Immunomodulatory Properties of Human Breast Milk: MicroRNA Contents and Potential Epigenetic Effects
Abstract
1. Introduction
2. The Physiological Basis of Breastfeeding and Milk Composition
3. HBM Composition
4. Breastfeeding and Immunity
5. Circulating miRNAs in HBM
5.1. Exosomal miRNAs
5.2. Sources of HBM miRNAs and the Effects of Different Conditions
5.3. Variability in miRNA Expressions in HBM
6. Immunoregulatory Roles of HBM-Derived miRNAs
7. Breastfeeding and Epigenetics
7.1. MiRNAs–Mediated Epigenetics and Immunity
7.2. Epigenetic Effects of HBM
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kramer, M.S. “Breast is best”: The evidence. Early Hum. Dev. 2010, 86, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Infant and Young Child Nutrition: Global Strategy on Infant and Young Child Feeding. Available online: https://apps.who.int/gb/archive/pdf_files/WHA55/ea5515.pdf (accessed on 10 March 2022).
- Labayen, I.; Ruiz, J.R.; Ortega, F.B.; Loit, H.M.; Harro, J.; Villa, I.; Veidebaum, T.; Sjostrom, M. Exclusive breastfeeding duration and cardiorespiratory fitness in children and adolescents. Am. J. Clin. Nutr. 2012, 95, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Boronat-Catalá, M.; Montiel-Company, J.M.; Bellot-Arcís, C.; Almerich-Silla, J.M.; Catalá-Pizarro, M. Association between duration of breastfeeding and malocclusions in primary and mixed dentition: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 5048. [Google Scholar] [CrossRef] [PubMed]
- Montgomery-Downs, H.E.; Crabtree, V.M.; Sans Capdevila, O.; Gozal, D. Infant-feeding methods and childhood sleep-disordered breathing. Pediatrics 2007, 120, 1030–1035. [Google Scholar] [CrossRef]
- Kalies, H.; Heinrich, J.; Borte, N.; Schaaf, B.; von Berg, A.; von Kries, R.; Wichmann, H.E.; Bolte, G. The effect of breastfeeding on weight gain in infants: Results of a birth cohort study. Eur. J. Med. Res. 2005, 10, 36–42. [Google Scholar]
- Brown Belfort, M. The Science of Breastfeeding and Brain Development. Breastfeed. Med. 2017, 12, 459–461. [Google Scholar] [CrossRef]
- Lund-Blix, N.A.; Dydensborg Sander, S.; Størdal, K.; Nybo Andersen, A.-M.; Rønningen, K.S.; Joner, G.; Skrivarhaug, T.; Njølstad, P.R.; Husby, S.; Stene, L.C. Infant Feeding and Risk of Type 1 Diabetes in Two Large Scandinavian Birth Cohorts. Diabetes Care 2017, 40, 920–927. [Google Scholar] [CrossRef]
- Biks, G.A.; Berhane, Y.; Worku, A.; Gete, Y.K. Exclusive breast feeding is the strongest predictor of infant survival in Northwest Ethiopia: A longitudinal study. J. Health Popul. Nutr. 2015, 34, 9. [Google Scholar] [CrossRef]
- Ozkan, H.; Tuzun, F.; Kumral, A.; Duman, N. Milk kinship hypothesis in light of epigenetic knowledge. Clin. Epigenetics 2012, 4, 14. [Google Scholar] [CrossRef]
- Moran, L.; Gilad, J. From folklore to scientific evidence: Breast-feeding and wet-nursing in islam and the case of non-puerperal lactation. Int. J. Biomed. Sci. IJBS 2007, 3, 251–257. [Google Scholar]
- Ozkan, H.; Tuzun, F.; Taheri, S.; Korhan, P.; Akokay, P.; Yılmaz, O.; Duman, N.; Özkul, Y.; Tufan, E.; Kumral, A.; et al. Epigenetic Programming Through Breast Milk and Its Impact on Milk-Siblings Mating. Front. Genet. 2020, 11, 569232. [Google Scholar] [CrossRef]
- Kramer, M.S.; Kakuma, R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst. Rev. 2012, 2012, Cd003517. [Google Scholar] [CrossRef]
- Delli Carpini, G.; Morini, S.; Papiccio, M.; Serri, M.; Damiani, V.; Grelloni, C.; Clemente, N.; Ciavattini, A. The association between childbirth, breastfeeding, and uterine fibroids: An observational study. Sci. Rep. 2019, 9, 10117. [Google Scholar] [CrossRef]
- Abedi, P.; Jahanfar, S.; Namvar, F.; Lee, J. Breastfeeding or nipple stimulation for reducing postpartum haemorrhage in the third stage of labour. Cochrane Database Syst. Rev. 2016, 2016, Cd010845. [Google Scholar] [CrossRef]
- Figueiredo, B.; Dias, C.C.; Brandão, S.; Canário, C.; Nunes-Costa, R. Breastfeeding and postpartum depression: State of the art review. J. Pediatr. 2013, 89, 332–338. [Google Scholar] [CrossRef]
- Ahn, S.; Corwin, E.J. The association between breastfeeding, the stress response, inflammation, and postpartum depression during the postpartum period: Prospective cohort study. Int. J. Nurs. Stud. 2015, 52, 1582–1590. [Google Scholar] [CrossRef]
- Gouveri, E.; Papanas, N.; Hatzitolios, A.I.; Maltezos, E. Breastfeeding and diabetes. Curr. Diabetes Rev. 2011, 7, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef]
- Thai, J.D.; Gregory, K.E. Bioactive Factors in Human Breast Milk Attenuate Intestinal Inflammation during Early Life. Nutrients 2020, 12, 581. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yi, D.Y. Components of human breast milk: From macronutrient to microbiome and microRNA. Clin. Exp. Pediatrics 2020, 63, 301–309. [Google Scholar] [CrossRef]
- Uauy, R.; Hoffman, D.R.; Peirano, P.; Birch, D.G.; Birch, E.E. Essential fatty acids in visual and brain development. Lipids 2001, 36, 885–895. [Google Scholar] [CrossRef]
- Haschke, F.; Haiden, N.; Thakkar, S.K. Nutritive and Bioactive Proteins in Breastmilk. Ann. Nutr. Metab. 2016, 69 (Suppl. S2), 17–26. [Google Scholar] [CrossRef]
- Berger, P.K.; Plows, J.F.; Demerath, E.W.; Fields, D.A. Carbohydrate composition in breast milk and its effect on infant health. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 277–281. [Google Scholar] [CrossRef]
- Young, B.E.; Westcott, J.; Kemp, J.; Allen, L.; Hampel, D.; Garcés, A.L.; Figueroa, L.; Goudar, S.S.; Dhaded, S.M.; Somannavar, M.; et al. B-Vitamins and Choline in Human Milk Are Not Impacted by a Preconception Lipid-Based Nutrient Supplement, but Differ Among Three Low-to-Middle Income Settings-Findings From the Women First Trial. Front. Nutr. 2021, 8, 750680. [Google Scholar] [CrossRef]
- de Vries, J.Y.; Pundir, S.; McKenzie, E.; Keijer, J.; Kussmann, M. Maternal Circulating Vitamin Status and Colostrum Vitamin Composition in Healthy Lactating Women-A Systematic Approach. Nutrients 2018, 10, 687. [Google Scholar] [CrossRef]
- Li, C.; Solomons, N.W.; Scott, M.E.; Koski, K.G. Minerals and Trace Elements in Human Breast Milk Are Associated with Guatemalan Infant Anthropometric Outcomes within the First 6 Months. J. Nutr. 2016, 146, 2067–2074. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Khayat, S.; Fanaei, H.; Ghanbarzehi, A. Minerals in Pregnancy and Lactation: A Review Article. J. Clin. Diagn. Res. 2017, 11, QE01–QE05. [Google Scholar] [CrossRef]
- Domellöf, M.; Lönnerdal, B.; Dewey, K.G.; Cohen, R.J.; Hernell, O. Iron, zinc, and copper concentrations in breast milk are independent of maternal mineral status. Am. J. Clin. Nutr. 2004, 79, 111–115. [Google Scholar] [CrossRef]
- Roumelioti, M.-E.; Glew, R.H.; Khitan, Z.J.; Rondon-Berrios, H.; Argyropoulos, C.P.; Malhotra, D.; Raj, D.S.; Agaba, E.I.; Rohrscheib, M.; Murata, G.H.; et al. Fluid balance concepts in medicine: Principles and practice. World J. Nephrol. 2018, 7, 1–28. [Google Scholar] [CrossRef]
- Moretti, A. What is the role of magnesium for skeletal muscle cramps? A Cochrane Review summary with commentary. J. Musculoskelet. Neuronal Interact. 2021, 21, 1–3. [Google Scholar]
- Mazzocchi, A.; Giannì, M.L.; Morniroli, D.; Leone, L.; Roggero, P.; Agostoni, C.; De Cosmi, V.; Mosca, F. Hormones in Breast Milk and Effect on Infants’ Growth: A Systematic Review. Nutrients 2019, 11, 1845. [Google Scholar] [CrossRef]
- Savino, F.; Liguori, S.A.; Fissore, M.F.; Oggero, R. Breast milk hormones and their protective effect on obesity. Int. J. Pediatr. Endocrinol. 2009, 2009, 327505. [Google Scholar] [CrossRef]
- Suwaydi, M.A.; Gridneva, Z.; Perrella, S.L.; Wlodek, M.E.; Lai, C.T.; Geddes, D.T. Human Milk Metabolic Hormones: Analytical Methods and Current Understanding. Int. J. Mol. Sci. 2021, 22, 8708. [Google Scholar] [CrossRef]
- Ilcol, Y.O.; Hizli, Z.B.; Ozkan, T. Leptin concentration in breast milk and its relationship to duration of lactation and hormonal status. Int. Breastfeed. J. 2006, 1, 21. [Google Scholar] [CrossRef]
- Pundir, S.; Gridneva, Z.; Pillai, A.; Thorstensen, E.B.; Wall, C.R.; Geddes, D.T.; Cameron-Smith, D. Human Milk Glucocorticoid Levels Are Associated with Infant Adiposity and Head Circumference Over the First Year of Life. Front. Nutr. 2020, 7, 166. [Google Scholar] [CrossRef]
- Quitadamo, P.A.; Comegna, L.; Cristalli, P. Anti-Infective, Anti-Inflammatory, and Immunomodulatory Properties of Breast Milk Factors for the Protection of Infants in the Pandemic From COVID-19. Front. Public Health 2020, 8, 589736. [Google Scholar] [CrossRef]
- Field, C.J. The immunological components of human milk and their effect on immune development in infants. J. Nutr. 2005, 135, 1–4. [Google Scholar] [CrossRef]
- Toscano, M.; De Grandi, R.; Grossi, E.; Drago, L. Role of the Human Breast Milk-Associated Microbiota on the Newborns’ Immune System: A Mini Review. Front. Microbiol. 2017, 8, 2100. [Google Scholar] [CrossRef]
- Soto, A.; Martín, V.; Jiménez, E.; Mader, I.; Rodríguez, J.M.; Fernández, L. Lactobacilli and bifidobacteria in human breast milk: Influence of antibiotherapy and other host and clinical factors. J. Pediatric Gastroenterol. Nutr. 2014, 59, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.R.; Brede, G.; Johansen, J.; Johnsen, R.; Storrø, O.; Sætrom, P.; Øien, T. Human Breast Milk miRNA, Maternal Probiotic Supplementation and Atopic Dermatitis in Offspring. PLoS ONE 2015, 10, e0143496. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human Milk Cells and Lipids Conserve Numerous Known and Novel miRNAs, Some of Which Are Differentially Expressed during Lactation. PLoS ONE 2016, 11, e0152610. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Jiang, X.; Li, R.; Chen, M.; Song, W.; Li, X. The levels of human milk microRNAs and their association with maternal weight characteristics. Eur. J. Clin. Nutr. 2016, 70, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Carney, M.C.; Tarasiuk, A.; DiAngelo, S.L.; Silveyra, P.; Podany, A.; Birch, L.L.; Paul, I.M.; Kelleher, S.; Hicks, S.D. Metabolism-related microRNAs in maternal breast milk are influenced by premature delivery. Pediatric Res. 2017, 82, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Du, X.; Li, J.; Lönnerdal, B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol. Nutr. Food Res. 2017, 61, 1700082. [Google Scholar] [CrossRef] [PubMed]
- Benmoussa, A.; Provost, P. Milk MicroRNAs in Health and Disease. Compr. Rev. Food Sci. Food Saf. 2019, 18, 703–722. [Google Scholar] [CrossRef]
- Kosaka, N.; Izumi, H.; Sekine, K.; Ochiya, T. microRNA as a new immune-regulatory agent in breast milk. Silence 2010, 1, 7. [Google Scholar] [CrossRef]
- Hicks, S.D.; Confair, A.; Warren, K.; Chandran, D. Levels of Breast Milk MicroRNAs and Other Non-Coding RNAs Are Impacted by Milk Maturity and Maternal Diet. Front. Immunol. 2021, 12, 785217. [Google Scholar] [CrossRef]
- Stephen, B.J.; Pareek, N.; Saeed, M.; Kausar, M.A.; Rahman, S.; Datta, M. Xeno-miRNA in Maternal-Infant Immune Crosstalk: An Aid to Disease Alleviation. Front. Immunol. 2020, 11, 404. [Google Scholar] [CrossRef]
- Carrillo-Lozano, E.; Sebastián-Valles, F.; Knott-Torcal, C. Circulating microRNAs in Breast Milk and Their Potential Impact on the Infant. Nutrients 2020, 12, 3066. [Google Scholar] [CrossRef]
- Ng, E.K.; Tsang, W.P.; Ng, S.S.; Jin, H.C.; Yu, J.; Li, J.J.; Röcken, C.; Ebert, M.P.; Kwok, T.T.; Sung, J.J. MicroRNA-143 targets DNA methyltransferases 3A in colorectal cancer. Br. J. Cancer 2009, 101, 699–706. [Google Scholar] [CrossRef]
- Huumonen, K.; Korkalainen, M.; Viluksela, M.; Lahtinen, T.; Naarala, J.; Juutilainen, J. Role of microRNAs and DNA Methyltransferases in Transmitting Induced Genomic Instability between Cell Generations. Front. Public Health 2014, 2, 139. [Google Scholar] [CrossRef]
- Macfarlane, L.-A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Navarro Quiroz, E.; Navarro Quiroz, R.; Pacheco Lugo, L.; Aroca Martínez, G.; Gómez Escorcia, L.; Gonzalez Torres, H.; Cadena Bonfanti, A.; Marmolejo, M.D.C.; Sanchez, E.; Villarreal Camacho, J.L.; et al. Integrated analysis of microRNA regulation and its interaction with mechanisms of epigenetic regulation in the etiology of systemic lupus erythematosus. PLoS ONE 2019, 14, e0218116. [Google Scholar] [CrossRef]
- Infant and Young Child Feeding: Model Chapter for Textbooks for Medical Students and Allied Health Professionals; World Health Organization: Geneva, Switzerland, 2009.
- Zucca-Matthes, G.; Urban, C.; Vallejo, A. Anatomy of the nipple and breast ducts. Gland Surg. 2016, 5, 32–36. [Google Scholar] [CrossRef]
- Uvnas Moberg, K.; Ekstrom-Bergstrom, A.; Buckley, S.; Massarotti, C.; Pajalic, Z.; Luegmair, K.; Kotlowska, A.; Lengler, L.; Olza, I.; Grylka-Baeschlin, S.; et al. Maternal plasma levels of oxytocin during breastfeeding-A systematic review. PLoS ONE 2020, 15, e0235806. [Google Scholar] [CrossRef]
- Macias, H.; Hinck, L. Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef]
- Colleluori, G.; Perugini, J.; Barbatelli, G.; Cinti, S. Mammary gland adipocytes in lactation cycle, obesity and breast cancer. Rev. Endocr. Metab. Disord. 2021, 22, 241–255. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Lawrence, R.M. Breastfeeding: A Guide for the Medical Profession; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Voogt, J.L. Control of hormone release during lactation. Clin. Obstet. Gynaecol. 1978, 5, 435–455. [Google Scholar] [CrossRef]
- Hartmann, P.E.; Owens, R.A.; Cox, D.B.; Kent, J.C. Breast Development and Control of Milk Synthesis. Food Nutr. Bull. 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Ostrom, K.M. A review of the hormone prolactin during lactation. Prog. Food Nutr. Sci. 1990, 14, 1–43. [Google Scholar] [PubMed]
- Saleem, M.; Martin, H.; Coates, P. Prolactin Biology and Laboratory Measurement: An Update on Physiology and Current Analytical Issues. Clin. Biochem. Rev. 2018, 39, 3–16. [Google Scholar]
- Kent, J.C.; Prime, D.K.; Garbin, C.P. Principles for maintaining or increasing breast milk production. J. Obstet. Gynecol. Neonatal Nurs. 2012, 41, 114–121. [Google Scholar] [CrossRef]
- Okatani, Y.; Sagara, Y. Role of melatonin in nocturnal prolactin secretion in women with normoprolactinemia and mild hyperprolactinemia. Am. J. Obstet. Gynecol. 1993, 168, 854–861. [Google Scholar] [CrossRef]
- Ramsay, D.T.; Kent, J.C.; Owens, R.A.; Hartmann, P.E. Ultrasound imaging of milk ejection in the breast of lactating women. Pediatrics 2004, 113, 361–367. [Google Scholar] [CrossRef]
- Moberg, K.U.; Prime, D.K. Oxytocin effects in mothers and infants during breastfeeding. Infant 2013, 9, 201–206. [Google Scholar]
- Erickson, E.N.; Emeis, C.L. Breastfeeding Outcomes After Oxytocin Use During Childbirth: An Integrative Review. J. Midwifery Women’s Health 2017, 62, 397–417. [Google Scholar] [CrossRef]
- Stuebe, A.M.; Grewen, K.; Meltzer-Brody, S. Association between maternal mood and oxytocin response to breastfeeding. J. Women’s Health 2013, 22, 352–361. [Google Scholar] [CrossRef]
- Oguchi, S.; Shinohara, K.; Yamashiro, Y.; Walker, W.A.; Sanderson, I.R. Growth factors in breast milk and their effect on gastrointestinal development. Zhonghua Minguo Xiao Er Ke Yi Xue Hui Za Zhi 1997, 38, 332–337. [Google Scholar]
- Moossavi, S.; Miliku, K.; Sepehri, S.; Khafipour, E.; Azad, M.B. The Prebiotic and Probiotic Properties of Human Milk: Implications for Infant Immune Development and Pediatric Asthma. Front. Pediatr. 2018, 6, 197. [Google Scholar] [CrossRef]
- Burch, J.; Karmaus, W.; Gangur, V.; Soto-Ramírez, N.; Yousefi, M.; Goetzl, L.M. Pre- and perinatal characteristics and breast milk immune markers. Pediatric Res. 2013, 74, 615–621. [Google Scholar] [CrossRef]
- Trend, S.; Strunk, T.; Hibbert, J.; Kok, C.H.; Zhang, G.; Doherty, D.A.; Richmond, P.; Burgner, D.; Simmer, K.; Davidson, D.J.; et al. Antimicrobial protein and Peptide concentrations and activity in human breast milk consumed by preterm infants at risk of late-onset neonatal sepsis. PLoS ONE 2015, 10, e0117038. [Google Scholar] [CrossRef]
- Xavier, A.M.; Rai, K.; Hegde, A.M. Total antioxidant concentrations of breastmilk--an eye-opener to the negligent. J. Health Popul. Nutr. 2011, 29, 605–611. [Google Scholar] [CrossRef]
- Schrezenmeir, J.; Korhonen, H.; Williams, C.; Gill, H.S.; Shah, N. Foreword. Br. J. Nutr. 2007, 84, 1. [Google Scholar] [CrossRef]
- Garofalo, R. Cytokines in human milk. J. Pediatr. 2010, 156, S36–S40. [Google Scholar] [CrossRef]
- Cavaletto, M.; Giuffrida, M.G.; Conti, A. The proteomic approach to analysis of human milk fat globule membrane. Clin. Chim. Acta 2004, 347, 41–48. [Google Scholar] [CrossRef]
- Jenness, R. The composition of human milk. Semin. Perinatol. 1979, 3, 225–239. [Google Scholar]
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef]
- Vizzari, G.; Morniroli, D.; Ceroni, F.; Verduci, E.; Consales, A.; Colombo, L.; Cerasani, J.; Mosca, F.; Gianni, M.L. Human Milk, More Than Simple Nourishment. Children 2021, 8, 863. [Google Scholar] [CrossRef] [PubMed]
- Golan, Y.; Assaraf, Y.G. Genetic and Physiological Factors Affecting Human Milk Production and Composition. Nutrients 2020, 12, 1500. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.E.; Walker, W.A. Immunologic Factors in Human Milk and Disease Prevention in the Preterm Infant. Curr. Pediatr. Rep. 2013, 1, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Gidrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatrics 2014, 14, 216. [Google Scholar] [CrossRef]
- Steinhoff, M.C.; Omer, S.B. A review of fetal and infant protection associated with antenatal influenza immunization. Am. J. Obstet. Gynecol. 2012, 207, S21–S27. [Google Scholar] [CrossRef]
- Chheda, S.; Keeney, S.E.; Goldman, A.S. Immunology of Human Milk and Host Immunity. Fetal Neonatal Physiol. 2004, 2, 1610–1620. [Google Scholar] [CrossRef]
- M’Rabet, L.; Vos, A.P.; Boehm, G.N.; Garssen, J. Breast-Feeding and Its Role in Early Development of the Immune System in Infants: Consequences for Health Later in Life. J. Nutr. 2008, 138, 1782S–1790S. [Google Scholar] [CrossRef]
- Jakaitis, B.M.; Denning, P.W. Human breast milk and the gastrointestinal innate immune system. Clin. Perinatol. 2014, 41, 423–435. [Google Scholar] [CrossRef]
- Henrick, B.M.; Yao, X.D.; Nasser, L.; Roozrogousheh, A.; Rosenthal, K.L. Breastfeeding Behaviors and the Innate Immune System of Human Milk: Working Together to Protect Infants against Inflammation, HIV-1, and Other Infections. Front. Immunol. 2017, 8, 1631. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Ye, Y.; Wang, F.; Chen, F.; Zheng, C. The Role of Microbiota in Infant Health: From Early Life to Adulthood. Front. Immunol. 2021, 12, 708472. [Google Scholar] [CrossRef]
- Zhuang, L.; Chen, H.; Zhang, S.; Zhuang, J.; Li, Q.; Feng, Z. Intestinal Microbiota in Early Life and Its Implications on Childhood Health. Genom. Proteom. Bioinform. 2019, 17, 13–25. [Google Scholar] [CrossRef]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J.; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Dieterich, C.M.; Felice, J.P.; O’Sullivan, E.; Rasmussen, K.M. Breastfeeding and health outcomes for the mother-infant dyad. Pediatr. Clin. N. Am. 2013, 60, 31–48. [Google Scholar] [CrossRef]
- Newburg, D.S. Innate immunity and human milk. J. Nutr. 2005, 135, 1308–1312. [Google Scholar] [CrossRef]
- Bobinski, R.; Bobinska, J. Fatty acids of human milk—A review. Int. J. Vitam. Nutr. Res. 2020, 1–12. [Google Scholar] [CrossRef]
- Visentainer, J.V.; Santos, O.O.; Maldaner, L.; Zappielo, C.; Neia, V.; Visentainer, L.; Pelissari, L.; Pizzo, J.; Rydlewski, A.; Silveira, R.; et al. Lipids and Fatty Acids in Human Milk: Benefits and Analysis. In Biochemistry and Health Benefits of Fatty Acids; Waisundara, V., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Dilika, F.; Bremner, P.D.; Meyer, J.J. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia 2000, 71, 450–452. [Google Scholar] [CrossRef]
- He, Y.Y.; Lawlor, N.T.; Newburg, D.S. Human Milk Components Modulate Toll-Like Receptor-Mediated Inflammation. Adv. Nutr. Int. Rev. J. 2016, 7, 102–111. [Google Scholar] [CrossRef]
- Kurakevich, E.; Hennet, T.; Hausmann, M.; Rogler, G.; Borsig, L. Milk oligosaccharide sialyl (α2,3) lactose activates intestinal CD11c+ cells through TLR4. Proc. Natl. Acad. Sci. USA 2013, 110, 17444. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Fontana, L.; Gil, A. Human Milk Oligosaccharides and Immune System Development. Nutrients 2018, 10, 1038. [Google Scholar] [CrossRef]
- Laucirica, D.R.; Triantis, V.; Schoemaker, R.; Estes, M.K.; Ramani, S. Milk Oligosaccharides Inhibit Human Rotavirus Infectivity in MA104 Cells. J. Nutr. 2017, 147, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhou, Y.; Teng, Z.; Du, C.L.; Tian, C. Preparation and antibacterial activity of oligosaccharides derived from dandelion. Int. J. Biol. Macromol. 2014, 64, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Lis-Kuberka, J.; Orczyk-Pawilowicz, M. Sialylated Oligosaccharides and Glycoconjugates of Human Milk. The Impact on Infant and Newborn Protection, Development and Well-Being. Nutrients 2019, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.; Kunz, C.; Muhly-Reinholz, M.; Mayer, K.; Seeger, W.; Rudloff, S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb. Haemost. 2004, 92, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Suligoj, T.; Vigsnaes, L.K.; Abbeele, P.V.D.; Apostolou, A.; Karalis, K.; Savva, G.M.; McConnell, B.; Juge, N. Effects of Human Milk Oligosaccharides on the Adult Gut Microbiota and Barrier Function. Nutrients 2020, 12, 2808. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.; Walker, A. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatric Res. 2007, 61, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Mykoniatis, A.; Anton, P.M.; Wlk, M.; Wang, C.C.; Ungsunan, L.; Blher, S.; Venihaki, M.; Simeonidis, S.; Zacks, J.; Zhao, D. Leptin mediates Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology 2003, 124, 683–691. [Google Scholar] [CrossRef]
- Soliman, A.T.; Yasin, M.; Kassem, A. Leptin in pediatrics: A hormone from adipocyte that wheels several functions in children. Indian J. Endocrinol. Metab. 2012, 16, S577–S587. [Google Scholar] [CrossRef]
- Smith, T.J. Insulin-like growth factor-I regulation of immune function: A potential therapeutic target in autoimmune diseases? Pharmacol. Rev. 2010, 62, 199–236. [Google Scholar] [CrossRef]
- Cacho, N.T.; Lawrence, R.M. Innate Immunity and Breast Milk. Front. Immunol. 2017, 8, 584. [Google Scholar] [CrossRef]
- Witkowska-Zimny, M.; Kaminska-El-Hassan, E. Cells of human breast milk. Cell. Mol. Biol. Lett. 2017, 22, 11. [Google Scholar] [CrossRef]
- Goldman, A.S.; Garza, C.; Nichols, B.L.; Goldblum, R.M. Immunologic factors in human milk during the first year of lactation. J. Pediatrics 1982, 100, 563–567. [Google Scholar] [CrossRef]
- Yilmaz, H.L.; Saygili-Yilmaz, E.S.; Gunesacar, R. Interleukin-10 and-12 in human milk at 3 stages of lactation: A longitudinal study. Adv. Ther. 2007, 24, 603–610. [Google Scholar] [CrossRef]
- Guo, M. Human Milk and Infant Formula. In Functional Foods; Guo, M., Ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 299–337. [Google Scholar]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of Human Milk Bioactives on Infants’ Gut and Immune Health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef]
- Kosciuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzalkowska, N.; Jozwik, A.; Horbanczuk, J.; Krzyzewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef]
- Zucali, J.R.; Broxmeyer, H.E.; Levy, D.; Morse, C. Lactoferrin decreases monocyte-induced fibroblast production of myeloid colony-stimulating activity by suppressing monocyte release of interleukin-1. Blood 1989, 74, 1531–1536. [Google Scholar] [CrossRef]
- Buescher, E.S. Anti-inflammatory characteristics of human milk. In Bioactive Components of Human Milk; Springer: Berlin/Heidelberg, Germany, 2001; pp. 207–222. [Google Scholar]
- Brock, J. Lactoferrin in human milk: Its role in iron absorption and protection against enteric infection in the newborn infant. Arch. Dis. Child. 1980, 55, 417. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Tang, B.; Liu, Y.; Guo, C.; Yang, P.; Yu, T.; Li, R.; Zhao, J.; Zhang, L.; et al. Characterization of bioactive recombinant human lysozyme expressed in milk of cloned transgenic cattle. PLoS ONE 2011, 6, e17593. [Google Scholar] [CrossRef]
- Malaczewska, J.; Kaczorek-Lukowska, E.; Wojcik, R.; Siwicki, A.K. Antiviral effects of nisin, lysozyme, lactoferrin and their mixtures against bovine viral diarrhoea virus. BMC Vet. Res. 2019, 15, 318. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Yang, L.; Guo, Z.; Yu, M.; Cai, X.; Mao, Y.; Tian, F.; Xu, W.; Liu, G.; Li, X.; Zhao, Y.; et al. Profile of Nucleotides in Chinese Mature Breast Milk from Six Regions. Nutrients 2022, 14, 1418. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, R.; Fanion, M. Technical aspects of micronutrient addition to foods. In Food Fortification and Supplementation; Ottaway, P.B., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 108–125. [Google Scholar]
- Tingo, L.; Ahlberg, E.; Johansson, L.; Pedersen, S.A.; Chawla, K.; Saetrom, P.; Cione, E.; Simpson, M.R. Non-Coding RNAs in Human Breast Milk: A Systematic Review. Front. Immunol. 2021, 12, 725323. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.M.; Daum, M.; Skaar, T.; Philips, S.; Miracle, D.; Renbarger, J.L. Human breast milk as a source of DNA for amplification. J. Clin. Pharmacol. 2011, 51, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Guo, M. Human Milk Biochemistry and Infant Formula Manufacturing Technology; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Lonnerdal, B. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003, 77, 1537S–1543S. [Google Scholar] [CrossRef]
- Verduci, E.; Banderali, G.; Barberi, S.; Radaelli, G.; Lops, A.; Betti, F.; Riva, E.; Giovannini, M. Epigenetic effects of human breast milk. Nutrients 2014, 6, 1711–1724. [Google Scholar] [CrossRef]
- Wagner, C.L.; Taylor, S.N.; Johnson, D. Host factors in amniotic fluid and breast milk that contribute to gut maturation. Clin. Rev. Allergy Immunol. 2008, 34, 191–204. [Google Scholar] [CrossRef]
- Chang, C.J.; Chao, J.C. Effect of human milk and epidermal growth factor on growth of human intestinal Caco-2 cells. J. Pediatr. Gastroenterol. Nutr. 2002, 34, 394–401. [Google Scholar] [CrossRef]
