New Treatment Targets and Innovative Lipid-Lowering Therapies in Very-High-Risk Patients with Cardiovascular Disease
Abstract
:1. Introduction
Novel Aspects of This Review
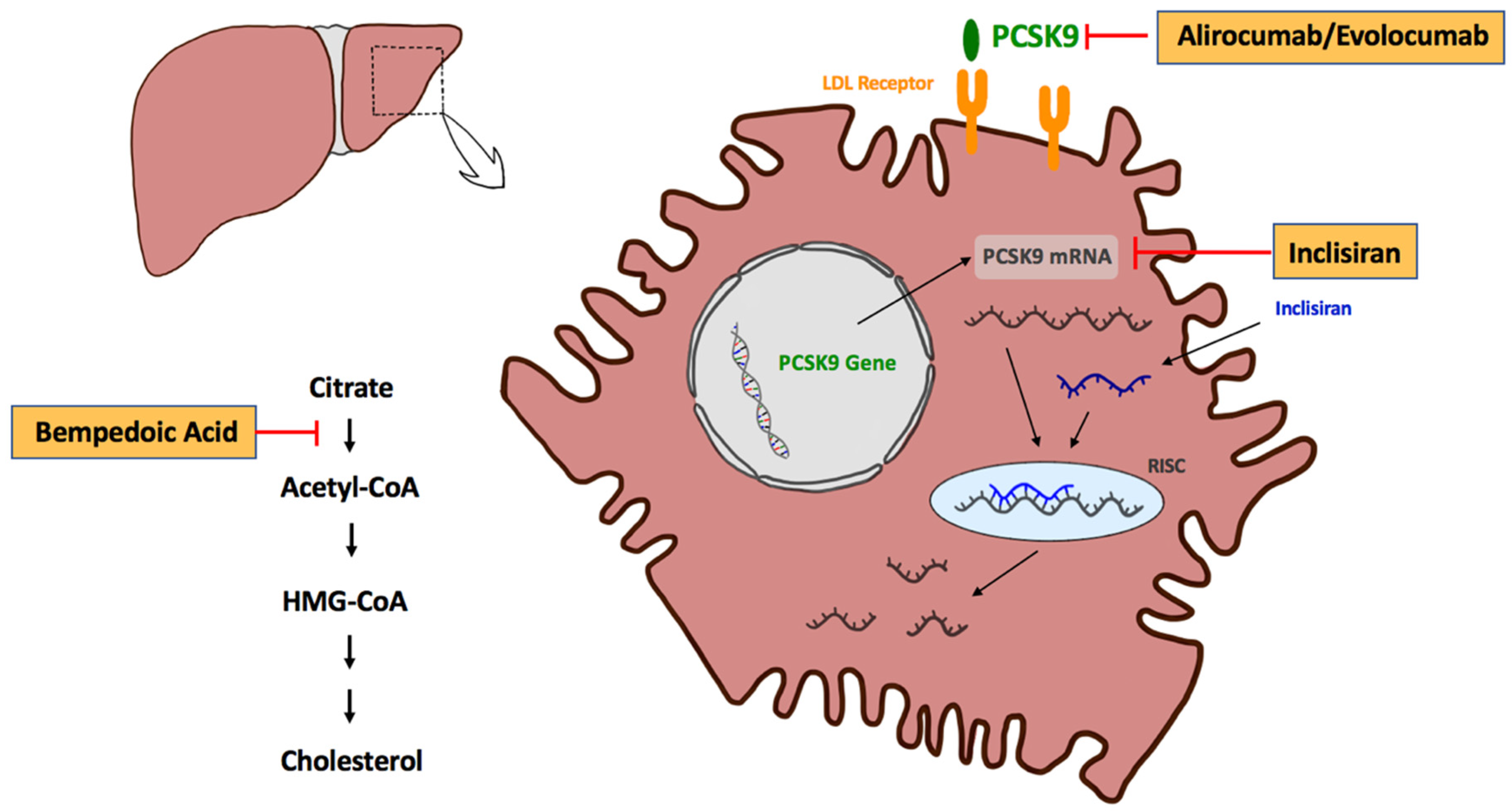
2. Inhibition of PCSK9 for LDL-C Reduction
3. Monoclonal Antibodies for PCSK9 Inhibition—Alirocumab and Evolocumab
4. Inclisiran
5. Bempedoic Acid
6. Pleiotropic Effects beyond LDL-C Reduction
7. LDL-C Treatment Targets—The Lower the Better
8. Importance of Early Reduction below Treatment Target—“Strike Effective and Strong”
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abbott, R.D.; Wilson, P.W.; Kannel, W.B.; Castelli, W.P. High density lipoprotein cholesterol, total cholesterol screening, and myocardial infarction. The Framingham Study. Arteriosclerosis 1988, 8, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Yoo, W.; Alesh, I.; Mahajan, N.; Mirowska, K.K.; Mewada, A.; Kahn, J.; Afonso, L.; Williams, K.A.; Flack, J.M. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: A Mendelian randomization analysis. J. Am. Coll. Cardiol. 2012, 60, 2631–2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Koskinas, K.C.; Siontis, G.C.M.; Piccolo, R.; Mavridis, D.; Räber, L.; Mach, F.; Windecker, S. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: A meta-analysis of randomized trials. Eur. Heart J. 2018, 39, 1172–1180. [Google Scholar] [CrossRef]
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389.
- Koren, M.J. Statin use in a “real-world” clinical setting: Aggressive lipid lowering compared with usual care in the Aggressive Lipid-Lowering Initiation Abates New Cardiac Events (ALLIANCE) trial. Am. J. Med. 2005, 118 (Suppl. S12), 16–21. [Google Scholar] [CrossRef] [PubMed]
