Knowledge Gap in Understanding the Steroidogenic Acute Regulatory Protein Regulation in Steroidogenesis Following Exposure to Bisphenol A and Its Analogues
Abstract
:1. Introduction
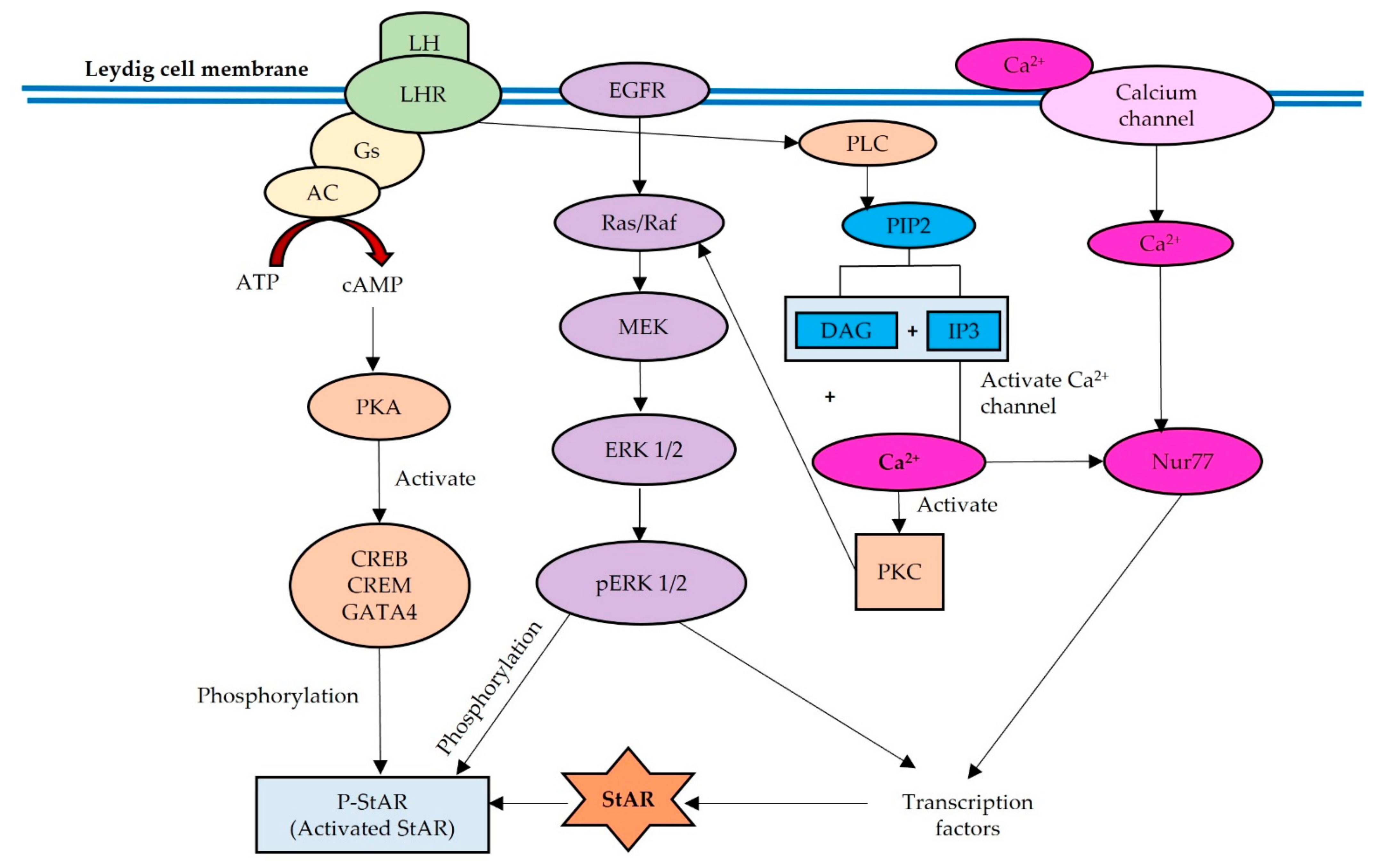
2. Regulation of StAR in Normal Physiology
3. Effects of BPA and Its Analogues on the LH-LHR, cAMP-PKA and PLC-PKC Signaling Pathways
4. Effects of BPA and Its Analogues on EGFR-MAPK-ERK Signaling Pathway
5. Effects of BPA and Its Analogues on the Ca2+ Signaling and the Involvement of Nur77 Transcription Factor
6. Effect of BPA and Its Analogues on StAR Protein Expression
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| ATP | adenosine 5′-triphosphate |
| BPA | bisphenol A |
| BPAF | bisphenol AF |
| BPAP | bisphenol AP |
| BPB | bisphenol B |
| BPC | bisphenol C |
| BPF | bisphenol F |
| BPS | bisphenol S |
| BPZ | bisphenol Z |
| cAMP | cyclic adenosine monophosphate |
| Ca2+ | Ion calcium |
| CREB | cAMP response element-binding |
| CREM | cAMP response element modulator |
| DAG | diacylglycerol |
| EDCs | endocrine disrupting chemicals |
| ERK | extracellular signal-regulated kinases |
| FSH | follicle-stimulating hormone |
| GATA4 | GATA Binding Protein 4 |
| HPG | hypothalamic–pituitary–gonadal axis |
| HSD | hydroxysteroid dehydrogenase |
| IP3 | inositol 1,4,5-trisphosphate |
| LH | luteinizing hormone |
| LHR | luteinizing hormone receptor |
| MAPK | mitogen-activated protein kinase |
| mRNA | messenger RNA |
| P | phosphorylate |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| PKA | protein kinase A |
| PKC | protein kinase C |
| PLC | phospholipase C |
| StAR | Steroidogenic acute regulatory |
References
- Delfosse, V.; Grimaldi, M.; Pons, J.L.; Boulahtouf, A.; Le Maire, A.; Cavailles, V.; Labesse, G.; Bourguet, W.; Balaguer, P. Structural and Mechanistic Insights into Bisphenols Action Provide Guidelines for Risk Assessment and Discovery of Bisphenol A Substitutes. Proc. Natl. Acad. Sci. USA 2012, 109, 14930–14935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyawaki, J.; Sakayama, K.; Kato, H.; Yamamoto, H.; Masuno, H. Perinatal and Postnatal Exposure to Bisphenol a Increase Adipose Tissue Mass and Serum Cholesterol Level in Mice. J. Atheroscler. Thromb. 2007, 14, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and Human Health: A Review of the Literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Rezg, R.; El-Fazaa, S.; Gharbi, N.; Mornagui, B. Bisphenol A and Human Chronic Diseases: Current Evidences, Possible Mechanisms, and Future Perspectives. Environ. Int. 2014, 64, 83–90. [Google Scholar] [CrossRef]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. Population to Bisphenol A and 4-Tertiary-Octylphenol: 2003-2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Fiege, H.; Voges, H.-W.; Hamamoto, T.; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D.; Paulus, W. Phenol Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Liao, C.; Liu, F.; Kannan, K. Bisphenol S, a New Bisphenol Analogue, in Paper Products and Currency Bills and Its Association with Bisphenol a Residues. Environ. Sci. Technol. 2012, 46, 6515–6522. [Google Scholar] [CrossRef]
- NTP. Chemical Information Profile for Bisphenol AF-Supporting Nomination for Toxicological Evaluation by the National Toxicology Program. Natl. Inst. Environ. Heal. Sci. 2008. Available online: https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/bisphenolaf_093008_508.pdf (accessed on 28 March 2022).
- Ohore, O.E.; Songhe, Z. Endocrine Disrupting Effects of Bisphenol a Exposure and Recent Advances on Its Removal by Water Treatment Systems. A Review. Sci. Afr. 2019, 5, e00135. [Google Scholar] [CrossRef]
- Shamhari, A.‘A.; Hamid, Z.A.; Budin, S.B.; Shamsudin, N.J.; Taib, I.S. Bisphenol a and Its Analogues Deteriorate the Hormones Physiological Function of the Male Reproductive System: A Mini-Review. Biomedicines 2021, 9, 1744. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, M.J.E.; van den Berg, M.; Bovee, T.F.H.; Piersma, A.H.; van Duursen, M.B. Structural Bisphenol Analogues Differentially Target Steroidogenesis in Murine MA-10 Leydig Cells as Well as the Glucocorticoid Receptor. Toxicology 2015, 329, 10–20. [Google Scholar] [CrossRef]