- Dvorak, B.; Fituch, C.C.; Williams, C.S.; Hurst, N.M.; Schanler, R.J. Increased epidermal growth factor levels in human milk of mothers with extremely premature infants. Pediatr. Res. 2003, 54, 15–19. [Google Scholar] [CrossRef]
- Munblit, D.; Sheth, S.; Abrol, P.; Treneva, M.; Peroni, D.G.; Chow, L.Y.; Boner, A.L.; Pampura, A.; Warner, J.O.; Boyle, R.J. Exposures influencing total IgA level in colostrum. J. Dev. Orig. Health Dis. 2016, 7, 61–67. [Google Scholar] [CrossRef]
- Twigger, A.J.; Hepworth, A.R.; Lai, C.T.; Chetwynd, E.; Stuebe, A.M.; Blancafort, P.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Gene expression in breastmilk cells is associated with maternal and infant characteristics. Sci. Rep. 2015, 5, 12933. [Google Scholar] [CrossRef]
- Agarwal, S.; Karmaus, W.; Davis, S.; Gangur, V. Immune markers in breast milk and fetal and maternal body fluids: A systematic review of perinatal concentrations. J. Hum. Lact. 2011, 27, 171–186. [Google Scholar] [CrossRef]
- Penttila, I.A. Milk-derived transforming growth factor-beta and the infant immune response. J. Pediatr. 2010, 156, S21–S25. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyata, M.; Ando, T.; Shimokawa, N.; Ohnuma, Y.; Katoh, R.; Ogawa, H.; Okumura, K.; Nakao, A. The latent form of transforming growth factor-beta administered orally is activated by gastric acid in mice. J. Nutr. 2009, 139, 1463–1468. [Google Scholar] [CrossRef]
- Musilova, S.; Rada, V.; Vlkova, E.; Bunesova, V. Beneficial effects of human milk oligosaccharides on gut microbiota. Benef. Microbes 2014, 5, 273–283. [Google Scholar] [CrossRef]
- Bezirtzoglou, E.; Tsiotsias, A.; Welling, G.W. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe 2011, 17, 478–482. [Google Scholar] [CrossRef]
- Lubiech, K.; Twaruzek, M. Lactobacillus Bacteria in Breast Milk. Nutrients 2020, 12, 3783. [Google Scholar] [CrossRef]
- Zhang, X.; Mushajiang, S.; Luo, B.; Tian, F.; Ni, Y.; Yan, W. The Composition and Concordance of Lactobacillus Populations of Infant Gut and the Corresponding Breast-Milk and Maternal Gut. Front. Microbiol. 2020, 11, 597911. [Google Scholar] [CrossRef]
- Oozeer, R.; van Limpt, K.; Ludwig, T.; Ben Amor, K.; Martin, R.; Wind, R.D.; Boehm, G.; Knol, J. Intestinal microbiology in early life: Specific prebiotics can have similar functionalities as human-milk oligosaccharides. Am. J. Clin. Nutr. 2013, 98, 561S–571S. [Google Scholar] [CrossRef]
- Chassard, C.; de Wouters, T.; Lacroix, C. Probiotics tailored to the infant: A window of opportunity. Curr. Opin. Biotechnol. 2014, 26, 141–147. [Google Scholar] [CrossRef]
- Zou, X.; Huang, J.; Jin, Q.; Guo, Z.; Liu, Y.; Cheong, L.; Xu, X.; Wang, X. Lipid composition analysis of milk fats from different mammalian species: Potential for use as human milk fat substitutes. J. Agric. Food Chem. 2013, 61, 7070–7080. [Google Scholar] [CrossRef]
- Luoto, R.; Ruuskanen, O.; Waris, M.; Kalliomaki, M.; Salminen, S.; Isolauri, E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2014, 133, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Ahanchian, H.; Jafari, S.A. Probiotics and Prebiotics for Prevention of Viral Respiratory Tract Infections. In Probiotics Prebiotics Synbiotics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 575–583. [Google Scholar] [CrossRef]
- Koromyslova, A.; Tripathi, S.; Morozov, V.; Schroten, H.; Hansman, G.S. Human norovirus inhibition by a human milk oligosaccharide. Virology 2017, 508, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Weichert, S.; Koromyslova, A.; Singh, B.K.; Hansman, S.; Jennewein, S.; Schroten, H.; Hansman, G.S. Structural Basis for Norovirus Inhibition by Human Milk Oligosaccharides. J. Virol. 2016, 90, 4843–4848. [Google Scholar] [CrossRef] [PubMed]
- Pichler, M.J.; Yamada, C.; Shuoker, B.; Alvarez-Silva, C.; Gotoh, A.; Leth, M.L.; Schoof, E.; Katoh, T.; Sakanaka, M.; Katayama, T.; et al. Butyrate producing colonic Clostridiales metabolise human milk oligosaccharides and cross feed on mucin via conserved pathways. Nat. Commun. 2020, 11, 3285. [Google Scholar] [CrossRef]
- Sela, D.A.; Garrido, D.; Lerno, L.; Wu, S.; Tan, K.; Eom, H.J.; Joachimiak, A.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subsp. infantis ATCC 15697 α-fucosidases are active on fucosylated human milk oligosaccharides. Appl. Environ. Microbiol. 2012, 78, 795–803. [Google Scholar] [CrossRef]
- Sakurama, H.; Kiyohara, M.; Wada, J.; Honda, Y.; Yamaguchi, M.; Fukiya, S.; Yokota, A.; Ashida, H.; Kumagai, H.; Kitaoka, M.; et al. Lacto-N-biosidase encoded by a novel gene of Bifidobacterium longum subspecies longum shows unique substrate specificity and requires a designated chaperone for its active expression. J. Biol. Chem. 2013, 288, 25194–25206. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Ismail, I.; Al-Shajrawi, O.M.; Ariff, T.M. Effect of stress on alteration of haematological parameters: A preliminary study on preclinical medical students in Malaysia. J. Cell. Neurosci. Oxidative Stress 2020, 11, 852–860. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Hatmal, M.M.; Sattar, K.; Ahmad, S.; Mustafa, M.Z.; Bittencourt, M.D.C.; Mohamud, R. Antiviral and Immunomodulatory Effects of Phytochemicals from Honey against COVID-19: Potential Mechanisms of Action and Future Directions. Molecules 2020, 25, 5017. [Google Scholar] [CrossRef]
- Gila-Diaz, A.; Arribas, S.M.; Algara, A.; Martin-Cabrejas, M.A.; Lopez de Pablo, A.L.; Saenz de Pipaon, M.; Ramiro-Cortijo, D. A Review of Bioactive Factors in Human Breastmilk: A Focus on Prematurity. Nutrients 2019, 11, 1307. [Google Scholar] [CrossRef]
- Castillo-Castaneda, P.C.; Garcia-Gonzalez, A.; Bencomo-Alvarez, A.E.; Barros-Nunez, P.; Gaxiola-Robles, R.; Mendez-Rodriguez, L.C.; Zenteno-Savin, T. Micronutrient content and antioxidant enzyme activities in human breast milk. J. Trace Elem. Med. Biol. 2019, 51, 36–41. [Google Scholar] [CrossRef]
- Ankrah, N.A.; Appiah-Opong, R.; Dzokoto, C. Human breastmilk storage and the glutathione content. J. Trop. Pediatr. 2000, 46, 111–113. [Google Scholar] [CrossRef]
- Goyal, M.M.; Basak, A. Human catalase: Looking for complete identity. Protein Cell 2010, 1, 888–897. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R. Tissue-specific functions of individual glutathione peroxidases. Free Radic. Biol. Med. 1999, 27, 951–965. [Google Scholar] [CrossRef]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef]
- Reiter, R.J. Antioxidant actions of melatonin. Adv. Pharmacol. 1997, 38, 103–117. [Google Scholar] [CrossRef]
- Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar]
- Ip, S.; Chung, M.; Raman, G. Tufts-New England Medical Center Evidence-based Practice Center. Breastfeeding and maternal and infant health outcomes in developed countries. Evid. Rep. Technol. Assess. 2007, 153, 1–186. [Google Scholar]
- Grulee, C.G.; Sanford, H.N.; Herron, P.H. Breast and artificial feeding: Influence on morbidity and mortality of twenty thousand infants. J. Am. Med. Assoc. 1934, 103, 735–739. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome-A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Turin, C.G.; Ochoa, T.J. The Role of Maternal Breast Milk in Preventing Infantile Diarrhea in the Developing World. Curr. Trop. Med. Rep. 2014, 1, 97–105. [Google Scholar] [CrossRef]
- Yahaya, T.; Shemishere, U. Association between Bioactive Molecules in Breast Milk and Type 1 Diabetes Mellitus. Sultan Qaboos Univ. Med. J. 2020, 20, e5–e12. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.-Y.; Chang, D.C.; Lin, S.-L. The microRNA (miRNA): Overview of the RNA genes that modulate gene function. Mol. Biotechnol. 2008, 38, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Du, R.; Edwards, A.; Flemington, E.K.; Zhang, K. The sequence structures of human microRNA molecules and their implications. PLoS ONE 2013, 8, e54215. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Rajewsky, K. MicroRNA control in the immune system: Basic principles. Cell 2009, 136, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from repression to activation: MicroRNAs can up-regulate translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002, 12, 735–739. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, P.H. Are circulating microRNAs peripheral biomarkers for Alzheimer’s disease? Biochim. Biophys. Acta 2016, 1862, 1617–1627. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Valinezhad Orang, A.; Safaralizadeh, R.; Kazemzadeh-Bavili, M. Mechanisms of miRNA-Mediated Gene Regulation from Common Downregulation to mRNA-Specific Upregulation. Int. J. Genom. 2014, 2014, 970607. [Google Scholar] [CrossRef]
- Webster, M.W.; Chen, Y.-H.; Stowell, J.A.W.; Alhusaini, N.; Sweet, T.; Graveley, B.R.; Coller, J.; Passmore, L.A. mRNA Deadenylation Is Coupled to Translation Rates by the Differential Activities of Ccr4-Not Nucleases. Mol. Cell 2018, 70, 1089–1100.e1088. [Google Scholar] [CrossRef]
- O’Carroll, D.; Schaefer, A. General Principals of miRNA Biogenesis and Regulation in the Brain. Neuropsychopharmacology 2013, 38, 39–54. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci. Rep. 2016, 6, 20680. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk cells contain numerous miRNAs that may change with milk removal and regulate multiple physiological processes. Int. J. Mol. Sci. 2016, 17, 956. [Google Scholar] [CrossRef]
- Alsaweed, M.; Hepworth, A.R.; Lefevre, C.; Hartmann, P.E.; Geddes, D.T.; Hassiotou, F. Human Milk MicroRNA and Total RNA Differ Depending on Milk Fractionation. J. Cell. Biochem. 2015, 116, 2397–2407. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhu, Q.; Zhou, X.; Wang, X.; Gao, X.; Li, X. Immune-related microRNAs are abundant in breast milk exosomes. Int. J. Biol. Sci. 2012, 8, 118–123. [Google Scholar] [CrossRef]
- Munch, E.M.; Harris, R.A.; Mohammad, M.; Benham, A.L.; Pejerrey, S.M.; Showalter, L.; Hu, M.; Shope, C.D.; Maningat, P.D.; Gunaratne, P.H.; et al. Transcriptome profiling of microRNA by Next-Gen deep sequencing reveals known and novel miRNA species in the lipid fraction of human breast milk. PLoS ONE 2013, 8, e50564. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.C.; Tavazoie, M.; Doetsch, F. Stem cells: From epigenetics to microRNAs. Neuron 2005, 46, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Pothof, J.; Verkaik, N.S.; Van Ijcken, W.; Wiemer, E.A.; Ta, V.T.; van der Horst, G.T.; Jaspers, N.G.; van Gent, D.C.; Hoeijmakers, J.H.; Persengiev, S.P. MicroRNA-mediated gene silencing modulates the UV-induced DNA-damage response. EMBO J. 2009, 28, 2090–2099. [Google Scholar] [CrossRef]
- Gao, Y.; Schug, J.; McKenna, L.B.; Le Lay, J.; Kaestner, K.H.; Greenbaum, L.E. Tissue-specific regulation of mouse microRNA genes in endoderm-derived tissues. Nucleic Acids Res. 2011, 39, 454–463. [Google Scholar] [CrossRef]
- Buescher, E.S.; Malinowska, I. Soluble receptors and cytokine antagonists in human milk. Pediatr. Res. 1996, 40, 839–844. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef]
- Kinouchi, T.; Koizumi, K.; Kuwata, T.; Yajima, T. Milk-borne insulin with trypsin inhibitor in milk induces pancreatic amylase development at the onset of weaning in rats. J. Pediatric Gastroenterol. Nutr. 2000, 30, 515–521. [Google Scholar] [CrossRef]
- Alsaweed, M.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. MicroRNAs in Breastmilk and the Lactating Breast: Potential Immunoprotectors and Developmental Regulators for the Infant and the Mother. Int. J. Environ. Res. Public Health 2015, 12, 13981–14020. [Google Scholar] [CrossRef]
- Saurabh, S.; Vidyarthi, A.S.; Prasad, D. RNA interference: Concept to reality in crop improvement. Planta 2014, 239, 543–564. [Google Scholar] [CrossRef]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: MicroRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef]
- Bentwich, I.; Avniel, A.; Karov, Y.; Aharonov, R.; Gilad, S.; Barad, O.; Barzilai, A.; Einat, P.; Einav, U.; Meiri, E.; et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 2005, 37, 766–770. [Google Scholar] [CrossRef]
- Jiang, X.; You, L.; Zhang, Z.; Cui, X.; Zhong, H.; Sun, X.; Ji, C.; Chi, X. Biological Properties of Milk-Derived Extracellular Vesicles and Their Physiological Functions in Infant. Front. Cell Dev. Biol. 2021, 9, 693534. [Google Scholar] [CrossRef]
- Tome-Carneiro, J.; Fernandez-Alonso, N.; Tomas-Zapico, C.; Visioli, F.; Iglesias-Gutierrez, E.; Davalos, A. Breast milk microRNAs harsh journey towards potential effects in infant development and maturation. Lipid encapsulation can help. Pharmacol. Res. 2018, 132, 21–32. [Google Scholar] [CrossRef]
- Leroux, C.; Chervet, M.L.; German, J.B. Perspective: Milk microRNAs as Important Players in Infant Physiology and Development. Adv. Nutr. 2021, 12, 1625–1635. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Zhu, S.; Si, M.L.; Wu, H.; Mo, Y.Y. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1). J. Biol. Chem. 2007, 282, 14328–14336. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Deng, J.J.; Gowda, P.S.; Rao, M.K.; Lin, C.L.; Chen, C.L.; Huang, T.; Sun, L.Z. Androgen receptor and microRNA-21 axis downregulates transforming growth factor beta receptor II (TGFBR2) expression in prostate cancer. Oncogene 2014, 33, 4097–4106. [Google Scholar] [CrossRef] [PubMed]
- Bhome, R.; Del Vecchio, F.; Lee, G.H.; Bullock, M.D.; Primrose, J.N.; Sayan, A.E.; Mirnezami, A.H. Exosomal microRNAs (exomiRs): Small molecules with a big role in cancer. Cancer Lett. 2018, 420, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef]
- Ji, L.; Chen, X. Regulation of small RNA stability: Methylation and beyond. Cell Res. 2012, 22, 624–636. [Google Scholar] [CrossRef]
- Wolf, T.; Baier, S.R.; Zempleni, J. The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells. J. Nutr. 2015, 145, 2201–2206. [Google Scholar] [CrossRef]
- Nazari-Jahantigh, M.; Egea, V.; Schober, A.; Weber, C. MicroRNA-specific regulatory mechanisms in atherosclerosis. J. Mol. Cell. Cardiol. 2015, 89, 35–41. [Google Scholar] [CrossRef]
- Ito, Y.; Taniguchi, K.; Kuranaga, Y.; Eid, N.; Inomata, Y.; Lee, S.W.; Uchiyama, K. Uptake of MicroRNAs from Exosome-Like Nanovesicles of Edible Plant Juice by Rat Enterocytes. Int. J. Mol. Sci. 2021, 22, 3749. [Google Scholar] [CrossRef]
- Blank, A.; Dekker, C.; Schieven, G.; Sugiyama, R.; Thelen, M. Human body fluid ribonucleases: Detection, interrelationships and significance. Nucleic Acids Symp. Ser. 1981, 203–209. [Google Scholar]
- Matos, R.G.; Barbas, A.; Arraiano, C.M. RNase R mutants elucidate the catalysis of structured RNA: RNA-binding domains select the RNAs targeted for degradation. Biochem. J. 2009, 423, 291–301. [Google Scholar] [CrossRef]
- Bernhardt, H.S.; Tate, W.P. Primordial soup or vinaigrette: Did the RNA world evolve at acidic pH? Biol. Direct 2012, 7, 4. [Google Scholar] [CrossRef]
- Levy, M.; Miller, S.L. The stability of the RNA bases: Implications for the origin of life. Proc. Natl. Acad. Sci. USA 1998, 95, 7933–7938. [Google Scholar] [CrossRef]
- Chiba, T.; Kooka, A.; Kowatari, K.; Yoshizawa, M.; Chiba, N.; Takaguri, A.; Fukushi, Y.; Hongo, F.; Sato, H.; Wada, S. Expression profiles of hsa-miR-148a-3p and hsa-miR-125b-5p in human breast milk and infant formulae. Int. Breastfeed. J. 2022, 17, 1. [Google Scholar] [CrossRef]
- Lukasik, A.; Brzozowska, I.; Zielenkiewicz, U.; Zielenkiewicz, P. Detection of Plant miRNAs Abundance in Human Breast Milk. Int. J. Mol. Sci. 2017, 19, 37. [Google Scholar] [CrossRef]
- Mar-Aguilar, F.; Arreola-Triana, A.; Mata-Cardona, D.; Gonzalez-Villasana, V.; Rodriguez-Padilla, C.; Resendez-Perez, D. Evidence of transfer of miRNAs from the diet to the blood still inconclusive. PeerJ 2020, 8, e9567. [Google Scholar] [CrossRef]
- Li, Z.; Xu, R.; Li, N. MicroRNAs from plants to animals, do they define a new messenger for communication? Nutr. Metab. 2018, 15, 68. [Google Scholar] [CrossRef]
- Hayek, H.; Kosmider, B.; Bahmed, K. The role of miRNAs in alveolar epithelial cells in emphysema. Biomed. Pharmacol. 2021, 143, 112216. [Google Scholar] [CrossRef]
- Diez-Sainz, E.; Lorente-Cebrian, S.; Aranaz, P.; Riezu-Boj, J.I.; Martinez, J.A.; Milagro, F.I. Potential Mechanisms Linking Food-Derived MicroRNAs, Gut Microbiota and Intestinal Barrier Functions in the Context of Nutrition and Human Health. Front. Nutr. 2021, 8, 586564. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Zhang, N.; Hu, M.; Zhang, H.; Joshi, T.; Xu, D. Evidence for plant-derived xenomiRs based on a large-scale analysis of public small RNA sequencing data from human samples. PLoS ONE 2018, 13, e0187519. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Chu, Q.; Sun, S.; Wu, Y.; Tong, Z.; Fang, W.; Timko, M.P.; Fan, L. Large-scale identification of extracellular plant miRNAs in mammals implicates their dietary intake. PLoS ONE 2021, 16, e0257878. [Google Scholar] [CrossRef]
- Sundaram, G.M. Dietary non-coding RNAs from plants: Fairy tale or treasure? Noncoding RNA Res. 2019, 4, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Peila, C.; Sottemano, S.; Cesare Marincola, F.; Stocchero, M.; Pusceddu, N.G.; Dessi, A.; Baraldi, E.; Fanos, V.; Bertino, E. NMR Metabonomic Profile of Preterm Human Milk in the First Month of Lactation: From Extreme to Moderate Prematurity. Foods 2022, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Zamanillo, R.; Sanchez, J.; Serra, F.; Palou, A. Breast Milk Supply of MicroRNA Associated with Leptin and Adiponectin Is Affected by Maternal Overweight/Obesity and Influences Infancy BMI. Nutrients 2019, 11, 2589. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.; Kim, Y.; Park, D.J.; Oh, S. Comparative analysis of dietary exosome-derived microRNAs from human, bovine and caprine colostrum and mature milk. J. Anim. Sci. Technol. 2021, 63, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, C.X.; Li, Y.S.; Lv, J.Y.; Ma, Y.; Shao, T.T.; Xu, L.D.; Wang, Y.Y.; Du, L.; Zhang, Y.P.; et al. MiRNA-miRNA synergistic network: Construction via co-regulating functional modules and disease miRNA topological features. Nucleic Acids Res. 2011, 39, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, W.; Yuan, Y.; Bai, Y.; Sun, Y.; Zhu, W.; Du, Z. MicroRNAs tend to synergistically control expression of genes encoding extensively-expressed proteins in humans. PeerJ 2017, 5, e3682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pham, V.V.H.; Liu, L.; Xu, T.; Truong, B.; Li, J.; Rao, N.; Le, T.D. Identifying miRNA synergism using multiple-intervention causal inference. BMC Bioinform. 2019, 20, 613. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, R.; Chen, J.; Qi, E.; Zhou, S.; Wang, Y.; Fu, Q.; Chen, R.; Fang, X. Let-7i-5p Regulation of Cell Morphology and Migration Through Distinct Signaling Pathways in Normal and Pathogenic Urethral Fibroblasts. Front. Bioeng. Biotechnol. 2020, 8, 428. [Google Scholar] [CrossRef]
- Xiang, W.; Tian, C.; Lin, J.; Wu, X.; Pang, G.; Zhou, L.; Pan, S.; Deng, Z. Plasma let-7i and miR-15a expression are associated with the effect of recombinant tissue plasminogen activator treatment in acute ischemic stroke patients. Thromb. Res. 2017, 158, 121–125. [Google Scholar] [CrossRef]
- Elkhadragy, L.; Chen, M.; Miller, K.; Yang, M.H.; Long, W. A regulatory BMI1/let-7i/ERK3 pathway controls the motility of head and neck cancer cells. Mol. Oncol. 2017, 11, 194–207. [Google Scholar] [CrossRef]
- Wolska-Gawron, K.; Bartosinska, J.; Rusek, M.; Kowal, M.; Raczkiewicz, D.; Krasowska, D. Circulating miRNA-181b-5p, miRNA-223-3p, miRNA-210-3p, let 7i-5p, miRNA-21-5p and miRNA-29a-3p in patients with localized scleroderma as potential biomarkers. Sci. Rep. 2020, 10, 20218. [Google Scholar] [CrossRef]
- Kimura, K.; Hohjoh, H.; Fukuoka, M.; Sato, W.; Oki, S.; Tomi, C.; Yamaguchi, H.; Kondo, T.; Takahashi, R.; Yamamura, T. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat. Commun. 2018, 9, 17. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, C.; Yao, S.; Tang, R.; Guo, W.; Cong, H.; Li, J.; Bao, L.; Wang, D.; Li, Y.; et al. Exosomal miRNA Profiling to Identify Nanoparticle Phagocytic Mechanisms. Small 2018, 14, e1704008. [Google Scholar] [CrossRef]
- You, B.; Zhang, P.; Gu, M.; Yin, H.; Fan, Y.; Yao, H.; Pan, S.; Xie, H.; Cheng, T.; Liu, H.; et al. Let-7i-5p promotes a malignant phenotype in nasopharyngeal carcinoma via inhibiting tumor-suppressive autophagy. Cancer Lett. 2022, 531, 14–26. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Hu, L.; Xu, C.; Liang, X. Let-7i-5p enhances cell proliferation, migration and invasion of ccRCC by targeting HABP4. BMC Urol. 2021, 21, 49. [Google Scholar] [CrossRef]
- Xu, X.; Shen, H.R.; Yu, M.; Du, M.R.; Li, X.L. MicroRNA let-7i inhibits granulosa-luteal cell proliferation and oestradiol biosynthesis by directly targeting IMP2. Reprod. Biomed. Online 2022, 44, 803–816. [Google Scholar] [CrossRef]
- Xu, X.; Shen, H.-R.; Yu, M.; Du, M.-R.; Li, X.-L. Let-7i Regulates KGN Proliferation and Estradiol Biosynthesis By Directly Targeting IMP2 in Polycystic Ovary Syndrome. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Sahu, M.; Mallick, B. Deciphering synergistic regulatory networks of microRNAs in hESCs and fibroblasts. Int. J. Biol. Macromol. 2018, 113, 1279–1286. [Google Scholar] [CrossRef]
- Banerjee, S.; Kalyani Yabalooru, S.R.; Karunagaran, D. Identification of mRNA and non-coding RNA hubs using network analysis in organ tropism regulated triple negative breast cancer metastasis. Comput. Biol. Med. 2020, 127, 104076. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Zhao, J.; Hao, J.; Fu, J.; Li, Y. MiR-423-5p may regulate ovarian response to ovulation induction via CSF1. Reprod. Biol. Endocrinol. 2020, 18, 26. [Google Scholar] [CrossRef]
- Li, S.; Zeng, A.; Hu, Q.; Yan, W.; Liu, Y.; You, Y. miR-423-5p contributes to a malignant phenotype and temozolomide chemoresistance in glioblastomas. Neuro Oncol. 2017, 19, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, J.; Feng, J.; Li, Z.; Liu, Q.; Lv, P.; Wang, F.; Gao, H.; Zhang, Y. Identification of Serum miRNA-423-5p Expression Signature in Somatotroph Adenomas. Int. J. Endocrinol. 2019, 2019, 8516858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, D.; Zhu, S.; Wang, F.; Sun, X.; Yang, S.; Wang, C. Plasma Exosomal Mir-423-5p Is Involved in the Occurrence and Development of Bicuspid Aortopathy via TGF-beta/SMAD2 Pathway. Front. Physiol. 2021, 12, 759035. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Yang, Q.; Shu, Y.; Sun, C.; Yin, L.; Zou, J.; Zhan, P.; Wang, Y.; Wu, M.; et al. Prostaglandin E2 Promotes Retinal Microvascular Endothelial cell-derived miR-423-5p-containing extracellular vesicles inducing Muller cell activation in diabetic retinopathy. Traffic 2022, 23, 305. [Google Scholar] [CrossRef]
- Dai, T.; Zhao, X.; Li, Y.; Yu, L.; Li, Y.; Zhou, X.; Gong, Q. miR-423 Promotes Breast Cancer Invasion by Activating NF-kappaB Signaling. Onco Targets Ther. 2020, 13, 5467–5478. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Wang, L.; Ma, W.; Sun, Q. miR-320b Is Down-Regulated in Psoriasis and Modulates Keratinocyte Proliferation by Targeting AKT3. Inflammation 2018, 41, 2160–2170. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Yu, J.; Xu, X.; Jing, H.; Li, N.; Tang, Y.; Wang, S.; Li, Y.; Cai, J.; et al. MiR-320b/RAD21 axis affects hepatocellular carcinoma radiosensitivity to ionizing radiation treatment through DNA damage repair signaling. Cancer Sci. 2021, 112, 575–588. [Google Scholar] [CrossRef]
- Laxman, N.; Mallmin, H.; Nilsson, O.; Kindmark, A. miR-203 and miR-320 Regulate Bone Morphogenetic Protein-2-Induced Osteoblast Differentiation by Targeting Distal-Less Homeobox 5 (Dlx5). Genes 2016, 8, 4. [Google Scholar] [CrossRef]
- Lu, X.; Yang, B.; Yang, H.; Wang, L.; Li, H.; Chen, S.; Lu, X.; Gu, D. MicroRNA-320b Modulates Cholesterol Efflux and Atherosclerosis. J. Atheroscler. Thromb. 2022, 29, 200–220. [Google Scholar] [CrossRef]
- Luo, Y.; Ji, H.; Cao, Y.; Ding, X.; Li, M.; Song, H.; Li, S.; WaTableng, C.; Wu, H.; Meng, J.; et al. miR-26b-5p/TCF-4 Controls the Adipogenic Differentiation of Human Adipose-derived Mesenchymal Stem Cells. Cell Transplant. 2020, 29, 963689720934418. [Google Scholar] [CrossRef]
- Jing, S.; Tian, J.; Zhang, Y.; Chen, X.; Zheng, S. Identification of a new pseudogenes/lncRNAs-hsa-miR-26b-5p-COL12A1 competing endogenous RNA network associated with prognosis of pancreatic cancer using bioinformatics analysis. Aging 2020, 12, 19107–19128. [Google Scholar] [CrossRef]
- Hu, P.; Dong, Z.S.; Zheng, S.; Guan, X.; Zhang, L.; Li, L.; Liu, Z. The effects of miR-26b-5p on fibroblast-like synovial cells in rheumatoid arthritis (RA-FLS) via targeting EZH2. Tissue Cell 2021, 72, 101591. [Google Scholar] [CrossRef]
- Zhang, M.F.; Yang, P.; Shen, M.Y.; Wang, X.; Gao, N.X.; Zhou, X.P.; Zhou, L.L.; Lu, Y. MicroRNA-26b-5p alleviates murine collagen-induced arthritis by modulating Th17 cell plasticity. Cell Immunol. 2021, 365, 104382. [Google Scholar] [CrossRef]
- Du, J.Y.; Wang, L.F.; Wang, Q.; Yu, L.D. miR-26b inhibits proliferation, migration, invasion and apoptosis induction via the downregulation of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 driven glycolysis in osteosarcoma cells. Oncol. Rep. 2015, 33, 1890–1898. [Google Scholar] [CrossRef]
- Xu, B.; Huang, Y.; Niu, X.; Tao, T.; Jiang, L.; Tong, N.; Chen, S.; Liu, N.; Zhu, W.; Chen, M. Hsa-miR-146a-5p modulates androgen-independent prostate cancer cells apoptosis by targeting ROCK1. Prostate 2015, 75, 1896–1903. [Google Scholar] [CrossRef]
- Pesce, S.; Squillario, M.; Greppi, M.; Loiacono, F.; Moretta, L.; Moretta, A.; Sivori, S.; Castagnola, P.; Barla, A.; Candiani, S.; et al. New miRNA Signature Heralds Human NK Cell Subsets at Different Maturation Steps: Involvement of miR-146a-5p in the Regulation of KIR Expression. Front. Immunol. 2018, 9, 2360. [Google Scholar] [CrossRef]
- Contreras, J.R.; Palanichamy, J.K.; Tran, T.M.; Fernando, T.R.; Rodriguez-Malave, N.I.; Goswami, N.; Arboleda, V.A.; Casero, D.; Rao, D.S. MicroRNA-146a modulates B-cell oncogenesis by regulating Egr1. Oncotarget 2015, 6, 11023–11037. [Google Scholar] [CrossRef][Green Version]
- Wotschofsky, Z.; Gummlich, L.; Liep, J.; Stephan, C.; Kilic, E.; Jung, K.; Billaud, J.N.; Meyer, H.A. Integrated microRNA and mRNA Signature Associated with the Transition from the Locally Confined to the Metastasized Clear Cell Renal Cell Carcinoma Exemplified by miR-146-5p. PLoS ONE 2016, 11, e0148746. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, J.; Zhang, C.Y.; Shen, Y.Q.; Wang, H.M.; Ding, L.; Gu, Y.C.; Lou, J.T.; Zhao, X.T.; Ma, Z.L.; et al. MiR-146a-5p inhibits cell proliferation and cell cycle progression in NSCLC cell lines by targeting CCND1 and CCND2. Oncotarget 2016, 7, 59287–59298. [Google Scholar] [CrossRef]
