Viability and Functionality of Neonatal Porcine Islet-like Cell Clusters Bioprinted in Alginate-Based Bioinks
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of NICC
2.2. Preparation and Bioprinting of the Bioinks
2.3. Characterisation of Cell-Laden Scaffolds
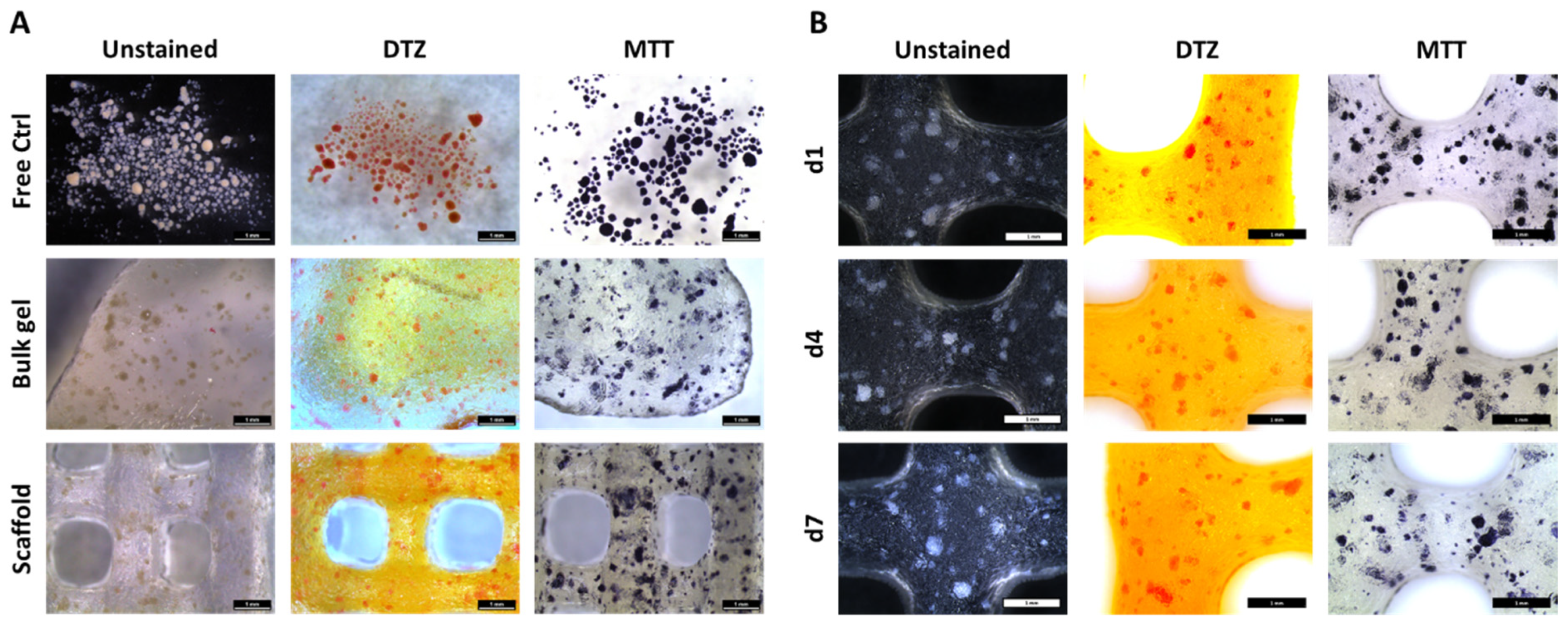
2.3.1. MTT and DTZ Staining
2.3.2. Live/Dead Staining
2.3.3. Immunofluorescence Staining
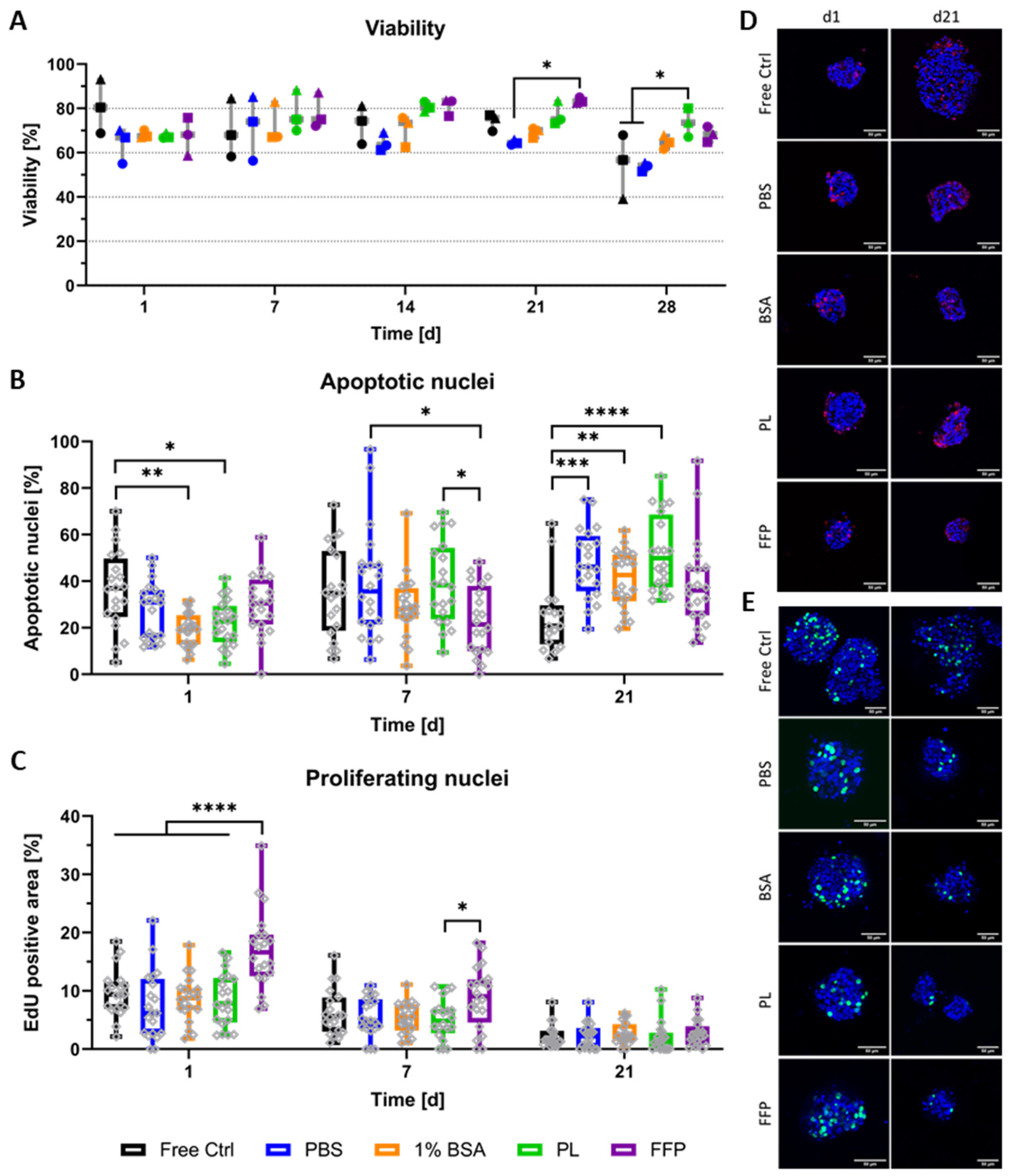
2.3.4. Staining for Apoptotic and Proliferating Nuclei
2.3.5. Quantification of DNA
2.3.6. Functional Analysis of Islets: Glucose Stimulated Insulin Release (GSIR)
2.4. Statistics
3. Results
3.1. Distribution and Viability of NICC Bioprinted in algMC
3.2. Distribution, Viability, and Proliferation of NICC Bioprinted in Supplemented algMC
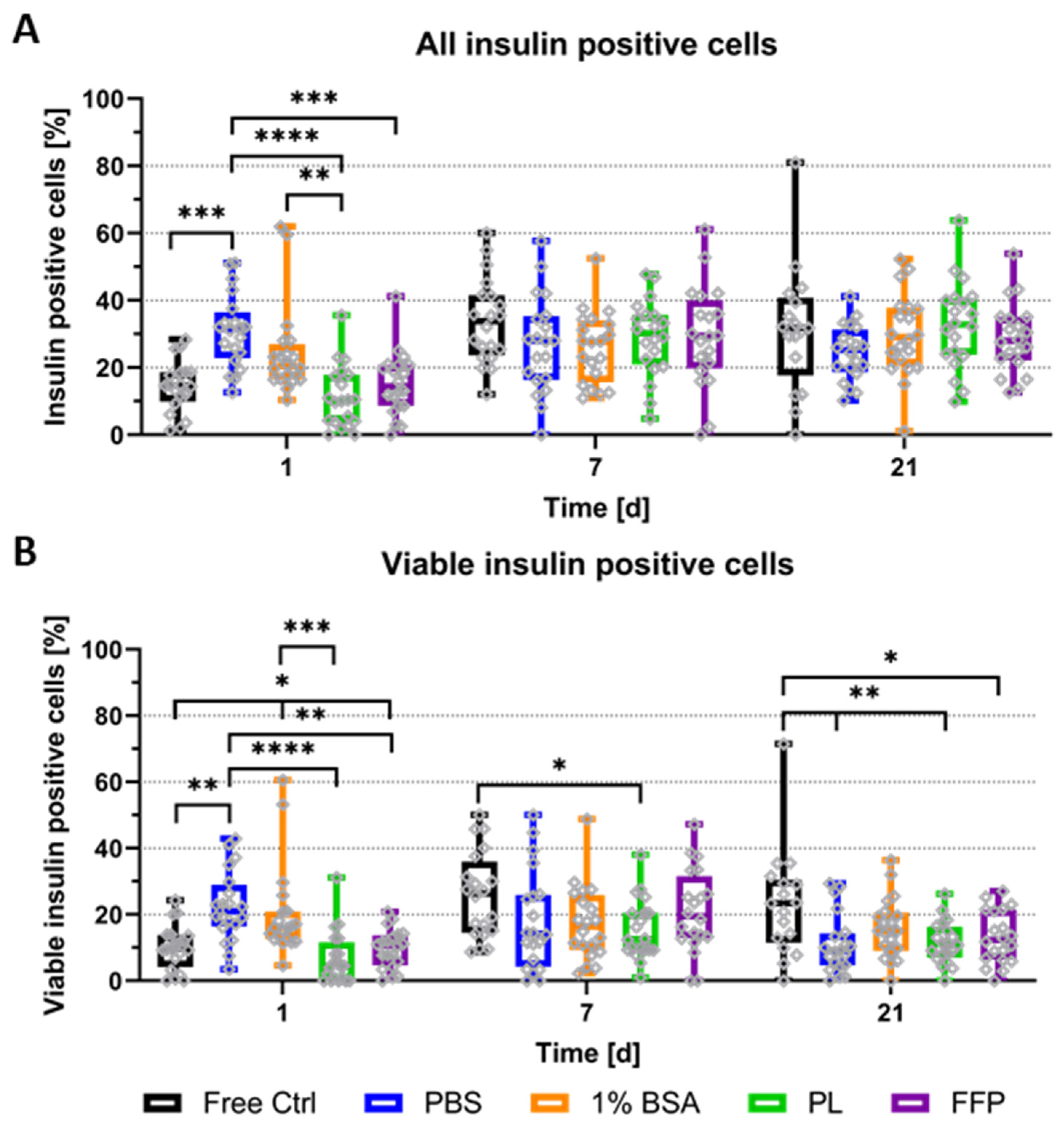
3.3. Functionality of Bioprinted NICC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foster, N.C.; Beck, R.W.; Miller, K.M.; Clements, M.A.; Rickels, M.R.; DiMeglio, L.A.; Maahs, D.M.; Tamborlane, W.V.; Bergenstal, R.; Smith, E.; et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol. Ther. 2019, 21, 66–72. [Google Scholar] [CrossRef] [PubMed]
- DiMeglio, A.L.; Evans-Molina, C.; Oram, R. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Livingstone, S.J.; Levin, D.; Looker, H.C.; Lindsay, R.; Wild, S.H.; Joss, N.; Leese, G.; Leslie, P.; McCrimmon, R.; Metcalfe, W.; et al. Estimated Life Expectancy in a Scottish Cohort with Type 1 Diabetes, 2008-2010. JAMA J. Am. Med. Assoc. 2015, 313, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.J.; Pokrywczynska, M.; Ricordi, A.M.J.S.M.P.C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 2016, 13, 268–277. [Google Scholar] [CrossRef]
- van der Windt, D.J.; Bottino, R.; Casu, A.; Campanile, N.; Cooper, D.K. Rapid loss of intraportally transplanted islets: An overview of pathophysiology and preventive strategies. Xenotransplantation 2007, 14, 288–297. [Google Scholar] [CrossRef]
- Pepper, A.R.; Bruni, A.; Shapiro, A.J. Clinical islet transplantation: Is the future finally now? Curr. Opin. Organ Transplant. 2018, 23, 428–439. [Google Scholar] [CrossRef]
- Emamaullee, J.A.; Shapiro, A.M.J.; Rajotte, R.V.; Korbutt, G.; Elliott, J.F. Neonatal Porcine Islets Exhibit Natural Resistance to Hypoxia-Induced Apoptosis. Transplantation 2006, 82, 945–952. [Google Scholar] [CrossRef]
- Welsch, C.A.; Rust, W.L.; Csete, M. Concise Review: Lessons Learned from Islet Transplant Clinical Trials in Developing Stem Cell Therapies for Type 1 Diabetes. Stem Cells Transl. Med. 2019, 8, 209–214. [Google Scholar] [CrossRef]
- Ludwig, B.; Ludwig, S.; Steffen, A.; Saeger, H.-D.; Bornstein, S.R. Islet Versus Pancreas Transplantation in Type 1 Diabetes: Competitive or Complementary? Curr. Diabetes Rep. 2010, 10, 506–511. [Google Scholar] [CrossRef]
- Warshauer, J.T.; Bluestone, J.A.; Anderson, M.S. New Frontiers in the Treatment of Type 1 Diabetes. Cell Metab. 2020, 31, 46–61. [Google Scholar] [CrossRef]