- Jambor, T.; Jana, B.; Hana, G.; Eva, T.; Norbert, L. Male Reproduction: One of the Primary Targets of Bisphenol. In Bisphenol A Exposure and Health Risks; InTech.: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Payne, A.H.; Quinn, P.G.; Stalvey, J.R.D. The Stimulation of Steroid Biosynthesis by Luteinizing Hormone. In Luteinizing Hormone Action and Receptors; CRC Press: Boca Raton, FL, USA, 2019; pp. 135–172. ISBN 9780429285684. [Google Scholar]
- Paz, C.; Cornejo Maciel, F.; Gorostizaga, A.; Castillo, A.F.; Mori Sequeiros García, M.M.; Maloberti, P.M.; Orlando, U.D.; Mele, P.G.; Poderoso, C.; Podesta, E.J. Role of Protein Phosphorylation and Tyrosine Phosphatases in the Adrenal Regulation of Steroid Synthesis and Mitochondrial Function. Front. Endocrinol. 2016, 7, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galano, M.; Li, Y.; Li, L.; Sottas, C.; Papadopoulos, V. Role of Constitutive Star in Leydig Cells. Int. J. Mol. Sci. 2021, 22, 2021. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Stetson, C.L.; Slominski, A.T.; Pruitt, K. Role of the Steroidogenic Acute Regulatory Protein in Health and Disease. Endocrine 2016, 51, 7–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, W.L. StAR Search-What We Know about How the Steroidogenic Acute Regulatory Protein Mediates Mitochondrial Cholesterol Import. Mol. Endocrinol. 2007, 21, 589–601. [Google Scholar] [CrossRef] [Green Version]
- Bose, H.S.; Lingappat, V.R.; Miller, W.L. Rapid Regulation of Steroidogenesis by Mitochondrial Protein Import. Nature 2002, 417, 87–91. [Google Scholar] [CrossRef]
- Kotrasová, V.; Keresztesová, B.; Ondrovičová, G.; Bauer, J.A.; Havalová, H.; Pevala, V.; Kutejová, E.; Kunová, N. Mitochondrial Kinases and the Role of Mitochondrial Protein Phosphorylation in Health and Disease. Life 2021, 11, 82. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Commentary: Molecular Mechanisms of Action of FSH. Front. Endocrinol. 2019, 10, 305. [Google Scholar] [CrossRef] [Green Version]
- Midzak, A.; Zirkin, B.; Papadopoulos, V. Translocator Protein: Pharmacology and Steroidogenesis. Biochem. Soc. Trans. 2015, 43, 572–578. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig Cells: Effects of Aging and Environmental Factors. Reproduction 2017, 154, R111–R122. [Google Scholar] [CrossRef] [Green Version]
- Manna, P.R.; Jo, Y.; Stocco, D.M. Regulation of Leydig Cell Steroidogenesis by Extracellular Signal-Regulated Kinase 1/2: Role of Protein Kinase A and Protein Kinase C Signaling. J. Endocrinol. 2007, 193, 53–63. [Google Scholar] [CrossRef]
- Manna, P.R.; Stocco, D.M. The Role of Specific Mitogen-Activated Protein Kinase Signaling Cascades in the Regulation of Steroidogenesis. J. Signal Transduct. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.B.; Walker, W.H. Hormone Signaling in the Testis. In Knobil and Neill’s Physiology of Reproduction: Two-Volume Set; Elsevier North-Holland, Inc.: Amsterdam, The Netherlands, 2015; Volume 1, pp. 637–690. ISBN 9780123977694. [Google Scholar]
- Liu, S.; Tang, Y.; Chen, B.; Zhao, Y.; Aguilar, Z.P.; Tao, X.; Xu, H. Inhibition of Testosterone Synthesis Induced by Oral TiO2NPs Is Associated with ROS-MAPK(ERK1/2)-StAR Signaling Pathway in SD Rat. Toxicol. Res. 2021, 10, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Seger, R.; Hanoch, T.; Rosenberg, R.; Dantes, A.; Merz, W.E.; Strauss, J.F.; Amsterdam, A. Inhibition of Gonadotropin-Stimulated Steroidogenesis by the Erk Cascade. bioRxiv 2019, 853648. [Google Scholar] [CrossRef]
