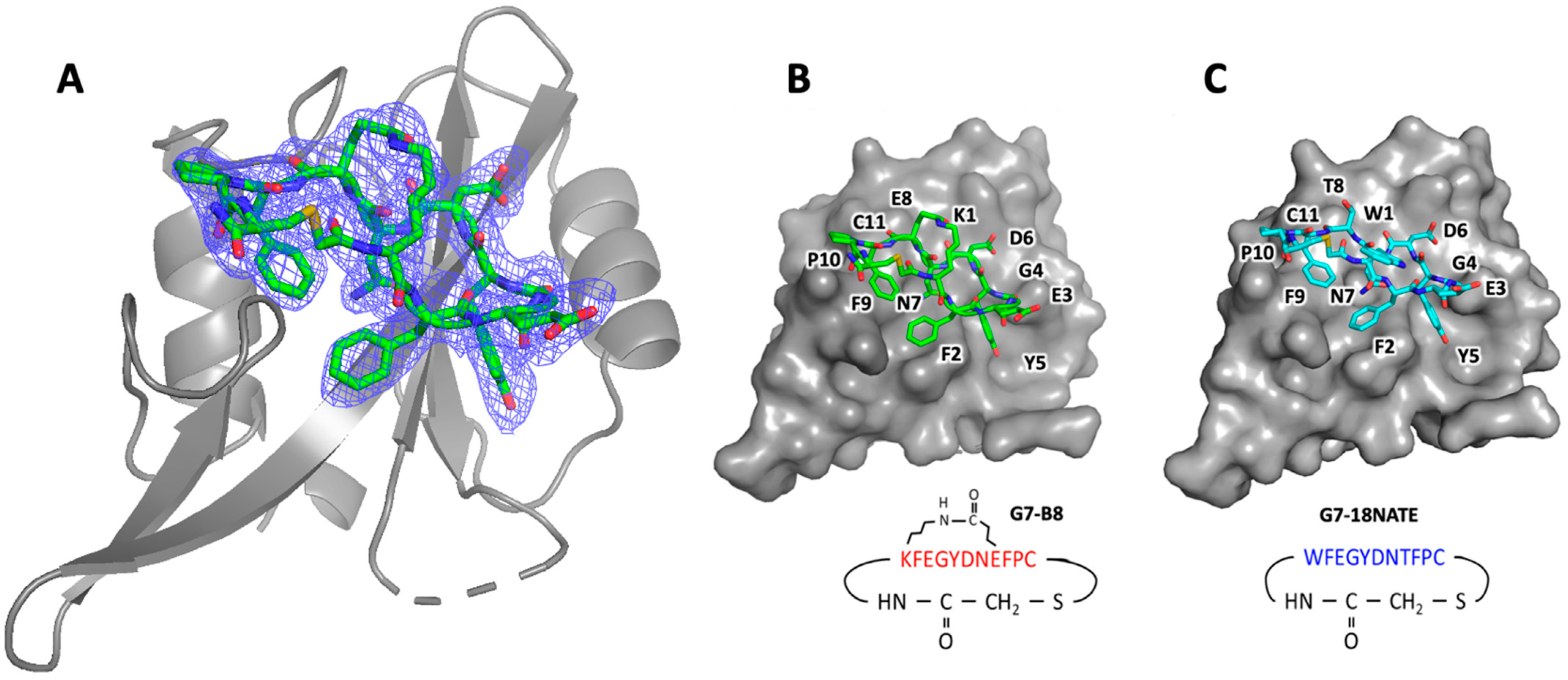
3.2. G7-B8 Binds Grb7-SH2 in a Similar Fashion to G7-18NATE
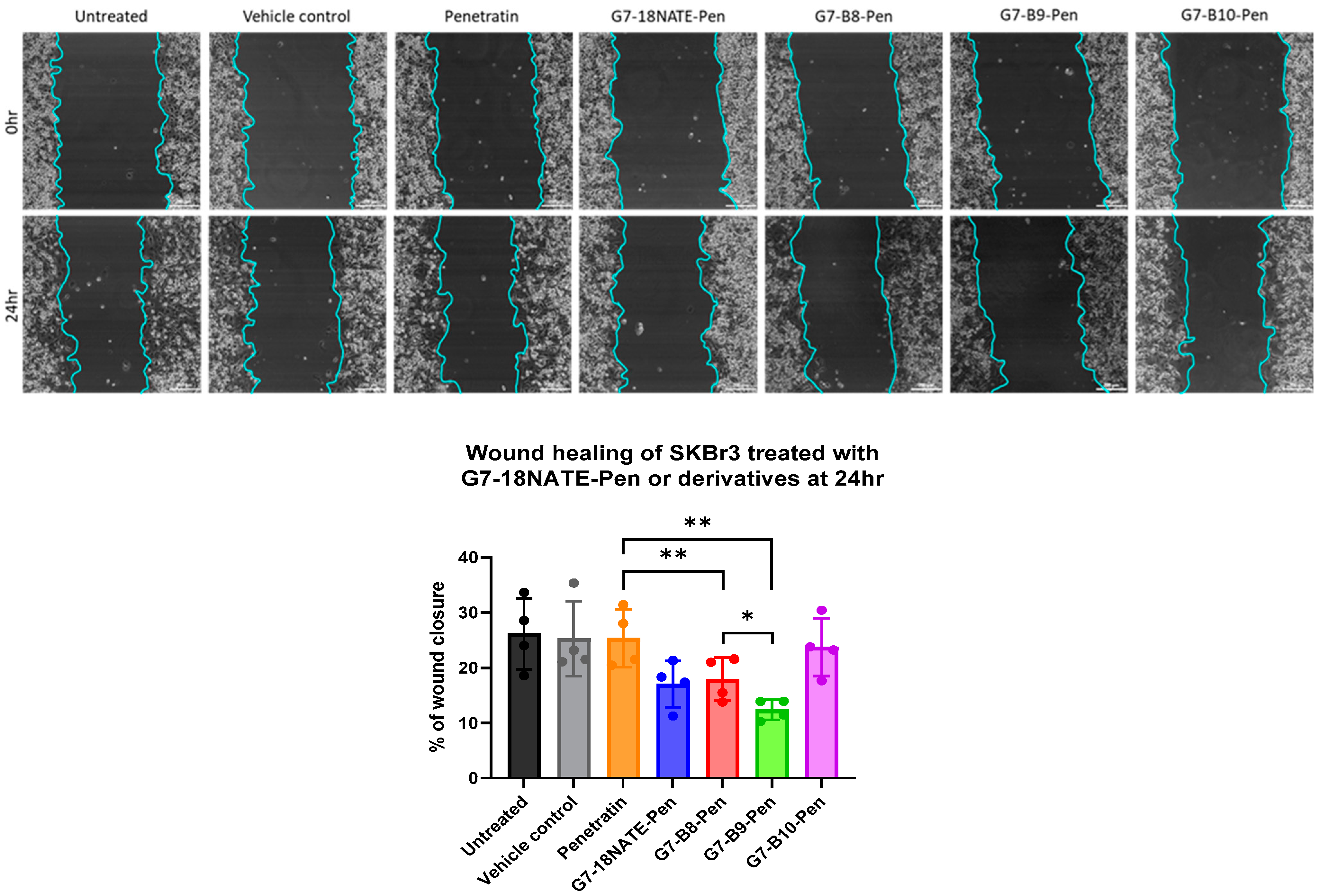
3.3. Impact of G7 Peptides on Migration of HER2+ Breast Cancer Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Ni, D.; Liu, Y.; Lu, S. Rational Design of Peptide-Based Inhibitors Disrupting Protein-Protein Interactions. Front. Chem. 2021, 9, 682675. [Google Scholar] [CrossRef]
- Ran, X.; Gestwicki, J.E. Inhibitors of protein-protein interactions (PPIs): An analysis of scaffold choices and buried surface area. Curr. Opin. Chem. Biol. 2018, 44, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Tang, R.; Hardie, J.; Farkas, M.E.; Rotello, V.M. Promises and Pitfalls of Intracellular Delivery of Proteins. Bioconjug. Chem. 2014, 25, 1602–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorko, M.; Jones, S.; Langel, Ü. Cell-penetrating peptides in protein mimicry and cancer therapeutics. Adv. Drug Deliv. Rev. 2022, 180, 114044. [Google Scholar] [CrossRef]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Lim, R.C.; Price, J.T.; Wilce, J.A. Context-dependent role of Grb7 in HER2+ve and triple-negative breast cancer cell lines. Breast Cancer Res. Treat. 2014, 143, 593–603. [Google Scholar] [CrossRef]
- Pradip, D.; Bouzyk, M.; Dey, N.; Leyland-Jones, B. Dissecting GRB7-mediated signals for proliferation and migration in HER2 overexpressing breast tumor cells: GTP-ase rules. Am. J. Cancer Res. 2013, 3, 173–195. [Google Scholar] [PubMed]
- Ramsey, B.; Bai, T.; Hanlon Newell, A.; Troxell, M.; Park, B.; Olson, S.; Keenan, E.; Luoh, S.W. GRB7 protein over-expression and clinical outcome in breast cancer. Breast Cancer Res. Treat. 2011, 127, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.; Wu, J.; Fuqua, S.A.; Roonprapunt, C.; Yajnik, V.; D’Eustachio, P.; Moskow, J.J.; Buchberg, A.M.; Osborne, C.K.; Margolis, B. The SH2 domain protein GRB-7 is co-amplified, overexpressed and in a tight complex with HER2 in breast cancer. EMBO J. 1994, 13, 1331–1340. [Google Scholar] [CrossRef]
- Chu, P.Y.; Tai, Y.L.; Shen, T.L. Grb7, a Critical Mediator of EGFR/ErbB Signaling, in Cancer Development and as a Potential Therapeutic Target. Cells 2019, 8, 435. [Google Scholar] [CrossRef] [Green Version]