- Klymiuk, N.; Ludwig, B.; Seissler, J.; Reichart, B.; Wolf, E. Current Concepts of Using Pigs as a Source for Beta-Cell Replacement Therapy of Type 1 Diabetes. Curr. Mol. Biol. Rep. 2016, 2, 73–82. [Google Scholar] [CrossRef][Green Version]
- Korbutt, G.S.; Elliott, J.F.; Ao, Z.; Smith, D.K.; Warnock, G.L.; Rajotte, R.V. Large scale isolation, growth, and function of porcine neonatal islet cells. J. Clin. Investig. 1996, 97, 2119–2129. [Google Scholar] [CrossRef]
- Pellegrini, S.; Cantarelli, E.; Sordi, V.; Nano, R.; Piemonti, L. The state of the art of islet transplantation and cell therapy in type 1 diabetes. Geol. Rundsch. 2016, 53, 683–691. [Google Scholar] [CrossRef]
- He, S.; Wang, C.; Du, X.; Chen, Y.; Zhao, J.; Tian, B.; Lu, H.; Zhang, Y.; Liu, J.; Yang, G.; et al. MSCs promote the development and improve the function of neonatal porcine islet grafts. FASEB J. 2018, 32, 3242–3253. [Google Scholar] [CrossRef]
- Köllmer, M.; Appel, A.A.; Somo, S.I.; Brey, E.M. Long-Term Function of Alginate-Encapsulated Islets. Tissue Eng. Part B Rev. 2016, 22, 34–46. [Google Scholar] [CrossRef]
- MacKenzie, D.A.; Hullett, D.A.; Sollinger, H.W. Xenogeneic transplantation of porcine islets: An overview. Transplantation 2003, 76, 887–891. [Google Scholar] [CrossRef]
- Elliott, R.B.; Escobar, L.; Tan, P.L.J.; Muzina, M.; Zwain, S.; Buchanan, C. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation 2007, 14, 157–161. [Google Scholar] [CrossRef]
- Li, X.; Meng, Q.; Zhang, L. Overcoming Immunobiological Barriers Against Porcine Islet Xenografts: What Should Be Done? Pancreas 2019, 48, 299–308. [Google Scholar] [CrossRef]
- Cantarelli, E.; Piemonti, L. Alternative Transplantation Sites for Pancreatic Islet Grafts. Curr. Diabetes Rep. 2011, 11, 364–374. [Google Scholar] [CrossRef]
- Cayabyab, F.; Nih, L.R.; Yoshihara, E. Advances in Pancreatic Islet Transplantation Sites for the Treatment of Diabetes. Front. Endocrinol. 2021, 12, 732431. [Google Scholar] [CrossRef]
- de Vos, P.; Marchetti, P. Encapsulation of pancreatic islets for transplantation in diabetes: The untouchable islets. Trends Mol. Med. 2002, 8, 363–366. [Google Scholar] [CrossRef]
- Scharp, D.W.; Marchetti, P. Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution. Adv. Drug Deliv. Rev. 2014, 67, 35–73. [Google Scholar] [CrossRef]
- Izeia, L.; Eufrasio-Da-Silva, T.; Dolatshahi-Pirouz, A.; Ostrovidov, S.; Paolone, G.; Peppas, N.A.; De Vos, P.; Emerich, D.; Orive, G. Cell-laden alginate hydrogels for the treatment of diabetes. Expert Opin. Drug Deliv. 2020, 17, 1113–1118. [Google Scholar] [CrossRef]
- Dufrane, D.; Goebbels, R.-M.; Saliez, A.; Guiot, Y.; Gianello, P. Six-Month Survival of Microencapsulated Pig Islets and Alginate Biocompatibility in Primates: Proof of Concept. Transplantation 2006, 81, 1345–1353. [Google Scholar] [CrossRef]