- Kotula-Balak, M.; Duliban, M.; Pawlicki, P.; Tuz, R.; Bilinska, B.; Plachno, B.J.; Arent, Z.J.; Krakowska, I.; Tarasiuk, K. The Meaning of Non-Classical Estrogen Receptors and Peroxisome Proliferator-Activated Receptor for Boar Leydig Cell of Immature Testis. Acta. Histochem. 2020, 122, 151526. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mu, X.; Ye, L.; Ze, Y.; Hong, F. Suppression of Testosterone Production by Nanoparticulate TiO 2 Is Associated with ERK1/2-PKA-PKC Signaling Pathways in Rat Primary Cultured Leydig Cells. Int. J. Nanomed. 2018, 13, 5909–5924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, A.; Castillo, A.F.; Podestá, E.J.; Poderoso, C. Mitochondrial Fusion and ERK Activity Regulate Steroidogenic Acute Regulatory Protein Localization in Mitochondria. PLoS ONE 2014, 9, e100387. [Google Scholar] [CrossRef] [Green Version]
- Castillo, A.F.; Orlando, U.; Helfenberger, K.E.; Poderoso, C.; Podesta, E.J. The Role of Mitochondrial Fusion and StAR Phosphorylation in the Regulation of StAR Activity and Steroidogenesis. Mol. Cell Endocrinol. 2014, 408, 73–79. [Google Scholar] [CrossRef]
- de Santi, F.; Beltrame, F.L.; Rodrigues, B.M.; Scaramele, N.F.; Lopes, F.L.; Cerri, P.S.; Sasso-Cerri, E. Venlafaxine-Induced Adrenergic Signaling Stimulates Leydig Cells Steroidogenesis via Nur77 Overexpression: A Possible Role of EGF. Life Sci. 2022, 289, 120069. [Google Scholar] [CrossRef]
- Ha, M.; Zhang, P.; Li, L.; Liu, C. Triclosan Suppresses Testicular Steroidogenesis via the MiR-6321/JNK/Nur77 Cascade. Cell Physiol. Biochem. 2018, 50, 2029–2045. [Google Scholar] [CrossRef]
- Kim, H.; Kumar, S.; Lee, K. FOXA3, a Negative Regulator of Nur77 Expression and Activity in Testicular Steroidogenesis. Int. J. Endocrinol. 2021, 2021, 6619447. [Google Scholar] [CrossRef]
- Wisniewski, P.; Romano, R.M.; Kizys, M.M.L.; Oliveira, K.C.; Kasamatsu, T.; Giannocco, G.; Chiamolera, M.I.; Dias-da-Silva, M.R.; Romano, M.A. Adult Exposure to Bisphenol A (BPA) in Wistar Rats Reduces Sperm Quality with Disruption of the Hypothalamic-Pituitary-Testicular Axis. Toxicology 2015, 329, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Wang, J.; Li, X.; Zhang, X. Echinacoside and Cistanche Tubulosa (Schenk) R. Wight Ameliorate Bisphenol A-Induced Testicular and Sperm Damage in Rats through Gonad Axis Regulated Steroidogenic Enzymes. J. Ethnopharmacol. 2016, 193, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C.; Jiang, Z.; Wang, M.; Jiang, H.; Zhang, X. Protective Effect of Cordyceps Militaris Extract against Bisphenol A Induced Reproductive Damage. Syst. Biol. Reprod. Med. 2016, 62, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Yang, X.; Liu, J.; Ren, W.; Chen, Y.; Shen, S. Effects of BPF on Steroid Hormone Homeostasis and Gene Expression in the Hypothalamic–Pituitary–Gonadal Axis of Zebrafish. Environ. Sci. Pollut. Res. 2017, 24, 21311–21322. [Google Scholar] [CrossRef] [PubMed]
- Akintunde, J.K.; Farouk, A.A.; Mogbojuri, O. Metabolic Treatment of Syndrome Linked with Parkinson’s Disease and Hypothalamus Pituitary Gonadal Hormones by Turmeric Curcumin in Bisphenol-A Induced Neuro-Testicular Dysfunction of Wistar Rat. Biochem. Biophys. Rep. 2019, 17, 97–107. [Google Scholar] [CrossRef]
- Majid, M.; Ijaz, F.; Baig, M.W.; Nasir, B.; Khan, M.R.; Haq, I.U. Scientific Validation of Ethnomedicinal Use of Ipomoea Batatas L. Lam. as Aphrodisiac and Gonadoprotective Agent against Bisphenol a Induced Testicular Toxicity in Male Sprague Dawley Rats. Biomed Res. Int. 2019, 2019, 8939854. [Google Scholar] [CrossRef]
- Alboghobeish, S.; Mahdavinia, M.; Zeidooni, L.; Samimi, A.; Oroojan, A.A.; Alizadeh, S.; Dehghani, M.A.; Ahangarpour, A.; Khorsandi, L. Efficiency of Naringin against Reproductive Toxicity and Testicular Damages Induced by Bisphenol A in Rats. Iran. J. Basic Med. Sci. 2019, 22, 315–323. [Google Scholar] [CrossRef]
- Zahra, Z.; Khan, M.R.; Majid, M.; Maryam, S.; Sajid, M. Gonadoprotective Ability of Vincetoxicum Arnottianum Extract against Bisphenol A-Induced Testicular Toxicity and Hormonal Imbalance in Male Sprague Dawley Rats. Andrologia 2020, 52, e13590. [Google Scholar] [CrossRef]
- Ullah, H.; Ullah, F.; Rehman, O.; Jahan, S.; Afsar, T.; Al-Disi, D.; Almajwal, A.; Razak, S. Chronic Exposure of Bisphenol S (BPS) Affect Hypothalamic-Pituitary-Testicular Activities in Adult Male Rats: Possible in Estrogenic Mode of Action. Environ. Health Prev. Med. 2021, 26, 1–11. [Google Scholar] [CrossRef]