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 7–22. [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Ray, K.K.; Bays, H.E.; Catapano, A.L.; Lalwani, N.D.; Bloedon, L.T.; Sterling, L.R.; Robinson, P.L.; Ballantyne, C.M. Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol. N. Engl. J. Med. 2019, 380, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Urban, D.; Pöss, J.; Böhm, M.; Laufs, U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J. Am. Coll. Cardiol. 2013, 62, 1401–1408. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, N.; Phan, B.A.; Ding, Y.; Fong, A.; Krauss, R.M. Proprotein convertase subtilisin/kexin type 9 inhibition: A new therapeutic mechanism for reducing cardiovascular disease risk. Circulation 2015, 132, 1648–1666. [Google Scholar] [CrossRef]
- Dietschy, J.M.; Turley, S.D.; Spady, D.K. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid Res. 1993, 34, 1637–1659. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lagace, T.A.; McNutt, M.C.; Horton, J.D.; Deisenhofer, J. Molecular basis for LDL receptor recognition by PCSK9. Proc. Natl. Acad. Sci. USA 2008, 105, 1820–1825. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Henry, L.; Ho, Y.K.; Pownall, H.J.; Rudenko, G. Mechanism of LDL binding and release probed by structure-based mutagenesis of the LDL receptor. J. Lipid Res. 2010, 51, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Nassoury, N.; Blasiole, D.A.; Tebon Oler, A.; Benjannet, S.; Hamelin, J.; Poupon, V.; McPherson, P.S.; Attie, A.D.; Prat, A.; Seidah, N.G. The cellular trafficking of the secretory proprotein convertase PCSK9 and its dependence on the LDLR. Traffic 2007, 8, 718–732. [Google Scholar] [CrossRef]
- Norata, G.D.; Tibolla, G.; Catapano, A.L. Targeting PCSK9 for hypercholesterolemia. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 273–293. [Google Scholar] [CrossRef] [PubMed]
- PRALUENT—Summary of Produc Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/praluent-epar-product-information_en.pdf (accessed on 12 March 2022).