- Nencioni, A.; Cea, M.; Garuti, A.; Passalacqua, M.; Raffaghello, L.; Soncini, D.; Moran, E.; Zoppoli, G.; Pistoia, V.; Patrone, F.; et al. Grb7 upregulation is a molecular adaptation to HER2 signaling inhibition due to removal of Akt-mediated gene repression. PLoS ONE 2010, 5, e9024. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.Y.; Li, T.K.; Ding, S.T.; Lai, I.R.; Shen, T.L. EGF-induced Grb7 recruits and promotes Ras activity essential for the tumorigenicity of Sk-Br3 breast cancer cells. J. Biol. Chem. 2010, 285, 29279–29285. [Google Scholar] [CrossRef] [Green Version]
- Fiddes, R.J.; Campbell, D.H.; Janes, P.W.; Sivertsen, S.P.; Sasaki, H.; Wallasch, C.; Daly, R.J. Analysis of Grb7 recruitment by heregulin-activated erbB receptors reveals a novel target selectivity for erbB3. J. Biol. Chem. 1998, 273, 7717–7724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, D.C.; Shen, T.L.; Guan, J.L. Role of Grb7 targeting to focal contacts and its phosphorylation by focal adhesion kinase in regulation of cell migration. J. Biol. Chem. 2000, 275, 28911–28917. [Google Scholar] [CrossRef] [Green Version]
- Chu, P.-Y.; Huang, L.-Y.; Hsu, C.-H.; Liang, C.-C.; Guan, J.-L.; Hung, T.-H.; Shen, T.-L. Tyrosine Phosphorylation of Growth Factor Receptor-bound Protein-7 by Focal Adhesion Kinase in the Regulation of Cell Migration, Proliferation, and Tumorigenesis. J. Biol. Chem. 2009, 284, 20215–20226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margolis, B.; Silvennoinen, O.; Comoglio, F.; Roonprapunt, C.; Skolnik, E.; Ullrich, A.; Schlessinger, J. High-efficiency expression/cloning of epidermal growth factor-receptor-binding proteins with Src homology 2 domains. Proc. Natl. Acad. Sci. USA 1992, 89, 8894–8898. [Google Scholar] [CrossRef] [Green Version]
- Janes, P.W.; Lackmann, M.; Church, W.B.; Sanderson, G.M.; Sutherland, R.L.; Daly, R.J. Structural determinants of the interaction between the erbB2 receptor and the Src homology 2 domain of Grb7. J. Biol. Chem. 1997, 272, 8490–8497. [Google Scholar] [CrossRef] [Green Version]
- Han, D.C.; Guan, J.-L. Association of Focal Adhesion Kinase with Grb7 and Its Role in Cell Migration. J. Biol. Chem. 1999, 274, 24425–24430. [Google Scholar] [CrossRef] [Green Version]
- Pero, S.C.; Oligino, L.; Daly, R.J.; Soden, A.L.; Liu, C.; Roller, P.P.; Li, P.; Krag, D.N. Identification of novel non-phosphorylated ligands, which bind selectively to the SH2 domain of Grb7. J. Biol. Chem. 2002, 277, 11918–11926. [Google Scholar] [CrossRef] [Green Version]