- Jahan, S.; Ain, Q.U.; Ullah, H. Therapeutic Effects of Quercetin against Bisphenol a Induced Testicular Damage in Male Sprague Dawley Rats. Syst. Biol. Reprod. Med. 2016, 62, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, I.M.; Romano, R.M.; De Campos, P.; Cavallin, M.D.; Oliveira, C.A.; Romano, M.A. Delayed Onset of Puberty in Male Offspring from Bisphenol A-Treated Dams Is Followed by the Modulation of Gene Expression in the Hypothalamic-Pituitary-Testis Axis in Adulthood. Reprod. Fertil. Dev. 2017, 29, 2496–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akingbemi, B.T.; Sottas, C.M.; Koulova, A.I.; Klinefelter, G.R.; Hardy, M.P. Inhibition of Testicular Steroidogenesis by the Xenoestrogen Bisphenol a Is Associated with Reduced Pituitary Luteinizing Hormone Secretion and Decreased Steroidogenic Enzyme Gene Expression in Rat Leydig Cells. Endocrinology 2004, 145, 592–603. [Google Scholar] [CrossRef]
- Savchuk, I.; Söder, O.; Svechnikov, K. Mouse Leydig Cells with Different Androgen Production Potential Are Resistant to Estrogenic Stimuli but Responsive to Bisphenol A Which Attenuates Testosterone Metabolism. PLoS ONE 2013, 8, e71722. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yin, J.; Jiao, Z.; Shi, J.; Li, M.; Shao, B. Bisphenol AF May Cause Testosterone Reduction by Directly Affecting Testis Function in Adult Male Rats. Toxicol. Lett. 2012, 211, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.D.; Klett, D.; Combarnous, Y. Estrogenic Compounds or Adiponectin Inhibit Cyclic AMP Response to Human Luteinizing Hormone in Mouse Leydig Tumor Cells. Biology 2019, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Nikula, H.; Talonpoika, T.; Kaleva, M.; Toppari, J. Inhibition of HCG-Stimulated Steroidogenesis in Cultured Mouse Leydig Tumor Cells by Bisphenol A and Octylphenols. Toxicol. Appl. Pharmacol. 1999, 157, 166–173. [Google Scholar] [CrossRef]
- Kim, J.Y.; Han, E.H.; Kim, H.G.; Oh, K.N.; Kim, S.K.; Lee, K.Y.; Jeong, H.G. Bisphenol A-Induced Aromatase Activation Is Mediated by Cyclooxygenase-2 up-Regulation in Rat Testicular Leydig Cells. Toxicol. Lett. 2010, 193, 200–208. [Google Scholar] [CrossRef]
- Budin, S.B.; Abdul Rahman, W.Z.; Jubaidi, F.F.; Mohammed Yusof, N.L.; Taib, I.S.; Zainalabidin, S. Roselle (Hibiscus Sabdiriffa) Polyphenol-Rich Extract Prevents Testicular Damage of Diabetic Rats. J. Appl. Pharm. Sci. 2018, 8, 065–070. [Google Scholar] [CrossRef] [Green Version]
- Akmar, K.; Noor, M.M. The Potential Effect of Gynura Procumbens Aqueous Extract as Anti-Hyperglycaemia, Pro-Fertility and Libido Agent Towards Diabetes-Induced Male Rats. Proceeding Int. Conf. Sci. Eng. 2020, 3, 103–108. [Google Scholar] [CrossRef]
- Jaffar, F.H.F.; Osman, K.; Hui, C.K.; Zulkefli, A.F.; Ibrahim, S.F. Edible Bird’s Nest Supplementation Improves Male Reproductive Parameters of Sprague Dawley Rat. Front. Pharmacol. 2021, 12, 466. [Google Scholar] [CrossRef]
- Kasim, R.M.; Jubaidi, F.F.; Taib, I.S.; Budin, S.B. Testicular Damage and Abnormal Sperm Characteristic Due to Chronic Hyperglycemia Exposure Restored by Polyphenol Rich Extract of Hibiscus Sabdariffa Linn. J. Adv. Res. Appl. Sci. Eng. Technol. 2021, 1, 43–55. [Google Scholar] [CrossRef]
- Baufeld, A.; Vanselow, J. Lactate-Induced Effects on Bovine Granulosa Cells Are Mediated via PKA Signaling. Cell Tissue Res. 2022, 388, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Darghouthi, M.; Rezg, R.; Boughmadi, O.; Mornagui, B. Low-Dose Bisphenol S Exposure Induces Hypospermatogenesis and Mitochondrial Dysfunction in Rats: A Possible Implication of StAR Protein. Reprod. Toxicol. 2022, 107, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Lanfranchi, B.; Rubia, R.F.; Gassmann, M.; Schuler, G.; Kowalewski, M.P. Transcriptional Regulation of HIF1α-Mediated STAR Expression in Murine KK1 Granulosa Cell Line Involves CJUN, CREB and CBP-Dependent Pathways. Gen. Comp. Endocrinol. 2022, 315, 113923. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Chang, F.; Zhou, R.; Jin, P.P.; Matsumoto, H.; Sokabe, M.; Chen, L. Increase of Anteroventral Periventricular Kisspeptin Neurons and Generation of E2-Induced LH-Surge System in Male Rats Exposed Perinatally to Environmental Dose of Bisphenol-A. Endocrinology 2011, 152, 1562–1571. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Pirzada, M.; Afsar, T.; Razak, S.; Almajwal, A.; Jahan, S. Effect of Bisphenol F, an Analog of Bisphenol A, on the Reproductive Functions of Male Rats. Environ. Health Prev. Med. 2019, 24, 1–11. [Google Scholar] [CrossRef]