- Liu, C.; Xue, J.; Xu, B.; Zhang, A.; Qin, L.; Liu, J.; Yang, Y. Exosomes Derived from miR-146a-5p-Enriched Mesenchymal Stem Cells Protect the Cardiomyocytes and Myocardial Tissues in the Polymicrobial Sepsis through Regulating MYBL1. Stem Cells Int. 2021, 2021, 1530445. [Google Scholar] [CrossRef]
- Peta, E.; Cappellesso, R.; Masi, G.; Sinigaglia, A.; Trevisan, M.; Grassi, A.; Di Camillo, B.; Vassarotto, E.; Fassina, A.; Palu, G.; et al. Down-regulation of microRNA-146a is associated with high-risk human papillomavirus infection and epidermal growth factor receptor overexpression in penile squamous cell carcinoma. Hum. Pathol. 2017, 61, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.X.; Li, L.; Dong, Y.J.; Li, P.H.; Su, Q.; Guo, Y.H.; Lu, Y.R.; Zhong, Y.; Jia, Y.; Cheng, J.Q. miR-146a-5p improves the decidual cytokine microenvironment by regulating the toll-like receptor signaling pathway in unexplained spontaneous abortion. Int. Immunopharmacol. 2020, 89, 107066. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guan, S.; Liu, F.; Chen, X.; Han, L.; Wang, D.; Nesa, E.U.; Wang, X.; Bao, C.; Wang, N.; et al. Prognostic and diagnostic potential of miR-146a in oesophageal squamous cell carcinoma. Br. J. Cancer 2016, 114, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, N.; Wang, X.; Tong, N.; Shao, N.; Tao, J.; Li, P.; Niu, X.; Feng, N.; Zhang, L.; et al. MiR-146a suppresses tumor growth and progression by targeting EGFR pathway and in a p-ERK-dependent manner in castration-resistant prostate cancer. Prostate 2012, 72, 1171–1178. [Google Scholar] [CrossRef]
- Mei, J.; Bachoo, R.; Zhang, C.L. MicroRNA-146a inhibits glioma development by targeting Notch1. Mol. Cell. Biol. 2011, 31, 3584–3592. [Google Scholar] [CrossRef]
- Cui, Y.; She, K.; Tian, D.; Zhang, P.; Xin, X. miR-146a Inhibits Proliferation and Enhances Chemosensitivity in Epithelial Ovarian Cancer via Reduction of SOD2. Oncol. Res. 2016, 23, 275–282. [Google Scholar] [CrossRef]
- Araki, Y.; Aiba, H.; Yoshida, T.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Miwa, S.; Igarashi, K.; Nguyen, T.D.; Ishii, K.A.; et al. Osteosarcoma-Derived Small Extracellular Vesicles Enhance Tumor Metastasis and Suppress Osteoclastogenesis by miR-146a-5p. Front. Oncol. 2021, 11, 667109. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, C.; Sun, Y.; Chen, Y.; Xue, D. Let-7c-5p Is Involved in Chronic Kidney Disease by Targeting TGF-beta Signaling. Biomed. Res. Int. 2020, 2020, 6960941. [Google Scholar] [CrossRef]
- Liu, G.X.; Ma, S.; Li, Y.; Yu, Y.; Zhou, Y.X.; Lu, Y.D.; Jin, L.; Wang, Z.L.; Yu, J.H. Hsa-let-7c controls the committed differentiation of IGF-1-treated mesenchymal stem cells derived from dental pulps by targeting IGF-1R via the MAPK pathways. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, H.; Wang, Q.; Li, S.; Lu, Y.; Masu, W.; Zhang, J.; Wang, T. Let-7c-5p/IGF2BP1 axis in Oral Squamous Cell Carcinoma represses progressions in vitro. Trop. J. Pharm. Res. 2021, 20, 2511–2518. [Google Scholar] [CrossRef]
- Rhodes, L.V.; Martin, E.C.; Segar, H.C.; Miller, D.F.; Buechlein, A.; Rusch, D.B.; Nephew, K.P.; Burow, M.E.; Collins-Burow, B.M. Dual regulation by microRNA-200b-3p and microRNA-200b-5p in the inhibition of epithelial-to-mesenchymal transition in triple-negative breast cancer. Oncotarget 2015, 6, 16638–16652. [Google Scholar] [CrossRef]
- Lee, K.H.; Lim, B.J.; Ferreira, V.H.; Min, S.Y.; Hong, Y.M.; Jo, J.H.; Han, S.H. Expression of human miR-200b-3p and -200c-3p in cytomegalovirus-infected tissues. Biosci. Rep. 2018, 38, BSR20180961. [Google Scholar] [CrossRef]
- Moh-Moh-Aung, A.; Fujisawa, M.; Ito, S.; Katayama, H.; Ohara, T.; Ota, Y.; Yoshimura, T.; Matsukawa, A. Decreased miR-200b-3p in cancer cells leads to angiogenesis in HCC by enhancing endothelial ERG expression. Sci. Rep. 2020, 10, 10418. [Google Scholar] [CrossRef]
- Nwaeburu, C.C.; Abukiwan, A.; Zhao, Z.; Herr, I. Quercetin-induced miR-200b-3p regulates the mode of self-renewing divisions in pancreatic cancer. Mol. Cancer 2017, 16, 23. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, N.; Du, J.; Zhang, H.; Zhang, C. MicroRNA-200b-3p promotes endothelial cell apoptosis by targeting HDAC4 in atherosclerosis. BMC Cardiovasc. Disord. 2021, 21, 172. [Google Scholar] [CrossRef]
- Li, C.; Qin, F.; Xue, M.; Lei, Y.; Hu, F.; Xu, H.; Sun, G.; Wang, T.; Guo, M. miR-429 and miR-424-5p inhibit cell proliferation and Ca2+ influx by downregulating CaSR in pulmonary artery smooth muscle cells. Am. J. Physiol. Cell Physiol. 2019, 316, C111–C120. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, J.; Li, X. lncRNA RUNDC3A-AS1 Regulates Proliferation and Apoptosis of Thyroid Cancer Cells via the miR-151b/SNRPB Axis. Int. J. Endocrinol. 2022, 2022, 9433434. [Google Scholar] [CrossRef]
- Wang, S.; Pan, Y.; Zhang, R.; Xu, T.; Wu, W.; Zhang, R.; Wang, C.; Huang, H.; Calin, C.A.; Yang, H.; et al. Hsa-miR-24-3p increases nasopharyngeal carcinoma radiosensitivity by targeting both the 3’UTR and 5’UTR of Jab1/CSN5. Oncogene 2016, 35, 6096–6108. [Google Scholar] [CrossRef]
- Dong, X.; Ding, W.; Ye, J.; Yan, D.; Xue, F.; Xu, L.; Yin, J.; Guo, W. MiR-24-3p enhances cell growth in hepatocellular carcinoma by targeting metallothionein 1M. Cell Biochem. Funct. 2016, 34, 491–496. [Google Scholar] [CrossRef]
- Jing, P.; Zhao, N.; Xie, N.; Ye, M.; Zhang, Y.; Zhang, Z.; Li, M.; Lai, X.; Zhang, J.; Gu, Z. miR-24-3p/FGFR3 Signaling as a Novel Axis Is Involved in Epithelial-Mesenchymal Transition and Regulates Lung Adenocarcinoma Progression. J. Immunol. Res. 2018, 2018, 2834109. [Google Scholar] [CrossRef]
- Kang, M.J.; Park, S.Y.; Han, J.S. MicroRNA-24-3p regulates neuronal differentiation by controlling hippocalcin expression. Cell Mol. Life Sci. 2019, 76, 4569–4580. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Gu, J.; Tao, T.; Zhang, J.; Wang, H.; Fan, Y. MiR-24-3p Inhibits the Progression of Pancreatic Ductal Adenocarcinoma Through LAMB3 Downregulation. Front. Oncol. 2019, 9, 1499. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Rho, J.G.; Kim, C.; Tak, H.; Lee, H.; Ji, E.; Ahn, S.; Shin, A.R.; Cho, H.I.; Huh, Y.H.; et al. The miR-24-3p/p130Cas: A novel axis regulating the migration and invasion of cancer cells. Sci. Rep. 2017, 7, 44847. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, N.; Wei, H.; Li, C.; Wu, J.; Yang, G. miR-24-3p Regulates Progression of Gastric Mucosal Lesions and Suppresses Proliferation and Invasiveness of N87 Via Peroxiredoxin 6. Dig. Dis. Sci. 2016, 61, 3486–3497. [Google Scholar] [CrossRef]
- Moncini, S.; Salvi, A.; Zuccotti, P.; Viero, G.; Quattrone, A.; Barlati, S.; De Petro, G.; Venturin, M.; Riva, P. The role of miR-103 and miR-107 in regulation of CDK5R1 expression and in cellular migration. PLoS ONE 2011, 6, e20038. [Google Scholar] [CrossRef]
- Wang, Z.; Zhuang, X.; Chen, B.; Feng, D.; Li, G.; Wei, M. The Role of miR-107 as a Potential Biomarker and Cellular Factor for Acute Aortic Dissection. DNA Cell Biol. 2020, 39, 1895–1906. [Google Scholar] [CrossRef]
- Luo, Y.; Hua, T.; You, X.; Lou, J.; Yang, X.; Tang, N. Effects of MiR-107 on The Chemo-drug Sensitivity of Breast Cancer Cells. Open Med. 2019, 14, 59–65. [Google Scholar] [CrossRef]
- Sharma, P.; Saini, N.; Sharma, R. miR-107 functions as a tumor suppressor in human esophageal squamous cell carcinoma and targets Cdc42. Oncol. Rep. 2017, 37, 3116–3127. [Google Scholar] [CrossRef]
- Rajabi, H.; Aslani, S.; Rahbarghazi, R. Level of miR-101a and miR-107 in Human Adipose Mesenchymal Stem Cells Committed to Insulin-producing Cells. Int. J. Mol. Cell. Med. 2021, 10, 68–74. [Google Scholar] [CrossRef]
- Tian, F.; Wang, J.; Zhang, Z.; Yang, J. miR-107 modulates chondrocyte proliferation, apoptosis, and extracellular matrix synthesis by targeting PTEN. Int. J. Clin. Exp. Pathol. 2019, 12, 488–497. [Google Scholar]
- Chen, L.; Chen, X.R.; Zhang, R.; Li, P.; Liu, Y.; Yan, K.; Jiang, X.D. MicroRNA-107 inhibits glioma cell migration and invasion by modulating Notch2 expression. J. Neurooncol. 2013, 112, 59–66. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Yin, Z.Y.; Fan, X.L.; Hu, B.; Wang, L.Q.; Zhang, D. miR-107 regulates cisplatin chemosensitivity of A549 non small cell lung cancer cell line by targeting cyclin dependent kinase 8. Int. J. Clin. Exp. Pathol. 2014, 7, 7236–7241. [Google Scholar]
- Ren, W.; Zhang, X.; Li, W.; Feng, Q.; Feng, H.; Tong, Y.; Rong, H.; Wang, W.; Zhang, D.; Zhang, Z.; et al. Exosomal miRNA-107 induces myeloid-derived suppressor cell expansion in gastric cancer. Cancer Manag. Res. 2019, 11, 4023–4040. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Wang, L.; Zhou, Q.; Li, Y.; Shen, C.; Zhang, X.; Wang, C. MicroRNA 107 partly inhibits endothelial progenitor cells differentiation via HIF-1beta. PLoS ONE 2012, 7, e40323. [Google Scholar] [CrossRef]
- Li, Y.; Mao, L.; Gao, Y.; Baral, S.; Zhou, Y.; Hu, B. MicroRNA-107 contributes to post-stroke angiogenesis by targeting Dicer-1. Sci. Rep. 2015, 5, 13316. [Google Scholar] [CrossRef]
- Wang, Y.C.; Xie, H.; Zhang, Y.C.; Meng, Q.H.; Xiong, M.M.; Jia, M.W.; Peng, F.; Tang, D.L. Exosomal miR-107 antagonizes profibrotic phenotypes of pericytes by targeting a pathway involving HIF-1alpha/Notch1/PDGFRbeta/YAP1/Twist1 axis in vitro. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H520–H534. [Google Scholar] [CrossRef]
- Wei, C.; Xiang, S.; Yu, Y.; Song, J.; Zheng, M.; Lian, F. miR-221-3p regulates apoptosis of ovarian granulosa cells via targeting FOXO1 in older women with diminished ovarian reserve (DOR). Mol. Reprod. Dev. 2021, 88, 251–260. [Google Scholar] [CrossRef]
- Li, X.; Tang, M. Exosomes released from M2 macrophages transfer miR-221-3p contributed to EOC progression through targeting CDKN1B. Cancer Med. 2020, 9, 5976–5988. [Google Scholar] [CrossRef]
- Tu, Y.; Wu, W.; Guo, Y.; Lu, F.; Li, X.; Xu, D.; Zou, D.; Tu, Y.; Chai, Y.; He, L. Up-regulation of hsa-miR-221-3p induced by UVB affects proliferation and apoptosis of keratinocytes via Bcl-xL/Bax pathway. Photodermatol. Photoimmunol. Photomed. 2021, 37, 269–277. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, Y.; Feng, Y.; Wu, W.; Zhang, H.; He, J.; Hu, Q.; Zhao, J.; Xu, Y.; Liu, Z.; et al. Decreased epithelial and sputum miR-221-3p associates with airway eosinophilic inflammation and CXCL17 expression in asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L253–L264. [Google Scholar] [CrossRef]
- Binas, S.; Knyrim, M.; Hupfeld, J.; Kloeckner, U.; Rabe, S.; Mildenberger, S.; Quarch, K.; Stratz, N.; Misiak, D.; Gekle, M.; et al. miR-221 and -222 target CACNA1C and KCNJ5 leading to altered cardiac ion channel expression and current density. Cell. Mol. Life Sci. 2020, 77, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cui, H.; Wang, X. Downregulation of EIF5A2 by miR-221-3p inhibits cell proliferation, promotes cell cycle arrest and apoptosis in medulloblastoma cells. Biosci. Biotechnol. Biochem. 2019, 83, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Kojima, S.; Nishikawa, R.; Kurozumi, A.; Kato, M.; Enokida, H.; Matsushita, R.; Yamazaki, K.; Ishida, Y.; Nakagawa, M.; et al. MicroRNA expression signature of castration-resistant prostate cancer: The microRNA-221/222 cluster functions as a tumour suppressor and disease progression marker. Br. J. Cancer 2015, 113, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Felli, N.; Fontana, L.; Pelosi, E.; Botta, R.; Bonci, D.; Facchiano, F.; Liuzzi, F.; Lulli, V.; Morsilli, O.; Santoro, S.; et al. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc. Natl. Acad. Sci. USA 2005, 102, 18081–18086. [Google Scholar] [CrossRef]
- Fornari, F.; Gramantieri, L.; Ferracin, M.; Veronese, A.; Sabbioni, S.; Calin, G.A.; Grazi, G.L.; Giovannini, C.; Croce, C.M.; Bolondi, L.; et al. MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene 2008, 27, 5651–5661. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.J.; Jung, K.H.; Eun, J.W.; Shen, Q.; Kim, H.S.; Park, S.J.; Shin, W.C.; Yang, H.D.; Park, W.S.; Lee, J.Y.; et al. MicroRNA-221 governs tumor suppressor HDAC6 to potentiate malignant progression of liver cancer. J. Hepatol. 2015, 63, 408–419. [Google Scholar] [CrossRef]
- Gits, C.M.; van Kuijk, P.F.; Jonkers, M.B.; Boersma, A.W.; van Ijcken, W.F.; Wozniak, A.; Sciot, R.; Rutkowski, P.; Schoffski, P.; Taguchi, T.; et al. MiR-17-92 and miR-221/222 cluster members target KIT and ETV1 in human gastrointestinal stromal tumours. Br. J. Cancer 2013, 109, 1625–1635. [Google Scholar] [CrossRef]
- Daugaard, I.; Sanders, K.J.; Idica, A.; Vittayarukskul, K.; Hamdorf, M.; Krog, J.D.; Chow, R.; Jury, D.; Hansen, L.L.; Hager, H.; et al. miR-151a induces partial EMT by regulating E-cadherin in NSCLC cells. Oncogenesis 2017, 6, e366. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, M. NORAD-sponged miR-378c alleviates malignant behaviors of stomach adenocarcinoma via targeting NRP1. Cancer Cell Int. 2022, 22, 79. [Google Scholar] [CrossRef]
- Shaer, A.; Azarpira, N.; Vahdati, A.; Karimi, M.H.; Shariati, M. miR-375 induces human decidua basalis-derived stromal cells to become insulin-producing cells. Cell. Mol. Biol. Lett. 2014, 19, 483–499. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Wang, X.; Li, Z.; Niu, D.; Wang, M.; Xiang, J.; Yue, Y.; Xia, Y.; Li, X. miR-375 Promotes Pancreatic Differentiation In Vitro by Affecting Different Target Genes at Different Stages. Stem Cells Int. 2021, 2021, 6642983. [Google Scholar] [CrossRef]
- Yi, J.; Jin, L.; Chen, J.; Feng, B.; He, Z.; Chen, L.; Song, H. MiR-375 suppresses invasion and metastasis by direct targeting of SHOX2 in esophageal squamous cell carcinoma. Acta Biochim. Biophys. Sin. 2017, 49, 159–169. [Google Scholar] [CrossRef]
- Chang, Y.; Yan, W.; He, X.; Zhang, L.; Li, C.; Huang, H.; Nace, G.; Geller, D.A.; Lin, J.; Tsung, A. miR-375 inhibits autophagy and reduces viability of hepatocellular carcinoma cells under hypoxic conditions. Gastroenterology 2012, 143, 177–187.e178. [Google Scholar] [CrossRef]
- Li, Q.; Huyan, T.; Cai, S.; Huang, Q.; Zhang, M.; Peng, H.; Zhang, Y.; Liu, N.; Zhang, W. The role of exosomal miR-375-3p: A potential suppressor in bladder cancer via the Wnt/beta-catenin pathway. FASEB J. 2020, 34, 12177–12196. [Google Scholar] [CrossRef]
- Jafarian, A.; Taghikani, M.; Abroun, S.; Allahverdi, A.; Lamei, M.; Lakpour, N.; Soleimani, M. The Generation of Insulin Producing Cells from Human Mesenchymal Stem Cells by MiR-375 and Anti-MiR-9. PLoS ONE 2015, 10, e0128650. [Google Scholar] [CrossRef]
- Nathan, G.; Kredo-Russo, S.; Geiger, T.; Lenz, A.; Kaspi, H.; Hornstein, E.; Efrat, S. MiR-375 promotes redifferentiation of adult human beta cells expanded in vitro. PLoS ONE 2015, 10, e0122108. [Google Scholar] [CrossRef]
- Jung, H.M.; Phillips, B.L.; Chan, E.K. miR-375 activates p21 and suppresses telomerase activity by coordinately regulating HPV E6/E7, E6AP, CIP2A, and 14-3-3zeta. Mol. Cancer 2014, 13, 80. [Google Scholar] [CrossRef]
- Chang, Z.K.; Meng, F.G.; Zhang, Z.Q.; Mao, G.P.; Huang, Z.Y.; Liao, W.M.; He, A.S. MicroRNA-193b-3p regulates matrix metalloproteinase 19 expression in interleukin-1beta-induced human chondrocytes. J. Cell. Biochem. 2018, 119, 4775–4782. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Zhang, Q.; Xu, Z.; Xu, F.; Li, D.; Li, Y. Chondrocyte sheet in vivo cartilage regeneration technique using miR-193b-3p to target MMP16. Aging 2019, 11, 7070–7082. [Google Scholar] [CrossRef]
- Meng, F.; Li, Z.; Zhang, Z.; Yang, Z.; Kang, Y.; Zhao, X.; Long, D.; Hu, S.; Gu, M.; He, S.; et al. MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3. Theranostics 2018, 8, 2862–2883. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, J.; Su, Y. miR-193b-3p possesses anti-tumor activity in ovarian carcinoma cells by targeting p21-activated kinase 3. Biomed. Pharmacol. 2017, 96, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.; Wu, D.; Liang, T.; Pan, P.; Yuan, G.; Li, X.; Li, H.; Shen, H.; Wang, Z.; Chen, G. Systemic exosomal miR-193b-3p delivery attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage in mice. J. Neuroinflamm. 2020, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, W.; Liu, Y.; Mei, B.; Liu, X.; Huang, Q. Osteoporosis genome-wide association study variant c.3781 C>A is regulated by a novel anti-osteogenic factor miR-345-5p. Hum. Mutat. 2020, 41, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Zhu, X.; Geng, Q.; Xia, L.; Sun, X.; Chen, Y.; Li, W.; Zhou, Z.; Zhan, Y.; Xu, D. The microRNA-423-3p-Bim Axis Promotes Cancer Progression and Activates Oncogenic Autophagy in Gastric Cancer. Mol. Ther. 2017, 25, 1027–1037. [Google Scholar] [CrossRef]
- Aghdam, R.; Habibi, M.; Taheri, G. Using informative features in machine learning based method for COVID-19 drug repurposing. J. Cheminform. 2021, 13, 70. [Google Scholar] [CrossRef]
- Yan, L.; Yu, M.C.; Gao, G.L.; Liang, H.W.; Zhou, X.Y.; Zhu, Z.T.; Zhang, C.Y.; Wang, Y.B.; Chen, X. MiR-125a-5p functions as a tumour suppressor in breast cancer by downregulating BAP1. J. Cell. Biochem. 2018, 119, 8773–8783. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Lv, J.; Wang, S.; Zhang, Q. MiR-125a-5p suppresses bladder cancer progression through targeting FUT4. Biomed. Pharmacol. 2018, 108, 1039–1047. [Google Scholar] [CrossRef]
- Qin, X.; Wan, Y.; Wang, S.; Xue, M. MicroRNA-125a-5p modulates human cervical carcinoma proliferation and migration by targeting ABL2. Drug Des. Dev. 2016, 10, 71–79. [Google Scholar] [CrossRef]
- Xu, X.; Tao, Y.; Niu, Y.; Wang, Z.; Zhang, C.; Yu, Y.; Ma, L. miR-125a-5p inhibits tumorigenesis in hepatocellular carcinoma. Aging 2019, 11, 7639–7662. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, S.; Tu, Y.; Zhang, G.; Liu, Y.; Li, D.; Xu, S.; Le, Z.; Xiong, J.; Zou, W.; et al. MicroRNA-125a-5p inhibits invasion and metastasis of gastric cancer cells by targeting BRMS1 expression. Oncol. Lett. 2018, 15, 5119–5130. [Google Scholar] [CrossRef]
- Tong, Z.; Liu, N.; Lin, L.; Guo, X.; Yang, D.; Zhang, Q. miR-125a-5p inhibits cell proliferation and induces apoptosis in colon cancer via targeting BCL2, BCL2L12 and MCL1. Biomed. Pharmacol. 2015, 75, 129–136. [Google Scholar] [CrossRef]
- Fu, Y.; Cao, F. MicroRNA-125a-5p regulates cancer cell proliferation and migration through NAIF1 in prostate carcinoma. Onco Targets Ther. 2015, 8, 3827–3835. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, J.; Kang, H.; Wang, Y.; Qian, J. miR-125a-5p suppresses colorectal cancer progression by targeting VEGFA. Cancer Manag. Res. 2018, 10, 5839–5853. [Google Scholar] [CrossRef]
- Jiang, L.; Chang, J.; Zhang, Q.; Sun, L.; Qiu, X. MicroRNA hsa-miR-125a-3p activates p53 and induces apoptosis in lung cancer cells. Cancer Investig. 2013, 31, 538–544. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, Q.; Chang, J.; Wang, E.; Qiu, X. MicroRNA HSA-miR-125a-5p induces apoptosis by activating p53 in lung cancer cells. Exp. Lung Res. 2011, 37, 387–398. [Google Scholar] [CrossRef]
- Li, G.; Li, J.; Li, C.; Qi, H.; Dong, P.; Zheng, J.; Yu, F. MicroRNA-125a-5p Contributes to Hepatic Stellate Cell Activation through Targeting FIH1. Cell. Physiol. Biochem. 2016, 38, 1544–1552. [Google Scholar] [CrossRef]
- Xueya, Z.; Yamei, L.; Sha, C.; Dan, C.; Hong, S.; Xingyu, Y.; Weiwei, C. Exosomal encapsulation of miR-125a-5p inhibited trophoblast cell migration and proliferation by regulating the expression of VEGFA in preeclampsia. Biochem. Biophys. Res. Commun. 2020, 525, 646–653. [Google Scholar] [CrossRef]
- Vonk, L.A.; Kragten, A.H.; Dhert, W.J.; Saris, D.B.; Creemers, L.B. Overexpression of hsa-miR-148a promotes cartilage production and inhibits cartilage degradation by osteoarthritic chondrocytes. Osteoarthr. Cartil. 2014, 22, 145–153. [Google Scholar] [CrossRef]
- Xuan, J.; Xu, H.; Li, H.; Chen, D.; Qiu, Y.; Chen, X.; Shao, M.; Xia, X. Extracellular Vesicles from miR-148a-5p-Enriched Bone Marrow Mesenchymal Stem Cells Relieve Hepatic Fibrosis by Targeting Smad4. Mol. Biotechnol. 2022, 64, 535–545. [Google Scholar] [CrossRef]
- Tan, Y.; Lu, X.; Cheng, Z.; Pan, G.; Liu, S.; Apiziaji, P.; Wang, H.; Zhang, J.; Abulimiti, Y. miR-148a Regulates the Stem Cell-Like Side Populations Distribution by Affecting the Expression of ACVR1 in Esophageal Squamous Cell Carcinoma. Onco Targets Ther. 2020, 13, 8079–8094. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, J.; Tian, X.; Wu, L. hsa-miR-29c-3p regulates biological function of colorectal cancer by targeting SPARC. Oncotarget 2017, 8, 104508–104524. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Feng, F.; Yang, Y.; Liu, K.; Duan, J.; Liu, H.; Yang, J.; Wu, M.; Liu, C.; Chang, Y. High-throughput screening identified miR-7-2-3p and miR-29c-3p as metastasis suppressors in gallbladder carcinoma. J. Gastroenterol. 2020, 55, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Chaolumen, Q.; Li, L.; Chen, B.; Su, Q.; Yang, Y.; Zhang, X. MiR-29b-3p cooperates with miR-29c-3p to affect the malignant biological behaviors in T-cell acute lymphoblastic leukemia via TFAP2C/GPX1 axis. Biochem. Biophys. Res. Commun. 2020, 527, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Luo, S.; Ji, C.; Shi, J. miR-29c-3p regulates proliferation and migration in ovarian cancer by targeting KIF4A. World J. Surg. Oncol. 2020, 18, 315. [Google Scholar] [CrossRef]
- Liu, J.; Tao, G.; Zhong, C.; Liu, X. Upregulation of miR-29c-3p Hinders Melanoma Progression by Inhibiting CDCA4 Expression. Biomed Res. Int. 2021, 2021, 7065963. [Google Scholar] [CrossRef]
- Harati, R.; Hammad, S.; Tlili, A.; Mahfood, M.; Mabondzo, A.; Hamoudi, R. miR-27a-3p regulates expression of intercellular junctions at the brain endothelium and controls the endothelial barrier permeability. PLoS ONE 2022, 17, e0262152. [Google Scholar] [CrossRef]
- Xu, Y.; Li, D.; Zhu, Z.; Li, L.; Jin, Y.; Ma, C.; Zhang, W. miR27a3p negatively regulates osteogenic differentiation of MC3T3E1 preosteoblasts by targeting osterix. Mol. Med. Rep. 2020, 22, 1717–1726. [Google Scholar] [CrossRef]
- Zeng, G.; Xun, W.; Wei, K.; Yang, Y.; Shen, H. MicroRNA-27a-3p regulates epithelial to mesenchymal transition via targeting YAP1 in oral squamous cell carcinoma cells. Oncol. Rep. 2016, 36, 1475–1482. [Google Scholar] [CrossRef]
- Li, J.M.; Zhou, J.; Xu, Z.; Huang, H.J.; Chen, M.J.; Ji, J.S. MicroRNA-27a-3p inhibits cell viability and migration through down-regulating DUSP16 in hepatocellular carcinoma. J. Cell. Biochem. 2018, 119, 5143–5152. [Google Scholar] [CrossRef]
- Chen, L.; Lu, Q.; Chen, J.; Feng, R.; Yang, C. Upregulating miR-27a-3p inhibits cell proliferation and inflammation of rheumatoid arthritis synovial fibroblasts through targeting toll-like receptor 5. Exp. Med. 2021, 22, 1227. [Google Scholar] [CrossRef]
- Wu, H.; Pula, T.; Tews, D.; Amri, E.Z.; Debatin, K.M.; Wabitsch, M.; Fischer-Posovszky, P.; Roos, J. microRNA-27a-3p but Not -5p Is a Crucial Mediator of Human Adipogenesis. Cells 2021, 10, 3205. [Google Scholar] [CrossRef]
- Yin, L.; Xiao, X.; Georgikou, C.; Yin, Y.; Liu, L.; Karakhanova, S.; Luo, Y.; Gladkich, J.; Fellenberg, J.; Sticht, C.; et al. MicroRNA-365a-3p inhibits c-Rel-mediated NF-kappaB signaling and the progression of pancreatic cancer. Cancer Lett. 2019, 452, 203–212. [Google Scholar] [CrossRef]
- Yang, R.; Liu, G.; Han, L.; Qiu, Y.; Wang, L.; Wang, M. MiR-365a-3p-Mediated Regulation of HELLS/GLUT1 Axis Suppresses Aerobic Glycolysis and Gastric Cancer Growth. Front. Oncol. 2021, 11, 616390. [Google Scholar] [CrossRef]
- Tian, Q.; Sun, H.F.; Wang, W.J.; Li, Q.; Ding, J.; Di, W. miRNA-365b promotes hepatocellular carcinoma cell migration and invasion by downregulating SGTB. Future Oncol. 2019, 15, 2019–2028. [Google Scholar] [CrossRef]
- Oliveira-Rizzo, C.; Ottati, M.C.; Fort, R.S.; Chavez, S.; Trinidad, J.M.; DiPaolo, A.; Garat, B.; Sotelo-Silveira, J.R.; Duhagon, M.A. Hsa-miR-183-5p Modulates Cell Adhesion by Repression of ITGB1 Expression in Prostate Cancer. Non-Coding RNA 2022, 8, 11. [Google Scholar] [CrossRef]
- Akbar, R.; Ullah, K.; Rahman, T.U.; Cheng, Y.; Pang, H.Y.; Jin, L.Y.; Wang, Q.J.; Huang, H.F.; Sheng, J.Z. miR-183-5p regulates uterine receptivity and enhances embryo implantation. J. Mol. Endocrinol. 2020, 64, 43–52. [Google Scholar] [CrossRef]
- Chen, J.; Gu, L.; Ni, J.; Hu, P.; Hu, K.; Shi, Y.L. MiR-183 Regulates ITGB1P Expression and Promotes Invasion of Endometrial Stromal Cells. Biomed. Res. Int. 2015, 2015, 340218. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Min, K.H.; Lee, W. MiR-183-5p induced by saturated fatty acids regulates the myogenic differentiation by directly targeting FHL1 in C2C12 myoblasts. BMB Rep. 2020, 53, 605–610. [Google Scholar] [CrossRef]
- Mollazadeh, S.; Fazly Bazzaz, B.S.; Neshati, V.; de Vries, A.A.F.; Naderi-Meshkin, H.; Mojarad, M.; Mirahmadi, M.; Neshati, Z.; Kerachian, M.A. Overexpression of MicroRNA-148b-3p stimulates osteogenesis of human bone marrow-derived mesenchymal stem cells: The role of MicroRNA-148b-3p in osteogenesis. BMC Med. Genet. 2019, 20, 117. [Google Scholar] [CrossRef]
- Wang, G.; Li, Z.; Tian, N.; Han, L.; Fu, Y.; Guo, Z.; Tian, Y. miR-148b-3p inhibits malignant biological behaviors of human glioma cells induced by high HOTAIR expression. Oncol. Lett. 2016, 12, 879–886. [Google Scholar] [CrossRef]
- Yan, S.; Chen, Y.; Yang, M.; Zhang, Q.; Ma, J.; Liu, B.; Yang, L.; Li, X. hsa-MicroRNA-28-5p Inhibits Diffuse Large B-Cell Lymphoma Cell Proliferation by Downregulating 14-3-3zeta Expression. Evid. Based Complement. Altern. Med. 2022, 2022, 4605329. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Niu, X.; Qiao, X.; Wang, X.; Liu, J.; Zhong, M. Upregulation of Neural Cell Adhesion Molecule 1 (NCAM1) by hsa-miR-141-3p Suppresses Ameloblastoma Cell Migration. Med. Sci. Monit. 2020, 26, e923491. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, P.; Liu, W.; Wang, N.; Lu, Z.; Feng, J.; Zeng, X.; Yang, J.; Wang, Y.; Zhao, W. miR-141-3p suppresses proliferation and promotes apoptosis by targeting GLI2 in osteosarcoma cells. Oncol. Rep. 2018, 39, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wu, L.; Qin, Y.; Li, Z.; Luo, S.; Qin, H.; Yang, Y.; Chen, J. Anti-proliferative role and prognostic implication of miR-141 in gastric cancer. Am. J. Transl. Res. 2016, 8, 3549–3557. [Google Scholar]