- Hwa, A.J.; Weir, G.C. Transplantation of Macroencapsulated Insulin-Producing Cells. Curr. Diabetes Rep. 2018, 18, 50. [Google Scholar] [CrossRef]
- Bochenek, M.A.; Veiseh, O.; Vegas, A.J.; McGarrigle, J.J.; Qi, M.; Marchese, E.; Omami, M.; Doloff, J.C.; Mendoza-Elias, J.; Nourmohammadzadeh, M.; et al. Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat. Biomed. Eng. 2019, 2, 810–821. [Google Scholar] [CrossRef]
- Schütz, K.; Placht, A.-M.; Paul, B.; Brüggemeier, S.; Gelinsky, M.; Lode, A. Three-dimensional plotting of a cell-laden alginate/methylcellulose blend: Towards biofabrication of tissue engineering constructs with clinically relevant dimensions. J. Tissue Eng. Regen. Med. 2017, 11, 1574–1587. [Google Scholar] [CrossRef]
- Shintaku, H.; Okitsu, T.; Kawano, S.; Matsumoto, S.; Suzuki, T.; Kanno, I.; Kotera, H. Effects of fluid dynamic stress on fracturing of cell-aggregated tissue during purification for islets of Langerhans transplantation. J. Phys. D Appl. Phys. 2008, 41, 115507. [Google Scholar] [CrossRef]
- Silva, P.N.; Green, B.J.; Altamentova, S.M.; Rocheleau, J.V. A microfluidic device designed to induce media flow throughout pancreatic islets while limiting shear-induced damage. Lab Chip 2013, 13, 4374–4384. [Google Scholar] [CrossRef]
- Klak, M.; Kowalska, P.; Dobrzański, T.; Tymicki, G.; Cywoniuk, P.; Gomółka, M.; Kosowska, K.; Bryniarski, T.; Berman, A.; Dobrzyń, A.; et al. Bionic Organs: Shear Forces Reduce Pancreatic Islet and Mammalian Cell Viability during the Process of 3D Bioprinting. Micromachines 2021, 12, 304. [Google Scholar] [CrossRef]
- Duin, S.; Schütz, K.; Ahlfeld, T.; Lehmann, S.; Lode, A.; Ludwig, B.; Gelinsky, M. 3D Bioprinting of Functional Islets of Langerhans in an Alginate/Methylcellulose Hydrogel Blend. Adv. Healthc. Mater. 2019, 8, 1801631. [Google Scholar] [CrossRef] [PubMed]
- Marchioli, G.; van Gurp, L.; Van Krieken, P.P.; Stamatialis, D.; Engelse, M.; Van Blitterswijk, C.A.; Karperien, M.; De Koning, E.; Alblas, J.; Moroni, L.; et al. Fabrication of three-dimensional bioplotted hydrogel scaffolds for islets of Langerhans transplantation. Biofabrication 2015, 7, 025009. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Carter, S.D.; Renes, M.J.; Kim, J.; Rojas-Canales, D.M.; Penko, D.; Angus, C.; Beirne, S.; Drogemuller, C.J.; Yue, Z.; et al. Development of a Coaxial 3D Printing Platform for Biofabrication of Implantable Islet-Containing Constructs. Adv. Healthc. Mater. 2019, 8, e1801181. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shim, I.K.; Hwang, D.G.; Na Lee, Y.; Kim, M.; Kim, H.; Kim, S.-W.; Lee, S.; Kim, S.C.; Cho, D.-W.; et al. 3D cell printing of islet-laden pancreatic tissue-derived extracellular matrix bioink constructs for enhancing pancreatic functions. J. Mater. Chem. B 2019, 7, 1773–1781. [Google Scholar] [CrossRef]