- Toth, P.P.; Worthy, G.; Gandra, S.R.; Sattar, N.; Bray, S.; Cheng, L.I.; Bridges, I.; Worth, G.M.; Dent, R.; Forbes, C.A.; et al. Systematic Review and Network Meta-Analysis on the Efficacy of Evolocumab and Other Therapies for the Management of Lipid Levels in Hyperlipidemia. J. Am. Heart Assoc. 2017, 6, e005367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Leiter, L.A.; Colhoun, H.M.; Thompson, D.; Bessac, L.; Pordy, R.; Toth, P.P. Alirocumab Treatment and Achievement of Non-High-Density Lipoprotein Cholesterol and Apolipoprotein B Goals in Patients With Hypercholesterolemia: Pooled Results From 10 Phase 3 ODYSSEY Trials. J. Am. Heart Assoc. 2017, 6, e005639. [Google Scholar] [CrossRef]
- Szarek, M.; Bittner, V.A.; Aylward, P.; Baccara-Dinet, M.; Bhatt, D.L.; Diaz, R.; Fras, Z.; Goodman, S.G.; Halvorsen, S.; Harrington, R.A.; et al. Lipoprotein(a) lowering by alirocumab reduces the total burden of cardiovascular events independent of low-density lipoprotein cholesterol lowering: ODYSSEY OUTCOMES trial. Eur. Heart J. 2020, 41, 4245–4255. [Google Scholar] [CrossRef]
- Jukema, J.W.; Zijlstra, L.E.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Drexel, H.; Goodman, S.G.; Kim, Y.U.; Pordy, R.; Reiner, Ž.; et al. Effect of Alirocumab on Stroke in ODYSSEY OUTCOMES. Circulation 2019, 140, 2054–2062. [Google Scholar] [CrossRef]
- Szarek, M.; White, H.D.; Schwartz, G.G.; Alings, M.; Bhatt, D.L.; Bittner, V.A.; Chiang, C.E.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; et al. Alirocumab Reduces Total Nonfatal Cardiovascular and Fatal Events: The ODYSSEY OUTCOMES Trial. J. Am. Coll. Cardiol. 2019, 73, 387–396. [Google Scholar] [CrossRef]
- REPATHA—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/repatha-epar-product-information_en.pdf (accessed on 12 March 2022).
- Koren, M.J.; Lundqvist, P.; Bolognese, M.; Neutel, J.M.; Monsalvo, M.L.; Yang, J.; Kim, J.B.; Scott, R.; Wasserman, S.M.; Bays, H. Anti-PCSK9 monotherapy for hypercholesterolemia: The MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J. Am. Coll. Cardiol. 2014, 63, 2531–2540. [Google Scholar] [CrossRef] [Green Version]
- Stroes, E.; Colquhoun, D.; Sullivan, D.; Civeira, F.; Rosenson, R.S.; Watts, G.F.; Bruckert, E.; Cho, L.; Dent, R.; Knusel, B.; et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: The GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J. Am. Coll. Cardiol. 2014, 63, 2541–2548. [Google Scholar] [CrossRef] [Green Version]
- Cannon, C.P.; Cariou, B.; Blom, D.; McKenney, J.M.; Lorenzato, C.; Pordy, R.; Chaudhari, U.; Colhoun, H.M. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: The ODYSSEY COMBO II randomized controlled trial. Eur. Heart J. 2015, 36, 1186–1194. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.G.; Nedergaard, B.S.; Rogers, W.J.; Fialkow, J.; Neutel, J.M.; Ramstad, D.; Somaratne, R.; Legg, J.C.; Nelson, P.; Scott, R.; et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: The LAPLACE-2 randomized clinical trial. JAMA 2014, 311, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Mach, F.; Zavitz, K.; Kurtz, C.; Im, K.; Kanevsky, E.; Schneider, J.; Wang, H.; Keech, A.; Pedersen, T.R.; et al. Cognitive Function in a Randomized Trial of Evolocumab. N. Engl. J. Med. 2017, 377, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Raccah, H.B.; Yanovsky, A.; Treves, N.; Rotshild, V.; Renoux, C.; Danenberg, H.; Eliaz, R.; Matok, I. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) inhibitors and the risk for neurocognitive adverse events: A systematic review, meta-analysis and meta-regression. Int. J. Cardiol. 2021, 335, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Fonarow, G.C.; Keech, A.C.; Pedersen, T.R.; Giugliano, R.P.; Sever, P.S.; Lindgren, P.; van Hout, B.; Villa, G.; Qian, Y.; Somaratne, R.; et al. Cost-effectiveness of Evolocumab Therapy for Reducing Cardiovascular Events in Patients With Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2017, 2, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Korman, M.; Wisløff, T. Modelling the cost-effectiveness of PCSK9 inhibitors vs. ezetimibe through LDL-C reductions in a Norwegian setting. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Fonarow, G.C.; van Hout, B.; Villa, G.; Arellano, J.; Lindgren, P. Updated Cost-effectiveness Analysis of Evolocumab in Patients With Very High-risk Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2019, 4, 691–695. [Google Scholar] [CrossRef] [Green Version]
- Kam, N.; Perera, K.; Zomer, E.; Liew, D.; Ademi, Z. Inclisiran as Adjunct Lipid-Lowering Therapy for Patients with Cardiovascular Disease: A Cost-Effectiveness Analysis. PharmacoEconomics 2020, 38, 1007–1020. [Google Scholar] [CrossRef]
- Azari, S.; Rezapour, A.; Omidi, N.; Alipour, V.; Behzadifar, M.; Safari, H.; Tajdini, M.; Bragazzi, N.L. Cost-effectiveness analysis of PCSK9 inhibitors in cardiovascular diseases: A systematic review. Heart Fail. Rev. 2020, 25, 1077–1088. [Google Scholar] [CrossRef]
- Fitzgerald, K.; Frank-Kamenetsky, M.; Shulga-Morskaya, S.; Liebow, A.; Bettencourt, B.R.; Sutherland, J.E.; Hutabarat, R.M.; Clausen, V.A.; Karsten, V.; Cehelsky, J.; et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: A randomised, single-blind, placebo-controlled, phase 1 trial. Lancet 2014, 383, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, K.; White, S.; Borodovsky, A.; Bettencourt, B.R.; Strahs, A.; Clausen, V.; Wijngaard, P.; Horton, J.D.; Taubel, J.; Brooks, A.; et al. A Highly Durable RNAi Therapeutic Inhibitor of PCSK9. N. Engl. J. Med. 2017, 376, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Frank-Kamenetsky, M.; Grefhorst, A.; Anderson, N.N.; Racie, T.S.; Bramlage, B.; Akinc, A.; Butler, D.; Charisse, K.; Dorkin, R.; Fan, Y.; et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc. Natl. Acad. Sci. USA 2008, 105, 11915–11920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.J.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- A Randomized Trial Assessing the Effects of Inclisiran on Clinical Outcomes among People with Cardiovascular Disease (ORION-4). ClinicalTrials.gov Identifier: NCT03705234. Available online: https://clinicaltrials.gov/ct2/show/NCT03705234 (accessed on 29 January 2022).
- Khan, S.A.; Naz, A.; Qamar Masood, M.; Shah, R. Meta-Analysis of Inclisiran for the Treatment of Hypercholesterolemia. Am. J. Cardiol. 2020, 134, 69–73. [Google Scholar] [CrossRef]
- An Extension Trial of Inclisiran Compared to Evolocumab in Participants with Cardiovascular Disease and High Cholesterol (ORION-3). ClinicalTrials.gov Identifier: NCT03060577. Available online: https://clinicaltrials.gov/ct2/show/NCT03060577 (accessed on 27 March 2022).