- Gunzburg, M.J.; Ambaye, N.D.; Del Borgo, M.P.; Pero, S.C.; Krag, D.N.; Wilce, M.C.; Wilce, J.A. Interaction of the non-phosphorylated peptide G7-18NATE with Grb7-SH2 domain requires phosphate for enhanced affinity and specificity. J. Mol. Recognit. 2012, 25, 57–67. [Google Scholar] [CrossRef]
- Tanaka, S.; Pero, S.C.; Taguchi, K.; Shimada, M.; Mori, M.; Krag, D.N.; Arii, S. Specific Peptide Ligand for Grb7 Signal Transduction Protein and Pancreatic Cancer Metastasis. JNCI J. Natl. Cancer Inst. 2006, 98, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, G.M.; Kulkarni, K.; Sang, J.; Ma, X.; Gunzburg, M.J.; Perlmutter, P.; Wilce, M.C.J.; Wilce, J.A. Discovery, Development, and Cellular Delivery of Potent and Selective Bicyclic Peptide Inhibitors of Grb7 Cancer Target. J. Med. Chem. 2017, 60, 9349–9359. [Google Scholar] [CrossRef]
- Gunzburg, M.J.; Kulkarni, K.; Watson, G.M.; Ambaye, N.D.; Del Borgo, M.P.; Brandt, R.; Pero, S.C.; Perlmutter, P.; Wilce, M.C.J.; Wilce, J.A. Unexpected involvement of staple leads to redesign of selective bicyclic peptide inhibitor of Grb7. Sci. Rep. 2016, 6, 27060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, J.; Kulkarni, K.; Watson, G.M.; Ma, X.; Craik, D.J.; Henriques, S.T.; Poth, A.G.; Benfield, A.H.; Wilce, J.A. Evaluation of Cyclic Peptide Inhibitors of the Grb7 Breast Cancer Target: Small Change in Cargo Results in Large Change in Cellular Activity. Molecules 2019, 24, 3739. [Google Scholar] [CrossRef] [Green Version]
- Bechara, C.; Sagan, S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013, 587, 1693–1702. [Google Scholar] [CrossRef]
- Watson, G.M.; Gunzburg, M.J.; Ambaye, N.D.; Lucas, W.A.; Traore, D.A.; Kulkarni, K.; Cergol, K.M.; Payne, R.J.; Panjikar, S.; Pero, S.C.; et al. Cyclic Peptides Incorporating Phosphotyrosine Mimetics as Potent and Specific Inhibitors of the Grb7 Breast Cancer Target. J. Med. Chem. 2015, 58, 7707–7718. [Google Scholar] [CrossRef] [PubMed]
- McPhillips, T.M.; McPhillips, S.E.; Chiu, H.J.; Cohen, A.E.; Deacon, A.M.; Ellis, P.J.; Garman, E.; Gonzalez, A.; Sauter, N.K.; Phizackerley, R.P.; et al. Blu-Ice and the Distributed Control System: Software for data acquisition and instrument control at macromolecular crystallography beamlines. J. Synchrotron Radiat. 2002, 9, 401–406. [Google Scholar] [CrossRef]