- Schmied, B.; Ulrich, A.; Matsuzaki, H.; Batra, S.; Pour, P.; Ding, X.; Adrian, T.; Ricordi, C.; Moyer, M. Maintenance of human islets in long term culture. Differentiation 2000, 66, 173–180. [Google Scholar] [CrossRef]
- Chen, X.-B. Influence of heme oxygenase-1 gene transfer on the viability and function of rat islets inin vitroculture. World J. Gastroenterol. 2007, 13, 1053–1059. [Google Scholar] [CrossRef]
- Andersson, M.; Axelsson, A.; Zacchi, G. Diffusion of glucose and insulin in a swelling N-isopropylacrylamide gel. Int. J. Pharm. 1997, 157, 199–208. [Google Scholar] [CrossRef]
- Idaszek, J.; Volpi, M.; Paradiso, A.; Quoc, M.N.; Górecka, Ż.; Klak, M.; Tymicki, G.; Berman, A.; Wierzbicki, M.; Jaworski, S.; et al. Alginate-based tissue-specific bioinks for multi-material 3D-bioprinting of pancreatic islets and blood vessels: A step towards vascularized pancreas grafts. Bioprinting 2021, 24, e00163. [Google Scholar] [CrossRef]
- Schneider, S.; Feilen, P.; Cramer, H.; Hillgärtner, M.; Brunnenmeier, F.; Zimmermann, H.; Weber, M.M.; Zimmermann, U. Beneficial effects of human serum albumin on stability and functionality of alginate microcapsules fabricated in different ways. J. Microencapsul. 2003, 20, 627–636. [Google Scholar] [CrossRef]
- El-Tahawy, N.; Rifaai, R.A.; Saber, E.A.; Saied, S.R.; Ibrahim, R.A. Effect of Platelet Rich Plasma (PRP) Injection on the Endocrine Pancreas of the Experimentally Induced Diabetes in Male Albino Rats: A Histological and Immunohistochemical Study. J. Diabetes Metab. 2017, 8, 2–9. [Google Scholar] [CrossRef]
- Zarin, M.; Karbalaei, N.; Keshtgar, S.; Nemati, M. Platelet-rich plasma improves impaired glucose hemostasis, disrupted insulin secretion, and pancreatic oxidative stress in streptozotocin-induced diabetic rat. Growth Factors 2019, 37, 226–237. [Google Scholar] [CrossRef]
- Kemter, E.; Cohrs, C.M.; Schäfer, M.; Schuster, M.; Steinmeyer, K.; Buerck, L.W.-V.; Wolf, A.; Wuensch, A.; Kurome, M.; Kessler, B.; et al. INS-eGFP transgenic pigs: A novel reporter system for studying maturation, growth and vascularisation of neonatal islet-like cell clusters. Diabetologia 2017, 60, 1152–1156. [Google Scholar] [CrossRef]
- Hodder, E.; Duin, S.; Kilian, D.; Ahlfeld, T.; Seidel, J.; Nachtigall, C.; Bush, P.; Covill, D.; Gelinsky, M.; Lode, A. Investigating the effect of sterilisation methods on the physical properties and cytocompatibility of methyl cellulose used in combination with alginate for 3D-bioplotting of chondrocytes. J. Mater. Sci. Mater. Med. 2019, 30, 10. [Google Scholar] [CrossRef]
- Barkai, U.; Weir, G.C.; Colton, C.K.; Ludwig, B.; Bornstein, S.R.; Brendel, M.D.; Neufeld, T.; Bremer, C.; Leon, A.; Evron, Y.; et al. Enhanced Oxygen Supply Improves Islet Viability in a New Bioartificial Pancreas. Cell Transplant. 2013, 22, 1463–1476. [Google Scholar] [CrossRef]