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.; Turner, T.; Visseren, F.L.; et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef] [Green Version]
- Pinkosky, S.L.; Newton, R.S.; Day, E.A.; Ford, R.J.; Lhotak, S.; Austin, R.C.; Birch, C.M.; Smith, B.K.; Filippov, S.; Groot, P.H.E.; et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat. Commun. 2016, 7, 13457. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, A.C.; Leiter, L.A.; Stroes, E.S.G.; Baum, S.J.; Hanselman, J.C.; Bloedon, L.T.; Lalwani, N.D.; Patel, P.M.; Zhao, X.; Duell, P.B. Effect of Bempedoic Acid vs Placebo Added to Maximally Tolerated Statins on Low-Density Lipoprotein Cholesterol in Patients at High Risk for Cardiovascular Disease: The CLEAR Wisdom Randomized Clinical Trial. JAMA 2019, 322, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Laufs, U.; Banach, M.; Mancini, G.B.J.; Gaudet, D.; Bloedon, L.T.; Sterling, L.R.; Kelly, S.; Stroes, E.S.G. Efficacy and Safety of Bempedoic Acid in Patients with Hypercholesterolemia and Statin Intolerance. J. Am. Heart Assoc. 2019, 8, e011662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballantyne, C.M.; Banach, M.; Mancini, G.B.J.; Lepor, N.E.; Hanselman, J.C.; Zhao, X.; Leiter, L.A. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: A randomized, placebo-controlled study. Atherosclerosis 2018, 277, 195–203. [Google Scholar] [CrossRef]
- Evaluation of Major Cardiovascular Events in Patients with, or at High Risk for, Cardiovascular Disease Who Are Statin Intolerant Treated with Bempedoic Acid (ETC-1002) or Placebo (CLEAR Outcomes). NCT02993406. Available online: https://clinicaltrials.gov/ct2/show/NCT02993406 (accessed on 28 February 2022).
- Satny, M.; Hubacek, J.A.; Vrablik, M. Statins and Inflammation. Curr. Atheroscler. Rep. 2021, 23, 80. [Google Scholar] [CrossRef]
- Davignon, J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004, 109 (Suppl. S1), Iii39–Iii43. [Google Scholar] [CrossRef] [Green Version]
- Montecucco, F.; Mach, F. Update on statin-mediated anti-inflammatory activities in atherosclerosis. Semin. Immunopathol. 2009, 31, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Diomede, L.; Albani, D.; Sottocorno, M.; Donati, M.B.; Bianchi, M.; Fruscella, P.; Salmona, M. In vivo anti-inflammatory effect of statins is mediated by nonsterol mevalonate products. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1327–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridker, P.M.; Cannon, C.P.; Morrow, D.; Rifai, N.; Rose, L.M.; McCabe, C.H.; Pfeffer, M.A.; Braunwald, E. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 2005, 352, 20–28. [Google Scholar] [CrossRef]
- Ruscica, M.; Tokgözoğlu, L.; Corsini, A.; Sirtori, C.R. PCSK9 inhibition and inflammation: A narrative review. Atherosclerosis 2019, 288, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Seidah, N.G.; Awan, Z.; Chrétien, M.; Mbikay, M. PCSK9: A key modulator of cardiovascular health. Circ. Res. 2014, 114, 1022–1036. [Google Scholar] [CrossRef]
- Li, S.; Guo, Y.L.; Xu, R.X.; Zhang, Y.; Zhu, C.G.; Sun, J.; Qing, P.; Wu, N.Q.; Jiang, L.X.; Li, J.J. Association of plasma PCSK9 levels with white blood cell count and its subsets in patients with stable coronary artery disease. Atherosclerosis 2014, 234, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Jiang, L.; Peng, J.; Ren, Z.; Wei, D.; Wu, C.; Pan, L.; Jiang, Z.; Liu, L. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-κB activation in THP-1-derived macrophages. Int. J. Mol. Med. 2012, 30, 931–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giunzioni, I.; Tavori, H.; Covarrubias, R.; Major, A.S.; Ding, L.; Zhang, Y.; DeVay, R.M.; Hong, L.; Fan, D.; Predazzi, I.M.; et al. Local effects of human PCSK9 on the atherosclerotic lesion. J. Pathol. 2016, 238, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Kühnast, S.; van der Hoorn, J.W.; Pieterman, E.J.; van den Hoek, A.M.; Sasiela, W.J.; Gusarova, V.; Peyman, A.; Schäfer, H.L.; Schwahn, U.; Jukema, J.W.; et al. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J. Lipid Res. 2014, 55, 2103–2112. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 2016, 316, 2373–2384. [Google Scholar] [CrossRef]
- Imaging of Coronary Plaques in Participants Treated with Evolocumab. NCT03570697. Available online: https://clinicaltrials.gov/ct2/show/results/NCT03570697 (accessed on 27 February 2022).