- Wilkenfeld, S.R.; Lin, C.; Frigo, D.E. Communication between Genomic and Non-Genomic Signaling Events Coordinate Steroid Hormone Actions. Steroids. 2018, 133, 2–7. [Google Scholar] [CrossRef]
- Falvo, S.; Santillo, A.; Chieffi Baccari, G.; Cioffi, F.; Di Fiore, M.M. D-Aspartate and N-Methyl-D-Aspartate Promote Proliferative Activity in Mouse Spermatocyte GC-2 Cells. Reprod. Biol. 2022, 22, 100601. [Google Scholar] [CrossRef]
- Nanjappa, M.K.; Simon, L.; Akingbemi, B.T. The Industrial Chemical Bisphenol A (BPA) Interferes with Proliferative Activity and Development of Steroidogenic Capacity in Rat Leydig Cells. Biol. Reprod. 2012, 86, 1–12. [Google Scholar] [CrossRef]
- Chen, Z.J.; Zhang, K.S.; Ge, L.C.; Liu, H.; Chen, L.K.; Du, J.; Wang, H.S. Signals Involved in the Effects of Bisphenol A (BPA) on Proliferation and Motility of Leydig Cells: A Comparative Proteomic Analysis. Toxicol. Res. 2016, 5, 1573–1584. [Google Scholar] [CrossRef] [Green Version]
- Ok, S.; Kang, J.S.; Kim, K.M. Testicular Antioxidant Mechanism of Cultivated Wild Ginseng Extracts. Mol. Cell. Toxicol. 2016, 12, 149–158. [Google Scholar] [CrossRef]
- Li, Y.; Yan, H.; Yu, Y.; Zou, C.; Tian, L.; Xin, X.; Zhang, S.; Li, Z.; Ma, F.; Ge, R.S. Bisphenol B Stimulates Leydig Cell Proliferation but Inhibits Maturation in Late Pubertal Rats. Food Chem. Toxicol. 2021, 153, 112248. [Google Scholar] [CrossRef] [PubMed]
- Thuillier, R.; Manku, G.; Wang, Y.; Culty, M. Changes in MAPK Pathway in Neonatal and Adult Testis Following Fetal Estrogen Exposure and Effects on Rat Testicular Cells. Microsc. Res. Tech. 2009, 72, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.W.; Yang, Z.J.; Huang, H.H.; Chang, A.A.; Cheng, Y.C.; Wu, G.J.; Lan, H.C. Low-Dose Bisphenol A Activates the ERK Signaling Pathway and Attenuates Steroidogenic Gene Expression in Human Placental Cells. Biol. Reprod. 2018, 98, 250–258. [Google Scholar] [CrossRef]
- Ham, J.; Lim, W.; You, S.; Song, G. Butylated Hydroxyanisole Induces Testicular Dysfunction in Mouse Testis Cells by Dysregulating Calcium Homeostasis and Stimulating Endoplasmic Reticulum Stress. Sci. Total Environ. 2020, 702, 134775. [Google Scholar] [CrossRef] [PubMed]
- Batista-Silva, H.; Rodrigues, K.; Sousa de Moura, K.R.; Van Der Kraak, G.; Delalande-Lecapitaine, C.; Mena Barreto Silva, F.R. Role of Bisphenol A on Calcium Influx and Its Potential Toxicity on the Testis of Danio Rerio. Ecotoxicol. Environ. Saf. 2020, 202, 110876. [Google Scholar] [CrossRef]
- Gonçalves, R.; Zanatta, A.P.; Cavalari, F.C.; do Nascimento, M.A.W.; Delalande-Lecapitaine, C.; Bouraïma-Lelong, H.; Silva, F.R.M.B. Acute Effect of Bisphenol A: Signaling Pathways on Calcium Influx in Immature Rat Testes. Reprod. Toxicol. 2018, 77, 94–102. [Google Scholar] [CrossRef]
- Pawlicki, P.; Duliban, M.; Tuz, R.; Ptak, A.; Milon, A.; Gorowska-Wojtowicz, E.; Tworzydlo, W.; Płachno, B.J.; Bilinska, B.; Knapczyk-Stwora, K.; et al. Do G-Protein Coupled Estrogen Receptor and Bisphenol A Analogs Influence on Leydig Cell Epigenetic Regulation in Immature Boar Testis Ex Vivo? Anim. Reprod. Sci. 2019, 207, 21–35. [Google Scholar] [CrossRef]
- Ahn, S.W.; Nedumaran, B.; Xie, Y.; Kim, D.K.; Kim, Y.D.; Choi, H.S. Bisphenol A Bis(2,3-Dihydroxypropyl) Ether (BADGE.2H2O) Induces Orphan Nuclear Receptor Nur77 Gene Expression and Increases Steroidogenesis in Mouse Testicular Leydig Cells. Mol. Cells 2008, 26, 74–80. [Google Scholar]
- Song, K.H.; Keesook, L.E.E.; Choi, H.S. Endocrine Disrupter Bisphenol A Induces Orphan Nuclear Receptor Nur77 Gene Expression and Steroidogenesis in Mouse Testicular Leydig Cells. Endocrinology 2002, 143, 2208–2215. [Google Scholar] [CrossRef]