- Karaoz, E.; Genç, Z.S.; Demircan, P.; Aksoy, A.; Duruksu, G. Protection of rat pancreatic islet function and viability by coculture with rat bone marrow-derived mesenchymal stem cells. Cell Death Dis. 2010, 1, e36. [Google Scholar] [CrossRef]
- Britt, L.D.; Stojeba, P.C.; Scharp, C.R.; Greider, M.H.; Scharp, D.W. Neonatal Pig Pseudo-Islets: A Product of Selective Aggregation. Diabetes 1981, 30, 580–583. [Google Scholar] [CrossRef]
- Mourad, N.I.; Perota, A.; Xhema, D.; Galli, C.; Gianello, P. Transgenic Expression of Glucagon-Like Peptide-1 (GLP-1) and Activated Muscarinic Receptor (M3R) Significantly Improves Pig Islet Secretory Function. Cell Transplant. 2017, 26, 901–911. [Google Scholar] [CrossRef]
- Taymour, R.; Kilian, D.; Ahlfeld, T.; Gelinsky, M.; Lode, A. 3D bioprinting of hepatocytes: Core–shell structured co-cultures with fibroblasts for enhanced functionality. Sci. Rep. 2021, 11, 5130. [Google Scholar] [CrossRef]
- Lode, A.; Krujatz, F.; Brüggemeier, S.; Quade, M.; Schütz, K.; Knaack, S.; Weber, J.; Bley, T.; Gelinsky, M. Green bioprinting: Fabrication of photosynthetic algae-laden hydrogel scaffolds for biotechnological and medical applications. Eng. Life Sci. 2015, 15, 177–183. [Google Scholar] [CrossRef]
- Kilian, D.; Ahlfeld, T.; Akkineni, A.R.; Bernhardt, A.; Gelinsky, M.; Lode, A. 3D Bioprinting of osteochondral tissue substitutes—In vitro-chondrogenesis in multi-layered mineralized constructs. Sci. Rep. 2020, 10, 8277. [Google Scholar] [CrossRef]
- Emmermacher, J.; Spura, D.; Cziommer, J.; Kilian, D.; Wollborn, T.; Fritsching, U.; Steingroewer, J.; Walther, T.; Gelinsky, M.; Lode, A. Engineering considerations on extrusion-based bioprinting: Interactions of material behavior, mechanical forces and cells in the printing needle. Biofabrication 2020, 12, 025022. [Google Scholar] [CrossRef]
- Nielsen, T.B.; Yderstraede, K.B.; Schrøder, H.D.; Holst, J.J.; Brusgaard, K.; Beck-Nielsen, H. Functional and Immunohistochemical Evaluation of Porcine Neonatal Islet-Like Cell Clusters. Cell Transplant. 2003, 12, 13–25. [Google Scholar] [CrossRef]
- Hassouna, T.; Seeberger, K.L.; Salama, B.; Korbutt, G.S. Functional Maturation and In Vitro Differentiation of Neonatal Porcine Islet Grafts. Transplantation 2018, 102, e413–e423. [Google Scholar] [CrossRef]
- Yoon, K.-H.; Quickel, R.R.; Tatarkiewicz, K.; Ulrich, T.R.; Hollister-Lock, J.; Trivedi, N.; Bonner-Weir, S.; Weir, G.C. Differentiation and expansion of beta cell mass in porcine neonatal pancreatic cell clusters transplanted into nude mice. Cell Transplant. 1999, 8, 673–689. [Google Scholar] [CrossRef]
- Luca, G.; Nastruzzi, C.; Calvitti, M.; Becchetti, E.; Baroni, T.; Neri, L.M.; Capitani, S.; Basta, G.; Brunetti, P.; Calafiore, R. Accelerated Functional Maturation of Isolated Neonatal Porcine Cell Clusters: In Vitro and In Vivo Results in NOD Mice. Cell Transplant. 2005, 14, 249–261. [Google Scholar] [CrossRef]