- Marques, P.; Domingo, E.; Rubio, A.; Martinez-Hervás, S.; Ascaso, J.F.; Piqueras, L.; Real, J.T.; Sanz, M.J. Beneficial effects of PCSK9 inhibition with alirocumab in familial hypercholesterolemia involve modulation of new immune players. Biomed. Pharmacother. 2022, 145, 112460. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.X.; Li, S.; Liu, H.H.; Li, J.J. Impact of PCSK9 monoclonal antibodies on circulating hs-CRP levels: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2018, 8, e022348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landmesser, U.; Haghikia, A.; Leiter, L.A.; Wright, R.S.; Kallend, D.; Wijngaard, P.; Stoekenbroek, R.; Kastelein, J.J.; Ray, K.K. Effect of inclisiran, the small-interfering RNA against proprotein convertase subtilisin/kexin type 9, on platelets, immune cells, and immunological biomarkers: A pre-specified analysis from ORION-1. Cardiovasc. Res. 2021, 117, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Hernandez, A.V.; Banach, M. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003121. [Google Scholar] [CrossRef] [PubMed]
- Pinkosky, S.L.; Filippov, S.; Srivastava, R.A.; Hanselman, J.C.; Bradshaw, C.D.; Hurley, T.R.; Cramer, C.T.; Spahr, M.A.; Brant, A.F.; Houghton, J.L.; et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J. Lipid Res. 2013, 54, 134–151. [Google Scholar] [CrossRef] [Green Version]
- Filippov, S.; Pinkosky, S.L.; Lister, R.J.; Pawloski, C.; Hanselman, J.C.; Cramer, C.T.; Srivastava, R.A.K.; Hurley, T.R.; Bradshaw, C.D.; Spahr, M.A.; et al. ETC-1002 regulates immune response, leukocyte homing, and adipose tissue inflammation via LKB1-dependent activation of macrophage AMPK. J. Lipid Res. 2013, 54, 2095–2108. [Google Scholar] [CrossRef] [Green Version]
- Sanjay, K.V.; Vishwakarma, S.; Zope, B.R.; Mane, V.S.; Mohire, S.; Dhakshinamoorthy, S. ATP citrate lyase inhibitor Bempedoic Acid alleviate long term HFD induced NASH through improvement in glycemic control, reduction of hepatic triglycerides & total cholesterol, modulation of inflammatory & fibrotic genes and improvement in NAS score. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100051. [Google Scholar] [CrossRef]
- Fulcher, J.; O’Connell, R.; Voysey, M.; Emberson, J.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; Colhoun, H.; et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Pedersen, T.R.; Park, J.G.; De Ferrari, G.M.; Gaciong, Z.A.; Ceska, R.; Toth, K.; Gouni-Berthold, I.; Lopez-Miranda, J.; Schiele, F.; et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: A prespecified secondary analysis of the FOURIER trial. Lancet 2017, 390, 1962–1971. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Gabriel Steg, P.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Goodman, S.G.; Jukema, J.W.; Kim, Y.U.; Li, Q.H.; Manvelian, G.; et al. Clinical Efficacy and Safety of Alirocumab After Acute Coronary Syndrome According to Achieved Level of Low-Density Lipoprotein Cholesterol: A Propensity Score-Matched Analysis of the ODYSSEY OUTCOMES Trial. Circulation 2021, 143, 1109–1122. [Google Scholar] [CrossRef]
- Filippone, E.J.; Foy, A. The J-curve revisited: A therapeutic dilemma. Cardiol. Rev. 2012, 20, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Robinson, J.G.; Kowalewski, M.; Kolodziejczak, M.; Andreotti, F.; Bliden, K.; Tantry, U.; Kubica, J.; Raggi, P.; Gurbel, P.A. Association Between Baseline LDL-C Level and Total and Cardiovascular Mortality After LDL-C Lowering: A Systematic Review and Meta-analysis. JAMA 2018, 319, 1566–1579. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; Baigent, C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zingel, R.; Bohlken, J.; Riedel-Heller, S.; Barth, S.; Kostev, K. Association Between Low-Density Lipoprotein Cholesterol Levels, Statin Use, and Dementia in Patients followed in German General Practices. J. Alzheimer’s Dis. 2021, 79, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Benn, M.; Nordestgaard, B.G.; Frikke-Schmidt, R.; Tybjærg-Hansen, A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer’s disease and Parkinson’s disease: Mendelian randomisation study. BMJ 2017, 357, j1648. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Liang, Y.; Zhang, X.; Xu, J.; Lin, J.; Zhang, R.; Kang, K.; Liu, C.; Zhao, C.; Zhao, M. Low-Density Lipoprotein Cholesterol and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2020, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Ference, B.A.; Robinson, J.G.; Brook, R.D.; Catapano, A.L.; Chapman, M.J.; Neff, D.R.; Voros, S.; Giugliano, R.P.; Smith, D.G.; Fazio, S.; et al. Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. N. Engl. J. Med. 2016, 375, 2144–2153. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Tuakli-Wosornu, Y.; Lagace, T.A.; Kinch, L.; Grishin, N.V.; Horton, J.D.; Cohen, J.C.; Hobbs, H.H. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am. J. Hum. Genet. 2006, 79, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Linton, M.F.; Farese, R.V., Jr.; Young, S.G. Familial hypobetalipoproteinemia. J. Lipid Res. 1993, 34, 521–541. [Google Scholar] [CrossRef]
- Sankatsing, R.R.; Fouchier, S.W.; de Haan, S.; Hutten, B.A.; de Groot, E.; Kastelein, J.J.; Stroes, E.S. Hepatic and cardiovascular consequences of familial hypobetalipoproteinemia. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1979–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benn, M.; Nordestgaard, B.G.; Grande, P.; Schnohr, P.; Tybjaerg-Hansen, A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J. Am. Coll. Cardiol. 2010, 55, 2833–2842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnefond, A.; Yengo, L.; Le May, C.; Fumeron, F.; Marre, M.; Balkau, B.; Charpentier, G.; Franc, S.; Froguel, P.; Cariou, B. The loss-of-function PCSK9 p.R46L genetic variant does not alter glucose homeostasis. Diabetologia 2015, 58, 2051–2055. [Google Scholar] [CrossRef] [Green Version]
- Ray, K.K.; Molemans, B.; Schoonen, W.M.; Giovas, P.; Bray, S.; Kiru, G.; Murphy, J.; Banach, M.; De Servi, S.; Gaita, D.; et al. EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: The DA VINCI study. Eur. J. Prev. Cardiol. 2021, 28, 1279–1289. [Google Scholar] [CrossRef]
- De Backer, G.; Jankowski, P.; Kotseva, K.; Mirrakhimov, E.; Reiner, Ž.; Rydén, L.; Tokgözoğlu, L.; Wood, D.; De Bacquer, D. Management of dyslipidaemia in patients with coronary heart disease: Results from the ESC-EORP EUROASPIRE V survey in 27 countries. Atherosclerosis 2019, 285, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef] [Green Version]
- Mazalin Protulipac, J.; Sonicki, Z.; Reiner, Ž. Cardiovascular disease (CVD) risk factors in older adults—Perception and reality. Arch. Gerontol. Geriatr. 2015, 61, 88–92. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Leiter, L.A.; Wiviott, S.D.; Giugliano, R.P.; Deedwania, P.; De Ferrari, G.M.; Murphy, S.A.; Kuder, J.F.; Gouni-Berthold, I.; Lewis, B.S.; et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: A prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 941–950. [Google Scholar] [CrossRef]
- Song, J.; Murugiah, K.; Hu, S.; Gao, Y.; Li, X.; Krumholz, H.M.; Zheng, X. Incidence, predictors, and prognostic impact of recurrent acute myocardial infarction in China. Heart 2020, 107, 313–318. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Wright, R.S.; Spencer, F.A.; Fredrick, P.D.; Dong, W.; Every, N.; French, W.J. Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am. J. Cardiol. 2005, 96, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Kowalewski, M.; Andreotti, F.; van Wely, M.; Camaro, C.; Kolodziejczak, M.; Gorny, B.; Wirianta, J.; Kubica, J.; Kelm, M.; et al. Meta-analysis of time-related benefits of statin therapy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am. J. Cardiol. 2014, 113, 1753–1764. [Google Scholar] [CrossRef]
