Thixotropic Red Microalgae Sulfated Polysaccharide-Peptide Composite Hydrogels as Scaffolds for Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
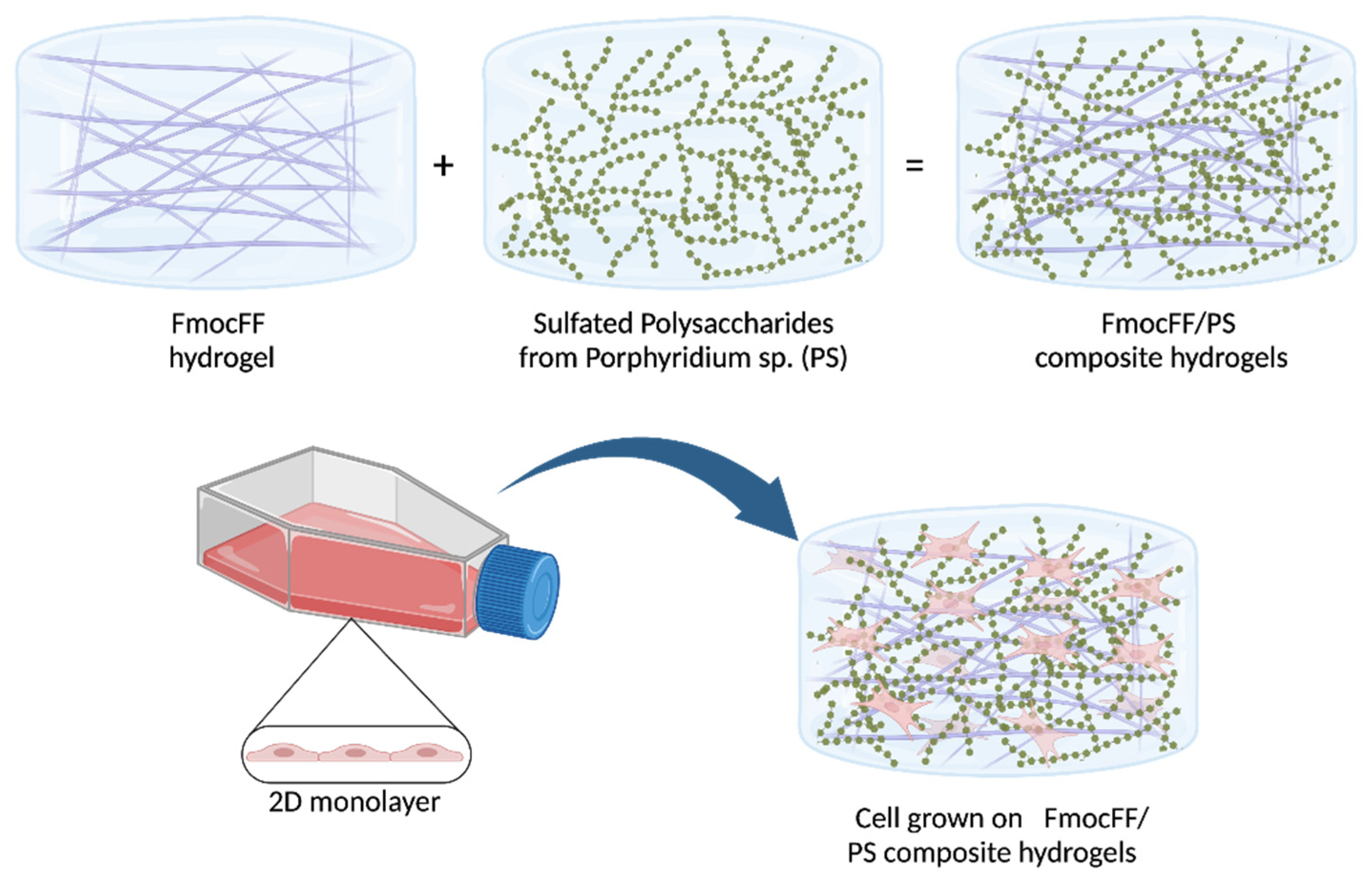
2.2.1. Preparation of FmocFF/PS Composite Hydrogels
2.2.2. Transmission Electron Microscopy (TEM)
2.2.3. Scanning Electron Microscopy (SEM)
2.2.4. Rheological Measurements
2.2.5. Fourier-Transform Infrared (FTIR) Spectroscopy
2.2.6. Swelling
2.2.7. Curcumin Release
2.2.8. Cell Viability Tests
2.2.9. Calcium Concentration Assay
2.2.10. Alizarin Red Mineralization Assay
2.2.11. Statistical Analysis
3. Results
3.1. Preparation and Structural Characterization of the Composite FmocFF/PS Hydrogels
3.2. Curcumin Release from the FmocFF/PS Composite Hydrogels
3.3. Biocompatibility of the FmocFF/PS Composite Hydrogels
3.4. Osteodifferentiation of MC3T3-E1 Preosteoblasts on FmocFF/PS Composite Hydrogels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354, S32–S34. [Google Scholar] [CrossRef]
- Heslop, J.A.; Hammond, T.G.; Santeramo, I.; Tort Piella, A.; Hopp, I.; Zhou, J.; Baty, R.; Graziano, E.I.; Proto Marco, B.; Caron, A.; et al. Concise review: Workshop review: Understanding and assessing the risks of stem cell-based therapies. Stem Cells Transl. Med. 2015, 4, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Zhang, M.; Wu, Z.F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010, 31, 6279–6308. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 42001. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, S.J.; Yang, D.H.; Chun, H.J. Chitosan for Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1077, 475–485. [Google Scholar]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive potential of natural biomaterials: Identification, retention and assessment of biological properties. Signal Transduct. Target Ther. 2021, 6, 122. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; Colliec-Jouault, S. Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [Green Version]
- Manlusoc, J.K.T.; Hsieh, C.-L.; Hsieh, C.-Y.; Salac, E.S.N.; Lee, Y.-T.; Tsai, P.-W. Pharmacologic Application Potentials of Sulfated Polysaccharide from Marine Algae. Polymers 2019, 11, 1163. [Google Scholar] [CrossRef] [Green Version]
- Raposo, M.F.d.J.; De Morais, R.M.S.C.; Bernardo de Morais, A.M.M. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [Green Version]
- Matsui, M.S.; Muizzuddin, N.; Arad, S.; Marenus, K. Sulfated polysaccharides from red microalgae have antiinflammatory properties in vitro and in vivo. Appl. Biochem. Biotechnol. 2003, 104, 13–22. [Google Scholar] [CrossRef]
- Geresh, S.; Arad Malis, S.; Levy-Ontman, O.; Zhang, W.; Tekoah, Y.; Glaser, R. Isolation and characterization of poly- and oligosaccharides from the red microalga Porphyridium sp. Carbohydr. Res. 2009, 344, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Geresh, S.; Arad, S. The extracellular polysaccharides of the red microalgae: Chemistry and rheology. Bioresour. Technol. 1991, 38, 195–201. [Google Scholar] [CrossRef]
- Arad, S.M.; Rapoport, L.; Moshkovich, A.; van Moppes, D.; Karpasas, M.; Golan, R.; Golan, Y. Superior biolubricant from a species of red microalga. Langmuir 2006, 22, 7313–7317. [Google Scholar] [CrossRef] [PubMed]