- Liang, Z.; Li, X.; Liu, S.; Li, C.; Wang, X.; Xing, J. MiR-141-3p inhibits cell proliferation, migration and invasion by targeting TRAF5 in colorectal cancer. Biochem. Biophys. Res. Commun. 2019, 514, 699–705. [Google Scholar] [CrossRef]
- Masoumi-Dehghi, S.; Babashah, S.; Sadeghizadeh, M. microRNA-141-3p-containing small extracellular vesicles derived from epithelial ovarian cancer cells promote endothelial cell angiogenesis through activating the JAK/STAT3 and NF-kappaB signaling pathways. J. Cell Commun. Signal. 2020, 14, 233–244. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Lee, W. MiR-141-3p regulates myogenic differentiation in C2C12 myoblasts via CFL2-YAP-mediated mechanotransduction. BMB Rep. 2022, 55, 104–109. [Google Scholar] [CrossRef]
- Siddiqui, S.; Johansson, K.; Joo, A.; Bonser, L.R.; Koh, K.D.; Le Tonqueze, O.; Bolourchi, S.; Bautista, R.A.; Zlock, L.; Roth, T.L.; et al. Epithelial miR-141 regulates IL-13-induced airway mucus production. JCI Insight 2021, 6, e139019. [Google Scholar] [CrossRef]
- Yu, K.R.; Lee, S.; Jung, J.W.; Hong, I.S.; Kim, H.S.; Seo, Y.; Shin, T.H.; Kang, K.S. MicroRNA-141-3p plays a role in human mesenchymal stem cell aging by directly targeting ZMPSTE24. J. Cell Sci. 2013, 126, 5422–5431. [Google Scholar] [CrossRef]
- Liang, H.; Chen, Y.; Li, H.; Yu, X.; Xia, C.; Ming, Z.; Zhong, C. miR-22-3p Suppresses Endothelial Progenitor Cell Proliferation and Migration via Inhibiting Onecut 1 (OC1)/Vascular Endothelial Growth Factor A (VEGFA) Signaling Pathway and Its Clinical Significance in Venous Thrombosis. Med. Sci. Monit. 2020, 26, e925482. [Google Scholar] [CrossRef]
- Saccomani, V.; Grassi, A.; Piovan, E.; Bongiovanni, D.; Di Martino, L.; Minuzzo, S.; Tosello, V.; Zanovello, P. miR-22-3p Negatively Affects Tumor Progression in T-Cell Acute Lymphoblastic Leukemia. Cells 2020, 9, 1726. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Kong, M.; Yang, J. MiR-22-3p suppresses sepsis-induced acute kidney injury by targeting PTEN. Biosci. Rep. 2020, 40, BSR20200527. [Google Scholar] [CrossRef]
- Xue, J.; Min, Z.; Xia, Z.; Cheng, B.; Lan, B.; Zhang, F.; Han, Y.; Wang, K.; Sun, J. The hsa-miR-181a-5p reduces oxidation resistance by controlling SECISBP2 in osteoarthritis. BMC Musculoskelet. Disord. 2018, 19, 355. [Google Scholar] [CrossRef]
- Shen, H.; Weng, X.D.; Liu, X.H.; Yang, D.; Wang, L.; Guo, J.; Wang, M.; Wang, X.; Diao, C.H. miR-181a-5p is downregulated and inhibits proliferation and the cell cycle in prostate cancer. Int. J. Clin. Exp. Pathol. 2018, 11, 3969–3976. [Google Scholar]
- He, S.; Zeng, S.; Zhou, Z.W.; He, Z.X.; Zhou, S.F. Hsa-microRNA-181a is a regulator of a number of cancer genes and a biomarker for endometrial carcinoma in patients: A bioinformatic and clinical study and the therapeutic implication. Drug Des. Dev. 2015, 9, 1103–1175. [Google Scholar] [CrossRef]
- Liu, K.; Xie, F.; Gao, A.; Zhang, R.; Zhang, L.; Xiao, Z.; Hu, Q.; Huang, W.; Huang, Q.; Lin, B.; et al. SOX2 regulates multiple malignant processes of breast cancer development through the SOX2/miR-181a-5p, miR-30e-5p/TUSC3 axis. Mol. Cancer 2017, 16, 62. [Google Scholar] [CrossRef]
- Wu, L.; Song, W.Y.; Xie, Y.; Hu, L.L.; Hou, X.M.; Wang, R.; Gao, Y.; Zhang, J.N.; Zhang, L.; Li, W.W.; et al. miR-181a-5p suppresses invasion and migration of HTR-8/SVneo cells by directly targeting IGF2BP2. Cell Death Dis. 2018, 9, 16. [Google Scholar] [CrossRef]
- Potenza, N.; Mosca, N.; Mondola, P.; Damiano, S.; Russo, A.; De Felice, B. Human miR-26a-5p regulates the glutamate transporter SLC1A1 (EAAT3) expression. Relevance in multiple sclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 317–323. [Google Scholar] [CrossRef]
- Rasheed, Z.; Al-Shobaili, H.A.; Rasheed, N.; Mahmood, A.; Khan, M.I. MicroRNA-26a-5p regulates the expression of inducible nitric oxide synthase via activation of NF-kappaB pathway in human osteoarthritis chondrocytes. Arch. Biochem. Biophys. 2016, 594, 61–67. [Google Scholar] [CrossRef]
- Huang, Z.M.; Ge, H.F.; Yang, C.C.; Cai, Y.; Chen, Z.; Tian, W.Z.; Tao, J.L. MicroRNA-26a-5p inhibits breast cancer cell growth by suppressing RNF6 expression. Kaohsiung J. Med. Sci. 2019, 35, 467–473. [Google Scholar] [CrossRef]
- Erdmann, K.; Kaulke, K.; Rieger, C.; Salomo, K.; Wirth, M.P.; Fuessel, S. MiR-26a and miR-138 block the G1/S transition by targeting the cell cycle regulating network in prostate cancer cells. J. Cancer Res. Clin. Oncol. 2016, 142, 2249–2261. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, Y.; Cai, J.; Cui, K.; Li, R.X.; Wang, H.; Shang, X.; Wei, D. MiR-30a-5p Suppresses Tumor Metastasis of Human Colorectal Cancer by Targeting ITGB3. Cell. Physiol. Biochem. 2016, 39, 1165–1176. [Google Scholar] [CrossRef]
- Yang, J.; Rao, S.; Cao, R.; Xiao, S.; Cui, X.; Ye, L. miR-30a-5p suppresses lung squamous cell carcinoma via ATG5-mediated autophagy. Aging 2021, 13, 17462–17472. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Chen, D.; Jin, L.; Su, Z.; Liu, J.; Duan, H.; Li, X.; Qi, Z.; Shi, M.; et al. miR30a5p in the tumorigenesis of renal cell carcinoma: A tumor suppressive microRNA. Mol. Med. Rep. 2016, 13, 4085–4094. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.L.; Yang, Y.J.; Tang, C.; Sheng, H.J.; Jiang, Y.; Han, K.; Ding, L.J. Estrogen combined with progesterone decreases cell proliferation and inhibits the expression of Bcl-2 via microRNA let-7a and miR-34b in ovarian cancer cells. Clin. Transl. Oncol. 2014, 16, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Guo, L. MicroRNA-148a-3p inhibits cancer progression and is a novel screening biomarker for gastric cancer. J. Clin. Lab. Anal. 2020, 34, e23454. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hou, Y.; Ma, W.; Xie, M.; Jin, Y.; Xu, L.; Li, C.; Wang, Y.; Chen, J.; Chen, W.; et al. A novel mechanism underlying alcohol dehydrogenase expression: Hsa-miR-148a-3p promotes ADH4 expression via an AGO1-dependent manner in control and ethanol-exposed hepatic cells. Biochem. Pharmacol. 2021, 189, 114458. [Google Scholar] [CrossRef]
- Kim, H.; Ko, Y.; Park, H.; Zhang, H.; Jeong, Y.; Kim, Y.; Noh, M.; Park, S.; Kim, Y.M.; Kwon, Y.G. MicroRNA-148a/b-3p regulates angiogenesis by targeting neuropilin-1 in endothelial cells. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef]
- Miao, W.; Li, N.; Gu, B.; Yi, G.; Su, Z.; Cheng, H. MiR-27b-3p suppresses glioma development via targeting YAP1. Biochem. Cell Biol. 2020, 98, 466–473. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, T.; Cao, Y.W.; Ding, X.Q. Antitumor effect of miR-27b-3p on lung cancer cells via targeting Fzd7. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4113–4123. [Google Scholar]
- Chen, Y.; Chen, G.; Zhang, B.; Liu, C.; Yu, Y.; Jin, Y. miR-27b-3p suppresses cell proliferation, migration and invasion by targeting LIMK1 in colorectal cancer. Int. J. Clin. Exp. Pathol. 2017, 10, 9251–9261. [Google Scholar]
- Rong, X.; Ge, D.; Shen, D.; Chen, X.; Wang, X.; Zhang, L.; Jia, C.; Zeng, J.; He, Y.; Qiu, H.; et al. miR-27b Suppresses Endothelial Cell Proliferation and Migration by Targeting Smad7 in Kawasaki Disease. Cell. Physiol. Biochem. 2018, 48, 1804–1814. [Google Scholar] [CrossRef]
- Peng, W.; Zhu, S.; Li, X.; Weng, J.; Chen, S. miR-27b-3p Suppressed Osteogenic Differentiation of Maxillary Sinus Membrane Stem Cells by Targeting Sp7. Implant Dent. 2017, 26, 492–499. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Dong, Y.; Fan, Y.; Li, Y.; Zhao, C.; Wang, C.; Liu, J.; Li, X.; Dong, M.; et al. MiR-146b-5p functions as a suppressor miRNA and prognosis predictor in non-small cell lung cancer. J. Cancer 2017, 8, 1704–1716. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Yu, L.; Sun, C.; Cheng, D.; Yu, S.; Wang, Q.; Yan, Y.; Kang, C.; Jin, S.; et al. miR-146b-5p inhibits glioma migration and invasion by targeting MMP16. Cancer Lett. 2013, 339, 260–269. [Google Scholar] [CrossRef]
- Garcia, A.I.; Buisson, M.; Bertrand, P.; Rimokh, R.; Rouleau, E.; Lopez, B.S.; Lidereau, R.; Mikaelian, I.; Mazoyer, S. Down-regulation of BRCA1 expression by miR-146a and miR-146b-5p in triple negative sporadic breast cancers. EMBO Mol. Med. 2011, 3, 279–290. [Google Scholar] [CrossRef]
- Kirchmeyer, M.; Servais, F.A.; Hamdorf, M.; Nazarov, P.V.; Ginolhac, A.; Halder, R.; Vallar, L.; Glanemann, M.; Rubie, C.; Lammert, F.; et al. Cytokine-mediated modulation of the hepatic miRNome: miR-146b-5p is an IL-6-inducible miRNA with multiple targets. J. Leukoc. Biol. 2018, 104, 987–1002. [Google Scholar] [CrossRef]
- Han, L.; Zhou, Y.; Zhang, R.; Wu, K.; Lu, Y.; Li, Y.; Duan, R.; Yao, Y.; Zhu, D.; Jia, Y. MicroRNA Let-7f-5p Promotes Bone Marrow Mesenchymal Stem Cells Survival by Targeting Caspase-3 in Alzheimer Disease Model. Front. Neurosci. 2018, 12, 333. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Xie, N.; Shao, L.; Sun, H.; Wei, Y.; Sun, Y.; Wang, P.; Yan, Y.; Xie, S.; et al. Anticancer roles of let-7f-1-3p in non-small cell lung cancer via direct targeting of integrin beta1. Exp. Med. 2021, 22, 1305. [Google Scholar] [CrossRef]
- Du, L.; Tao, X.; Shen, X. Human umbilical cord mesenchymal stem cell-derived exosomes inhibit migration and invasion of breast cancer cells via miR-21-5p/ZNF367 pathway. Breast Cancer 2021, 28, 829–837. [Google Scholar] [CrossRef]
- He, Q.; Ye, A.; Ye, W.; Liao, X.; Qin, G.; Xu, Y.; Yin, Y.; Luo, H.; Yi, M.; Xian, L.; et al. Cancer-secreted exosomal miR-21-5p induces angiogenesis and vascular permeability by targeting KRIT1. Cell Death Dis. 2021, 12, 576. [Google Scholar] [CrossRef] [PubMed]
- De Santi, C.; Vencken, S.; Blake, J.; Haase, B.; Benes, V.; Gemignani, F.; Landi, S.; Greene, C.M. Identification of MiR-21-5p as a Functional Regulator of Mesothelin Expression Using MicroRNA Capture Affinity Coupled with Next Generation Sequencing. PLoS ONE 2017, 12, e0170999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, L.; Liu, Y.; Wang, S.; Hou, Z.; Zhou, J. miR-21-5p promotes cell proliferation by targeting BCL11B in Thp-1 cells. Oncol. Lett. 2021, 21, 119. [Google Scholar] [CrossRef]
- Yan, L.; Cao, R.; Liu, Y.; Wang, L.; Pan, B.; Lv, X.; Jiao, H.; Zhuang, Q.; Sun, X.; Xiao, R. MiR-21-5p Links Epithelial-Mesenchymal Transition Phenotype with Stem-Like Cell Signatures via AKT Signaling in Keloid Keratinocytes. Sci. Rep. 2016, 6, 28281. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.B.; Zhang, X.; Deng, L.; Jiang, L.; Meng, W.; Lu, Z.; Wang, X. MiR-92a mediates AZD6244 induced apoptosis and G1-phase arrest of lymphoma cells by targeting Bim. Cell Biol. Int. 2014, 38, 435–443. [Google Scholar] [CrossRef]
- Li, M.; Guan, X.; Sun, Y.; Mi, J.; Shu, X.; Liu, F.; Li, C. miR-92a family and their target genes in tumorigenesis and metastasis. Exp. Cell Res. 2014, 323, 1–6. [Google Scholar] [CrossRef]
- Kalinina, N.; Klink, G.; Glukhanyuk, E.; Lopatina, T.; Efimenko, A.; Akopyan, Z.; Tkachuk, V. miR-92a regulates angiogenic activity of adipose-derived mesenchymal stromal cells. Exp. Cell Res. 2015, 339, 61–66. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhang, C.; Zhang, C.; Yang, H.; Gao, R.; Tong, Z. Plasma Hsa-miR-92a-3p in correlation with lipocalin-2 is associated with sepsis-induced coagulopathy. BMC Infect. Dis. 2020, 20, 155. [Google Scholar] [CrossRef]
- Mao, G.; Zhang, Z.; Huang, Z.; Chen, W.; Huang, G.; Meng, F.; Zhang, Z.; Kang, Y. MicroRNA-92a-3p regulates the expression of cartilage-specific genes by directly targeting histone deacetylase 2 in chondrogenesis and degradation. Osteoarthr. Cartil. 2017, 25, 521–532. [Google Scholar] [CrossRef]
- Mao, G.; Wu, P.; Zhang, Z.; Zhang, Z.; Liao, W.; Li, Y.; Kang, Y. MicroRNA-92a-3p Regulates Aggrecanase-1 and Aggrecanase-2 Expression in Chondrogenesis and IL-1beta-Induced Catabolism in Human Articular Chondrocytes. Cell. Physiol. Biochem. 2017, 44, 38–52. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, L.; Li, F.; Yang, J.; Wan, X.; Ouyang, M. microRNA-16-5p-containing exosomes derived from bone marrow-derived mesenchymal stem cells inhibit proliferation, migration, and invasion, while promoting apoptosis of colorectal cancer cells by downregulating ITGA2. J. Cell. Physiol. 2019, 234, 21380–21394. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, K.; Ren, T.; Huang, Y.; Tang, X.; Guo, W. miR-16-5p inhibits chordoma cell proliferation, invasion and metastasis by targeting Smad3. Cell Death Dis. 2018, 9, 680. [Google Scholar] [CrossRef]
- Chava, S.; Reynolds, C.P.; Pathania, A.S.; Gorantla, S.; Poluektova, L.Y.; Coulter, D.W.; Gupta, S.C.; Pandey, M.K.; Challagundla, K.B. miR-15a-5p, miR-15b-5p, and miR-16-5p inhibit tumor progression by directly targeting MYCN in neuroblastoma. Mol. Oncol. 2020, 14, 180–196. [Google Scholar] [CrossRef]
- Ruan, L.; Qian, X. MiR-16-5p inhibits breast cancer by reducing AKT3 to restrain NF-kappaB pathway. Biosci. Rep. 2019, 39, BSR20191611. [Google Scholar] [CrossRef]
- Calderon-Dominguez, M.; Mangas, A.; Belmonte, T.; Quezada-Feijoo, M.; Ramos, M.; Toro, R. Ischemic dilated cardiomyopathy pathophysiology through microRNA-16-5p. Rev. Esp. Cardiol. 2021, 74, 740–749. [Google Scholar] [CrossRef]
- Wang, F.; Mao, A.; Tang, J.; Zhang, Q.; Yan, J.; Wang, Y.; Di, C.; Gan, L.; Sun, C.; Zhang, H. microRNA-16-5p enhances radiosensitivity through modulating Cyclin D1/E1-pRb-E2F1 pathway in prostate cancer cells. J. Cell. Physiol. 2019, 234, 13182–13190. [Google Scholar] [CrossRef]
- Zhang, N.; Li, W.W.; Lv, C.M.; Gao, Y.W.; Liu, X.L.; Zhao, L. miR-16-5p and miR-19b-3p prevent amyloid beta-induced injury by targeting BACE1 in SH-SY5Y cells. Neuroreport 2020, 31, 205–212. [Google Scholar] [CrossRef]
- Dong, L.X.; Bao, H.L.; Zhang, Y.Y.; Liu, Y.; Zhang, G.W.; An, F.M. MicroRNA-16-5p/BTG2 axis affects neurological function, autophagy and apoptosis of hippocampal neurons in Alzheimer’s disease. Brain Res. Bull. 2021, 175, 254–262. [Google Scholar] [CrossRef]
- Li, L.; Jia, J.; Liu, X.; Yang, S.; Ye, S.; Yang, W.; Zhang, Y. MicroRNA-16-5p Controls Development of Osteoarthritis by Targeting SMAD3 in Chondrocytes. Curr. Pharm. Des. 2015, 21, 5160–5167. [Google Scholar] [CrossRef]
- Yao, Q.; Xing, Y.; Wang, Z.; Liang, J.; Lin, Q.; Huang, M.; Chen, Y.; Lin, B.; Xu, X.; Chen, W. MiR-16-5p suppresses myofibroblast activation in systemic sclerosis by inhibiting NOTCH signaling. Aging 2020, 13, 2640–2654. [Google Scholar] [CrossRef]
- Cai, B.; Ma, M.; Chen, B.; Li, Z.; Abdalla, B.A.; Nie, Q.; Zhang, X. MiR-16-5p targets SESN1 to regulate the p53 signaling pathway, affecting myoblast proliferation and apoptosis, and is involved in myoblast differentiation. Cell Death Dis. 2018, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; You, X.; Zhou, H.; He, W.; Li, Z.; Li, B.; Xia, J.; Zhu, H.; Zhao, Y.; Yu, G.; et al. MiR-16-5p regulates postmenopausal osteoporosis by directly targeting VEGFA. Aging 2020, 12, 9500–9514. [Google Scholar] [CrossRef] [PubMed]
- Normann, L.S.; Haugen, M.H.; Aure, M.R.; Kristensen, V.N.; Maelandsmo, G.M.; Sahlberg, K.K. miR-101-5p Acts as a Tumor Suppressor in HER2-Positive Breast Cancer Cells and Improves Targeted Therapy. Breast Cancer (Dove Med. Press) 2022, 14, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Shan, X.; Chen, K.; Liu, Y.; Yu, G.; Chen, Q.; Zeng, T.; Zhu, L.; Dang, H.; Chen, F.; et al. LINC00052/miR-101-3p axis inhibits cell proliferation and metastasis by targeting SOX9 in hepatocellular carcinoma. Gene 2018, 679, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Ding, S.; Chen, K.; Chen, J.; Wang, S.; Zou, C.; Zhang, J.; Cao, Y.; Huang, A.; Tang, H. Functional analysis of miR-101-3p and Rap1b involved in hepatitis B virus-related hepatocellular carcinoma pathogenesis. Biochem. Cell Biol. 2014, 92, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shao, M.Y.; Zou, S.C.; Xiao, Z.F.; Chen, Z.C. MiR-101-3p inhibits EMT to attenuate proliferation and metastasis in glioblastoma by targeting TRIM44. J. Neurooncol. 2019, 141, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, B. miR-101-3p induces autophagy in endometrial carcinoma cells by targeting EZH2. Arch. Gynecol. Obstet. 2018, 297, 1539–1548. [Google Scholar] [CrossRef]
- Guo, X.; Chen, M.; Cao, L.; Hu, Y.; Li, X.; Zhang, Q.; Ren, Y.; Wu, X.; Meng, Z.; Xu, K. Cancer-Associated Fibroblasts Promote Migration and Invasion of Non-Small Cell Lung Cancer Cells via miR-101-3p Mediated VEGFA Secretion and AKT/eNOS Pathway. Front. Cell Dev. Biol. 2021, 9, 764151. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, Y.; Xie, T.; Liu, H.; Zhan, X.; Xu, Y. miR-101-3p Serves as a Tumor Suppressor for Renal Cell Carcinoma and Inhibits Its Invasion and Metastasis by Targeting EZH2. Biomed Res. Int. 2021, 2021, 9950749. [Google Scholar] [CrossRef]
- Luo, C.; Merz, P.R.; Chen, Y.; Dickes, E.; Pscherer, A.; Schadendorf, D.; Eichmuller, S.B. MiR-101 inhibits melanoma cell invasion and proliferation by targeting MITF and EZH2. Cancer Lett. 2013, 341, 240–247. [Google Scholar] [CrossRef]
- Ziemann, M.; Lim, S.C.; Kang, Y.; Samuel, S.; Sanchez, I.L.; Gantier, M.; Stojanovski, D.; McKenzie, M. MicroRNA-101-3p Modulates Mitochondrial Metabolism via the Regulation of Complex II Assembly. J. Mol. Biol. 2022, 434, 167361. [Google Scholar] [CrossRef]
- Chen, Q.; Li, X.; Kong, L.; Xu, Q.; Wang, Z.; Lv, Q. miR-101-3p induces vascular endothelial cell dysfunction by targeting tet methylcytosine dioxygenase 2. Acta Biochim. Biophys. Sin. 2020, 52, 180–191. [Google Scholar] [CrossRef]
- Kaneda-Ikeda, E.; Iwata, T.; Mizuno, N.; Nagahara, T.; Kajiya, M.; Ouhara, K.; Yoshioka, M.; Ishida, S.; Kawaguchi, H.; Kurihara, H. Regulation of osteogenesis via miR-101-3p in mesenchymal stem cells by human gingival fibroblasts. J. Bone Miner. Metab. 2020, 38, 442–455. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Yu, Y.; Li, J.; Hu, Q.; Xue, C.; Chen, J.; Shen, S.; Luo, Y.; Ren, F.; et al. LDHA is a direct target of miR-30d-5p and contributes to aggressive progression of gallbladder carcinoma. Mol. Carcinog. 2018, 57, 772–783. [Google Scholar] [CrossRef]
- Bjornetro, T.; Redalen, K.R.; Meltzer, S.; Thusyanthan, N.S.; Samiappan, R.; Jegerschold, C.; Handeland, K.R.; Ree, A.H. An experimental strategy unveiling exosomal microRNAs 486-5p, 181a-5p and 30d-5p from hypoxic tumour cells as circulating indicators of high-risk rectal cancer. J. Extracell. Vesicles 2019, 8, 1567219. [Google Scholar] [CrossRef]
- Jacob, H.; Stanisavljevic, L.; Storli, K.E.; Hestetun, K.E.; Dahl, O.; Myklebust, M.P. Identification of a sixteen-microRNA signature as prognostic biomarker for stage II and III colon cancer. Oncotarget 2017, 8, 87837–87847. [Google Scholar] [CrossRef]
- Song, Y.; Song, C.; Yang, S. Tumor-Suppressive Function of miR-30d-5p in Prostate Cancer Cell Proliferation and Migration by Targeting NT5E. Cancer Biother. Radiopharm. 2018, 33, 203–211. [Google Scholar] [CrossRef]
- Wang, G.; Feng, B.; Niu, Y.; Wu, J.; Yang, Y.; Shen, S.; Guo, Y.; Liang, J.; Guo, W.; Dong, Z. A novel long noncoding RNA, LOC440173, promotes the progression of esophageal squamous cell carcinoma by modulating the miR-30d-5p/HDAC9 axis and the epithelial-mesenchymal transition. Mol. Carcinog. 2020, 59, 1392–1408. [Google Scholar] [CrossRef]
- Liang, L.; Yang, Z.; Deng, Q.; Jiang, Y.; Cheng, Y.; Sun, Y.; Li, L. miR-30d-5p suppresses proliferation and autophagy by targeting ATG5 in renal cell carcinoma. FEBS Open Bio 2021, 11, 529–540. [Google Scholar] [CrossRef]
- Zheng, H.; Ding, B.; Xue, K.; Yu, J.; Lou, W. Construction of a lncRNA/pseudogene-hsa-miR-30d-5p-GJA1 regulatory network related to metastasis of pancreatic cancer. Genomics 2021, 113, 1742–1753. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Liu, H.; Pan, Z. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod. Biol. Endocrinol. 2019, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Kang, Z.; Guo, L.; Qu, F.; Qu, R. The Role of LINC00284 in the Development of Thyroid Cancer via Its Regulation of the MicroRNA-30d-5p-Mediated ADAM12/Notch Axis. Front. Oncol. 2021, 11, 643039. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Hou, Y.; Qi, L. miR-30d-5p represses the proliferation, migration, and invasion of lung squamous cell carcinoma via targeting DBF4. J. Environ. Sci. Health C Toxicol. Carcinog. 2021, 39, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yin, Y.; Liu, X.; Xi, X.; Xue, W.; Qu, Y. Non-small cell lung cancer associated microRNA expression signature: Integrated bioinformatics analysis, validation and clinical significance. Oncotarget 2017, 8, 24564–24578. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Soltani, B.M.; Tavallaei, M.; Mowla, S.J.; Tafsiri, E.; Bagheri, A.; Khorshid, H.R.K. Clinically Significant Dysregulation of hsa-miR-30d-5p and hsa-let-7b Expression in Patients with Surgically Resected Non-Small Cell Lung Cancer. Avicenna J. Med. Biotechnol. 2018, 10, 98–104. [Google Scholar]
- Chen, D.; Guo, W.; Qiu, Z.; Wang, Q.; Li, Y.; Liang, L.; Liu, L.; Huang, S.; Zhao, Y.; He, X. MicroRNA-30d-5p inhibits tumour cell proliferation and motility by directly targeting CCNE2 in non-small cell lung cancer. Cancer Lett. 2015, 362, 208–217. [Google Scholar] [CrossRef]
- Krist, B.; Florczyk, U.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The Role of miR-378a in Metabolism, Angiogenesis, and Muscle Biology. Int. J. Endocrinol. 2015, 2015, 281756. [Google Scholar] [CrossRef]
- Guo, X.B.; Zhang, X.C.; Chen, P.; Ma, L.M.; Shen, Z.Q. miR378a3p inhibits cellular proliferation and migration in glioblastoma multiforme by targeting tetraspanin 17. Oncol. Rep. 2019, 42, 1957–1971. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, J.J.; Feng, Y.; Yang, J.L.; Huang, H.; Chung, W.Y.; Hu, Y.L.; Xue, W.J. DNMT1-induced miR-378a-3p silencing promotes angiogenesis via the NF-kappaB signaling pathway by targeting TRAF1 in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 352. [Google Scholar] [CrossRef]
- Chu, X.; Zheng, W.; Wang, J.; Zhang, J.; Pan, Y.; Shao, C. CDK6 inhibition targeted by miR-378a-3p protects against intestinal injury induced by ionizing radiation. Biochem. Biophys. Res. Commun. 2020, 531, 328–334. [Google Scholar] [CrossRef]
- Soonthornchai, W.; Tangtanatakul, P.; Meesilpavikkai, K.; Dalm, V.; Kueanjinda, P.; Wongpiyabovorn, J. MicroRNA-378a-3p is overexpressed in psoriasis and modulates cell cycle arrest in keratinocytes via targeting BMP2 gene. Sci. Rep. 2021, 11, 14186. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, Z.; Jin, C.; Chen, C.; Wu, N. hsa-miR-191-5p inhibits replication of human immunodeficiency virus type 1 by downregulating the expression of NUP50. Arch. Virol. 2021, 166, 755–766. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Zhang, Z.; Ren, F.; Chen, L.; Lan, Z. miR10a5p inhibits osteogenic differentiation of bone marrowderived mesenchymal stem cells. Mol. Med. Rep. 2020, 22, 135–144. [Google Scholar] [CrossRef]
- Vaher, H.; Runnel, T.; Urgard, E.; Aab, A.; Carreras Badosa, G.; Maslovskaja, J.; Abram, K.; Raam, L.; Kaldvee, B.; Annilo, T.; et al. miR-10a-5p is increased in atopic dermatitis and has capacity to inhibit keratinocyte proliferation. Allergy 2019, 74, 2146–2156. [Google Scholar] [CrossRef]
- Bin, S.; Xin, L.; Lin, Z.; Jinhua, Z.; Rui, G.; Xiang, Z. Targeting miR-10a-5p/IL-6R axis for reducing IL-6-induced cartilage cell ferroptosis. Exp. Mol. Pathol. 2021, 118, 104570. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, J.; Kong, W.; Fang, Z.; Li, Y.; Huang, Z.; Wen, J.; Wang, Y. Roles of miR-10a-5p and miR-103a-3p, Regulators of BDNF Expression in Follicular Fluid, in the Outcomes of IVF-ET. Front. Endocrinol. 2021, 12, 637384. [Google Scholar] [CrossRef]
- Arai, T.; Okato, A.; Kojima, S.; Idichi, T.; Koshizuka, K.; Kurozumi, A.; Kato, M.; Yamazaki, K.; Ishida, Y.; Naya, Y.; et al. Regulation of spindle and kinetochore-associated protein 1 by antitumor miR-10a-5p in renal cell carcinoma. Cancer Sci. 2017, 108, 2088–2101. [Google Scholar] [CrossRef]
- Zhang, S.; Al-Maghout, T.; Cao, H.; Pelzl, L.; Salker, M.S.; Veldhoen, M.; Cheng, A.; Lang, F.; Singh, Y. Gut Bacterial Metabolite Urolithin A (UA) Mitigates Ca2+ Entry in T Cells by Regulating miR-10a-5p. Front. Immunol. 2019, 10, 1737. [Google Scholar] [CrossRef]
- Zhu, J.; Du, S.; Zhang, J.; Huang, G.; Dong, L.; Ren, E.; Liu, D. microRNA-10a-5p from gastric cancer cell-derived exosomes enhances viability and migration of human umbilical vein endothelial cells by targeting zinc finger MYND-type containing 11. Bioengineered 2022, 13, 496–507. [Google Scholar] [CrossRef]
- Liu, T.W.; Liu, F.; Kang, J. Let-7b-5p is involved in the response of endoplasmic reticulum stress in acute pulmonary embolism through upregulating the expression of stress-associated endoplasmic reticulum protein 1. IUBMB Life 2020, 72, 1725–1736. [Google Scholar] [CrossRef]
- Aday, S.; Hazan-Halevy, I.; Chamorro-Jorganes, A.; Anwar, M.; Goldsmith, M.; Beazley-Long, N.; Sahoo, S.; Dogra, N.; Sweaad, W.; Catapano, F.; et al. Bioinspired artificial exosomes based on lipid nanoparticles carrying let-7b-5p promote angiogenesis in vitro and in vivo. Mol. Ther. 2021, 29, 2239–2252. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.S.; Zheng, C.F.; Zeng, Y.P.; Lin, J.; Chen, J.L.; Shi, S.J. [Hsa-let-7b-5p Inhibits Proliferation of Human Leukemia THP-1 Cells via FTO/m(6)A/MYC Signaling Pathway]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2020, 28, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Shakespear, N.; Ogura, M.; Yamaki, J.; Homma, Y. Astrocyte-Derived Exosomal microRNA miR-200a-3p Prevents MPP(+)-Induced Apoptotic Cell Death Through Down-Regulation of MKK4. Neurochem. Res. 2020, 45, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Bao, H.; Zhang, S.; Li, R.; Chen, L.; Zhu, Y. miR-186-5p Promotes Apoptosis by Targeting IGF-1 in SH-SY5Y OGD/R Model. Int. J. Biol. Sci. 2018, 14, 1791–1799. [Google Scholar] [CrossRef]
- Bayat, Z.; Ghaemi, Z.; Behmanesh, M.; Soltani, B.M. Hsa-miR-186-5p regulates TGFbeta signaling pathway through expression suppression of SMAD6 and SMAD7 genes in colorectal cancer. Biol. Chem. 2021, 402, 469–480. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, H.; Wang, Z.; Wu, Y.; Wang, J.; Duan, G.; Guo, Q.; Zhang, Y. miR-320a/SP1 negative reciprocal interaction contributes to cell growth and invasion in colorectal cancer. Cancer Cell Int. 2021, 21, 175. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, T.; Zhou, H.; Wang, L.; Huang, A.; Feng, B.; Quan, Y.; Jin, R.; Zhang, W.; Sun, J.; et al. miR-320a suppresses colorectal cancer progression by targeting Rac1. Carcinogenesis 2014, 35, 886–895. [Google Scholar] [CrossRef]