- Park, S.J.; Shin, S.; Koo, O.J.; Moon, J.H.; Jang, G.; Ahn, C.; Lee, B.C.; Yoo, Y.J. Functional improvement of porcine neonatal pancreatic cell clustersviaconformal encapsulation using an air-driven encapsulator. Exp. Mol. Med. 2012, 44, 20–25. [Google Scholar] [CrossRef][Green Version]
- Harb, G.; Korbutt, G.S. Effect of prolonged in vitro exposure to high glucose on neonatal porcine pancreatic islets. J. Endocrinol. 2006, 191, 37–44. [Google Scholar] [CrossRef]
- Kitzmann, J.P.; Law, L.; Shome, A.; Muzina, M.; Elliott, R.B.; Mueller, K.R.; Schuurman, H.-J.; Papas, K.K. Real-time assessment of encapsulated neonatal porcine islets prior to clinical xenotransplantation. Xenotransplantation 2012, 19, 333–336. [Google Scholar] [CrossRef]
- Nemati, M.; Karbalaei, N.; Mokarram, P.; Dehghani, F. Effects of platelet-rich plasma on the pancreatic islet survival and function, islet transplantation outcome and pancreatic pdx1 and insulin gene expression in streptozotocin-induced diabetic rats. Growth Factors 2020, 38, 137–151. [Google Scholar] [CrossRef]
- Schneider, S.; Feilen, P.J.; Brunnenmeier, F.; Minnemann, T.; Zimmermann, H.; Zimmermann, U.; Weber, M.M. Long-Term Graft Function of Adult Rat and Human Islets Encapsulated in Novel Alginate-Based Microcapsules After Transplantation in Immunocompetent Diabetic Mice. Diabetes 2005, 54, 687–693. [Google Scholar] [CrossRef]
- Bertera, S.; Balamurugan, A.N.; Bottino, R.; He, J.; Trucco, M. Increased Yield and Improved Transplantation Outcome of Mouse Islets with Bovine Serum Albumin. J. Transplant. 2012, 2012, 856386. [Google Scholar] [CrossRef] [PubMed]
- El-Haroun, H.; Salama, R.M. Comparative study on the therapeutic effects of bone marrow mesenchymal stem cells versus platelet rich plasma on the pancreas of adult male albino rats with streptozotocin-induced type 1 diabetes mellitus. Folia Morphol. 2022, 81, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Enderami, S.E.; Mortazavi, Y.; Soleimani, M.; Nadri, S.; Biglari, A.; Mansour, R.N. Generation of Insulin-Producing Cells from Human-Induced Pluripotent Stem Cells Using a Stepwise Differentiation Protocol Optimized with Platelet-Rich Plasma. J. Cell. Physiol. 2017, 232, 2878–2886. [Google Scholar] [CrossRef] [PubMed]
- Duruksu, G.; Polat, S.; Kayiş, L.; Gürcan, N.E.; Gacar, G.; Yazir, Y. Improvement of the insulin secretion from beta cells encapsulated in alginate/poly-L- histidine/alginate microbeads by platelet-rich plasma. Turk. J. Biol. 2018, 42, 297–306. [Google Scholar] [CrossRef]
- Zhang, L.; Miao, H.; Wang, D.; Qiu, H.; Zhu, Y.; Yao, X.; Guo, Y.; Wang, Z. Pancreatic extracellular matrix and platelet-rich plasma constructing injectable hydrogel for pancreas tissue engineering. Artif. Organs 2020, 44, e532–e551. [Google Scholar] [CrossRef]