- Reddy, A.T.; Lakshmi, S.P.; Banno, A.; Jadhav, S.K.; Kadamberi, I.P.; Kim, S.C.; Reddy, R.C. Cigarette Smoke Downregulates Nur77 to Exacerbate Inflammation in Chronic Obstructive Pulmonary Disease (COPD). PLoS ONE 2020, 15, e0229256. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, L.; Ma, T.; Gao, L.; Yang, L.; Wu, M.; Pang, Z.; Wang, X.; Yao, Q.; Xiao, Y.; et al. Bisphenol A Attenuates Testosterone Production in Leydig Cells via the Inhibition of NR1D1 Signaling. Chemosphere 2021, 263, 128020. [Google Scholar] [CrossRef] [PubMed]
- Wieckowski, M.; Ranga, S.; Moison, D.; Messiaen, S.; Abdallah, S.; Granon, S.; Habert, R.; Rouiller-Fabre, V.; Livera, G.; Guerquin, M.J. Unexpected Interacting Effects of Physical (Radiation) and Chemical (Bisphenol a) Treatments on Male Reproductive Functions in Mice. Int. J. Mol. Sci. 2021, 22, 11808. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.K.; Wang, X.; Vom Saal, F.S.; Tillitt, D.E. Transcriptome Analysis of Testis Reveals the Effects of Developmental Exposure to Bisphenol a or 17α-Ethinylestradiol in Medaka (Oryzias Latipes). Aquat. Toxicol. 2020, 225, 105553. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Y.; Li, L.; Xie, L.; Chen, X.; Liu, J.; Li, X.; Jin, L.; Li, X.; Ge, R.S. Bisphenol A Stimulates Differentiation of Rat Stem Leydig Cells in Vivo and in Vitro. Mol. Cell. Endocrinol. 2018, 474, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Whorton, A.E.; Sekulovski, N.; Maclean, J.A.; Hayashi, K. Prenatal Exposure to Bisphenol A, E, and S Induces Transgenerational Effects on Male Reproductive Functions in Mice. Toxicol. Sci. 2019, 172, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Sidorkiewicz, I.; Czerniecki, J.; Jarząbek, K.; Zbucka-Krętowska, M.; Wołczyński, S. Cellular, Transcriptomic and Methylome Effects of Individual and Combined Exposure to BPA, BPF, BPS on Mouse Spermatocyte GC-2 Cell Line. Toxicol. Appl. Pharmacol. 2018, 359, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, W.Y.; Do, J.T.; Park, C.; Song, H. Evaluation of Testicular Toxicity upon Fetal Exposure to Bisphenol A Using an Organ Culture Method. Chemosphere 2021, 270, 129445. [Google Scholar] [CrossRef]
- Bertero, E.; Maack, C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef]

| Bisphenol | Chemical Structure | Usage | Detection Matrices |
|---|---|---|---|
| Bisphenol A |  | Hard plastic items (baby bottles, reusable water bottles, food containers, pitchers, tableware and other storage containers); polycarbonate plastic (eyeglass lenses, CDs, DVDs, computers, appliances, sports safety equipment); epoxy resin linings coat the inside of metal products (foo d cans, bottle tops and water supply pipes). | Air, dust, water, blood, urine, sediment, food, municipal sewage sludge |
| Bisphenol B |  | Food-contact coatings, polymers | Food, dust, sediment, blood, urine |
| Bisphenol F |  | Epoxy resins, polycarbonates (lining of solid/high built systems); thermal receipt | Food, dust, sediment, receipts, urine, PCP, municipal sewage sludge |
| Bisphenol S |  | Wash fastening agent, electroplating solvent, thermal receipt papers | Blood, food, dust, sediment, receipts, urine |
| Bisphenol AP |  | Polycarbonates, epoxy resins, polyarylates, polyethers, polyetherimides, polyphenylene ethers, copolymers | Food, dust, sediment, receipts |
| Bisphenol AF |  | Crosslinker (specialty fluoro-elastomers synthesis) | Food, dust, sediment, municipal sewage sludge |
| Bisphenol C |  | Production of fire-resistant polymers | Receipts |
| Type of Bisphenol | Purity (Manufacturer) | Dose (Route) | Animal Cells | Duration of Exposure | Findings | Author |
|---|---|---|---|---|---|---|
| BPA | Sigma–Aldrich | 0.01 μM– 10 μM | Mouse Leydig Tumor Cells (mLTC) | 1 h of preincubation | 100 μM: ↓ cAMP | [50] |
| BPS | Sigma–Aldrich | (0, 0.5, 5, and 50 μg/L BPS mg/kg body weight/day) of BPS | Weaning Sprague–Dawley rats at postnatal day 22 (PND) | 48 weeks | ↓ LH | [44] |