- Guo, T.; Feng, Y.; Liu, Q.; Yang, X.; Jiang, T.; Chen, Y.; Zhang, Q. MicroRNA-320a suppresses in GBM patients and modulates glioma cell functions by targeting IGF-1R. Tumor Biol. 2014, 35, 11269–11275. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, J.; Pan, J.; Geng, X.; Li, L.; Wu, J.; Song, P.; Wang, Y.; Liu, J.; Wang, L. MiR-320a inhibits gastric carcinoma by targeting activity in the FoxM1-P27KIP1 axis. Oncotarget 2016, 7, 29275–29286. [Google Scholar] [CrossRef]
- Boldt, P.; Knechtle, B.; Nikolaidis, P.; Lechleitner, C.; Wirnitzer, G.; Leitzmann, C.; Rosemann, T.; Wirnitzer, K. Quality of life of female and male vegetarian and vegan endurance runners compared to omnivores-results from the NURMI study (step 2). J. Int. Soc. Sports Nutr. 2018, 15, 33. [Google Scholar] [CrossRef]
- Xishan, Z.; Ziying, L.; Jing, D.; Gang, L. MicroRNA-320a acts as a tumor suppressor by targeting BCR/ABL oncogene in chronic myeloid leukemia. Sci. Rep. 2015, 5, 12460. [Google Scholar] [CrossRef]
- Pisanu, C.; Merkouri Papadima, E.; Melis, C.; Congiu, D.; Loizedda, A.; Orru, N.; Calza, S.; Orru, S.; Carcassi, C.; Severino, G.; et al. Whole Genome Expression Analyses of miRNAs and mRNAs Suggest the Involvement of miR-320a and miR-155-3p and their Targeted Genes in Lithium Response in Bipolar Disorder. Int. J. Mol. Sci. 2019, 20, 6040. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Hu, C.; Zhou, J.; Xu, D.; Hou, Y.; Wu, C.; Zhao, J.; Li, M.; Zeng, X.; et al. MicroRNA-320a: An important regulator in the fibrotic process in interstitial lung disease of systemic sclerosis. Arthritis Res. 2021, 23, 21. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, D.; Wang, J.; Li, Y.; Chai, X.; Kang, Q. miR-320a regulates cell proliferation and apoptosis in multiple myeloma by targeting pre-B-cell leukemia transcription factor 3. Biochem. Biophys. Res. Commun. 2016, 473, 1315–1320. [Google Scholar] [CrossRef]
- Mittal, S.P.; Mathai, J.; Kulkarni, A.P.; Pal, J.K.; Chattopadhyay, S. miR-320a regulates erythroid differentiation through MAR binding protein SMAR1. Int. J. Biochem. Cell Biol. 2013, 45, 2519–2529. [Google Scholar] [CrossRef]
- Jo, S.; Xu, G.; Jing, G.; Chen, J.; Shalev, A. Human Glucagon Expression Is under the Control of miR-320a. Endocrinology 2021, 162, bqaa238. [Google Scholar] [CrossRef]
- Berkhout, R.P.; Keijser, R.; Repping, S.; Lambalk, C.B.; Afink, G.B.; Mastenbroek, S.; Hamer, G. High-quality human preimplantation embryos stimulate endometrial stromal cell migration via secretion of microRNA hsa-miR-320a. Hum. Reprod. 2020, 35, 1797–1807. [Google Scholar] [CrossRef]
- Dahlmans, D.; Houzelle, A.; Andreux, P.; Jorgensen, J.A.; Wang, X.; de Windt, L.J.; Schrauwen, P.; Auwerx, J.; Hoeks, J. An unbiased silencing screen in muscle cells identifies miR-320a, miR-150, miR-196b, and miR-34c as regulators of skeletal muscle mitochondrial metabolism. Mol. Metab. 2017, 6, 1429–1442. [Google Scholar] [CrossRef]
- Li, F.J.; Zhang, C.L.; Luo, X.J.; Peng, J.; Yang, T.L. Involvement of the MiR-181b-5p/HMGB1 Pathway in Ang II-induced Phenotypic Transformation of Smooth Muscle Cells in Hypertension. Aging Dis. 2019, 10, 231–248. [Google Scholar] [CrossRef]
- Di Marco, M.; Veschi, S.; Lanuti, P.; Ramassone, A.; Pacillo, S.; Pagotto, S.; Pepe, F.; George-William, J.N.; Curcio, C.; Marchisio, M.; et al. Enhanced Expression of miR-181b in B Cells of CLL Improves the Anti-Tumor Cytotoxic T Cell Response. Cancers 2021, 13, 257. [Google Scholar] [CrossRef]
- Miao, J.; Zhu, Y.; Xu, L.; Huang, X.; Zhou, X. miR181b5p inhibits trophoblast cell migration and invasion through targeting S1PR1 in multiple abnormal trophoblast invasionrelated events. Mol. Med. Rep. 2020, 22, 4442–4451. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.; Holbert, J.; Nothnick, W.B. miR-181b-5p Modulates Cell Migratory Proteins, Tissue Inhibitor of Metalloproteinase 3, and Annexin A2 During In Vitro Decidualization in a Human Endometrial Stromal Cell Line. Reprod. Sci. 2017, 24, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Yang, P.; Hu, L.; Zhang, C. MiR-181b suppresses the progression of epilepsy by regulation of lncRNA ZNF883. Am. J. Transl. Res. 2020, 12, 2769–2780. [Google Scholar] [PubMed]
- Wu, K.; Huang, J.; Xu, T.; Ye, Z.; Jin, F.; Li, N.; Lv, B. MicroRNA-181b blocks gensenoside Rg3-mediated tumor suppression of gallbladder carcinoma by promoting autophagy flux via CREBRF/CREB3 pathway. Am. J. Transl. Res. 2019, 11, 5776–5787. [Google Scholar]
- Dieter, C.; Assmann, T.S.; Costa, A.R.; Canani, L.H.; de Souza, B.M.; Bauer, A.C.; Crispim, D. MiR-30e-5p and MiR-15a-5p Expressions in Plasma and Urine of Type 1 Diabetic Patients with Diabetic Kidney Disease. Front. Genet. 2019, 10, 563. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Sun, L.; Chen, L.; Li, Y.; Huang, B.; Liu, Y.; Jiang, C. miR-30e-5p Regulates Autophagy and Apoptosis by Targeting Beclin1 Involved in Contrast-induced Acute Kidney Injury. Curr. Med. Chem. 2021, 28, 7974–7984. [Google Scholar] [CrossRef]
- Xu, G.; Cai, J.; Wang, L.; Jiang, L.; Huang, J.; Hu, R.; Ding, F. MicroRNA-30e-5p suppresses non-small cell lung cancer tumorigenesis by regulating USP22-mediated Sirt1/JAK/STAT3 signaling. Exp. Cell Res. 2018, 362, 268–278. [Google Scholar] [CrossRef]
- Zhang, S.; Li, G.; Liu, C.; Lu, S.; Jing, Q.; Chen, X.; Zheng, H.; Ma, H.; Zhang, D.; Ren, S.; et al. miR-30e-5p represses angiogenesis and metastasis by directly targeting AEG-1 in squamous cell carcinoma of the head and neck. Cancer Sci. 2020, 111, 356–368. [Google Scholar] [CrossRef]
- Mishra, R.; Bhattacharya, S.; Rawat, B.S.; Kumar, A.; Kumar, A.; Niraj, K.; Chande, A.; Gandhi, P.; Khetan, D.; Aggarwal, A.; et al. MicroRNA-30e-5p has an Integrated Role in the Regulation of the Innate Immune Response during Virus Infection and Systemic Lupus Erythematosus. iScience 2020, 23, 101322. [Google Scholar] [CrossRef]
- Laudato, S.; Patil, N.; Abba, M.L.; Leupold, J.H.; Benner, A.; Gaiser, T.; Marx, A.; Allgayer, H. P53-induced miR-30e-5p inhibits colorectal cancer invasion and metastasis by targeting ITGA6 and ITGB1. Int. J. Cancer 2017, 141, 1879–1890. [Google Scholar] [CrossRef]
- Ge, J.; Mao, L.; Xu, W.; Fang, W.; Wang, N.; Ye, D.; Dong, Z.; Guan, H.; Guan, C. miR-103a-3p Suppresses Cell Proliferation and Invasion by Targeting Tumor Protein D52 in Prostate Cancer. J. Investig. Surg. 2021, 34, 984–992. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Q.; Pang, H.; Zhang, M.; Wei, W. MiR-103a-3p aggravates renal cell carcinoma by targeting TMEM33. Am. J. Transl. Res. 2021, 13, 12694–12703. [Google Scholar]
- Fasihi, A.; MSoltani, B.; Atashi, A.; Nasiri, S. Introduction of hsa-miR-103a and hsa-miR-1827 and hsa-miR-137 as new regulators of Wnt signaling pathway and their relation to colorectal carcinoma. J. Cell. Biochem. 2018, 119, 5104–5117. [Google Scholar] [CrossRef]
- Moskwa, P.; Buffa, F.M.; Pan, Y.; Panchakshari, R.; Gottipati, P.; Muschel, R.J.; Beech, J.; Kulshrestha, R.; Abdelmohsen, K.; Weinstock, D.M.; et al. miR-182-mediated downregulation of BRCA1 impacts DNA repair and sensitivity to PARP inhibitors. Mol. Cell 2011, 41, 210–220. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, Z.L.; Huang, Y.; Zhang, K.N.; Xiong, B. MiR-182-5p inhibited proliferation and metastasis of colorectal cancer by targeting MTDH. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1494–1501. [Google Scholar] [CrossRef]
- Xu, X.; Wu, J.; Li, S.; Hu, Z.; Xu, X.; Zhu, Y.; Liang, Z.; Wang, X.; Lin, Y.; Mao, Y.; et al. Downregulation of microRNA-182-5p contributes to renal cell carcinoma proliferation via activating the AKT/FOXO3a signaling pathway. Mol. Cancer 2014, 13, 109. [Google Scholar] [CrossRef]
- Wang, F.; Wu, D.; Xu, Z.; Chen, J.; Zhang, J.; Li, X.; Chen, S.; He, F.; Xu, J.; Su, L.; et al. miR-182-5p affects human bladder cancer cell proliferation, migration and invasion through regulating Cofilin 1. Cancer Cell Int. 2019, 19, 42. [Google Scholar] [CrossRef]
- Souza, M.F.; Colus, I.M.S.; Fonseca, A.S.; Antunes, V.C.; Kumar, D.; Cavalli, L.R. MiR-182-5p Modulates Prostate Cancer Aggressive Phenotypes by Targeting EMT Associated Pathways. Biomolecules 2022, 12, 187. [Google Scholar] [CrossRef]
- Roser, A.E.; Caldi Gomes, L.; Halder, R.; Jain, G.; Maass, F.; Tonges, L.; Tatenhorst, L.; Bahr, M.; Fischer, A.; Lingor, P. miR-182-5p and miR-183-5p Act as GDNF Mimics in Dopaminergic Midbrain Neurons. Mol. Nucleic Acids 2018, 11, 9–22. [Google Scholar] [CrossRef]
- Kulkarni, P.; Dasgupta, P.; Bhat, N.S.; Shahryari, V.; Shiina, M.; Hashimoto, Y.; Majid, S.; Deng, G.; Saini, S.; Tabatabai, Z.L.; et al. Elevated miR-182-5p Associates with Renal Cancer Cell Mitotic Arrest through Diminished MALAT-1 Expression. Mol. Cancer Res. 2018, 16, 1750–1760. [Google Scholar] [CrossRef]
- Jury, D.; Daugaard, I.; Sanders, K.J.; Hansen, L.L.; Agalliu, D.; Pedersen, I.M. miR-151a enhances Slug dependent angiogenesis. Oncotarget 2020, 11, 2160–2171. [Google Scholar] [CrossRef] [PubMed]
- Tornero-Esteban, P.; Rodriguez-Rodriguez, L.; Abasolo, L.; Tome, M.; Lopez-Romero, P.; Herranz, E.; Gonzalez, M.A.; Marco, F.; Moro, E.; Fernandez-Gutierrez, B.; et al. Signature of microRNA expression during osteogenic differentiation of bone marrow MSCs reveals a putative role of miR-335-5p in osteoarthritis. BMC Musculoskelet. Disord. 2015, 16, 182. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Borquez, A.; Polakovicova, I.; Carrasco-Veliz, N.; Lobos-Gonzalez, L.; Riquelme, I.; Carrasco-Avino, G.; Bizama, C.; Norero, E.; Owen, G.I.; Roa, J.C.; et al. MicroRNA-335-5p is a potential suppressor of metastasis and invasion in gastric cancer. Clin. Epigenet. 2017, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, Y.; Chen, L.; Sun, Y.; Fan, S. miR-335-5p Inhibits Progression of Uterine Leiomyoma by Targeting ARGLU1. Comput. Math. Methods Med. 2022, 2022, 2329576. [Google Scholar] [CrossRef] [PubMed]
- Suyal, G.; Pandey, P.; Saraya, A.; Sharma, R. Tumour suppressor role of microRNA-335-5p in esophageal squamous cell carcinoma by targeting TTK (Mps1). Exp. Mol. Pathol. 2022, 124, 104738. [Google Scholar] [CrossRef]
- Kay, M.; Soltani, B.M.; Aghdaei, F.H.; Ansari, H.; Baharvand, H. Hsa-miR-335 regulates cardiac mesoderm and progenitor cell differentiation. Stem Cell Res. 2019, 10, 191. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, D.; Tolleson, W.H.; Knox, B.; Wang, Y.; Chen, S.; Ren, Z.; Deng, H.; Guo, Y.; Ning, B. MicroRNA hsa-miR-25-3p suppresses the expression and drug induction of CYP2B6 in human hepatocytes. Biochem. Pharmacol. 2016, 113, 88–96. [Google Scholar] [CrossRef]
- Yu, C.; Wu, D.; Zhao, C.; Wu, C. CircRNA TGFBR2/MiR-25-3p/TWIST1 axis regulates osteoblast differentiation of human aortic valve interstitial cells. J. Bone Miner. Metab. 2021, 39, 360–371. [Google Scholar] [CrossRef]
- Jia, W.Q.; Zhu, J.W.; Yang, C.Y.; Ma, J.; Pu, T.Y.; Han, G.Q.; Zou, M.M.; Xu, R.X. Verbascoside inhibits progression of glioblastoma cells by promoting Let-7g-5p and down-regulating HMGA2 via Wnt/beta-catenin signalling blockade. J. Cell. Mol. Med. 2020, 24, 2901–2916. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, M.; Wang, X.; Xu, A.L.; Shen, M.; Jiang, B.; Zhou, X.; Zhou, L. MicroRNA let-7g-5p alleviates murine collagen-induced arthritis by inhibiting Th17 cell differentiation. Biochem. Pharmacol. 2020, 174, 113822. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Qin, Y.; Chen, C.; Yang, J.; Song, N.; Gu, M. MicroRNA-200c-3p/ZEB2 loop plays a crucial role in the tumor progression of prostate carcinoma. Ann. Transl. Med. 2019, 7, 141. [Google Scholar] [CrossRef]
- Maolakuerban, N.; Azhati, B.; Tusong, H.; Abula, A.; Yasheng, A.; Xireyazidan, A. MiR-200c-3p inhibits cell migration and invasion of clear cell renal cell carcinoma via regulating SLC6A1. Cancer Biol. 2018, 19, 282–291. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Messina, E.; Sanavia, T.; Mundo, L.; Farinella, F.; Lazzi, S.; Megiorni, F.; Ceccarelli, S.; Pontecorvi, P.; Marampon, F.; et al. MiR-200c-3p Contrasts PD-L1 Induction by Combinatorial Therapies and Slows Proliferation of Epithelial Ovarian Cancer through Downregulation of beta-Catenin and c-Myc. Cells 2021, 10, 519. [Google Scholar] [CrossRef]
- Obeng, G.; Park, E.J.; Appiah, M.G.; Kawamoto, E.; Gaowa, A.; Shimaoka, M. miRNA-200c-3p targets talin-1 to regulate integrin-mediated cell adhesion. Sci. Rep. 2021, 11, 21597. [Google Scholar] [CrossRef]
- Raue, R.; Frank, A.C.; Fuhrmann, D.C.; de la Cruz-Ojeda, P.; Rosser, S.; Bauer, R.; Cardamone, G.; Weigert, A.; Syed, S.N.; Schmid, T.; et al. MicroRNA-200c Attenuates the Tumor-Infiltrating Capacity of Macrophages. Biology 2022, 11, 349. [Google Scholar] [CrossRef]
- Cao, J.M.; Li, G.Z.; Han, M.; Xu, H.L.; Huang, K.M. MiR-30c-5p suppresses migration, invasion and epithelial to mesenchymal transition of gastric cancer via targeting MTA1. Biomed. Pharmacol. 2017, 93, 554–560. [Google Scholar] [CrossRef]
- Wang, Y.; Pei, X.; Xu, P.; Tan, Z.; Zhu, Z.; Zhang, G.; Jiang, Z.; Deng, Z. E2F7, regulated by miR30c, inhibits apoptosis and promotes cell cycle of prostate cancer cells. Oncol. Rep. 2020, 44, 849–862. [Google Scholar] [CrossRef]
- Zou, Y.F.; Liao, W.T.; Fu, Z.J.; Zhao, Q.; Chen, Y.X.; Zhang, W. MicroRNA-30c-5p ameliorates hypoxia-reoxygenation-induced tubular epithelial cell injury via HIF1alpha stabilization by targeting SOCS3. Oncotarget 2017, 8, 92801–92814. [Google Scholar] [CrossRef]
- Zietzer, A.; Hosen, M.R.; Wang, H.; Goody, P.R.; Sylvester, M.; Latz, E.; Nickenig, G.; Werner, N.; Jansen, F. The RNA-binding protein hnRNPU regulates the sorting of microRNA-30c-5p into large extracellular vesicles. J. Extracell. Vesicles 2020, 9, 1786967. [Google Scholar] [CrossRef]
- Bartoszewska, S.; Kochan, K.; Piotrowski, A.; Kamysz, W.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. The hypoxia-inducible miR-429 regulates hypoxia-inducible factor-1alpha expression in human endothelial cells through a negative feedback loop. FASEB J. 2015, 29, 1467–1479. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Mu, Q.; Zhou, D.; Li, H.; Zhang, B.; Yin, C. MiR-429 suppresses proliferation and invasion of breast cancer via inhibiting the Wnt/beta-catenin signaling pathway. Thorac. Cancer 2020, 11, 3126–3138. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Luan, F.; Zeng, L.; Zhang, Y.; Ma, K. MiR-429 suppresses the progression and metastasis of osteosarcoma by targeting ZEB1. EXCLI J. 2017, 16, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Samantarrai, D.; Mallick, B. miR-429 inhibits metastasis by targeting KIAA0101 in Soft Tissue Sarcoma. Exp. Cell Res. 2017, 357, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.Y.; Fan, Y.J.; Wang, X.L.; Xie, H.; Gao, H.J.; Zhang, Y.; Liu, M.; Tang, H. miR-429 is involved in regulation of NF-kappaBactivity by targeting IKKbeta and suppresses oncogenic activity in cervical cancer cells. FEBS Lett. 2017, 591, 118–128. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, B.B.; Lu, M.; Zheng, M.J.; Chen, H.; Ding, J.Z.; Xu, A.M.; Xu, Y.H. miR-429 functions as a tumor suppressor by targeting FSCN1 in gastric cancer cells. Onco Targets Ther. 2016, 9, 1123–1133. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, Z.J.; Liu, M.; Liu, H.M.; Zhang, J.Z.; Xiong, T.; Tang, Y.Y.; Huang, Z.P. hsamiR429 targets CBX8 to promote cell apoptosis in diffuse large Bcell lymphoma. Mol. Med. Rep. 2021, 24, 857. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Zang, W.; Ma, Y.; Wang, N.; Li, P.; Wang, T.; Zhao, G. MiR-429 up-regulation induces apoptosis and suppresses invasion by targeting Bcl-2 and SP-1 in esophageal carcinoma. Cell. Oncol. 2013, 36, 385–394. [Google Scholar] [CrossRef]
- Guo, C.; Zhao, D.; Zhang, Q.; Liu, S.; Sun, M.Z. miR-429 suppresses tumor migration and invasion by targeting CRKL in hepatocellular carcinoma via inhibiting Raf/MEK/ERK pathway and epithelial-mesenchymal transition. Sci. Rep. 2018, 8, 2375. [Google Scholar] [CrossRef]
- Xue, H.; Tian, G.Y. MiR-429 regulates the metastasis and EMT of HCC cells through targeting RAB23. Arch. Biochem. Biophys. 2018, 637, 48–55. [Google Scholar] [CrossRef]
- Dong, H.; Hao, X.; Cui, B.; Guo, M. MiR-429 suppresses glioblastoma multiforme by targeting SOX2. Cell Biochem. Funct. 2017, 35, 260–268. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Z.; Lin, Z.; Luo, Y.; Liang, Z.; Zhang, C.; Chen, J.; Peng, P. miR-429 suppresses cell proliferation, migration and invasion in nasopharyngeal carcinoma by downregulation of TLN1. Cancer Cell Int. 2019, 19, 115. [Google Scholar] [CrossRef]
- Liu, D.; Song, L.; Dai, Z.; Guan, H.; Kang, H.; Zhang, Y.; Yan, W.; Zhao, X.; Zhang, S. MiR-429 suppresses neurotrophin-3 to alleviate perineural invasion of pancreatic cancer. Biochem. Biophys. Res. Commun. 2018, 505, 1077–1083. [Google Scholar] [CrossRef]
- Wu, G.; Zheng, H.; Xu, J.; Guo, Y.; Zheng, G.; Ma, C.; Hao, S.; Liu, X.; Chen, H.; Wei, S.; et al. miR-429 suppresses cell growth and induces apoptosis of human thyroid cancer cell by targeting ZEB1. Artif. Cells Nanomed. Biotechnol. 2019, 47, 548–554. [Google Scholar] [CrossRef]
- Lei, W.; Liu, Y.E.; Zheng, Y.; Qu, L. MiR-429 inhibits oral squamous cell carcinoma growth by targeting ZEB1. Med. Sci. Monit. 2015, 21, 383–389. [Google Scholar] [CrossRef]
- Qiu, M.; Liang, Z.; Chen, L.; Tan, G.; Wang, K.; Liu, L.; Liu, J.; Chen, H. MicroRNA-429 suppresses cell proliferation, epithelial-mesenchymal transition, and metastasis by direct targeting of BMI1 and E2F3 in renal cell carcinoma. Urol. Oncol. 2015, 33, 332.e9–332.e18. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, X.; Zhao, Z.; Yuan, Z.; Ma, P.; Ye, Z.; Guo, L.; Xu, S.; Xu, L.; Liu, T.; et al. MicroRNA-429 inhibits bone metastasis in breast cancer by regulating CrkL and MMP-9. Bone 2020, 130, 115139. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Bartoszewska, S.; Kochan, K.; Piotrowski, A.; Kalinowski, L.; Kamysz, W.; Ochocka, R.J.; Bartoszewski, R.; Collawn, J.F. miR-429 regulates the transition between Hypoxia-Inducible Factor (HIF)1A and HIF3A expression in human endothelial cells. Sci. Rep. 2016, 6, 22775. [Google Scholar] [CrossRef]
- Zacharewicz, E.; Kalanon, M.; Murphy, R.M.; Russell, A.P.; Lamon, S. MicroRNA-99b-5p downregulates protein synthesis in human primary myotubes. Am. J. Physiol. Cell Physiol. 2020, 319, C432–C440. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, Y.; Wang, Y.Y. MicroRNA-99b suppresses human cervical cancer cell activity by inhibiting the PI3K/AKT/mTOR signaling pathway. J. Cell. Physiol. 2019, 234, 9577–9591. [Google Scholar] [CrossRef]
- Yu, D.; Green, B.; Tolleson, W.H.; Jin, Y.; Mei, N.; Guo, Y.; Deng, H.; Pogribny, I.; Ning, B. MicroRNA hsa-miR-29a-3p modulates CYP2C19 in human liver cells. Biochem. Pharmacol. 2015, 98, 215–223. [Google Scholar] [CrossRef]
- Ma, J.H.; Bu, X.; Wang, J.J.; Xie, Y.X. MicroRNA-29-3p Regulates Hepatocellular Carcinoma Progression Through NF-kappaB Pathway. Clin. Lab. 2019, 65. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Yang, Y.L.; Wang, F.S. The Role of miR-29a in the Regulation, Function, and Signaling of Liver Fibrosis. Int. J. Mol. Sci. 2018, 19, 1889. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Chang, Y.H.; Li, C.J.; Huang, Y.H.; Tsai, M.C.; Chu, P.Y.; Lin, H.Y. New Insights into the Role of miR-29a in Hepatocellular Carcinoma: Implications in Mechanisms and Theragnostics. J. Pers. Med. 2021, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, L.; Song, W.; Cui, H.; Chen, G.; Qiao, F.; Hu, J.; Zhou, R.; Fan, H. Reduced miR-29a-3p expression is linked to the cell proliferation and cell migration in gastric cancer. World J. Surg. Oncol. 2015, 13, 101. [Google Scholar] [CrossRef]
- He, H.; Wang, N.; Yi, X.; Tang, C.; Wang, D. Long non-coding RNA H19 regulates E2F1 expression by competitively sponging endogenous miR-29a-3p in clear cell renal cell carcinoma. Cell Biosci. 2017, 7, 65. [Google Scholar] [CrossRef]
- Deng, X.; Chu, X.; Wang, P.; Ma, X.; Wei, C.; Sun, C.; Yang, J.; Li, Y. MicroRNA-29a-3p Reduces TNFalpha-Induced Endothelial Dysfunction by Targeting Tumor Necrosis Factor Receptor 1. Mol. Nucleic Acids 2019, 18, 903–915. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Wei, C.; Zhao, C.; Wang, S.; Gao, J. High expression of SETDB1 mediated by miR-29a-3p associates with poor prognosis and immune invasion in breast invasive carcinoma. Transl. Cancer Res. 2021, 10, 5065–5075. [Google Scholar] [CrossRef]
- Zhou, R.; Ren, S.; Li, C.; Zhang, X.; Zhang, W. miR-29a is a potential protective factor for fibrogenesis in gluteal muscle contracture. Physiol. Res. 2020, 69, 467–479. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Z.F.; Liu, F.F.; Xu, J.Z.; Liu, Q.; Lan, J. MicroRNA-29a-3p regulates osteoblast differentiation and peri-implant osseointegration in a rat model of hyperlipidemia by modulating Frizzled 4 expression. Hua Xi Kou Qiang Yi Xue Za Zhi 2019, 37, 200–207. [Google Scholar] [CrossRef]
- Lv, B.; Liu, Z.; Wang, S.; Liu, F.; Yang, X.; Hou, J.; Hou, Z.; Chen, B. MiR-29a promotes intestinal epithelial apoptosis in ulcerative colitis by down-regulating Mcl-1. Int. J. Clin. Exp. Pathol. 2014, 7, 8542–8552. [Google Scholar]
- Widlansky, M.E.; Jensen, D.M.; Wang, J.; Liu, Y.; Geurts, A.M.; Kriegel, A.J.; Liu, P.; Ying, R.; Zhang, G.; Casati, M.; et al. miR-29 contributes to normal endothelial function and can restore it in cardiometabolic disorders. EMBO Mol. Med. 2018, 10, e8046. [Google Scholar] [CrossRef]
- Glantschnig, C.; Koenen, M.; Gil-Lozano, M.; Karbiener, M.; Pickrahn, I.; Williams-Dautovich, J.; Patel, R.; Cummins, C.L.; Giroud, M.; Hartleben, G.; et al. A miR-29a-driven negative feedback loop regulates peripheral glucocorticoid receptor signaling. FASEB J. 2019, 33, 5924–5941. [Google Scholar] [CrossRef]
- He, P.Y.; Yip, W.K.; Chai, B.L.; Chai, B.Y.; Jabar, M.F.; Dusa, N.; Mohtarrudin, N.; Seow, H.F. Inhibition of cell migration and invasion by miR29a3p in a colorectal cancer cell line through suppression of CDC42BPA mRNA expression. Oncol. Rep. 2017, 38, 3554–3566. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, N.; Duan, S. MiR-29a Inhibits Glioma Tumorigenesis through a Negative Feedback Loop of TRAF4/Akt Signaling. Biomed Res. Int. 2018, 2018, 2461363. [Google Scholar] [CrossRef]
- Fukumoto, I.; Kikkawa, N.; Matsushita, R.; Kato, M.; Kurozumi, A.; Nishikawa, R.; Goto, Y.; Koshizuka, K.; Hanazawa, T.; Enokida, H.; et al. Tumor-suppressive microRNAs (miR-26a/b, miR-29a/b/c and miR-218) concertedly suppressed metastasis-promoting LOXL2 in head and neck squamous cell carcinoma. J. Hum. Genet. 2016, 61, 109–118. [Google Scholar] [CrossRef]
- Li, J.; Wan, X.; Qiang, W.; Li, T.; Huang, W.; Huang, S.; Wu, D.; Li, Y. MiR-29a suppresses prostate cell proliferation and induces apoptosis via KDM5B protein regulation. Int. J. Clin. Exp. Med. 2015, 8, 5329–5339. [Google Scholar]
- Ma, Y.; Sun, Y. miR-29a-3p inhibits growth, proliferation, and invasion of papillary thyroid carcinoma by suppressing NF-kappaB signaling via direct targeting of OTUB2. Cancer Manag. Res. 2019, 11, 13–23. [Google Scholar] [CrossRef]
- Yan, Y.; Du, C.; Duan, X.; Yao, X.; Wan, J.; Jiang, Z.; Qin, Z.; Li, W.; Pan, L.; Gu, Z.; et al. Inhibiting collagen I production and tumor cell colonization in the lung via miR-29a-3p loading of exosome-/liposome-based nanovesicles. Acta Pharm. Sin. B 2022, 12, 939–951. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Yan, L.; Zheng, P.; Li, J. miR-29a-3p directly targets Smad nuclear interacting protein 1 and inhibits the migration and proliferation of cervical cancer HeLa cells. PeerJ 2020, 8, e10148. [Google Scholar] [CrossRef]
- Geng, A.; Luo, L.; Ren, F.; Zhang, L.; Zhou, H.; Gao, X. miR-29a-3p inhibits endometrial cancer cell proliferation, migration and invasion by targeting VEGFA/CD C42/PAK1. BMC Cancer 2021, 21, 843. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, F.; Xiao, S.; He, L.; Wu, W.; Zhao, Q. miR-29a-3p enhances the radiosensitivity of oral squamous cell carcinoma cells by inhibiting ADAM12. Eur. J. Histochem. 2021, 65, 3295. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Tolleson, W.H.; Knox, B.; Jin, Y.; Guo, L.; Guo, Y.; Kadlubar, S.A.; Ning, B. Modulation of ALDH5A1 and SLC22A7 by microRNA hsa-miR-29a-3p in human liver cells. Biochem. Pharmacol. 2015, 98, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wei, X.; Zhang, A.; Li, C.; Bai, J.; Dong, J. Long non-coding RNA HNF1A-AS1 functioned as an oncogene and autophagy promoter in hepatocellular carcinoma through sponging hsa-miR-30b-5p. Biochem. Biophys. Res. Commun. 2016, 473, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, X.F.; Dong, M.Z.; Tan, J.; Zhang, J.; Zhuang, L.K.; Liu, S.S.; Xin, Y.N. MiR-30b-5p regulates the lipid metabolism by targeting PPARGC1A in Huh-7 cell line. Lipids Health Dis. 2020, 19, 76. [Google Scholar] [CrossRef]
- Woo, C.C.; Liu, W.; Lin, X.Y.; Dorajoo, R.; Lee, K.W.; Richards, A.M.; Lee, C.N.; Wongsurawat, T.; Nookaew, I.; Sorokin, V. The interaction between 30b-5p miRNA and MBNL1 mRNA is involved in vascular smooth muscle cell differentiation in patients with coronary atherosclerosis. Int. J. Mol. Sci. 2019, 21, 11. [Google Scholar] [CrossRef]
- Li, F.; Chen, Q.; Song, X.; Zhou, L.; Zhang, J. MiR-30b Is Involved in the Homocysteine-Induced Apoptosis in Human Coronary Artery Endothelial Cells by Regulating the Expression of Caspase 3. Int. J. Mol. Sci. 2015, 16, 17682–17695. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, F.; Wu, X.; Li, Y.; Ye, B. miR-30b-5p inhibits osteoblast differentiation through targeting BCL6. Cell Cycle 2022, 21, 630–640. [Google Scholar] [CrossRef]
- Xu, C.; He, Z.; Lin, C.; Shen, Z. MiR-30b-5p inhibits proliferation and promotes apoptosis of medulloblastoma cells via targeting MYB proto-oncogene like 2 (MYBL2). J. Investig. Med. 2020, 68, 1179–1185. [Google Scholar] [CrossRef]
- Park, G.; Son, B.; Kang, J.; Lee, S.; Jeon, J.; Kim, J.H.; Yi, G.R.; Youn, H.; Moon, C.; Nam, S.Y.; et al. LDR-Induced miR-30a and miR-30b Target the PAI-1 Pathway to Control Adverse Effects of NSCLC Radiotherapy. Mol. Ther. 2019, 27, 342–354. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.; Wang, J.X.; Chen, M.H.; Xu, J.J.; Jiang, M.H.; Feng, Y.L.; Gu, Y.F. miR-30-5p-mediated ferroptosis of trophoblasts is implicated in the pathogenesis of preeclampsia. Redox Biol. 2020, 29, 101402. [Google Scholar] [CrossRef]