- Cubo, N.; Garcia, M.; del Cañizo, J.F.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2016, 9, 015006. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Cubo-Mateo, N.; Cometta, S.; Guduric, V.; Vater, C.; Bernhardt, A.; Akkineni, A.R.; Lode, A.; Gelinsky, M. A Novel Plasma-Based Bioink Stimulates Cell Proliferation and Differentiation in Bioprinted, Mineralized Constructs. ACS Appl. Mater. Interfaces 2020, 12, 12557–12572. [Google Scholar] [CrossRef]
- Delila, L.; Wu, Y.-W.; Nebie, O.; Widyaningrum, R.; Chou, M.-L.; Devos, D.; Burnouf, T. Extensive characterization of the composition and functional activities of five preparations of human platelet lysates for dedicated clinical uses. Platelets 2021, 32, 259–272. [Google Scholar] [CrossRef]
- Burnouf, P.; Juan, P.; Su, C.; Kuo, Y.; Chou, M.; Su, C.; Tseng, Y.; Lin, C.; Burnouf, T. A novel virally inactivated human platelet lysate preparation rich in TGF-β, EGF and IGF, and depleted of PDGF and VEGF. Biotechnol. Appl. Biochem. 2010, 56, 151–160. [Google Scholar] [CrossRef]
- Ewalenko, P.; Deloof, T.; Peeters, J. Composition of fresh frozen plasma. Crit. Care Med. 1986, 14, 145–146. [Google Scholar] [CrossRef]
- Lemoine, D.; Wauters, F.; Bouchend’Homme, S.; Préat, V. Preparation and characterization of alginate microspheres containing a model antigen. Int. J. Pharm. 1998, 176, 9–19. [Google Scholar] [CrossRef]
- Tanaka, H.; Matsumura, M.; Veliky, I.A. Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnol. Bioeng. 1984, 26, 53–58. [Google Scholar] [CrossRef]
- Sayyar, B.; Dodd, M.; Wen, J.; Ma, S.; Marquez-Curtis, L.; Janowska-Wieczorek, A.; Hortelano, G. Encapsulation of factor IX–engineered mesenchymal stem cells in fibrinogen–alginate microcapsules enhances their viability and transgene secretion. J. Tissue Eng. 2012, 3, 2041731412462018. [Google Scholar] [CrossRef]
- MacGregor, R.R.; Williams, S.J.; Tong, P.Y.; Kover, K.; Moore, W.V.; Stehno-Bittel, L. Small rat islets are superior to large islets in in vitro function and in transplantation outcomes. Am. J. Physiol. Metab. 2006, 290, E771–E779. [Google Scholar] [CrossRef]
- Nielsen, T.B.; Yderstraede, K.B.; Rasmussen, H.J.; Beck-Nielsen, H. Isolation of Neonatal Porcine Islet Tissue and Transplantation into Diabetic Mice. A Methodological Evaluation. Scand. J. Lab. Anim. Sci. 2000, 27, 129–137. [Google Scholar] [CrossRef]
- Omer, A.; Duvivier-Kali, V.F.; Trivedi, N.; Wilmot, K.; Bonner-Weir, S.; Weir, G.C. Survival and Maturation of Microencapsulated Porcine Neonatal Pancreatic Cell Clusters Transplanted into Immunocompetent Diabetic Mice. Diabetes 2003, 52, 69–75. [Google Scholar] [CrossRef]
- Trivedi, N.; Keegan, M.; Steil, G.M.; Hollister-Lock, J.; Hasenkamp, W.M.; Colton, C.K.; Bonner-Weir, S.; Weir, G.C. Islets in alginate macrobeads reverse diabetes despite minimal acute insulin secretory responses1. Transplantation 2001, 71, 203–211. [Google Scholar] [CrossRef]
- Cooper, D.K.; Matsumoto, S.; Abalovich, A.; Itoh, T.; Mourad, N.I.; Gianello, P.; Wolf, E.; Cozzi, E. Progress in Clinical Encapsulated Islet Xenotransplantation. Transplantation 2016, 100, 2301–2308. [Google Scholar] [CrossRef]