- Beilsten-Edmands, J.; Winter, G.; Gildea, R.; Parkhurst, J.; Waterman, D.; Evans, G. Scaling diffraction data in the DIALS software package: Algorithms and new approaches for multi-crystal scaling. Acta Crystallogr. Sect. D Struct. Biol. 2020, 76, 385–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, P.R.; Murshudov, G.N. How good are my data and what is the resolution? Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 1204–1214. [Google Scholar] [CrossRef]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Vagin, A.; Teplyakov, A. Molecular replacement with MOLREP. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Afonine, P.V.; Grosse-Kunstleve, R.W.; Echols, N.; Headd, J.J.; Moriarty, N.W.; Mustyakimov, M.; Terwilliger, T.C.; Urzhumtsev, A.; Zwart, P.H.; Adams, P.D. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 352–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, G.M.; Lucas, W.A.H.; Gunzburg, M.J.; Wilce, J.A. Insight into the Selectivity of the G7-18NATE Inhibitor Peptide for the Grb7-SH2 Domain Target. Front. Mol. Biosci. 2017, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Gunzburg, M.J.; Ambaye, N.D.; Del Borgo, M.P.; Perlmutter, P.; Wilce, J.A. Design and testing of bicyclic inhibitors of Grb7—Are two cycles better than one? Biopolymers 2013, 100, 543–549. [Google Scholar] [CrossRef]
- Ambaye, N.D.; Pero, S.C.; Gunzburg, M.J.; Yap, M.; Clayton, D.J.; Del Borgo, M.P.; Perlmutter, P.; Aguilar, M.I.; Shukla, G.S.; Peletskaya, E.; et al. Structural basis of binding by cyclic nonphosphorylated peptide antagonists of Grb7 implicated in breast cancer progression. J. Mol. Biol. 2011, 412, 397–411. [Google Scholar] [CrossRef]
- Nadler, Y.; Gonzalez, A.M.; Camp, R.L.; Rimm, D.L.; Kluger, H.M.; Kluger, Y. Growth factor receptor-bound protein-7 (Grb7) as a prognostic marker and therapeutic target in breast cancer. Ann. Oncol. 2010, 21, 466–473. [Google Scholar] [CrossRef]
- Sparano, J.A.; Goldstein, L.J.; Childs, B.H.; Shak, S.; Brassard, D.; Badve, S.; Baehner, F.L.; Bugarini, R.; Rowley, S.; Perez, E.A.; et al. Relationship between quantitative GRB7 RNA expression and recurrence after adjuvant anthracycline chemotherapy in triple-negative breast cancer. Clin. Cancer Res. 2011, 17, 7194–7203. [Google Scholar] [CrossRef] [Green Version]
- Giricz, O.; Calvo, V.; Pero, S.C.; Krag, D.N.; Sparano, J.A.; Kenny, P.A. GRB7 is required for triple-negative breast cancer cell invasion and survival. Breast Cancer Res. Treat. 2012, 133, 607–615. [Google Scholar] [CrossRef]
- Dougherty, P.G.; Sahni, A.; Pei, D. Understanding Cell Penetration of Cyclic Peptides. Chem. Rev. 2019, 119, 10241–10287. [Google Scholar] [CrossRef]