| BPAF | (99%) Tokyo Chemical Industry | 0, 2, 10, 50 and 200 mg/kg/day | Male Sprague–Dawley rats aged 7 weeks | 14 days | 200 mg/kg: ↓ LHR ↑ LH, FSH | [49] |
| BPA | Sigma–Aldrich | low (2.4 or 10 g/kg/D BPA) and high (100 or 200 mg/kg/d BPA) doses 0, 0.01, 0.1, 1, 10, 100, and 1000 nm BPA | Long–Evans strain of rat (Charles River, Wilmington, MA, USA) Adult Leydig cells obtained from 90-day-old rats with 0, 0.01, 0.1, 1, 10, 100, and 1000 nm BPA for 18 h |
15 days of oral gavage 18 h incubation for adult Leydig cell | ↑ LH | [47] |
| BPA | Sigma–Aldrich (natural exposure from polycarbonate cage) | 10−8 mol/liter | Leydig tumor cells (mLTC-1 cells) | preincubation of mLTC-1 cells for 48 h | ↓ cAMP | [51] |
| BPA | Sigma–Aldrich | 0.1 nM | rat Leydig R2C cells | 24 h | ↑ CREB ↑ cAMP ↑ PKA phosphorylation | [52] |
| BPA | Sigma–Aldrich | 10 mM | CBA/Lac, C57BL/6j, BALB/c and 129S2 mouse strains | 17 h | ↓ LHR ↑ LH | [48] |
| BPA | Sigma–Aldrich | 2 mg/kg/bw (s.c) | Offspring male Sprague Dawley | Perinatal exposure day 10 of gestation until day 7 of lactation | ↑ LH | [60] |
| BPF | J&K Scientific Ltd. | 0.1 and 1 mg/Lin aquarium water | Male Zebrafish | 21 days | ↑ LHR | [39] |
| BPA | Sigma-Aldrich | 5 or 25 mg/kg/bw (oral gavage) | Adult male Wistar rats | 40 days | ↓ LH | [36] |
| BPA | Sigma–Aldrich | 50 mg/kg/bw (oral gavage) | Adult male Wistar rats | 14 days | ↓ LH | [40] |
| BPA | Sigma-Aldrich | 50 mg/kg/bw (oral gavage) | Adult male Wistar rats | 30 days | ↓ LH | [42] |
| BPA | Sigma-Aldrich | 200 mg/kg (oral gavage) | Adult male SD rat | 42 days | ↓ LH | [37] |
| BPA | - | 25 mg/kg/bw (i.p.) | Adult male SD rats | Alternate day for 30 days | ↓ LH | [43] |
| BPA | Gracia chengdu chemical technology co | 200 mg/kg (oral gavage) | Adult male SD rats | 28 days | ↓ LH | [38] |
| BPF | Santa Cruz Biotechnologies | 1, 5, 25, 50, and 100 mg/kg/bw (Oral gavage) | Adult male SD rats | 28 days | ↓ LH | [61] |
| Type of Bisphenol | Purity (Manufacturer) | Dose (Route) | Animal/ Cells | Duration of Exposure | Findings | Author |
|---|---|---|---|---|---|---|
| BPA | Sigma-Aldrich | 2.5 or 25 ug/kg bw (oral gavage) | Long-Evans (LC) | GD 12 through weaning at PND 21 Assessment of Leydig cells differentiation at 21, 35, and 90 days of age of male pups | ↑ EGFR ↑ MAPK | [64] |
| BPA | Sigma-Aldrich | 10−8 to 10−3 M | LC TM3 | 24, 48, or 72 h | ↑ phosphorylation of ERK1/2 and Akt | [65] |
| BPB | (>98%) Tokyo Chemical Industry | 10, 100, and 200 mg/kg/bw (oral gavage) | Male Sprague-Dawley (35 days old) rats | 21 days | ↑ the phosphorylation of AKT1, AKT2, and ERK1/2 at 100 and 200 mg/kg ↓ Testosterone | [67] |
| BPA | Sigma-Aldrich | (0–200 µM) | JEG-3, a human choriocarcinoma cell line | 24 h (expose for 48, 72, 96 h) | ↑ phosphorylated ERK ↓ progesterone | [69] |
| BPA | Sigma-Aldrich | 0.1, 1, 10 nM | Rat testicular Leydig R2C | 30 min | ↑ phosphorylation of ERK1/2 ↑ aromatase activity | [52] |
| BPA | Sigma-Aldrich | 100 μM in 0.1% DMSO 50 mg/kg body weight/week | TM3 LC Male SD rats (i.p) | 24 h 7 days | ↑ phosphorylation of ERK1/2 ↑ phosphorylation of ERK1/2 | [66] |
| BPA | (>98%) Sigma-Aldrich | 0.1 to 200 mg/kg/day | Pregnant SD Male offspring at postnatal day (PND) 3, 21, or 60 | (GD14) to birth (D0) | ↑ ERK1, p-ERK1 (More prominent in Sertoli cell) ↑ Raf1 (more prominent in Leydig cell) | [68] |
| Type of Bisphenol | Purity (Manufacturer) | Dosage (Route) | Animal/ Cells | Duration of Exposure | Findings | Author |
|---|---|---|---|---|---|---|
| BPA | Sigma–Aldrich | 10 pM, 10 nM & 10 µM | D. rerio (Ex vivo) | 5, 10, 15, 20, and 30 min incubation | 5, 10, 15, 20: No effect 30 min: BPA treatment at 10 pM and 10 nM stimulated Ca2+ influx | [71] |
| BPA | Sigma–Aldrich | 0.1 pM, 1 pM & 10 nM | Thirty-day-old male Wistar rats of testes (Ex vivo) | 5 min incubation | BPA induces Ca2+ influx involved PKC activation in rat PLC inhibitors terminate the effect of BPA-induced Ca2+ influx. | [72] |
| BPA TBBPA | Sigma-Aldrich Santa Cruz | 10 nM | immature boar testis (Ex vivo) | 48 h | BPA and TBBPA ↑ (p < 0.01) of Ca2+ concentration | [73] |