- Mourad, N.I.; Gianello, P. Long-term culture and in vitro maturation of macroencapsulated adult and neonatal porcine islets. Xenotransplantation 2018, 26, e12461. [Google Scholar] [CrossRef]
- Murakami, M.; Satou, H.; Kimura, T.; Kobayashi, T.; Yamaguchi, A.; Nakagawara, G.; Iwata, H. Effects of micro-encapsulation on morphology and endocrine function of cryopreserved neonatal porcine islet-like cell clusters. Transplantation 2000, 70, 1143–1148. [Google Scholar] [CrossRef]
- Tatarkiewicz, K.; Garcia, M.; Lopez-Avalos, M.; Bonner-Weir, S.; Weir, G.C. Porcine neonatal pancreatic cell clusters in tissue culture: Benefits of serum and immobilization in alginate hydrogel1. Transplantation 2001, 71, 1518–1526. [Google Scholar] [CrossRef]
- Mouré, A.; Bekir, S.; Bacou, E.; Pruvost, Q.; Haurogné, K.; Allard, M.; De Beaurepaire, L.; Bosch, S.; Riochet, D.; Gauthier, O.; et al. Optimization of an O2-balanced bioartificial pancreas for type 1 diabetes using statistical design of experiment. Sci. Rep. 2022, 12, 4681. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Nociti, F.H.; Stefani, C.M.; Machado, M.A.N.; Sallum, E.A.; Toledo, S.; Sallum, A.W. Histometric Evaluation of Bone Regeneration Around Immediate Implants Partially in Contact with Bone. Implant Dent. 2000, 9, 321–325. [Google Scholar] [CrossRef]
- Schaschkow, A.; Sigrist, S.; Mura, C.; Barthes, J.; Vrana, N.E.; Czuba, E.; Lemaire, F.; Neidl, R.; Dissaux, C.; Lejay, A.; et al. Glycaemic control in diabetic rats treated with islet transplantation using plasma combined with hydroxypropylmethyl cellulose hydrogel. Acta Biomater. 2020, 102, 259–272. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duin, S.; Bhandarkar, S.; Lehmann, S.; Kemter, E.; Wolf, E.; Gelinsky, M.; Ludwig, B.; Lode, A. Viability and Functionality of Neonatal Porcine Islet-like Cell Clusters Bioprinted in Alginate-Based Bioinks. Biomedicines 2022, 10, 1420. https://doi.org/10.3390/biomedicines10061420
Duin S, Bhandarkar S, Lehmann S, Kemter E, Wolf E, Gelinsky M, Ludwig B, Lode A. Viability and Functionality of Neonatal Porcine Islet-like Cell Clusters Bioprinted in Alginate-Based Bioinks. Biomedicines. 2022; 10(6):1420. https://doi.org/10.3390/biomedicines10061420
Chicago/Turabian StyleDuin, Sarah, Shreya Bhandarkar, Susann Lehmann, Elisabeth Kemter, Eckhard Wolf, Michael Gelinsky, Barbara Ludwig, and Anja Lode. 2022. "Viability and Functionality of Neonatal Porcine Islet-like Cell Clusters Bioprinted in Alginate-Based Bioinks" Biomedicines 10, no. 6: 1420. https://doi.org/10.3390/biomedicines10061420
APA StyleDuin, S., Bhandarkar, S., Lehmann, S., Kemter, E., Wolf, E., Gelinsky, M., Ludwig, B., & Lode, A. (2022). Viability and Functionality of Neonatal Porcine Islet-like Cell Clusters Bioprinted in Alginate-Based Bioinks. Biomedicines, 10(6), 1420. https://doi.org/10.3390/biomedicines10061420