- Schubert, J.; Lindahl, B.; Melhus, H.; Renlund, H.; Leosdottir, M.; Yari, A.; Ueda, P.; James, S.; Reading, S.R.; Dluzniewski, P.J.; et al. Low-density lipoprotein cholesterol reduction and statin intensity in myocardial infarction patients and major adverse outcomes: A Swedish nationwide cohort study. Eur. Heart J. 2021, 42, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Berwanger, O.; Santucci, E.V.; de Barros, E.S.P.G.M.; Jesuíno, I.A.; Damiani, L.P.; Barbosa, L.M.; Santos, R.H.N.; Laranjeira, L.N.; Egydio, F.M.; de Oliveira, B.J.A.; et al. Effect of Loading Dose of Atorvastatin Prior to Planned Percutaneous Coronary Intervention on Major Adverse Cardiovascular Events in Acute Coronary Syndrome: The SECURE-PCI Randomized Clinical Trial. JAMA 2018, 319, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; de Barros, E.S.P.G.M.; de Andrade Jesuíno, I.; Santucci, E.V.; Barbosa, L.M.; Damiani, L.P.; Santos, N.R.H.; Laranjeira, L.N.; Orto, D.F.T.C.; de Andrade, B.P.; et al. Timing of Loading Dose of Atorvastatin in Patients Undergoing Percutaneous Coronary Intervention for Acute Coronary Syndromes: Insights From the SECURE-PCI Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 1113–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koskinas, K.C.; Windecker, S.; Pedrazzini, G.; Mueller, C.; Cook, S.; Matter, C.M.; Muller, O.; Häner, J.; Gencer, B.; Crljenica, C.; et al. Evolocumab for Early Reduction of LDL Cholesterol Levels in Patients With Acute Coronary Syndromes (EVOPACS). J. Am. Coll. Cardiol. 2019, 74, 2452–2462. [Google Scholar] [CrossRef]
- Leucker, T.M.; Blaha, M.J.; Jones, S.R.; Vavuranakis, M.A.; Williams, M.S.; Lai, H.; Schindler, T.H.; Latina, J.; Schulman, S.P.; Gerstenblith, G. Effect of Evolocumab on Atherogenic Lipoproteins During the Peri- and Early Postinfarction Period: A Placebo-Controlled, Randomized Trial. Circulation 2020, 142, 419–421. [Google Scholar] [CrossRef]
- Evolocumab or Normal Strategies to Reach LDL Objectives in Acute Myocardial Infarction Upbound to PCI (AMUNDSEN). NCT04951856. Available online: https://clinicaltrials.gov/ct2/show/NCT04951856 (accessed on 28 February 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burger, A.L.; Pogran, E.; Muthspiel, M.; Kaufmann, C.C.; Jäger, B.; Huber, K. New Treatment Targets and Innovative Lipid-Lowering Therapies in Very-High-Risk Patients with Cardiovascular Disease. Biomedicines 2022, 10, 970. https://doi.org/10.3390/biomedicines10050970
Burger AL, Pogran E, Muthspiel M, Kaufmann CC, Jäger B, Huber K. New Treatment Targets and Innovative Lipid-Lowering Therapies in Very-High-Risk Patients with Cardiovascular Disease. Biomedicines. 2022; 10(5):970. https://doi.org/10.3390/biomedicines10050970
Chicago/Turabian StyleBurger, Achim Leo, Edita Pogran, Marie Muthspiel, Christoph Clemens Kaufmann, Bernhard Jäger, and Kurt Huber. 2022. "New Treatment Targets and Innovative Lipid-Lowering Therapies in Very-High-Risk Patients with Cardiovascular Disease" Biomedicines 10, no. 5: 970. https://doi.org/10.3390/biomedicines10050970
APA StyleBurger, A. L., Pogran, E., Muthspiel, M., Kaufmann, C. C., Jäger, B., & Huber, K. (2022). New Treatment Targets and Innovative Lipid-Lowering Therapies in Very-High-Risk Patients with Cardiovascular Disease. Biomedicines, 10(5), 970. https://doi.org/10.3390/biomedicines10050970