- Netanel Liberman, G.; Ochbaum, G.; Mejubovsky-Mikhelis, M.; Bitton, R.; Arad Malis, S. Physico-chemical characteristics of the sulfated polysaccharides of the red microalgae Dixoniella grisea and Porphyridium aerugineum. Int. J. Biol. Macromol. 2020, 145, 1171–1179. [Google Scholar] [CrossRef]
- Netanel Liberman, G.; Ochbaum, G.; Bitton, R.; Arad Malis, S. Antimicrobial hydrogels composed of chitosan and sulfated polysaccharides of red microalgae. Polymer 2021, 215, 123353. [Google Scholar] [CrossRef]
- Netanel Liberman, G.; Ochbaum, G.; Malis Arad, S.; Bitton, R. The sulfated polysaccharide from a marine red microalga as a platform for the incorporation of zinc ions. Carbohydr. Polym. 2016, 152, 658–664. [Google Scholar] [CrossRef]
- Eteshola, E.; Karpasas, M.; Arad, S.; Gottlieb, M. Red microalga exopolysaccharides: 2. Study of the rheology, morphology and thermal gelation of aqueous preparations. Acta Polym. 1998, 49, 549–556. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Kyomugasho, C.; Van Looveren, N.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.G.; Van Loey, A.M. Molecular and rheological characterization of different cell wall fractions of Porphyridium cruentum. Carbohydr. Polym. 2018, 195, 542–550. [Google Scholar] [CrossRef]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling Cell Behavior through the Design of Biomaterial Surfaces: A Focus on Surface Modification Techniques. Adv. Mater. Interfaces 2019, 6, 1900572. [Google Scholar] [CrossRef] [Green Version]
- Aviv, M.; Halperin-Sternfeld, M.; Grigoriants, I.; Buzhansky, L.; Mironi-Harpaz, I.; Seliktar, D.; Einav, S.; Nevo, Z.; Adler-Abramovich, L. Improving the Mechanical Rigidity of Hyaluronic Acid by Integration of a Supramolecular Peptide Matrix. ACS Appl. Mater. Interfaces 2018, 10, 41883–41891. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Halperin-Sternfeld, M.; Grinberg, I.; Adler-Abramovich, L. Injectable Alginate-Peptide Composite Hydrogel as a Scaffold for Bone Tissue Regeneration. Nanomaterials 2019, 9, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachmiel, D.; Anconina, I.; Rudnick-Glick, S.; Halperin-Sternfeld, M.; Adler-Abramovich, L.; Sitt, A. Hyaluronic Acid and a Short Peptide Improve the Performance of a PCL Electrospun Fibrous Scaffold Designed for Bone Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 2425. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Çelik, E.; Bayram, C.; Akçapınar, R.; Türk, M.; Denkbaş, E.B. The effect of calcium chloride concentration on alginate/Fmoc-diphenylalanine hydrogel networks. Mater. Sci. Eng. C 2016, 66, 221–229. [Google Scholar] [CrossRef]
- Gong, X.; Branford-White, C.; Tao, L.; Li, S.; Quan, J.; Nie, H.; Zhu, L. Preparation and characterization of a novel sodium alginate incorporated self-assembled Fmoc-FF composite hydrogel. Mater. Sci. Eng. C 2016, 58, 478–486. [Google Scholar] [CrossRef]