- Wang-Renault, S.F.; Boudaoud, S.; Nocturne, G.; Roche, E.; Sigrist, N.; Daviaud, C.; Bugge Tinggaard, A.; Renault, V.; Deleuze, J.F.; Mariette, X.; et al. Deregulation of microRNA expression in purified T and B lymphocytes from patients with primary Sjogren’s syndrome. Ann. Rheum. Dis. 2018, 77, 133–140. [Google Scholar] [CrossRef]
- Osip’yants, A.I.; Knyazev, E.N.; Galatenko, A.V.; Nyushko, K.M.; Galatenko, V.V.; Shkurnikov, M.Y.; Alekseev, B.Y. Changes in the Level of Circulating hsa-miR-297 and hsa-miR-19b-3p miRNA Are Associated with Generalization of Prostate Cancer. Bull. Exp. Biol. Med. 2017, 162, 379–382. [Google Scholar] [CrossRef]
- Ma, Y.; Tian, S.; He, S.; Chen, Q.; Wang, Z.; Xiao, X.; Fu, L.; Lei, X. The mechanism of action of FXR1P-related miR-19b-3p in SH-SY5Y. Gene 2016, 588, 62–68. [Google Scholar] [CrossRef]
- Ye, L.; Morse, L.R.; Falci, S.P.; Olson, J.K.; Shrivastava, M.; Nguyen, N.; Linnman, C.; Troy, K.L.; Battaglino, R.A. hsa-MiR-19a-3p and hsa-MiR-19b-3p Are Associated with Spinal Cord Injury-Induced Neuropathic Pain: Findings from a Genome-Wide MicroRNA Expression Profiling Screen. Neurotrauma Rep. 2021, 2, 424–439. [Google Scholar] [CrossRef]
- Mullany, L.E.; Herrick, J.S.; Sakoda, L.C.; Samowitz, W.; Stevens, J.R.; Wolff, R.K.; Slattery, M.L. miRNA involvement in cell cycle regulation in colorectal cancer cases. Genes Cancer 2018, 9, 53–65. [Google Scholar] [CrossRef]
- Wu, F.; Wang, J.Y.; Chao, W.; Sims, C.; Kozar, R.A. miR-19b targets pulmonary endothelial syndecan-1 following hemorrhagic shock. Sci. Rep. 2020, 10, 15811. [Google Scholar] [CrossRef]
- Rivas, D.A.; Peng, F.; Benard, T.; Ramos da Silva, A.S.; Fielding, R.A.; Margolis, L.M. miR-19b-3p is associated with a diametric response to resistance exercise in older adults and regulates skeletal muscle anabolism via PTEN inhibition. Am. J. Physiol. Cell Physiol. 2021, 321, C977–C991. [Google Scholar] [CrossRef]
- Guo, C.; Pengli, H.; Keru, C.; Mingchuang, L.; Qingsong, Z.; Zheng, C.; Hanhua, D.; Yuejun, Q.; Jing, L. Molecular mechanism of miR-19b-3p regulating apoptosis of medullary thyroid cancer cells by targeting MTUS1. China Oncol. 2021, 31, 390–396. [Google Scholar] [CrossRef]
- Cui, L.; Du, W.; Xu, N.; Dong, J.; Xia, B.; Ma, J.; Yan, R.; Wang, L.; Feng, F. Impact of MicroRNAs in Interaction with Environmental Factors on Autism Spectrum Disorder: An Exploratory Pilot Study. Front. Psychiatry 2021, 12, 715481. [Google Scholar] [CrossRef]
- Li, K.; Ya, X.; Duan, X.; Li, Y.; Lin, X. miRNA-19b-3p Stimulates Cardiomyocyte Apoptosis Induced by Myocardial Ischemia Reperfusion via Downregulating PTEN. Dis. Markers 2021, 2021, 9956666. [Google Scholar] [CrossRef]
- Hassiotou, F.; Hepworth, A.R.; Williams, T.M.; Twigger, A.-J.; Perrella, S.; Lai, C.T.; Filgueira, L.; Geddes, D.T.; Hartmann, P.E. Breastmilk cell and fat contents respond similarly to removal of breastmilk by the infant. PLoS ONE 2013, 8, e78232. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hepworth, A.R.; Prime, D.K.; Lai, C.T.; Trengove, N.J.; Hartmann, P.E. Variation in fat, lactose, and protein composition in breast milk over 24 hours: Associations with infant feeding patterns. J. Hum. Lact. 2013, 29, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Geddes, D.T.; Hartmann, P.E. Cells in human milk: State of the science. J. Hum. Lact. 2013, 29, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Sang, X.; Hong, Z. Beyond nutrients: Food-derived microRNAs provide cross-kingdom regulation. Bioessays 2012, 34, 280–284. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Ferraro, L.; Ravo, M.; Nassa, G.; Tarallo, R.; De Filippo, M.R.; Giurato, G.; Cirillo, F.; Stellato, C.; Silvestro, S.; Cantarella, C. Effects of oestrogen on microRNA expression in hormone-responsive breast cancer cells. Horm. Cancer 2012, 3, 65–78. [Google Scholar] [CrossRef]
- Cochrane, D.R.; Spoelstra, N.S.; Richer, J.K. The role of miRNAs in progesterone action. Mol. Cell. Endocrinol. 2012, 357, 50–59. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Didelot, X.; Bruce, K.D.; Cagampang, F.R.; Vatish, M.; Hanson, M.; Lehnert, H.; Ceriello, A.; Byrne, C.D. Maternal high fat diet during pregnancy and lactation alters hepatic expression of insulin like growth factor-2 and key microRNAs in the adult offspring. BMC Genom. 2009, 10, 478. [Google Scholar] [CrossRef]
- Dall, S.R.; Boyd, I.L. Evolution of mammals: Lactation helps mothers to cope with unreliable food supplies. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2004, 271, 2049–2057. [Google Scholar] [CrossRef]
- Aagaard-Tillery, K.M.; Grove, K.; Bishop, J.; Ke, X.; Fu, Q.; McKnight, R.; Lane, R.H. Developmental origins of disease and determinants of chromatin structure: Maternal diet modifies the primate fetal epigenome. J. Mol. Endocrinol. 2008, 41, 91. [Google Scholar] [CrossRef]
- Suter, M.; Bocock, P.; Showalter, L.; Hu, M.; Shope, C.; McKnight, R.; Grove, K.; Lane, R.; Aagaard-Tillery, K. Epigenomics: Maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. FASEB J. 2011, 25, 714–726. [Google Scholar] [CrossRef]
- Saarela, T.; Kokkonen, J.; Koivisto, M. Macronutrient and energy contents of human milk fractions during the first six months of lactation. Acta Paediatr. 2005, 94, 1176–1181. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, X.J.; Zou, Q.; Wang, S.P.; Tang, S.M.; Zhang, G.Z. Biological functions of microRNAs: A review. J. Physiol. Biochem. 2011, 67, 129–139. [Google Scholar] [CrossRef]
- Lu, L.F.; Liston, A. MicroRNA in the immune system, microRNA as an immune system. Immunology 2009, 127, 291–298. [Google Scholar] [CrossRef]
- Asirvatham, A.J.; Magner, W.J.; Tomasi, T.B. miRNA regulation of cytokine genes. Cytokine 2009, 45, 58–69. [Google Scholar] [CrossRef]
- Ha, T.-Y. The Role of MicroRNAs in Regulatory T Cells and in the Immune Response. Immune Netw. 2011, 11, 11–41. [Google Scholar] [CrossRef]
- Liu, C.; Li, N.; Liu, G. The Role of MicroRNAs in Regulatory T Cells. J. Immunol. Res. 2020, 2020, 3232061. [Google Scholar] [CrossRef]
- Vigorito, E.; Perks, K.L.; Abreu-Goodger, C.; Bunting, S.; Xiang, Z.; Kohlhaas, S.; Das, P.P.; Miska, E.A.; Rodriguez, A.; Bradley, A.; et al. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity 2007, 27, 847–859. [Google Scholar] [CrossRef]
- Coffre, M.; Benhamou, D.; Rieß, D.; Blumenberg, L.; Snetkova, V.; Hines, M.J.; Chakraborty, T.; Bajwa, S.; Jensen, K.; Chong, M.M.W.; et al. miRNAs Are Essential for the Regulation of the PI3K/AKT/FOXO Pathway and Receptor Editing during B Cell Maturation. Cell Rep. 2016, 17, 2271–2285. [Google Scholar] [CrossRef]
- Xiao, C.; Nemazee, D.; Gonzalez-Martin, A. MicroRNA control of B cell tolerance, autoimmunity and cancer. Semin Cancer Biol. 2020, 64, 102–107. [Google Scholar] [CrossRef]
- Li, F.; Huang, Y.; Huang, Y.Y.; Kuang, Y.S.; Wei, Y.J.; Xiang, L.; Zhang, X.J.; Jia, Z.C.; Jiang, S.; Li, J.Y.; et al. MicroRNA-146a promotes IgE class switch in B cells via upregulating 14-3-3σ expression. Mol. Immunol. 2017, 92, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, L.C.; Bray, P.F. MicroRNAs in platelet production and activation. Blood 2011, 117, 5289–5296. [Google Scholar] [CrossRef] [PubMed]
- Czajka, P.; Fitas, A.; Jakubik, D.; Eyileten, C.; Gasecka, A.; Wicik, Z.; Siller-Matula, J.M.; Filipiak, K.J.; Postula, M. MicroRNA as Potential Biomarkers of Platelet Function on Antiplatelet Therapy: A Review. Front. Physiol. 2021, 12, 652579. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, O.; Sonmez, M. Role of platelets in immune system and inflammation. Porto Biomed. J. 2017, 2, 311–314. [Google Scholar] [CrossRef]
- Anderson, H.L.; Brodsky, I.E.; Mangalmurti, N.S. The Evolving Erythrocyte: Red Blood Cells as Modulators of Innate Immunity. J. Immunol. 2018, 201, 1343–1351. [Google Scholar] [CrossRef]
- Rivkin, N.; Chapnik, E.; Mildner, A.; Barshtein, G.; Porat, Z.; Kartvelishvily, E.; Dadosh, T.; Birger, Y.; Amir, G.; Yedgar, S.; et al. Erythrocyte survival is controlled by microRNA-142. Haematologica 2017, 102, 676–685. [Google Scholar] [CrossRef]
- Lindsay, M.A. microRNAs and the immune response. Trends Immunol. 2008, 29, 343–351. [Google Scholar] [CrossRef]
- Ventura, A.; Young, A.G.; Winslow, M.M.; Lintault, L.; Meissner, A.; Erkeland, S.J.; Newman, J.; Bronson, R.T.; Crowley, D.; Stone, J.R.; et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008, 132, 875–886. [Google Scholar] [CrossRef]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef]
- Jing, Q.; Huang, S.; Guth, S.; Zarubin, T.; Motoyama, A.; Chen, J.; Di Padova, F.; Lin, S.C.; Gram, H.; Han, J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 2005, 120, 623–634. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Taganov, K.D.; Boldin, M.P.; Cheng, G.; Baltimore, D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA 2007, 104, 1604–1609. [Google Scholar] [CrossRef]
- Altorki, S. Milk-Kinship in Arab Society: An Unexplored Problem in the Ethnography of Marriage. Ethnology 1980, 19, 233–244. [Google Scholar] [CrossRef]
- Koralov, S.B.; Muljo, S.A.; Galler, G.R.; Krek, A.; Chakraborty, T.; Kanellopoulou, C.; Jensen, K.; Cobb, B.S.; Merkenschlager, M.; Rajewsky, N.; et al. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell 2008, 132, 860–874. [Google Scholar] [CrossRef]
- Gaziel-Sovran, A.; Segura, M.F.; Di Micco, R.; Collins, M.K.; Hanniford, D.; Vega-Saenz de Miera, E.; Rakus, J.F.; Dankert, J.F.; Shang, S.; Kerbel, R.S.; et al. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell 2011, 20, 104–118. [Google Scholar] [CrossRef]
- Stittrich, A.-B.; Haftmann, C.; Sgouroudis, E.; Kühl, A.A.; Hegazy, A.N.; Panse, I.; Riedel, R.; Flossdorf, M.; Dong, J.; Fuhrmann, F. The microRNA miR-182 is induced by IL-2 and promotes clonal expansion of activated helper T lymphocytes. Nat. Immunol. 2010, 11, 1057–1062. [Google Scholar] [CrossRef]
- Navarro, A.; Gaya, A.; Martinez, A.; Urbano-Ispizua, A.; Pons, A.; Balague, O.; Gel, B.; Abrisqueta, P.; Lopez-Guillermo, A.; Artells, R.; et al. MicroRNA expression profiling in classic Hodgkin lymphoma. Blood 2008, 111, 2825–2832. [Google Scholar] [CrossRef]
- Vian, L.; Di Carlo, M.; Pelosi, E.; Fazi, F.; Santoro, S.; Cerio, A.M.; Boe, A.; Rotilio, V.; Billi, M.; Racanicchi, S.; et al. Transcriptional fine-tuning of microRNA-223 levels directs lineage choice of human hematopoietic progenitors. Cell Death Differ. 2014, 21, 290–301. [Google Scholar] [CrossRef]
- Perri, M.; Lucente, M.; Cannataro, R.; De Luca, I.F.; Gallelli, L.; Moro, G.; De Sarro, G.; Caroleo, M.C.; Cione, E. Variation in Immune-Related microRNAs Profile in Human Milk Amongst Lactating Women. Microrna 2018, 7, 107–114. [Google Scholar] [CrossRef]
- Roffel, M.P.; Bracke, K.R.; Heijink, I.H.; Maes, T. miR-223: A Key Regulator in the Innate Immune Response in Asthma and COPD. Front. Med. 2020, 7, 196. [Google Scholar] [CrossRef]
- Dong, J.; Liu, Y.; Liao, W.; Liu, R.; Shi, P.; Wang, L. miRNA-223 is a potential diagnostic and prognostic marker for osteosarcoma. J. Bone Oncol. 2016, 5, 74–79. [Google Scholar] [CrossRef]
- Johnnidis, J.B.; Harris, M.H.; Wheeler, R.T.; Stehling-Sun, S.; Lam, M.H.; Kirak, O.; Brummelkamp, T.R.; Fleming, M.D.; Camargo, F.D. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 2008, 451, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- de Yebenes, V.G.; Belver, L.; Pisano, D.G.; Gonzalez, S.; Villasante, A.; Croce, C.; He, L.; Ramiro, A.R. miR-181b negatively regulates activation-induced cytidine deaminase in B cells. J. Exp. Med. 2008, 205, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Quinn, S.R.; Mangan, N.E.; Caffrey, B.E.; Gantier, M.P.; Williams, B.R.; Hertzog, P.J.; McCoy, C.E.; O’Neill, L.A. The role of Ets2 transcription factor in the induction of microRNA-155 (miR-155) by lipopolysaccharide and its targeting by interleukin-10. J Biol. Chem. 2014, 289, 4316–4325. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, S.; Mayr, C.; Bartel, D.P.; Lodish, H.F. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc. Natl. Acad. Sci. USA 2007, 104, 7080–7085. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Calado, D.P.; Galler, G.; Thai, T.H.; Patterson, H.C.; Wang, J.; Rajewsky, N.; Bender, T.P.; Rajewsky, K. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell 2007, 131, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Srinivasan, L.; Calado, D.P.; Patterson, H.C.; Zhang, B.; Wang, J.; Henderson, J.M.; Kutok, J.L.; Rajewsky, K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat. Immunol. 2008, 9, 405–414. [Google Scholar] [CrossRef]
- Chen, C.Z.; Li, L.; Lodish, H.F.; Bartel, D.P. MicroRNAs modulate hematopoietic lineage differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef]
- van den Berg, A.; Kroesen, B.J.; Kooistra, K.; de Jong, D.; Briggs, J.; Blokzijl, T.; Jacobs, S.; Kluiver, J.; Diepstra, A.; Maggio, E.; et al. High expression of B-cell receptor inducible gene BIC in all subtypes of Hodgkin lymphoma. Genes Chromosomes Cancer 2003, 37, 20–28. [Google Scholar] [CrossRef]
- Metzler, M.; Wilda, M.; Busch, K.; Viehmann, S.; Borkhardt, A. High expression of precursor microRNA-155/BIC RNA in children with Burkitt lymphoma. Genes Chromosomes Cancer 2004, 39, 167–169. [Google Scholar] [CrossRef]
- Costinean, S.; Zanesi, N.; Pekarsky, Y.; Tili, E.; Volinia, S.; Heerema, N.; Croce, C.M. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc. Natl. Acad. Sci. USA 2006, 103, 7024–7029. [Google Scholar] [CrossRef]
- Lee, Y.S.; Nakahara, K.; Pham, J.W.; Kim, K.; He, Z.; Sontheimer, E.J.; Carthew, R.W. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 2004, 117, 69–81. [Google Scholar] [CrossRef]
- Cobb, B.S.; Nesterova, T.B.; Thompson, E.; Hertweck, A.; O’Connor, E.; Godwin, J.; Wilson, C.B.; Brockdorff, N.; Fisher, A.G.; Smale, S.T.; et al. T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J. Exp. Med. 2005, 201, 1367–1373. [Google Scholar] [CrossRef]
- Muljo, S.A.; Ansel, K.M.; Kanellopoulou, C.; Livingston, D.M.; Rao, A.; Rajewsky, K. Aberrant T cell differentiation in the absence of Dicer. J. Exp. Med. 2005, 202, 261–269. [Google Scholar] [CrossRef]
- Li, Q.J.; Chau, J.; Ebert, P.J.; Sylvester, G.; Min, H.; Liu, G.; Braich, R.; Manoharan, M.; Soutschek, J.; Skare, P.; et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell 2007, 129, 147–161. [Google Scholar] [CrossRef]
- Yu, D.; Tan, A.H.; Hu, X.; Athanasopoulos, V.; Simpson, N.; Silva, D.G.; Hutloff, A.; Giles, K.M.; Leedman, P.J.; Lam, K.P.; et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature 2007, 450, 299–303. [Google Scholar] [CrossRef]
- Lu, L.F.; Thai, T.H.; Calado, D.P.; Chaudhry, A.; Kubo, M.; Tanaka, K.; Loeb, G.B.; Lee, H.; Yoshimura, A.; Rajewsky, K.; et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity 2009, 30, 80–91. [Google Scholar] [CrossRef]
- Fazi, F.; Rosa, A.; Fatica, A.; Gelmetti, V.; De Marchis, M.L.; Nervi, C.; Bozzoni, I. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell 2005, 123, 819–831. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.J.; Cimino, A.; Costinean, S.; Dumitru, C.D.; Adair, B.; Fabbri, M.; Alder, H.; Liu, C.G.; Calin, G.A.; et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J. Immunol. 2007, 179, 5082–5089. [Google Scholar] [CrossRef]
- Taganov, K.D.; Boldin, M.P.; Chang, K.-J.; Baltimore, D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef]
- Mathieu, J.; Ruohola-Baker, H. Regulation of stem cell populations by microRNAs. In Transcriptional and Translational Regulation of Stem Cells; Springer: Berlin/Heidelberg, Germany, 2013; pp. 329–351. [Google Scholar]
- Zhou, X.; Li, X.; Wu, M. miRNAs reshape immunity and inflammatory responses in bacterial infection. Signal Transduct. Target. Ther. 2018, 3, 14. [Google Scholar] [CrossRef]
- Ma, F.; Xu, S.; Liu, X.; Zhang, Q.; Xu, X.; Liu, M.; Hua, M.; Li, N.; Yao, H.; Cao, X. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat. Immunol. 2011, 12, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Rolls, A.; Shechter, R.; London, A.; Ziv, Y.; Ronen, A.; Levy, R.; Schwartz, M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat. Cell Biol. 2007, 9, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.K.; Mansell, A. Toll-like receptors: The swiss army knife of immunity and vaccine development. Clin. Transl. Immunol. 2016, 5, e85. [Google Scholar] [CrossRef]
- Chen, C.; Schaffert, S.; Fragoso, R.; Loh, C. Regulation of immune responses and tolerance: The microRNA perspective. Immunol. Rev. 2013, 253, 112–128. [Google Scholar] [CrossRef]
- Pauley, K.M.; Cha, S.; Chan, E.K. MicroRNA in autoimmunity and autoimmune diseases. J. Autoimmun. 2009, 32, 189–194. [Google Scholar] [CrossRef]
- Lu, T.X.; Munitz, A.; Rothenberg, M.E. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J. Immunol. 2009, 182, 4994–5002. [Google Scholar] [CrossRef]
- Oddy, W.H. The long-term effects of breast-feeding on asthma and atopic disease. In Breast-Feeding: Early Influences on Later Health; Springer: Berlin/Heidelberg, Germany, 2009; pp. 237–251. [Google Scholar]
- Dupont, C.; Armant, D.R.; Brenner, C.A. Epigenetics: Definition, mechanisms and clinical perspective. Semin. Reprod. Med. 2009, 27, 351–357. [Google Scholar] [CrossRef]
- Hotchkiss, R.D. The quantitative separation of purines, pyrimidines, and nucleosides by paper chromatography. J. Biol. Chem. 1948, 175, 315–332. [Google Scholar] [CrossRef]
- Sinsheimer, R.L. The action of pancreatic deoxyribonuclease. II. Isomeric dinucleotides. J. Biol. Chem. 1955, 215, 579–583. [Google Scholar] [CrossRef]
- Nan, X.; Ng, H.H.; Johnson, C.A.; Laherty, C.D.; Turner, B.M.; Eisenman, R.N.; Bird, A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 1998, 393, 386–389. [Google Scholar] [CrossRef]
- Clapier, C.R.; Cairns, B.R. The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 2009, 78, 273–304. [Google Scholar] [CrossRef]
- Isles, A.R.; Wilkinson, L.S. Epigenetics: What is it and why is it important to mental disease? Br. Med. Bull. 2008, 85, 35–45. [Google Scholar] [CrossRef]
- Lipps, H.J.; Postberg, J.; Jackson, D.A.; Silahtaroglu, A.; Stenvang, J. MicroRNAs, epigenetics and disease. Essays Biochem. 2010, 48, 165–185. [Google Scholar] [CrossRef]
- Ahmad, A.; Ginnebaugh, K.R.; Yin, S.; Bollig-Fischer, A.; Reddy, K.B.; Sarkar, F.H. Functional role of miR-10b in tamoxifen resistance of ER-positive breast cancer cells through down-regulation of HDAC4. BMC Cancer 2015, 15, 540. [Google Scholar] [CrossRef]
- El-Awady, R.A.; Hersi, F.; Al-Tunaiji, H.; Saleh, E.M.; Abdel-Wahab, A.-H.A.; Al Homssi, A.; Suhail, M.; El-Serafi, A.; Al-Tel, T. Epigenetics and miRNA as predictive markers and targets for lung cancer chemotherapy. Cancer Biol. Ther. 2015, 16, 1056–1070. [Google Scholar] [CrossRef]
- Memari, F.; Joneidi, Z.; Taheri, B.; Aval, S.F.; Roointan, A.; Zarghami, N. Epigenetics and Epi-miRNAs: Potential markers/therapeutics in leukemia. Biomed. Pharmacol. 2018, 106, 1668–1677. [Google Scholar] [CrossRef]
- Benetti, R.; Gonzalo, S.; Jaco, I.; Munoz, P.; Gonzalez, S.; Schoeftner, S.; Murchison, E.; Andl, T.; Chen, T.; Klatt, P.; et al. A mammalian microRNA cluster controls DNA methylation and telomere recombination via Rbl2-dependent regulation of DNA methyltransferases. Nat. Struct. Mol. Biol. 2008, 15, 268–279. [Google Scholar] [CrossRef]
- Khraiwesh, B.; Arif, M.A.; Seumel, G.I.; Ossowski, S.; Weigel, D.; Reski, R.; Frank, W. Transcriptional control of gene expression by microRNAs. Cell 2010, 140, 111–122. [Google Scholar] [CrossRef]
- Tuddenham, L.; Wheeler, G.; Ntounia-Fousara, S.; Waters, J.; Hajihosseini, M.K.; Clark, I.; Dalmay, T. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett. 2006, 580, 4214–4217. [Google Scholar] [CrossRef]
- Chen, J.-F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.-Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.H.; Valdez, G.; Moresi, V.; Qi, X.; McAnally, J.; Elliott, J.L.; Bassel-Duby, R.; Sanes, J.R.; Olson, E.N. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science 2009, 326, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.C.; Jones, P.A. Epigenetics and MicroRNAs. Pediatric Res. 2007, 61, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Denis, H.; Van Grembergen, O.; Delatte, B.; Dedeurwaerder, S.; Putmans, P.; Calonne, E.; Rothé, F.; Sotiriou, C.; Fuks, F.; Deplus, R. MicroRNAs regulate KDM5 histone demethylases in breast cancer cells. Mol. Biosyst. 2016, 12, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Renzini, A.; Adamo, S.; Moresi, V. Coordinated Actions of MicroRNAs with other Epigenetic Factors Regulate Skeletal Muscle Development and Adaptation. Int. J. Mol. Sci. 2017, 18, 840. [Google Scholar] [CrossRef]
- Meng, S.; Zhou, H.; Feng, Z.; Xu, Z.; Tang, Y.; Wu, M. Epigenetics in Neurodevelopment: Emerging Role of Circular RNA. Front Cell Neurosci. 2019, 13, 327. [Google Scholar] [CrossRef]
- Magistri, M.; Faghihi, M.A.; St Laurent, G., 3rd; Wahlestedt, C. Regulation of chromatin structure by long noncoding RNAs: Focus on natural antisense transcripts. Trends Genet 2012, 28, 389–396. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; EAR, E.N.S.; Boer, J.C.; Ferji, K.; Six, J.L.; Chen, X.; Elkord, E.; Plebanski, M.; Mohamud, R. Synergistic Effects of Nanomedicine Targeting TNFR2 and DNA Demethylation Inhibitor-An Opportunity for Cancer Treatment. Cells 2019, 9, 33. [Google Scholar] [CrossRef]
- Celarain, N.; Tomas-Roig, J. Aberrant DNA methylation profile exacerbates inflammation and neurodegeneration in multiple sclerosis patients. J. Neuroinflamm. 2020, 17, 21. [Google Scholar] [CrossRef]
- Wang, S.; Wu, W.; Claret, F.X. Mutual regulation of microRNAs and DNA methylation in human cancers. Epigenetics 2017, 12, 187–197. [Google Scholar] [CrossRef]
- Busslinger, M.; Tarakhovsky, A. Epigenetic control of immunity. Cold Spring Harb. Perspect. Biol. 2014, 6, a019307. [Google Scholar] [CrossRef]
- Bezu, L.; Chuang, A.W.; Liu, P.; Kroemer, G.; Kepp, O. Immunological Effects of Epigenetic Modifiers. Cancers 2019, 11, 1911. [Google Scholar] [CrossRef]
- Hirahara, K.; Vahedi, G.; Ghoreschi, K.; Yang, X.P.; Nakayamada, S.; Kanno, Y.; O’Shea, J.J.; Laurence, A. Helper T-cell differentiation and plasticity: Insights from epigenetics. Immunology 2011, 134, 235–245. [Google Scholar] [CrossRef]
- Aune, T.M.; Collins, P.L.; Collier, S.P.; Henderson, M.A.; Chang, S. Epigenetic Activation and Silencing of the Gene that Encodes IFN-gamma. Front. Immunol. 2013, 4, 112. [Google Scholar] [CrossRef]
- Chomyk, A.M.; Volsko, C.; Tripathi, A.; Deckard, S.A.; Trapp, B.D.; Fox, R.J.; Dutta, R. DNA methylation in demyelinated multiple sclerosis hippocampus. Sci. Rep. 2017, 7, 8696. [Google Scholar] [CrossRef]
- Greer, J.M.; McCombe, P.A. The role of epigenetic mechanisms and processes in autoimmune disorders. Biologics 2012, 6, 307–327. [Google Scholar] [CrossRef]
- Lovinsky-Desir, S.; Miller, R.L. Epigenetics, asthma, and allergic diseases: A review of the latest advancements. Curr. Allergy Asthma Rep. 2012, 12, 211–220. [Google Scholar] [CrossRef]
- Cookson, W. The alliance of genes and environment in asthma and allergy. Nature 1999, 402, B5-11. [Google Scholar] [CrossRef]
- Barrett, E.G. Maternal influence in the transmission of asthma susceptibility. Pulm. Pharmacol. 2008, 21, 474–484. [Google Scholar] [CrossRef]
- Suarez-Alvarez, B.; Baragano Raneros, A.; Ortega, F.; Lopez-Larrea, C. Epigenetic modulation of the immune function: A potential target for tolerance. Epigenetics 2013, 8, 694–702. [Google Scholar] [CrossRef]
- Ehrlich, M.; Gama-Sosa, M.A.; Huang, L.H.; Midgett, R.M.; Kuo, K.C.; McCune, R.A.; Gehrke, C. Amount and distribution of 5-methylcytosine in human DNA from different types of tissues or cells. Nucleic Acids Res. 1982, 10, 2709–2721. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L. Methylated cytosine and the brain: A new base for neuroscience. Neuron 2001, 30, 649–652. [Google Scholar] [CrossRef]
- Cedar, H.; Bergman, Y. Linking DNA methylation and histone modification: Patterns and paradigms. Nat. Rev. Genet. 2009, 10, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Hlady, R.A.; Novakova, S.; Opavska, J.; Klinkebiel, D.; Peters, S.L.; Bies, J.; Hannah, J.; Iqbal, J.; Anderson, K.M.; Siebler, H.M. Loss of Dnmt3b function upregulates the tumor modifier Ment and accelerates mouse lymphomagenesis. J. Clin. Investig. 2012, 122, 163–177. [Google Scholar] [CrossRef]
- Tammen, S.A.; Friso, S.; Choi, S.W. Epigenetics: The link between nature and nurture. Mol. Asp. Med. 2013, 34, 753–764. [Google Scholar] [CrossRef]
- You, D.; Nilsson, E.; Tenen, D.E.; Lyubetskaya, A.; Lo, J.C.; Jiang, R.; Deng, J.; Dawes, B.A.; Vaag, A.; Ling, C.; et al. Dnmt3a is an epigenetic mediator of adipose insulin resistance. Elife 2017, 6, 30766. [Google Scholar] [CrossRef]
- Hatazawa, Y.; Ono, Y.; Hirose, Y.; Kanai, S.; Fujii, N.L.; Machida, S.; Nishino, I.; Shimizu, T.; Okano, M.; Kamei, Y.; et al. Reduced Dnmt3a increases Gdf5 expression with suppressed satellite cell differentiation and impaired skeletal muscle regeneration. FASEB J. 2018, 32, 1452–1467. [Google Scholar] [CrossRef]
- Kutay, H.; Klepper, C.; Wang, B.; Hsu, S.H.; Datta, J.; Yu, L.; Zhang, X.; Majumder, S.; Motiwala, T.; Khan, N.; et al. Reduced susceptibility of DNA methyltransferase 1 hypomorphic (Dnmt1N/+) mice to hepatic steatosis upon feeding liquid alcohol diet. PLoS ONE 2012, 7, e41949. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. Milk’s Role as an Epigenetic Regulator in Health and Disease. Diseases 2017, 5, 12. [Google Scholar] [CrossRef]
- Smyczynska, U.; Bartlomiejczyk, M.A.; Stanczak, M.M.; Sztromwasser, P.; Wesolowska, A.; Barbarska, O.; Pawlikowska, E.; Fendler, W. Impact of processing method on donated human breast milk microRNA content. PLoS ONE 2020, 15, e0236126. [Google Scholar] [CrossRef]
- Melnik, B.C.; Stremmel, W.; Weiskirchen, R.; John, S.M.; Schmitz, G. Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development. Biomolecules 2021, 11, 851. [Google Scholar] [CrossRef]
- Fernandez Vallone, V.; Leprovots, M.; Ribatallada-Soriano, D.; Gerbier, R.; Lefort, A.; Libert, F.; Vassart, G.; Garcia, M.I. LGR5 controls extracellular matrix production by stem cells in the developing intestine. EMBO Rep. 2020, 21, e49224. [Google Scholar] [CrossRef]