| BPA & BADGE.2H2O | Sigma-Aldrich | 1 µM | K28 mouse Leydig tumor cell line | 24 h | BADGE.2H2O and BPA treatment ↑ Nur77 mRNA expression BADGE.2H2O treatment decrease the StAR expression | [74] |
| BPA | Sigma-Aldrich | 1 μM | K28 mouse Leydig tumor cell line | 24 h | BPA specifically induces Nur77 gene expression in a time- and dose-dependent manner No changes of Nur77 after PKC inhibitor. BPA ↑ Nur77 gene promoter activity and its transactivation | [75] |
| Type of Bisphenol | Purity (Manufacturer) | Dose (Route) | Animal/ Cells | Duration of Exposure | Findings | Author |
|---|---|---|---|---|---|---|
| BPA | Sigma-Aldrich | in vivo: 20 mg/kg (i.p.) ex-vivo: 0, 5, 10, 20, 40, and 80 mM of BPA | C57BL/6J male wildtype (WT) mice Primary LCs | 7 days 24 h | ↓ StAR ↓ serum testosterone level | [77] |
| BPA | >99% (Merck) | 10 µM BPA (diluted in 0.1% ethanol) in drinking water | Pregnant mice (testes embryo) | 10.5 days post-coïtum (dpc) to 18.5 dpc. | Combination of radiation and BPA: ↓ gene expression (combination of StAR, Hsd3b1 and Hsd17b3) | [78] |
| BPA | >99% (Sigma-Aldrich) | In vivo: 10, 100 or 1000 pmol/testis (intratesticular injection) In vitro: 1, 10, 100, and 1000 nmol/L | SD (60 days) | In vivo: Post-EDS days 7–28 for 21 days | In vivo: 100 and 1000 pmol/testis of BPA from post-EDS day 14–28: ↑ serum testosterone ↑ Leydig cell-specific gene (Lhr, Star and their protein expression levels. No alteration: LH, FSH & proliferative capacity of Leydig cells in vivo. In vitro: 100 nmol/L stimulated the differentiation of stem Leydig cells by: ↑ testosterone levels up-regulating Leydig cell-specific Lhr gene and proteins but did not affect their proliferation. | [80] |
| BPAF | >99% (Tokyo Chemical Industry) | 0, 0.1, 1, 10, 30, 50, and 70 mM | mLTC-1 cell | 24 h | ↓ StAR after exposure to 70 mM BPAF. No alteration of StAR after treated with 22R-hydroxycholesterol | [49] |
| BPB | >98% (Tokyo Chemical Industry) | 10, 100 and 200 mg/kg/day (Oral gavage) | Male SD | (PND) 35 to PND 56 | No effects of expression of StAR | [67] |
| BPA | NA | BPA (10 μg/L) (tank) | Male medaka fish | from 8 h post-fertilization (as embryos) to adulthood 50 days post fertilization (dpf) | ↑ expression StAR gene pattern | [79] |
| BPA, BPF and BPS | Sigma Aldrich | BPA: 10−8 M BPF: 10−8 M BPS: 10−8 M | Germ cell line | 24, 48, 72 h | ↑ StAR gene expression at 24, 48 and 72 h exposure | [82] |
| BPA, BPE and BPS | Sigma Aldrich | 0.5 or 50 mg/kg/day | CD-1 mice | GD7 to birth | ↑ relative mRNA expression of Star in BPS | [81] |
| BPA | Sigma Aldrich | 10 µM 100 µM | Fetal testis | 5 days | ↑ StAR gene expression in BPA-treated fetal & BPA exposed testes | [83] |
| BPS | 98% (Sigma Aldrich) | 50 μg/L (drinking water) | Male Wistar rats on the post-natal day (PND) 21 | 10 weeks | In silico docking: illustrate BPS binds with StAR protein | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jefferi, N.E.S.; Shamhari, A.‘A.; Hamid, Z.A.; Budin, S.B.; Zulkifly, A.M.Z.; Roslan, F.N.; Taib, I.S. Knowledge Gap in Understanding the Steroidogenic Acute Regulatory Protein Regulation in Steroidogenesis Following Exposure to Bisphenol A and Its Analogues. Biomedicines 2022, 10, 1281. https://doi.org/10.3390/biomedicines10061281
Jefferi NES, Shamhari A‘A, Hamid ZA, Budin SB, Zulkifly AMZ, Roslan FN, Taib IS. Knowledge Gap in Understanding the Steroidogenic Acute Regulatory Protein Regulation in Steroidogenesis Following Exposure to Bisphenol A and Its Analogues. Biomedicines. 2022; 10(6):1281. https://doi.org/10.3390/biomedicines10061281
Chicago/Turabian StyleJefferi, Nur Erysha Sabrina, Asma’ ‘Afifah Shamhari, Zariyantey Abd Hamid, Siti Balkis Budin, Adam Muhammad Zackry Zulkifly, Fatin Norisha Roslan, and Izatus Shima Taib. 2022. "Knowledge Gap in Understanding the Steroidogenic Acute Regulatory Protein Regulation in Steroidogenesis Following Exposure to Bisphenol A and Its Analogues" Biomedicines 10, no. 6: 1281. https://doi.org/10.3390/biomedicines10061281