- Jobin, M.L.; Blanchet, M.; Henry, S.; Chaignepain, S.; Manigand, C.; Castano, S.; Lecomte, S.; Burlina, F.; Sagan, S.; Alves, I.D. The role of tryptophans on the cellular uptake and membrane interaction of arginine-rich cell penetrating peptides. Biochim. Biophys. Acta 2015, 1848, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Walrant, A.; Bauzá, A.; Girardet, C.; Alves, I.D.; Lecomte, S.; Illien, F.; Cardon, S.; Chaianantakul, N.; Pallerla, M.; Burlina, F.; et al. Ionpair-π interactions favor cell penetration of arginine/tryptophan-rich cell-penetrating peptides. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183098. [Google Scholar] [CrossRef] [PubMed]
| Grb7-SH2/G7-B8 | |
|---|---|
| Data collection | |
| Wavelength (Å) | 0.954 |
| Space group | P1 |
| Unit cell dimensions | |
| a, b, c (Å) | 42.16, 53.12, 54.81 |
| α, β, γ (°) | 103.98, 102.00, 100.05 |
| Resolution (Å) | 42.5–2.55 (2.64–2.55) |
| † Rmerge (%) | 0.2832 (0.7805) |
| Wilson B factor | 35.42 |
| CC (1/2) (%) | 0.912 (0.564) |
| I/σI | 8.33 (1.28) |
| Unique reflections measured | 14071 (1408) |
| Completeness (%) | 98.18% (97.56%) |
| Multiplicity | 4.2 (4.3) |
| Refinement | |
| Rwork (%) | 0.22 (0.27) |
| Rfree (%) | 0.26 (0.38) |
| No. of atoms | |
| Macromolecules | 3193 |
| Ligands | 20 |
| Solvent | 52 |
| Mean B-factors (Å2) | |
| Macromolecules | 40.56 |
| Ligands | 37.22 |
| Solvent | 35.62 |
| RMSDs | |
| Bond lengths (Å) | 0.002 |
| Bond angles (°) | 0.51 |
| Ramachandran plot (%) | |
| Favoured regions | 99.00 |
| Outliers | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sturre, N.P.; Colson, R.N.; Shah, N.; Watson, G.M.; Yang, X.; Wilce, M.C.J.; Price, J.T.; Wilce, J.A. Enhancing the Bioactivity of Bicyclic Peptides Targeted to Grb7-SH2 by Restoring Cell Permeability. Biomedicines 2022, 10, 1145. https://doi.org/10.3390/biomedicines10051145
Sturre NP, Colson RN, Shah N, Watson GM, Yang X, Wilce MCJ, Price JT, Wilce JA. Enhancing the Bioactivity of Bicyclic Peptides Targeted to Grb7-SH2 by Restoring Cell Permeability. Biomedicines. 2022; 10(5):1145. https://doi.org/10.3390/biomedicines10051145
Chicago/Turabian StyleSturre, Natasha P., Rhys N. Colson, Neelam Shah, Gabrielle M. Watson, Xue Yang, Matthew C. J. Wilce, John T. Price, and Jacqueline A. Wilce. 2022. "Enhancing the Bioactivity of Bicyclic Peptides Targeted to Grb7-SH2 by Restoring Cell Permeability" Biomedicines 10, no. 5: 1145. https://doi.org/10.3390/biomedicines10051145