- Kusuma, R.J.; Manca, S.; Friemel, T.; Sukreet, S.; Nguyen, C.; Zempleni, J. Human vascular endothelial cells transport foreign exosomes from cow’s milk by endocytosis. Am. J. Physiol. Cell Physiol. 2016, 310, C800–C807. [Google Scholar] [CrossRef]
- Melnik, B.C.; John, S.M.; Schmitz, G. Milk is not just food but most likely a genetic transfection system activating mTORC1 signaling for postnatal growth. Nutr. J. 2013, 12, 103. [Google Scholar] [CrossRef]
- Melnik, B.C.; Kakulas, F.; Geddes, D.T.; Hartmann, P.E.; John, S.M.; Carrera-Bastos, P.; Cordain, L.; Schmitz, G. Milk miRNAs: Simple nutrients or systemic functional regulators? Nutr. Metab. 2016, 13, 42. [Google Scholar] [CrossRef]
- Cao, H.; Wang, L.; Chen, B.; Zheng, P.; He, Y.; Ding, Y.; Deng, Y.; Lu, X.; Guo, X.; Zhang, Y.; et al. DNA Demethylation Upregulated Nrf2 Expression in Alzheimer’s Disease Cellular Model. Front. Aging Neurosci. 2015, 7, 244. [Google Scholar] [CrossRef]
- Fabbri, M.; Garzon, R.; Cimmino, A.; Liu, Z.; Zanesi, N.; Callegari, E.; Liu, S.; Alder, H.; Costinean, S.; Fernandez-Cymering, C.; et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. USA 2007, 104, 15805–15810. [Google Scholar] [CrossRef]
- Kuroda, A.; Rauch, T.A.; Todorov, I.; Ku, H.T.; Al-Abdullah, I.H.; Kandeel, F.; Mullen, Y.; Pfeifer, G.P.; Ferreri, K. Insulin gene expression is regulated by DNA methylation. PLoS ONE 2009, 4, e6953. [Google Scholar] [CrossRef]
- Qin, L.Q.; He, K.; Xu, J.Y. Milk consumption and circulating insulin-like growth factor-I level: A systematic literature review. Int. J. Food. Sci. Nutr. 2009, 60 (Suppl. S7), 330–340. [Google Scholar] [CrossRef]
- Ouni, M.; Gunes, Y.; Belot, M.-P.; Castell, A.-L.; Fradin, D.; Bougnères, P. The IGF1 P2 promoter is an epigenetic QTL for circulating IGF1 and human growth. Clin. Epigenet. 2015, 7, 22. [Google Scholar] [CrossRef]
- Palacios-Ortega, S.; Varela-Guruceaga, M.; Milagro, F.I.; Martinez, J.A.; de Miguel, C. Expression of Caveolin 1 is enhanced by DNA demethylation during adipocyte differentiation. status of insulin signaling. PLoS ONE 2014, 9, e95100. [Google Scholar] [CrossRef] [PubMed]
- Braud, M.; Magee, D.A.; Park, S.D.; Sonstegard, T.S.; Waters, S.M.; MacHugh, D.E.; Spillane, C. Genome-Wide microRNA Binding Site Variation between Extinct Wild Aurochs and Modern Cattle Identifies Candidate microRNA-Regulated Domestication Genes. Front. Genet. 2017, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Labrie, V.; Buske, O.J.; Oh, E.; Jeremian, R.; Ptak, C.; Gasiunas, G.; Maleckas, A.; Petereit, R.; Zvirbliene, A.; Adamonis, K.; et al. Lactase nonpersistence is directed by DNA-variation-dependent epigenetic aging. Nat. Struct. Mol. Biol. 2016, 23, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.G.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Auphan, N.; DiDonato, J.A.; Rosette, C.; Helmberg, A.; Karin, M. Immunosuppression by glucocorticoids: Inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 1995, 270, 286–290. [Google Scholar] [CrossRef]
- Scheinman, R.I.; Cogswell, P.C.; Lofquist, A.K.; Baldwin, A.S., Jr. Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science 1995, 270, 283–286. [Google Scholar] [CrossRef]
- Almawi, W.Y.; Melemedjian, O.K. Negative regulation of nuclear factor-kappaB activation and function by glucocorticoids. J. Mol. Endocrinol. 2002, 28, 69–78. [Google Scholar] [CrossRef]
- Mao, S.-Q.; Cuesta, S.M.; Tannahill, D.; Balasubramanian, S. Genome-wide DNA Methylation Signatures Are Determined by DNMT3A/B Sequence Preferences. Biochemistry 2020, 59, 2541–2550. [Google Scholar] [CrossRef]
- Sun, L.; Huang, L.; Nguyen, P.; Bisht, K.S.; Bar-Sela, G.; Ho, A.S.; Bradbury, C.M.; Yu, W.; Cui, H.; Lee, S.; et al. DNA methyltransferase 1 and 3B activate BAG-1 expression via recruitment of CTCFL/BORIS and modulation of promoter histone methylation. Cancer Res. 2008, 68, 2726–2735. [Google Scholar] [CrossRef]
- Deplus, R.; Brenner, C.; Burgers, W.A.; Putmans, P.; Kouzarides, T.; de Launoit, Y.; Fuks, F. Dnmt3L is a transcriptional repressor that recruits histone deacetylase. Nucleic Acids Res. 2002, 30, 3831–3838. [Google Scholar] [CrossRef]
- Viré, E.; Brenner, C.; Deplus, R.; Blanchon, L.; Fraga, M.; Didelot, C.; Morey, L.; Van Eynde, A.; Bernard, D.; Vanderwinden, J.-M.; et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature 2006, 439, 871–874. [Google Scholar] [CrossRef]
- Tan, J.Z.; Yan, Y.; Wang, X.X.; Jiang, Y.; Xu, H.E. EZH2: Biology, disease, and structure-based drug discovery. Acta Pharmacol. Sin. 2014, 35, 161–174. [Google Scholar] [CrossRef]
- Lee, B.; Muller, M.T. SUMOylation enhances DNA methyltransferase 1 activity. Biochem. J. 2009, 421, 449–461. [Google Scholar] [CrossRef]
- Ponnaluri, V.K.C.; Esteve, P.O.; Ruse, C.I.; Pradhan, S. S-adenosylhomocysteine Hydrolase Participates in DNA Methylation Inheritance. J. Mol. Biol. 2018, 430, 2051–2065. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, H.; Zhang, D.; Li, M.; Sun, X.; Wan, L.; Yu, D.; Tian, Y.; Jin, H.; Lin, A.; et al. Integrated analyses of multi-omics reveal global patterns of methylation and hydroxymethylation and screen the tumor suppressive roles of HADHB in colorectal cancer. Clin. Epigenet. 2018, 10, 30. [Google Scholar] [CrossRef]
- Obermann-Borst, S.A.; Eilers, P.H.C.; Tobi, E.W.; de Jong, F.H.; Slagboom, P.E.; Heijmans, B.T.; Steegers-Theunissen, R.P.M. Duration of breastfeeding and gender are associated with methylation of the LEPTIN gene in very young children. Pediatric Res. 2013, 74, 344–349. [Google Scholar] [CrossRef]
- Tao, M.H.; Marian, C.; Shields, P.G.; Potischman, N.; Nie, J.; Krishnan, S.S.; Berry, D.L.; Kallakury, B.V.; Ambrosone, C.; Edge, S.B.; et al. Exposures in early life: Associations with DNA promoter methylation in breast tumors. J. Dev. Orig. Health Dis. 2013, 4, 182–190. [Google Scholar] [CrossRef]
- Rossnerova, A.; Tulupova, E.; Tabashidze, N.; Schmuczerova, J.; Dostal, M.; Rossner, P., Jr.; Gmuender, H.; Sram, R.J. Factors affecting the 27K DNA methylation pattern in asthmatic and healthy children from locations with various environments. Mutat. Res. 2013, 741–742, 18–26. [Google Scholar] [CrossRef]
- Soto-Ramirez, N.; Arshad, S.H.; Holloway, J.W.; Zhang, H.; Schauberger, E.; Ewart, S.; Patil, V.; Karmaus, W. The interaction of genetic variants and DNA methylation of the interleukin-4 receptor gene increase the risk of asthma at age 18 years. Clin. Epigenet. 2013, 5, 1. [Google Scholar] [CrossRef]
- Naumova, O.Y.; Odintsova, V.V.; Arincina, I.A.; Rychkov, S.Y.; Muhamedrahimov, R.J.; Shneider, Y.V.; Grosheva, A.N.; Zhukova, O.V.; Grigorenko, E.L. A Study of the Association between Breastfeeding and DNA Methylation in Peripheral Blood Cells of Infants. Russ. J. Genet. 2019, 55, 749–755. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Loret de Mola, C.; Davies, N.M.; Victora, C.G.; Relton, C.L. Breastfeeding effects on DNA methylation in the offspring: A systematic literature review. PLoS ONE 2017, 12, e0173070. [Google Scholar] [CrossRef]
- Sherwood, W.B.; Bion, V.; Lockett, G.A.; Ziyab, A.H.; Soto-Ramírez, N.; Mukherjee, N.; Kurukulaaratchy, R.J.; Ewart, S.; Zhang, H.; Arshad, S.H.; et al. Duration of breastfeeding is associated with leptin (LEP) DNA methylation profiles and BMI in 10-year-old children. Clin. Epigenet. 2019, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, S.; Symons, L.; Vanautgaerden, E.L.; Ghosh, M.; Duca, R.C.; Bekaert, B.; Freson, K.; Huybrechts, I.; Langie, S.A.S.; Koppen, G.; et al. The Influence of the Duration of Breastfeeding on the Infant’s Metabolic Epigenome. Nutrients 2019, 11, 1408. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, W.B.; Kothalawala, D.M.; Kadalayil, L.; Ewart, S.; Zhang, H.; Karmaus, W.; Arshad, S.H.; Holloway, J.W.; Rezwan, F.I. Epigenome-Wide Association Study Reveals Duration of Breastfeeding Is Associated with Epigenetic Differences in Children. Int. J. Environ. Res. Public Health 2020, 17, 3569. [Google Scholar] [CrossRef]
- Acevedo, N.; Alashkar Alhamwe, B.; Caraballo, L.; Ding, M.; Ferrante, A.; Garn, H.; Garssen, J.; Hii, C.S.; Irvine, J.; Llinas-Caballero, K.; et al. Perinatal and Early-Life Nutrition, Epigenetics, and Allergy. Nutrients 2021, 13, 724. [Google Scholar] [CrossRef]
- Hao, X.; Wang, Y.; Ren, F.; Zhu, S.; Ren, Y.; Jia, B.; Li, Y.P.; Shi, Y.; Chang, Z. SNX25 regulates TGF-β signaling by enhancing the receptor degradation. Cell. Signal. 2011, 23, 935–946. [Google Scholar] [CrossRef]
- Oddy, W.H.; Rosales, F. A systematic review of the importance of milk TGF-beta on immunological outcomes in the infant and young child. Pediatric Allergy Immunol. Off. Publ. Eur. Soc. Pediatric Allergy Immunol. 2010, 21, 47–59. [Google Scholar] [CrossRef]
- Tirado-Rodriguez, B.; Ortega, E.; Segura-Medina, P.; Huerta-Yepez, S. TGF- β: An important mediator of allergic disease and a molecule with dual activity in cancer development. J. Immunol. Res. 2014, 2014, 318481. [Google Scholar] [CrossRef]
- Briollais, L.; Rustand, D.; Allard, C.; Wu, Y.; Xu, J.; Rajan, S.G.; Hivert, M.F.; Doyon, M.; Bouchard, L.; McGowan, P.O.; et al. DNA methylation mediates the association between breastfeeding and early-life growth trajectories. Clin. Epigenet. 2021, 13, 231. [Google Scholar] [CrossRef]
- Simpkin, A.J.; Suderman, M.; Gaunt, T.R.; Lyttleton, O.; McArdle, W.L.; Ring, S.M.; Tilling, K.; Davey Smith, G.; Relton, C.L. Longitudinal analysis of DNA methylation associated with birth weight and gestational age. Hum. Mol. Genet. 2015, 24, 3752–3763. [Google Scholar] [CrossRef]
- Fransquet, P.D.; Hutchinson, D.; Olsson, C.A.; Wilson, J.; Allsop, S.; Najman, J.; Elliott, E.; Mattick, R.P.; Saffery, R.; Ryan, J.; et al. Perinatal maternal alcohol consumption and methylation of the dopamine receptor DRD4 in the offspring: The Triple B study. Environ. Epigenet. 2016, 2, dvw023. [Google Scholar] [CrossRef]
- Genna, C.W. Epigenetics, Methylation, and Breastfeeding. Clin. Lact. 2018, 9, 144–147. [Google Scholar] [CrossRef]
- Mallisetty, Y.; Mukherjee, N.; Jiang, Y.; Chen, S.; Ewart, S.; Arshad, S.H.; Holloway, J.W.; Zhang, H.; Karmaus, W. Epigenome-Wide Association of Infant Feeding and Changes in DNA Methylation from Birth to 10 Years. Nutrients 2020, 13, 99. [Google Scholar] [CrossRef]
- Odintsova, V.V.; Hagenbeek, F.A.; Suderman, M.; Caramaschi, D.; van Beijsterveldt, C.E.M.; Kallsen, N.A.; Ehli, E.A.; Davies, G.E.; Sukhikh, G.T.; Fanos, V.; et al. DNA Methylation Signatures of Breastfeeding in Buccal Cells Collected in Mid-Childhood. Nutrients 2019, 11, 2804. [Google Scholar] [CrossRef]
- Hatmal, M.M.; Issa, N.N.; Alshaer, W.; Al-Ameer, H.J.; Abuyaman, O.; Tayyem, R.; Hijjawi, N.S. Association of Breastfeeding Duration with Susceptibility to Allergy, Influenza, and Methylation Status of TLR1 Gene. Medicina 2019, 55, 535. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Davey Smith, G.; Simpkin, A.J.; Victora, C.G.; Relton, C.L.; Caramaschi, D. Association between Breastfeeding and DNA Methylation over the Life Course: Findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Nutrients 2020, 12, 3309. [Google Scholar] [CrossRef]
- Taylor, R.M.; Smith, R.; Collins, C.E.; Mossman, D.; Wong-Brown, M.W.; Chan, E.C.; Evans, T.J.; Attia, J.R.; Buckley, N.; Drysdale, K.; et al. Global DNA methylation and cognitive and behavioral outcomes at 4 years of age: A cross-sectional study. Brain Behav. 2020, 10, e01579. [Google Scholar] [CrossRef]
- Castilhos, J.K.d.; Campagnolo, P.D.B.; Almeida, S.d.; Vitolo, M.R.; Mattevi, V.S. Impact of maternal dietary counseling in the first year of life on DNA methylation in a cohort of children. Genet. Mol. Biol. 2021, 44, e20200330. [Google Scholar] [CrossRef]
- Anderson, C.M.; Gillespie, S.L.; Thiele, D.K.; Ralph, J.L.; Ohm, J.E. Effects of Maternal Vitamin D Supplementation on the Maternal and Infant Epigenome. Breastfeed. Med. 2018, 13, 371–380. [Google Scholar] [CrossRef]
- Chatterjee, O.; Patil, K.; Sahu, A.; Gopalakrishnan, L.; Mol, P.; Advani, J.; Mukherjee, S.; Christopher, R.; Prasad, T.S. An overview of the oxytocin-oxytocin receptor signaling network. J. Cell Commun. Signal. 2016, 10, 355–360. [Google Scholar] [CrossRef]
- Neville, M.C.; Anderson, S.M.; McManaman, J.L.; Badger, T.M.; Bunik, M.; Contractor, N.; Crume, T.; Dabelea, D.; Donovan, S.M.; Forman, N.; et al. Lactation and neonatal nutrition: Defining and refining the critical questions. J. Mammary Gland Biol. Neoplasia 2012, 17, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, S.; Martin, D.; Hagios, C.; Chiquet-Ehrismann, R. Tenm, a Drosophila gene related to tenascin, is a new pair-rule gene. EMBO J. 1994, 13, 3728–3740. [Google Scholar] [CrossRef] [PubMed]
- Oohashi, T.; Zhou, X.H.; Feng, K.; Richter, B.; Morgelin, M.; Perez, M.T.; Su, W.D.; Chiquet-Ehrismann, R.; Rauch, U.; Fassler, R. Mouse ten-m/Odz is a new family of dimeric type II transmembrane proteins expressed in many tissues. J. Cell Biol. 1999, 145, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Michelson, Y.; Freger, V.; Avraham, Z.; Venken, K.J.; Bellen, H.J.; Justice, M.J.; Wides, R. Drosophila Ten-m and filamin affect motor neuron growth cone guidance. PLoS ONE 2011, 6, e22956. [Google Scholar] [CrossRef]
- Chen, Y.; Siegel, F.; Kipschull, S.; Haas, B.; Frohlich, H.; Meister, G.; Pfeifer, A. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat. Commun. 2013, 4, 1769. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Li, H.; Huang, L.; Sun, Q.; Dong, Y.; Tian, C.; Gao, S.; Dong, H.; Guan, D.; et al. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res. 2010, 20, 1128–1137. [Google Scholar] [CrossRef]
- He, A.; Zhu, L.; Gupta, N.; Chang, Y.; Fang, F. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol. Endocrinol. 2007, 21, 2785–2794. [Google Scholar] [CrossRef]
- Dehwah, M.A.; Xu, A.; Huang, Q. MicroRNAs and type 2 diabetes/obesity. J Genet Genom. 2012, 39, 11–18. [Google Scholar] [CrossRef]
- Hassiotou, F.; Geddes, D.T. Programming of appetite control during breastfeeding as a preventative strategy against the obesity epidemic. J. Hum. Lact. 2014, 30, 136–142. [Google Scholar] [CrossRef]
- Meng, F.; Henson, R.; Wehbe-Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef]
- Olivieri, F.; Spazzafumo, L.; Santini, G.; Lazzarini, R.; Albertini, M.C.; Rippo, M.R.; Galeazzi, R.; Abbatecola, A.M.; Marcheselli, F.; Monti, D.; et al. Age-related differences in the expression of circulating microRNAs: miR-21 as a new circulating marker of inflammaging. Mech. Ageing Dev. 2012, 133, 675–685. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hwang, S.J.; Bae, Y.C.; Jung, J.S. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells 2009, 27, 3093–3102. [Google Scholar] [CrossRef]
- Hammond, S.M. MicroRNAs as tumor suppressors. Nat. Genet. 2007, 39, 582–583. [Google Scholar] [CrossRef]
- Johnson, S.M.; Grosshans, H.; Shingara, J.; Byrom, M.; Jarvis, R.; Cheng, A.; Labourier, E.; Reinert, K.L.; Brown, D.; Slack, F.J. RAS is regulated by the let-7 microRNA family. Cell 2005, 120, 635–647. [Google Scholar] [CrossRef]
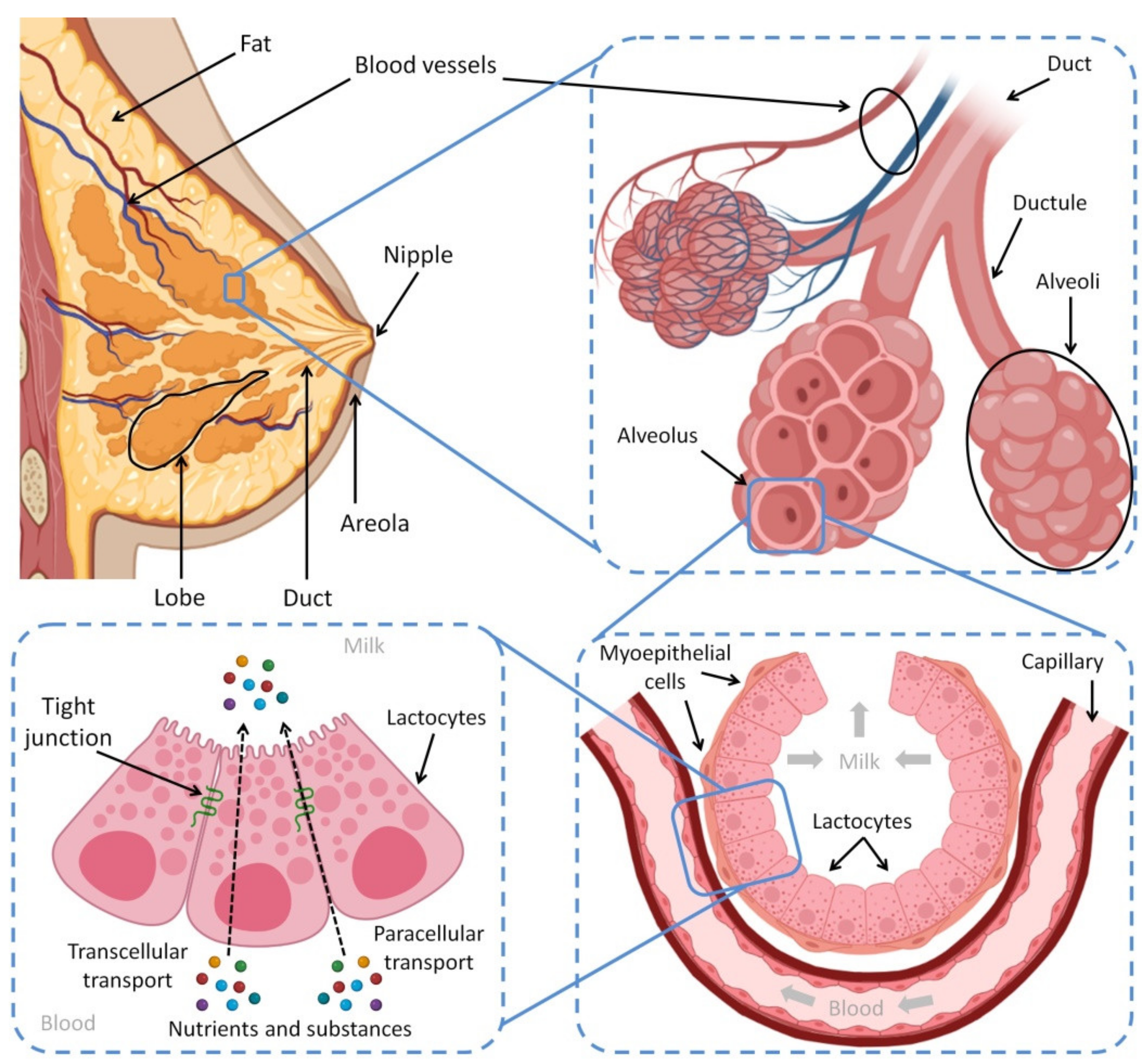
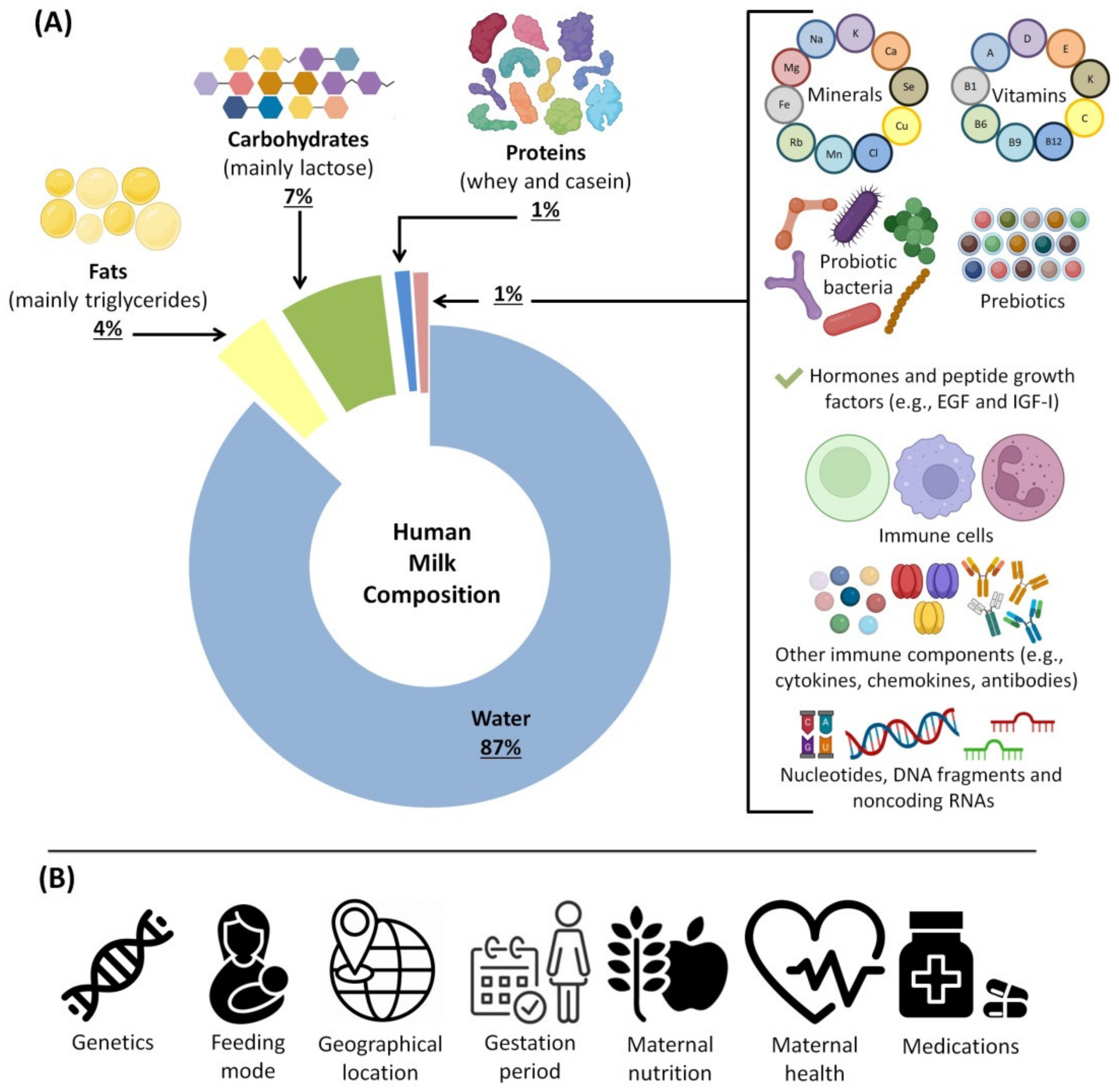


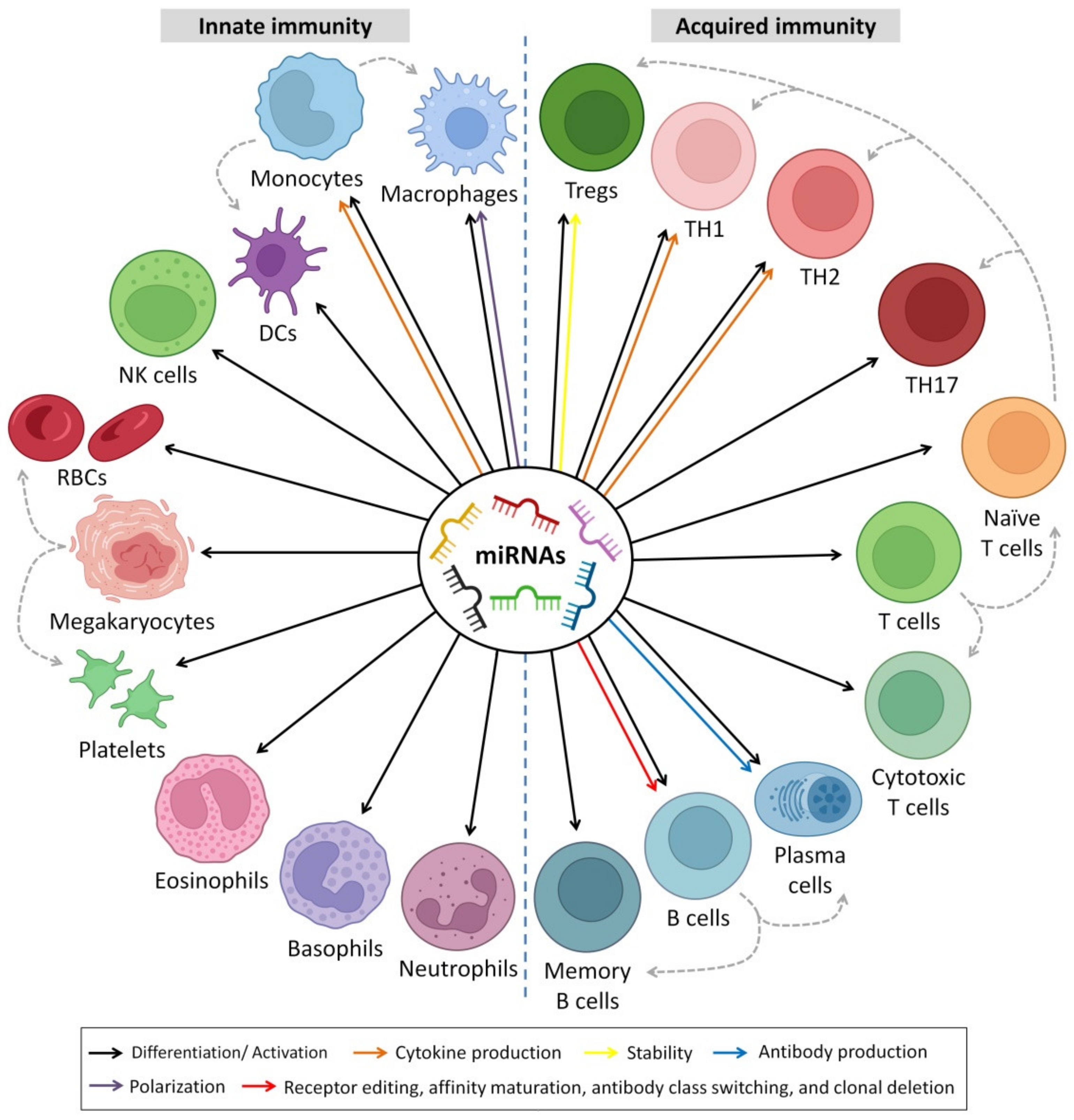

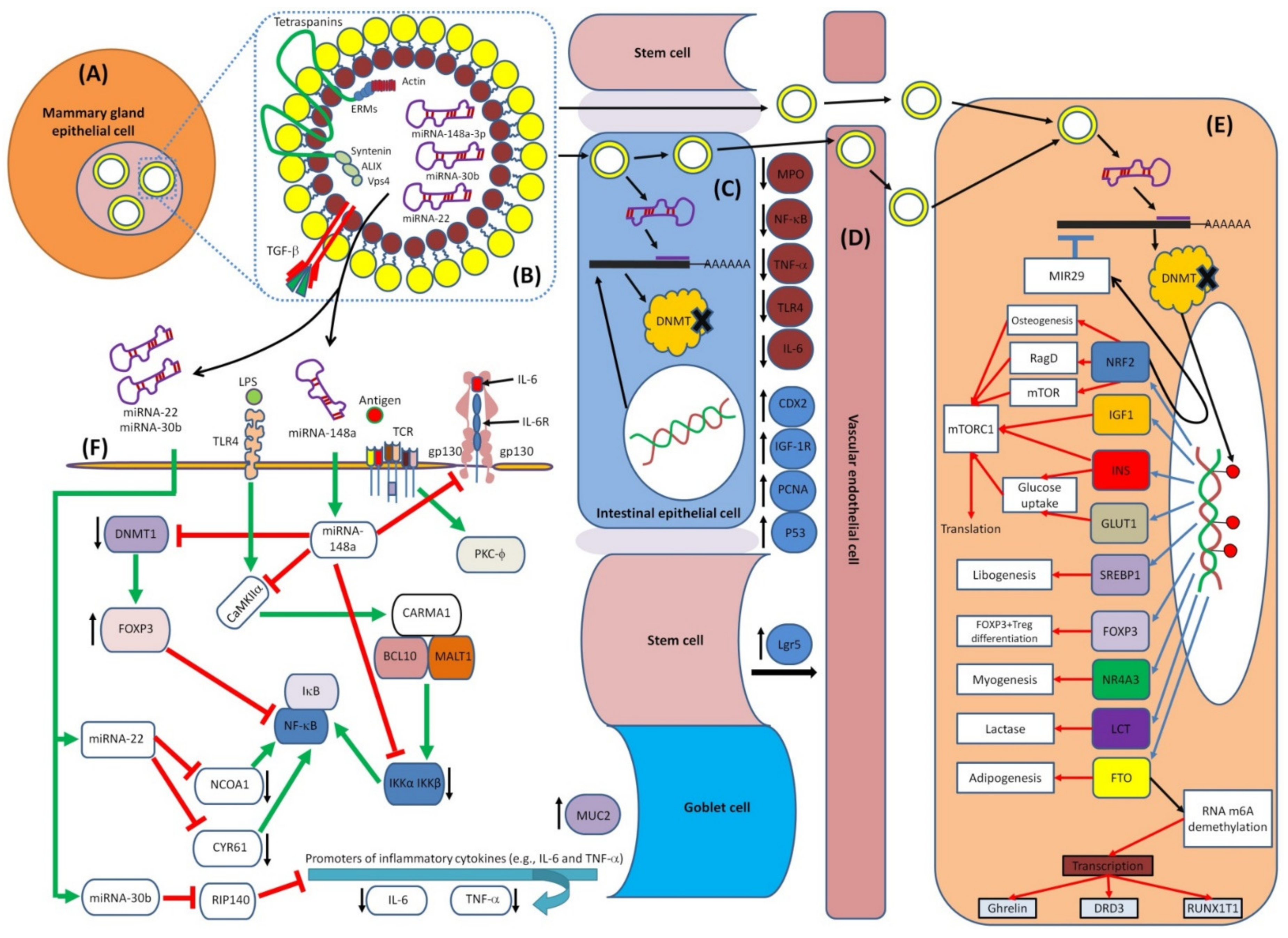

| Component | Types | Major Immune-Related Functions | Reference |
|---|---|---|---|
| Fatty acids | - Monounsaturated (42%) - Medium-chain (42%) - Long-chain polyunsaturated (16%); linoleic acid (10%), arachidonic acid (1%), α-linolenic acid (>1%), eicosapentaenoic acid (0.1%), docosahexaenoic acid (0.4%), and others (3.7%) | - Maturation of immune system - Modulate the acquired immunological responses that affect the balance between Th1 and Th2 cells and Treg and Teff cells - Regulate the production of immunomodulatory cytokines (e.g., TGFs) - Enhance level of innate immune response (i.e., soluble CD14) and adaptative immune response (i.e., IgA) - Act as antiviral, antibacterial and antiprotozoal agents | [98,99,100,101] |
| Oligosaccharides | - Fucosylated (35% to 50%) - Sialylated (12–14%) - Nonfucosylated neutral (42–55%) | - Influence the expression of chemokines (e.g., CX3CL1, CCL5, CXCL2, CXCL3), cytokines (e.g., IL-4, IL-17C, IL-8, IL-1β, IL-10, IFN-γ), cellular receptors (IFNGR1), cell adhesion molecules (e.g., ICAM-1/2) - Reduce the infectivity of rotavirus, norovirus and influenza viruses - Exhibit antimicrobial and antibiofilm activities against S. agalactiae, E. coli, B. subtilis and S. aureus - Shape the gut microbiota in infants - Inhibit leukocyte adhesion to endothelial cells - Modulate TLR-4 signaling - Induce the production of cytokines (i.e., IL-4 and IFN-γ) required for expansion of Th1 and Th17 implicated in the pathogenesis of enterocolitis - Increase serum levels of IgG1 and IgG - Increase the expression of CD27 on splenic B-cells - Inhibit the adherence and binding of specific pathogens to the host cells in intestine | [19,102,103,104,105,106,107,108,109] |
| Hormones | Leptin, erythropoietin, adiponectin, ghrelin, IGFs, resistin and obestatin | - Erythropoietin prevents HIV transmission from mother to child - Adiponectin reduces inflammation, regulates infant metabolism (reducing later-life obesity) and inhibits production of TNF-α in intestinal epithelium and macrophages - Leptin regulates proinflammatory cytokines (e.g., TNF-α and IL-6) and Th1 responses; it also promotes proliferation and activation of monocytes and NK cells, neutrophils chemotaxis and T cell survival by modulating the expression of anti-apoptotic proteins (e.g., Bcl-xL) - IGFs play vital roles in the development and function of T cells - Resistin is involved in the anti-infection immune process by interacting with a variety of immune cells; can either directly or indirectly promote infiltration, adhesion and migration of monocytes, neutrophils and CD4+ T cells | [19,35,110,111,112,113] |
| Cells | Leukocytes (i.e., lymphocytes, neutrophils and macrophages), hematopoietic stem cells and hematopoietic progenitor cells | - Maternal leukocytes provide active immunity by fighting pathogens via phagocytosis and intracellular killing, produce microbicidal molecules, present antigens; also play vital role in shaping infant’s immune system, promoting development of immunocompetence and altering gut bacterial colonization | [19,114,115] |
| Proteins, glycoproteins and peptides | Cytokines, chemokines, soluble receptors, receptor agonists and antagonists, growth factors, immunoglobulin and others | - They enhance defense against pathogenic bacteria, viruses and yeasts and promote gut development and immune function. Cytokines and chemokines are the most redundant secreted proteins that provide active immunity to infants. For example, TGF-β prevents diseases induced by allergy and controls wound repair and inflammation; G-CSF plays a role in sepsis treatment and enhances cell prefoliation, crypt depth and villi; IL-6 (a key circulating pyrogen) activates CNS mechanisms in fever during infection and inflammation; IL-7 helps develop thymic; IL-8 protects from TNF-α-induced damage; IL-10 has anti-inflammatory activity; IFN-γ has pro-inflammatory activity as it inhibits the Th2/allergic response while increases the Th1/inflammation response - HBM contains glycoprotein cytokine receptors that modulate immune responses. For example, sTNFR1/2 and IL1Ra suppress pro-inflammatory TNF-α and IL-1 activity, respectively, decrease stimulation of IL-8 secretion from the intestinal epithelial of neonates and reduce necrotizing enterocolitis; TLR1-9 agonist and antagonist protect the infant from infections - Growth factors: TGF-β induces regulatory T cell production; epidermal GF inhibits apoptosis in intestinal cells; NGF enhances the outgrowth and survival of neurons; IGF alters intestinal atrophy, induces erythropoiesis; VEGF plays a role in angiogenesis regulation, decreasing the burden of premature retinopathy - Immunoglobulins: IgG, IgM and IgA account for 90% of HBM immunoglobulins and provide passive immunity to the newborn. The major function of IgA and IgG is neutralization of pathogens by binding to them and preventing them from binding to the epithelial cells in the gut mucosa. Moreover, by opsonizing the antigen for complement fixation and destruction, IgM suppresses microbial infections - Bile salt-dependent lipase blocks viral infection (such as HIV) by binding to the pathogen receptor DC-SIGN - Mucin1/4 protect infant from viral (e.g., rotavirus, norovirus and HIV) and bacterial (e.g., E. coli and S. enterica) infections - Cathelicidin-derived antimicrobial peptides produced by cells in breast milk protect infant from autoimmune diseases and have broad antimicrobial activities against Gram-positive and Gram-negative bacteria - α-lactalbumin is the major protein found in HBM that is converted in the stomach to HAMLET. In the presence of free oleic acid, HAMLET reduces the volume of >95% of skin papilloma - Soluble CD14 sensitizes the innate mucosal immune system to Gram-negative bacteria, such as E. coli, and mediates TLR4 binding to lipopolysaccharide of Gram-negative bacteria; inhibits TLR2 signaling and attenuates TLR4 signaling - HβD-2 is a peptide that inhibits TLR7 signaling and has antibacterial activities against Salmonella spp., E. coli and P. aeruginosa - Lactoferrin is a glycoprotein that has capacity against various fungi, viruses and bacteria; inhibitory effects reported against V. cholera and E. coli; responsible for sequestering iron needed by bacteria for growth and survival; influences TLR4 signaling - Lactadherin is a glycoprotein that protects neonates from rotavirus infection, mediates phagocytosis of apoptotic cells, blocks NF-κB and TLR4 signaling leading to a signaling cascade that reduces inflammation | [19,98,110,116,117,118,119,120,121,122,123] |
| Lysozymes | - Lysozymes hinder growth of many bacterial species by disrupting the proteoglycan layer of the cell wall - Lysozymes are characterized by a positive charge which can facilitate electrostatic interactions with the viral capsid blocking the viral fusion proteins (especially in HSV and HIV) | [124,125,126] | |
| Nucleotides | CMP, UMP, GMP, AMP | - Enhance immune responses and promote the development of a less pathogenic intestinal flora in infant | [127,128] |
| Nucleic acids | - DNA fragments - ncRNAs, including miRNA, siRNA, lncRNA, circRNA, piRNA, rRNA and tRNA | - miRNAs have direct impacts on immunological regulation, such as suppressing the production of essential transcription factors in immune cell polarization or altering the epigenetic state of immune cell lineages - Other ncRNAs are less studied than miRNA but have been found to be functionally involved in several regulatory mechanisms related to miRNA mechanisms and mRNA translation process | [129,130] |
| Condition | Breastfeeding (Months) | Comments * | OR ** |
|---|---|---|---|
| Otitis media | Any | - | 0.77 |
| ≥3 | Exclusive BF | 0.50 | |
| Upper RTI | >6 | Exclusive BF | 0.30 |
| Lower RTI | ≥4 | Exclusive BF | 0.28 |
| Asthma | ≥3 | Atopic family history | 0.60 |
| No atopic family history | 0.74 | ||
| RSV bronchiolitis | >4 | - | 0.26 |
| NEC | NICU stay | Preterm infants with exclusive HBM | 0.23 |
| Atopic dermatitis | >3 | Exclusive BF negative family history | 0.84 |
| Exclusive BF positive family history | 0.58 | ||
| Gastroenteritis | Any | - | 0.36 |
| IBD | Any | - | 0.69 |
| Obesity | Any | - | 0.76 |
| Celiac disease | >2 | Gluten exposure when BF | 0.48 |
| T2D | >3 | Exclusive BF | 0.71 |
| Any | - | 0.61 | |
| ALL | >6 | - | 0.80 |
| - | 0.85 | ||
| SIDS | Any | - | 0.64 |
| miRNA [Sequence] | Function [Reference] |
|---|---|
| Colostrum-specific miRNAs | |
| hsa-let-7i-5p [UGAGGUAGUAGUUUGUGCUGUU] | Regulates cell morphology and migration through distinct signaling pathways in normal and pathogenic urethral fibroblasts [237]; protects against acute ischemic stroke [238]; controls the migration of head and neck cancer cells through downregulation of BMI1 protein [239]; inactivates localized scleroderma [240]; regulates MS pathogenesis by suppressing induction Treg by targeting IGF1R and TGFβR1 [241]; protects against pneumoconiosis caused by nanoparticles inhalation [242]; acts as an autophagy suppressor by targeting ATG10 and ATG16L1 in NPC and may represent a promising therapeutic target for NPC treatment [243]; targets HABP4 gene and functions as a tumor promoter in ccRCC, and thus offers a potential target for treatment [244]; inhibits granulosa-luteal cell proliferation and oestradiol biosynthesis by directly targeting IMP2 [245]; inhibits KGN proliferation and decreases estradiol production in an IMP2-dependent manner, providing insights into the pathogenesis of PCOS [246]; promotes differentiation of hESCs [247]; inhibits the metastasis of TNBC [248]. |
| hsa-miR-423-5p [AAAAGCUGGGUUGAGAGGGCAA] | Regulates ovarian response to ovulation [249]; targets ING-4 and upregulates signaling molecules such as p-AKT and p-ERK1/2, which support miR-423-5p functions as an oncogene in glioma and suggests targeting it as therapeutic potential for glioma [250]; targets PTTG1 and SYT1 mRNAs, thus induces cell apoptosis, inhibits cell proliferation and reduces growth hormone release and migration of GH3 cells [251]; regulates TGF-β signaling by targeting SMAD2, thus functions in the development of bicuspid aortic valve BAV disease and its complication, bicuspid aortopathy [252]; induces silencing of the nerve growth factor, which promotes retinal microvascular dysfunction, demonstrating the potential for miRNA-based therapy for treating diabetic retinopathy [253]; promotes BC invasion [254]. |
| hsa-miR-320b [UUCAAGUAAUUCAGGAUAGGU] | Negatively regulates normal human epidermal keratinocyte proliferation by targeting AKT3 to regulate the STAT3 and SAPK/JNK pathways, thus might participate in the pathogenesis of psoriasis, may act as a novel diagnostic marker or therapeutic target for this disease [255]; affects HCC radiosensitivity to ionizing radiation treatment through DNA damage repair signaling [256]; regulates osteoblast differentiation [257]; modulates cholesterol efflux and atherosclerosis [258]. |
| hsa-miR-26b-5p [UUCAAGUAAUUCAGGAUAGGU] | Controls the adipogenic differentiation of hADMSC [259]; acts as a tumor suppressor in PC [260]; affects cytokine secretion in RA [261]; modulates Th17 cell plasticity in RA [262]; inhibits proliferation, migration, invasion and apoptosis induction of osteosarcoma cells [263]. |