- Cohen, E.; Arad Malis, S. A closed system for outdoor cultivation of Porphyridium. Biomass 1989, 18, 59–67. [Google Scholar] [CrossRef]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, P.; Ghosh, M.; Schnaider, L.; Adadi, N.; Ji, W.; Bychenko, D.; Dvir, T.; Adler-Abramovich, L.; Gazit, E. Composite of Peptide-Supramolecular Polymer and Covalent Polymer Comprises a New Multifunctional, Bio-Inspired Soft Material. Macromol. Rapid Commun. 2019, 40, 1900175. [Google Scholar] [CrossRef]
- Di Foggia, M.; Taddei, P.; Torreggiani, A.; Dettin, M.; Tinti, A. Self-Assembling Peptides for Biomedical Applications: IR and Raman Spectroscopies for the Study of Secondary Structure. Proteom. Res. J. 2011, 2, 231. [Google Scholar]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Myung, D.; Waters, D.; Wiseman, M.; Duhamel, P.E.; Noolandi, J.; Ta, C.N.; Frank, C.W. Progress in the development of interpenetrating polymer network hydrogels. Polym. Adv. Technol. 2008, 19, 647–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.L.; Lin, S.P.; Nelli, S.R.; Zhan, F.K.; Cheng, H.; Lai, T.S.; Yeh, M.Y.; Lin, H.C.; Hung, S.C. Self-Assembled Peptide-Based Hydrogels as Scaffolds for Proliferation and Multi-Differentiation of Mesenchymal Stem Cells. Macromol. Biosci. 2017, 17, 1600192. [Google Scholar] [CrossRef]
- Alakpa, E.V.; Jayawarna, V.; Lampel, A.; Burgess, K.V.; West, C.C.; Bakker, S.C.J.; Roy, S.; Javid, N.; Fleming, S.; Lamprou, D.A.; et al. Tunable Supramolecular Hydrogels for Selection of Lineage-Guiding Metabolites in Stem Cell Cultures. Chem 2016, 1, 298–319. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, P.; Roy, B.; Bairi, P.; Nandi, A.K. Improved mechanical and photophysical properties of chitosan incorporated folic acid gel possessing the characteristics of dye and metal ion absorption. J. Mater. Chem. 2012, 22, 20291–20298. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, S.-h. Synthesis and Biomedical Applications of Self-healing Hydrogels. Front. Chem. 2018, 6, 449. [Google Scholar] [CrossRef] [Green Version]
- Phadke, A.; Zhang, C.; Arman, B.; Hsu, C.-C.; Mashelkar, R.A.; Lele, A.K.; Tauber, M.J.; Arya, G.; Varghese, S. Rapid self-healing hydrogels. Proc. Natl. Acad. Sci. USA 2012, 109, 4383–4388. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halperin-Sternfeld, M.; Netanel Liberman, G.; Kannan, R.; Netti, F.; Ma, P.X.; Arad, S.M.; Adler-Abramovich, L. Thixotropic Red Microalgae Sulfated Polysaccharide-Peptide Composite Hydrogels as Scaffolds for Tissue Engineering. Biomedicines 2022, 10, 1388. https://doi.org/10.3390/biomedicines10061388
Halperin-Sternfeld M, Netanel Liberman G, Kannan R, Netti F, Ma PX, Arad SM, Adler-Abramovich L. Thixotropic Red Microalgae Sulfated Polysaccharide-Peptide Composite Hydrogels as Scaffolds for Tissue Engineering. Biomedicines. 2022; 10(6):1388. https://doi.org/10.3390/biomedicines10061388
Chicago/Turabian StyleHalperin-Sternfeld, Michal, Gal Netanel Liberman, Raha Kannan, Francesca Netti, Peter X. Ma, Shoshana Malis Arad, and Lihi Adler-Abramovich. 2022. "Thixotropic Red Microalgae Sulfated Polysaccharide-Peptide Composite Hydrogels as Scaffolds for Tissue Engineering" Biomedicines 10, no. 6: 1388. https://doi.org/10.3390/biomedicines10061388






_Arad.jpg)