| hsa-miR-146a-5p [UGAGAACUGAAUUCCAUGGGUU] | Modulates androgen-independent PC cell apoptosis [264]; regulates KIR expression [265]; acts as a tumor suppressor in B-cell malignancies [266]; inhibits the metastasis of ccRCC [267]; inhibits NSCLC proliferation [268]; protects cardiomyocytes and myocardial tissues in polymicrobial sepsis [269]; associated with low-risk human PSCCs [270]; improves the decidual cytokine microenvironment [271]; acts as tumor suppressor in esophageal, prostatic, glioma and ovarian cancers [272,273,274,275]; suppresses osteoclastogenesis [276]. |
| hsa-let-7c-5p [UGAGGUAGUAGGUUGUAUGGUU] | Targets TGF-β signaling and contributes to the pathogenesis of renal fibrosis [277]; inhibits osteo/odontogenic differentiation of IGF-1-treated DPMSCs by targeting IGF-1 [278]; inhibits MAP4K4 expression; inhibits OSCC cell proliferation and migration [279]. |
| hsa-miR-200b-3p [UAAUACUGCCUGGUAAUGAUGA] | Inhibits epithelial-to-mesenchymal transition in TNBC [280]; inhibits human cytomegalovirus replication [281]; acts as a tumor suppressor in HCC [282]; regulates self-renewing divisions in PC cells by inducing less Notch signaling and promotes daughter cells to become asymmetric [283]; promotes endothelial cell apoptosis by targeting HDAC4 in atherosclerosis [284]; inhibits cell proliferation and Ca2+ influx in PASMCs [285]. |
| hsa-miR-151b [UCGAGGAGCUCACAGUCU] | Controls expression of GHR [189] and regulates proliferation and apoptosis of THCA cells through SNRPB axis [286]. |
| hsa-miR-24-3p [UGGCUCAGUUCAGCAGGAACAG] | Enhances NPC radiosensitivity by targeting both the 3’UTR and 5’UTR of Jab1/CSN5 [287]; enhances cell growth in HCC by targeting metallothionein 1M [288]; regulates lung adenocarcinoma progression through FGFR3 signaling [289]; regulates neuronal differentiation by regulating hippocalcin expression [290]; inhibits progression of pancreatic ductal adenocarcinoma through LAMB3 downregulation [291]; regulates proliferation, migration and invasion of cancer cells by directly targeting p130Cas [292]; suppresses proliferation and invasiveness of gastric mucosal lesions [293]. |
| hsa-miR-107 [AGCAGCAUUGUACAGGGCUAUCA] | Regulates cellular migration by inducing CDK5 activity and the associated molecular pathways [294]; inhibits acute aortic dissection progression [295]; regulates chemo-drug sensitivity in BC cell by targeting TRIAP1 [296]; downregulates Cdc42 3’UTR and suppresses ESCC proliferation, migration and invasion [297]; modulates NeuroD1 and SOX6 genes affecting MSCs commitment toward insulin-producing cells [298]; modulates chondrocyte proliferation [299]; inhibits glioma cell migration and invasion [300]; regulates cisplatin chemosensitivity in NSLCC [301]; promotes tumor suppressor in GC [302]; inhibits endothelial progenitor cell differentiation [303]; contributes to post-stroke angiogenesis [304]; antagonizes profibrotic phenotypes of pericytes [305]. |
| hsa-miR-221-3p [AGCUACAUUGUCUGCUGGGUUUC] | Regulates apoptosis in ovarian granulosa cells [306]; regulates epithelial ovarian cancer progression [307]; affects proliferation and apoptosis of keratinocytes [308]; reduces airway eosinophilia and CXCL17 expression in asthma [309]; targets CACNA1C and KCNJ5 and alters cardiac ion channel expression [310]; downregulates EIF5A2 and inhibits cell proliferation in medulloblastoma [311], acts as a tumor suppressor and disease progression marker in prostate cancer [312]; down-modulates KIT receptor, which suggests a potential role in cancer therapy [313]; regulates CDKN1C/p57 and CDKN1B/p27 expression in HCC [314], suppresses HDAC6 providing a new target for the treatment of liver malignancies [315], targets KIT and ETV1 in gastrointestinal stromal tumors [316]. |
| hsa-miR-151a-5p [UCGAGGAGCUCACAGUCUAGU] | Regulates E-cadherin in NSCLC cells, which promotes partial EMT and thus acts as a therapeutic target [317]. |
| hsa-miR-378c [ACUGGACUUGGAGUCAGAAGAGUGG] | Suppresses stomach adenocarcinoma cell proliferation, migration, invasion and epithelial-mesenchymal transition [318]. |
| Mature milk-specific miRNAs | |
| hsa-miR-375 [CCCCGCGACGAGCCCCUCGCACAAACCGGACCUGAGCGUUUUGUUCGUUCGGCUCGCGUGAGGC] | Induces generation of insulin-producing cells from human decidua basalis-derived stromal cells [319]; promotes pancreatic cell differentiation [320]; suppresses ESCC by direct targeting of SHOX2 [321], reduces viability of HCC under hypoxic conditions [322], suppresses bladder cancer via the Wnt/beta-catenin pathway [323]; enhances generation of insulin-producing cells from human MSCs [324]; promotes redifferentiation of adult human β cells [325]; enhances infant growth and development [189]; regulates expression of JAK2 [189]; activates p21; suppresses telomerase activity [326]. |
| hsa-miR-193b-3p [AACUGGCCCUCAAAGUCCCGCU] | Regulates matrix metalloproteinase in chondrocytes [327]; regulates chondrogenesis [328] and chondrocyte metabolism [329]; acts as tumor suppressor in ovarian carcinoma cells [330]; attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage [331]. |
| hsa-miR-345-5p [GCUGACUCCUAGUCCAGGGCUC] | Acts as anti-osteogenic factor [332]. |
| hsa-miR-423-3p [AGCUCGGUCUGAGGCCCCUCAGU] | Activates oncogenic autophagy in GC [333] and enhances tumor growth in lung adenocarcinoma [334]. |
| hsa-miR-125a-5p [UCCCUGAGACCCUUUAACCUGUGA] | Decreases sensitivity of Treg cells toward IL-6-mediated conversion [332]; suppresses breast cancer by downregulating BAP1 [335,336]; suppresses bladder cancer by targeting FUT4 [97]; suppresses cervical carcinoma [337], HCC [338], GC [339] colon cancer [340], prostate carcinoma [341], bladder cancer [336] and CRC [342]; activates p53 and induces apoptosis in lung cancer cells [343,344]; contributes to hepatic stellate cell activation [345]; inhibits trophoblast cell migration and proliferation in preeclampsia [346]. |
| hsa-miR-148a-5p [AAAGUUCUGAGACACUCCGACU] | Regulates expression of SOCS-7 [189]; controls ATPase expression [189]; regulates triacylglycerol and long-chain acyl-CoA fatty acid synthesis [189]; regulates lactose synthesis [189]; promotes cartilage production [347]; relieves hepatic fibrosis [348]; regulates the stem cell-like side population distribution in ESCC [349]. |
| hsa-miR-29c-3p [UAGCACCAUUUGAAAUCGGUUA] | Regulates biological function of CRC [350]; suppresses gallbladder carcinoma [351], T-cell acute lymphoblastic leukemia [352], ovarian cancer [353] and melanoma [354]. |
| hsa-miR-27a-3p [UUCACAGUGGCUAAGUUCCGC] | Regulates expression of intercellular junctions at the brain endothelium and controls the endothelial barrier permeability [355]; suppresses osteoblastogenesis [356]; suppresses OSCCs [357] and HCC [358]; inhibits cell proliferation and inflammation of RA in synovial fibroblasts [356,359]; mediates of human adipogenesis [360]. |
| hsa-miR-365a-3p [UAAUGCCCCUAAAAAUCCUUAU] | Suppresses progression of PC [361] and GC [362]. |
| hsa-miR-365b-3p [UAAUGCCCCUAAAAAUCCUUAU] | Promotes HCC cell migration and invasion [363]. |
| hsa-miR-183-5p [UAUGGCACUGGUAGAAUUCACU] | Modulates cell adhesion [364]; regulates uterine receptivity and enhances embryo implantation [365]; promotes invasion of endometrial stromal cells [366]; regulates myogenic differentiation [367]. |
| hsa-miR-148b-3p [UCAGUGCAUCACAGAACUUUGU] | Stimulates osteogenesis [368] and suppresses glioma cells [369]. |
| hsa-miR-28-3p [CACUAGAUUGUGAGCUCCUGGA] | Inhibits diffuse large B-Cell lymphoma cell proliferation [370]. |
| Common miRNAs | |
| hsa-miR-141-3p [UAACACUGUCUGGUAAAGAUGG] | Suppresses ameloblastoma cell migration [371]; suppresses osteosarcoma cells [372], GC [373] and CRC [374]; promotes endothelial cell angiogenesis [375]; regulates myogenic differentiation in myoblasts [376]; regulates IL-13-induced airway mucus production [377]; regulates mesenchymal stem cell aging [378]. |
| hsa-miR-22-3p [AAGCUGCCAGUUGAAGAACUGU] | Suppresses endothelial progenitor cell proliferation and migration in venous thrombosis [379]; suppresses T-cells in ALL [380]; suppresses sepsis-induced acute kidney injury [381]. |
| hsa-miR-181a-5p [AACAUUCAACGCUGUCGGUGAGU] | Reduces oxidation resistance in osteoarthritis [382]; suppresses prostate cancer [383]; regulates several cancer genes [384]; regulates multiple malignant processes of breast cancer [385]; suppresses invasion and migration of HTR-8/SVneo in pre-eclampsia [386]. |
| hsa-miR-26a-5p [UUCAAGUAAUCCAGGAUAGGCU] | Regulates the glutamate transporter in multiple sclerosis [387]; regulates fatty acid and sterol metabolism in nonalcoholic fatty liver disease; regulates the expression of inducible nitric oxide synthase in human osteoarthritis chondrocytes [388]; suppresses breast cancer [389] and prostate cancer [390]. |
| hsa-miR-30a-5p [UGUAAACAUCCUCGACUGGAAG] | Suppresses CRC [391], lung squamous cell carcinoma [392], and renal cell carcinoma [393]. |
| hsa-let-7a-5p [UGAGGUAGUAGGUUGUAUAGUU] | Decreases cell proliferation and inhibits the expression of Bcl-2 in ovarian cancer cells [394]. |
| hsa-miR-148a-3p [UCAGUGCACUACAGAACUUUGU] | Suppresses GC [395]; promotes ADH4 expression [396]; regulates angiogenesis [397]. |
| hsa-miR-27b-3p [UUCACAGUGGCUAAGUUCUGC] | Suppresses glioma [398], lung cancer [399], CRC [400]; endothelial cell proliferation and migration in Kawasaki Disease [401]; suppresses Osteogenic differentiation of maxillary sinus membrane stem cells by targeting Sp7 [402]. |
| hsa-miR-146b-5p [UGAGAACUGAAUUCCAUAGGCUG] | Suppresses NSCLC [403], and glioma [404]; down-regulates BRCA1 expression in TNBC [405]; induces IL-6 [406]. |
| hsa-let-7f-5p [UGAGGUAGUAGAUUGUAUAGUU] | Promotes bone marrow MSCs survival in AD [407] and suppresses NSCLC [408]. |
| hsa-miR-21-5p [UAGCUUAUCAGACUGAUGUUGA] | Suppresses breast cancer cells [409]; induces angiogenesis [410]; regulates mesothelin expression [411]; promotes ThP-1 cell proliferation [412]; links EMT in keloid keratinocytes [413]. |
| hsa-miR-92a-3p [UAUUGCACUUGUCCCGGCCUGU] | Suppresses lymphoma [414]; promotes cell proliferation, invasion and metastasis, inhibiting cell apoptosis and serving as predictive biomarkers for tumor diagnosis or chemoresistance [415]; regulates angiogenesis in stromal cells [416]; relates to activated partial thromboplastin time, prothrombin activity and plasma lipocalin-2 level [417]; regulates cartilage-specific gene expression in chondrogenesis [418]; regulates aggrecanase-1 and 2 expressions in human articular chondrocytes [419]. |
| hsa-miR-16-5p [UAGCAGCACGUAAAUAUUGGCG] | Suppresses CRC [420], chordoma [421], neuroblastoma [422] and breast cancer [423]; involved in dilation of ischemic cardiomyopathy [424]; enhances radiosensitivity in prostate cancer [425]; prevents amyloid β-induce injury [426]; affects neurological function, autophagy and apoptosis of hippocampal neurons in AD [427]; controls development of osteoarthritis in chondrocytes [428]; suppresses myofibroblast activation in systemic sclerosis [429]; regulates the p53 signaling pathway in myoblast differentiation [430]; regulates postmenopausal osteoporosis [431]. |
| hsa-miR-101-3p [UACAGUACUGUGAUAACUGAA] | Suppresses HER2-positive BC [432], HCC [433,434], glioblastoma [435], endometrial carcinoma [436], NSCLC [437], renal cell carcinoma [438] and melanoma [439]; regulates cancer proliferation [440]; regulates mitochondrial metabolic function [440]; induces vascular endothelial cell dysfunction [441]; regulates osteogenesis [442]. |
| hsa-miR-30d-5p [UGUAAACAUCCCCGACUGGAAG] | Suppresses gallbladder carcinoma [443], rectal cancer [444], colon cancer [445], prostate cancer [446], ESCC [447], renal carcinoma [448], PC [449], HCC [450], THCA [451], LSCC [452] and NSCLC [453,454,455]. |
| hsa-miR-378a-3p [ACUGGACUUGGAGUCAGAAGGC] | Controls metabolism, muscle differentiation/regeneration and angiogenesis [456]; suppresses glioblastoma [457] and HCC [458]; protects against intestinal injury [459]; modulates keratinocytes cell cycle arrest in psoriasis keratinocytes [460]. |
| hsa-miR-191-5p [CAACGGAAUCCCAAAAGCAGCUG] | Inhibits replication of human immunodeficiency virus type 1 (HIV-1) [461]. |
| hsa-miR-10a-5p [UACCCUGUAGAUCCGAAUUUGUG] | Inhibits osteogenic differentiation [462]; inhibits keratinocyte proliferation in atopic dermatitis [463]; reduces IL-6-induced cartilage cell ferroptosis [464]; regulates BDNF expression in follicular fluid [465]; suppresses renal cell carcinoma [466]; mitigates Ca2+ entry in T cells through gut bacterial metabolite urolithin [467]; enhances viability and migration of human umbilical vein endothelial cells [468]. |
| hsa-let-7b-5p [UGAGGUAGUAGGUUGUGUGGUU] | Promotes protein processing in endoplasmic reticulum in acute pulmonary embolism [469]; promotes angiogenesis [470]; inhibits proliferation of leukemia [471]; inhibits proliferation of leukemia THP-1 Cells [471]. |
| hsa-miR-200a-3p [UAACACUGUCUGGUAACGAUGU] | Prevents MPP+-induced apoptotic cell death [472]. |
| hsa-miR-186-5p [CAAAGAAUUCUCCUUUUGGGCU] | Promotes apoptosis [473] and suppresses CRC [474]. |
| hsa-miR-320a [CUCCCCUCCGCCUUCUCUUCCCGGUUCUUCCCGGAGUCGGGAAAAGCUG] | Suppresses CRC [475,476], glioblastoma [477], GC [478], salivary adenoid cystic carcinoma [479] and CML [480]; targets genes in lithium response in bipolar disorder [481]; regulates fibrotic process in interstitial lung disease of systemic sclerosis [482]; regulates cell proliferation and apoptosis in multiple myeloma [483]; regulates erythroid differentiation [484]; controls glucagon expression [485]; regulates cell proliferation and apoptosis in multiple myeloma [483]; stimulates endometrial stromal cell migration during preimplantation embryo stage [486]; improves skeletal muscle mitochondrial metabolism [487]. |
| hsa-miR-181b-5p [AACAUUCAUUGCUGUCGGUGGGU] | Involved in Ang II-induced phenotypic transformation of smooth muscle cells in hypertension [488]; suppresses starvation-induced cardiomyocyte autophagy [488]; improves anti-tumor cytotoxic T cell response in B cells of CLL [489]; inhibits trophoblast cell migration and invasion in multiple abnormal trophoblast invasion [490]; modulates cell migratory proteins in endometrial stromal cells [491]; suppresses the progression of epilepsy [492]; suppresses gallbladder carcinoma [493]. |
| hsa-miR-30e-5p [UGUAAACAUCCUUGACUGGAAG] | Regulates angiogenesis, apoptosis, cell differentiation, oxidative stress and hypoxia [494]; regulates autophagy and apoptosis in contrast-induced acute kidney injury [495]; suppresses NSCLC [496] and squamous cell carcinoma of the head and neck [497]; enhances innate immune responses [498]; suppresses cancer cell adhesion, migration and invasion, and considered as a potential target for curbing metastatic spread in P53-deficient tumors [499]. |
| hsa-miR-103a-3p [AGCAGCAUUGUACAGGGCUAUGA] | Regulates BDNF expression in follicular fluid [465]; suppresses PC [500]; aggravates renal cell carcinoma [501]; regulates Wnt signaling pathway in colorectal carcinoma [502]. |
| hsa-miR-182-5p [UUUGGCAAUGGUAGAACUCACACU] | Mediates downregulation of BRCA1, impacting DNA repair and sensitivity to PARP inhibitors [503]; suppresses CRC [504], renal cell carcinoma [505], bladder cancer [506] and prostate cancer [507]; acts as glial cell line-derived neurotrophic factor GDNF mimics dopaminergic midbrain neurons [508]; associates with renal cancer cell mitotic arrest [509]. |
| hsa-miR-151a-3p [CUAGACUGAAGCUCCUUGAGG] | Enhances slug-dependent angiogenesis and regulates multiple functions in the lung, such as cell growth, motility, partial EMT and angiogenesis [510]. |
| hsa-miR-335-5p [UCAAGAGCAAUAACGAAAAAUGU] | Regulates bone homeostasis [511]; suppresses GC [512], uterine leiomyoma [513] and ESCC [514]; regulates cardiac mesoderm and progenitor cell differentiation [515]. |
| hsa-miR-25-3p [CAUUGCACUUGUCUCGGUCUGA] | Suppresses hepatocytes [516] and regulates osteoblast differentiation of human aortic valve interstitial cells [517]. |
| hsa-let-7g-5p [UGAGGUAGUAGUUUGUACAGUU] | Suppresses epithelial ovarian cancer [517] and glioblastoma [518]; and alleviates murine collagen-induced arthritis by inhibiting Th17 cell differentiation [519]. |
| hsa-miR-200c-3p [UAAUACUGCCGGGUAAUGAUGGA] | Suppresses prostate carcinoma [520], renal cell carcinoma [521] and epithelial ovarian cancer [522]; regulates integrin-mediated cell adhesion [523]; attenuates the tumor-infiltrating capacity of macrophages [524]. |
| hsa-miR-30c-5p [UGUAAACAUCCUACACUCUCAGC] | Suppresses GC [525] and prostate cancer [526]; protects cells from hypoxia-reoxygenation-induced apoptosis and induces cell proliferation and anti-apoptotic and proliferative effects [527]; reduces cellular migration and pro-angiogenic gene expression in extracellular vesicle EV-recipient cells [528]. |
| hsa-miR-429 [UAAUACUGUCUGGUAAAACCGU] | Inhibits cell proliferation and Ca2+ influx by pulmonary artery smooth muscle cells [285]; regulates hypoxia [529]; suppresses breast cancer [530], osteosarcoma [531], THCA, soft tissue sarcoma [532], cervical cancer [533], GC [534], diffuse large B-cell lymphoma [535], esophageal carcinoma [536], HCC [537,538], glioblastoma [539], NPC [540], PC cancer [541], THCA [542], OSCC [543] and renal cell carcinoma [544]; inhibits bone metastasis in breast cancer [545]; regulates the transition between HIF1A and HIF3A expression in human endothelial cells [546]. |
| hsa-miR-99b-5p [CACCCGUAGAACCGACCUUGCG] | Suppresses primary myotubes [547], epidermal keratinocytes and cervical cancer cells [548]. |
| hsa-miR-29a-3p [UAGCACCAUCUGAAAUCGGUUA] | Modulates CYP2C19 in human liver cells [549]; suppresses cell proliferation [550]; activates hepatic stellate cells, which moderate their profibrogenic phenotype, supporting the use of miR-29a agonists for treating liver fibrosis [551]; regulates tumorigenicity and TME development [552]; involved in the progression of HCC, which elucidates its potential theragnostic implications [552]; suppresses GC [553] and renal cell carcinoma by regulating E2F1 expression by long non-coding RNA H19 [554]; induces TNFα in endothelial dysfunction [555]; mediates tumor immune infiltration in breast invasive carcinoma [556]; acts as a protective factor for fibrogenesis in gluteal muscle contracture [557]; regulates osteoblast differentiation and peri-implant osseointegration [558]; promotes intestinal epithelial apoptosis in ulcerative colitis [559]; regulates and restores endothelial function in normal people and cardiometabolic disorders, respectively [560]; regulates peripheral glucocorticoid receptor signaling [561]; suppresses CRC [562], gliomas [563], head and neck squamous cell carcinoma [564], prostate cancer [565], GC [553], PTC [566], lung cancer [567], cervical cancer [568] and endometrial cancer [569]; enhances the radiosensitivity of OSCC cells [570]; modulates ALDH5A1 and SLC22A7 in human liver cells [571]. |
| hsa-miR-30b-5p [UGUAAACAUCCUACACUCAGCU] | Suppresses HCC, which is sponged by long non-coding RNA HNF1A-AS1 oncogene [572]; inhibits GC cell migration [572]; regulates lipid metabolism [573]; involved in vascular smooth muscle cell differentiation [574]; involved in homocysteine-induced apoptosis in human coronary artery endothelial cells [575]; inhibits osteoblast differentiation [576]; inhibits proliferation and promotes apoptosis of medulloblastoma cells [577]; controls adverse effects of non-small cell lung cancer NSCLC radiotherapy [578]; mediates ferroptosis of trophoblasts, which is involved in the pathogenesis of preeclampsia [579]; suppresses expression of B-cell activating factor mRNA primary in Sjögren’s syndrome [580]. |
| hsa-miR-19b-3p [UGUGCAAAUCCAUGCAAAACUGA] | Suppresses cell mobility [581]; involved in proliferation, apoptosis and cycle of SH-SY5Y cells [582]; regulates neuropathic pain and neuroinflammation [583]; regulates cell cycle in CRC [584]; induces endothelial dysfunction and decreases lung injury, inflammation and permeability and improved hemodynamics [585]; regulates skeletal muscle anabolism [586]; regulates apoptosis in THCA [587]; interacts with environmental factors, such as maternal stress during pregnancy, neonatal jaundice and family psychiatric history, to impact risk of ASD [588]; stimulates cardiomyocyte apoptosis [589]. |
| Disease/Condition | Type of Study | Target(s) | Study Criteria and Participants | Main Findings | Reference |
|---|---|---|---|---|---|
| Cancer | Case-control | The p53 gene (the guardian of the genome) | In archived tumor blocks from 803 cases, the promoter methylation of the p16 gene in connection to breastfeeding was examined | The p53 gene promoter was nearly three times more likely to be methylated in premenopausal women who had never been breastfed | [695] |
| Metabolism and growth | Cross-sectional | LEP gene | 120 Dutch children (50 girls) were included with average age of 1.4 years | Children who were breastfed for at least 1 to 3 months had lower LEP promoter methylation in white blood cells and higher serum levels of leptin than children who were never breastfed | [727] |
| Prospective observational cohort | RXRA and LEP genes | The effects of breastfeeding duration on infant growth and methylation in obesity-related genes of buccal cells (n = 101) were assessed | At 12 months, breastfeeding duration was associated with epigenetic changes in RXRA and LEP genes, as well as infant biometry and growth | [734] | |
| Cohort | LEP gene | 23 CpGs in the LEP gene in 297 samples of 10-year-old and 16 CpGs in 305 samples of 18-year-old were tested for association with breastfeeding duration | Breastfeeding length is associated with LEP methylation at 10 years of age and BMI trajectory; despite the small sample size, LEP DNA methylation is related to BMI trajectories throughout childhood | [733] | |
| Cohort | The protein coding genes FDFT1 and SNX25, as well as the ncRNA gene LINC00840 | A comprehensive Epigenome-Wide Association Study to identify associations between breastfeeding and DNA methylation patterns in childhood (at birth, 10, 18 and 26 years) was performed. Breastfeeding durations of >3 months and >6 months, as well as exclusive breastfeeding durations of >3 months, were used to categorize the feeding. | In 10-year-old children who were breastfed for more than three months, a substantial differentially methylated region covering the gene FDFT1 was discovered | [735] | |
| BMI and weight | Cohort | A total of 2 CpG sites in boys (NREP and IL16) and 13 CpG sites in girls (ATP6V0A1, DHX15/PPARGC1A, LINC00398/ALOX5AP, FAM238C, miR-21, SNAPC3, NATP/NAT2, CUX1, TRAPPC9, OSBPL1A, ZNF185, FAM84A, PDPK1) were investigated | The study comprised 15,454 pregnancies, with 15,589 known fetuses, 14,901 of whom were alive at one year. A total of 12,761 children were available for study after twins (n = 201) and children missing anthropometric measurements or age information were removed. The kids were tracked for more than two decades | CpG sites were shown to be enriched in miRNAs and critical pathways (AMPK signaling, insulin signaling and endocytosis). When compared to no breastfeeding, DNA methylation variation corresponding to 3 to 5 months of exclusive breastfeeding was linked to lower BMI growth in the first 6 years of life | [740] |
| Lung diseases and asthma | Cohort | This gene is linked to asthma risk | Blood samples were collected from 245 females at age 18 years randomly selected for methylation analysis from a birth cohort (n = 1456) | The number of weeks of breastfeeding had minor impacts on methylation of the interleukin-4 receptor gene’s relevant CpG island | [730] |
| Neurological disorder | Cohort | DRD4 gene | The data came from a large population-based triple B pregnancy cohort study (n = 844) that included thorough information on maternal alcohol intake throughout pregnancy and in the early postpartum period. The methylation of the DRD4 promoter DNA was investigated | The methylation of a DRD4 (a key dopamine receptor) in cheek cells was higher in eight-week-old children whose moms drank moderate amounts of alcohol during breastfeeding compared to those who did not drink | [742] |
| Cohort (bioinformatics study) | Many genes, particularly those involved in oxytocin signaling pathway | Investigating the association of breastfeeding and DNA methylation in the peripheral blood cells of 37 children aged 9 months to 4 years | In response to breastfeeding, the oxytocin signaling pathway serves a unique role as a possible activator of coordinated epigenetic alterations in genes essential to CNS function | [751] | |
| Immunity and allergy | Cross-sectional | TLR1 gene | In 57 adult adults, DNA methylation at two locations in the promoter of the TLR1 gene, as well as the relationship between DNA methylation of the TLR1 gene and illness susceptibility, were studied | The promoter of the TLR1 gene showed a considerable reduction in DNA methylation. There was no link discovered between DNA methylation and illness vulnerability | [746] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatmal, M.M.; Al-Hatamleh, M.A.I.; Olaimat, A.N.; Alshaer, W.; Hasan, H.; Albakri, K.A.; Alkhafaji, E.; Issa, N.N.; Al-Holy, M.A.; Abderrahman, S.M.; et al. Immunomodulatory Properties of Human Breast Milk: MicroRNA Contents and Potential Epigenetic Effects. Biomedicines 2022, 10, 1219. https://doi.org/10.3390/biomedicines10061219
Hatmal MM, Al-Hatamleh MAI, Olaimat AN, Alshaer W, Hasan H, Albakri KA, Alkhafaji E, Issa NN, Al-Holy MA, Abderrahman SM, et al. Immunomodulatory Properties of Human Breast Milk: MicroRNA Contents and Potential Epigenetic Effects. Biomedicines. 2022; 10(6):1219. https://doi.org/10.3390/biomedicines10061219
Chicago/Turabian StyleHatmal, Ma’mon M., Mohammad A. I. Al-Hatamleh, Amin N. Olaimat, Walhan Alshaer, Hanan Hasan, Khaled A. Albakri, Enas Alkhafaji, Nada N. Issa, Murad A. Al-Holy, Salim M. Abderrahman, and et al. 2022. "Immunomodulatory Properties of Human Breast Milk: MicroRNA Contents and Potential Epigenetic Effects" Biomedicines 10, no. 6: 1219. https://doi.org/10.3390/biomedicines10061219
APA StyleHatmal, M. M., Al-Hatamleh, M. A. I., Olaimat, A. N., Alshaer, W., Hasan, H., Albakri, K. A., Alkhafaji, E., Issa, N. N., Al-Holy, M. A., Abderrahman, S. M., Abdallah, A. M., & Mohamud, R. (2022). Immunomodulatory Properties of Human Breast Milk: MicroRNA Contents and Potential Epigenetic Effects. Biomedicines, 10(6), 1219. https://doi.org/10.3390/biomedicines10061219








