Ldlr-Deficient Mice with an Atherosclerosis-Resistant Background Develop Severe Hyperglycemia and Type 2 Diabetes on a Western-Type Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Measurements of Plasma Lipids, Glucose and Insulin
2.3. Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
2.4. Statistical Analysis
3. Results
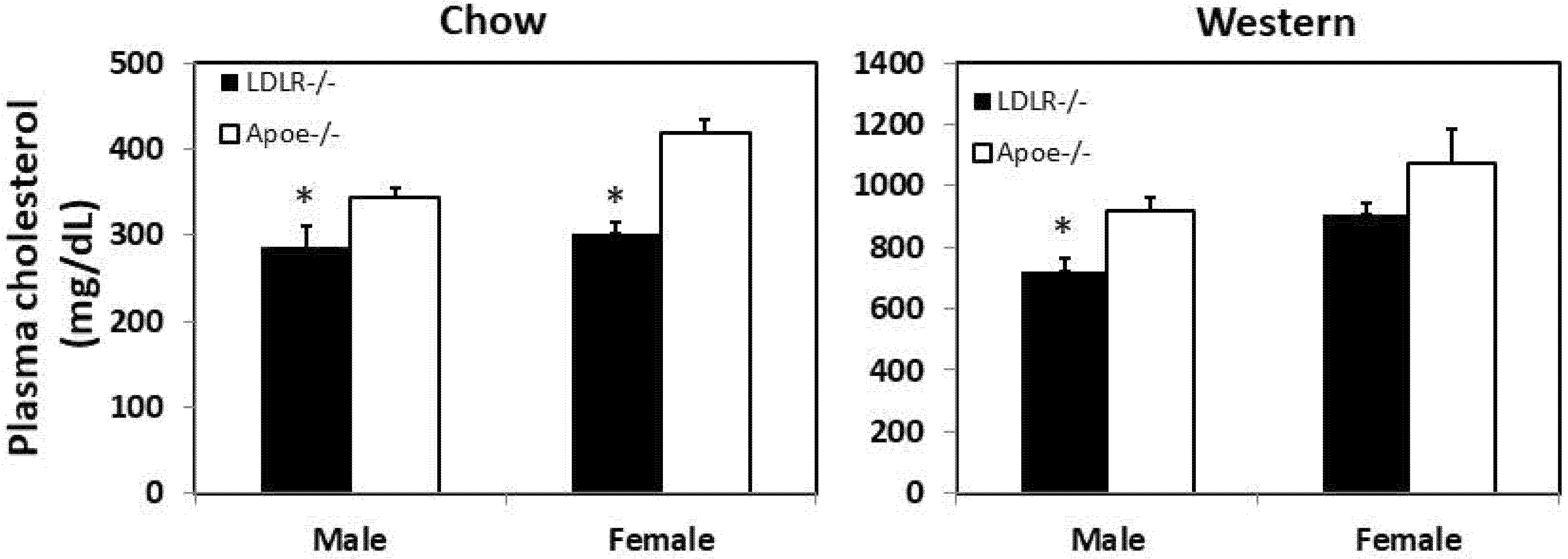
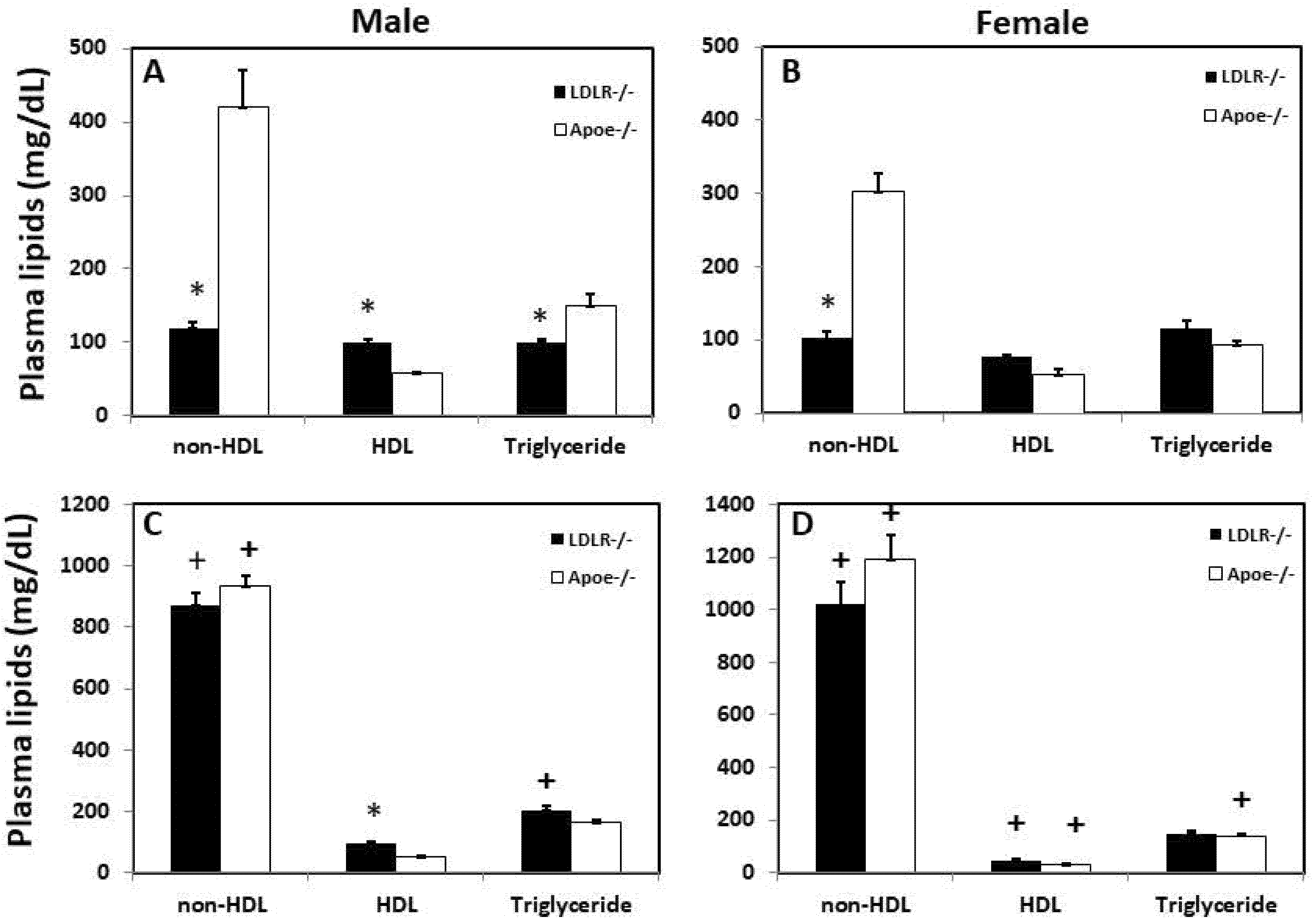
3.1. Fasting Plasma Lipid Levels
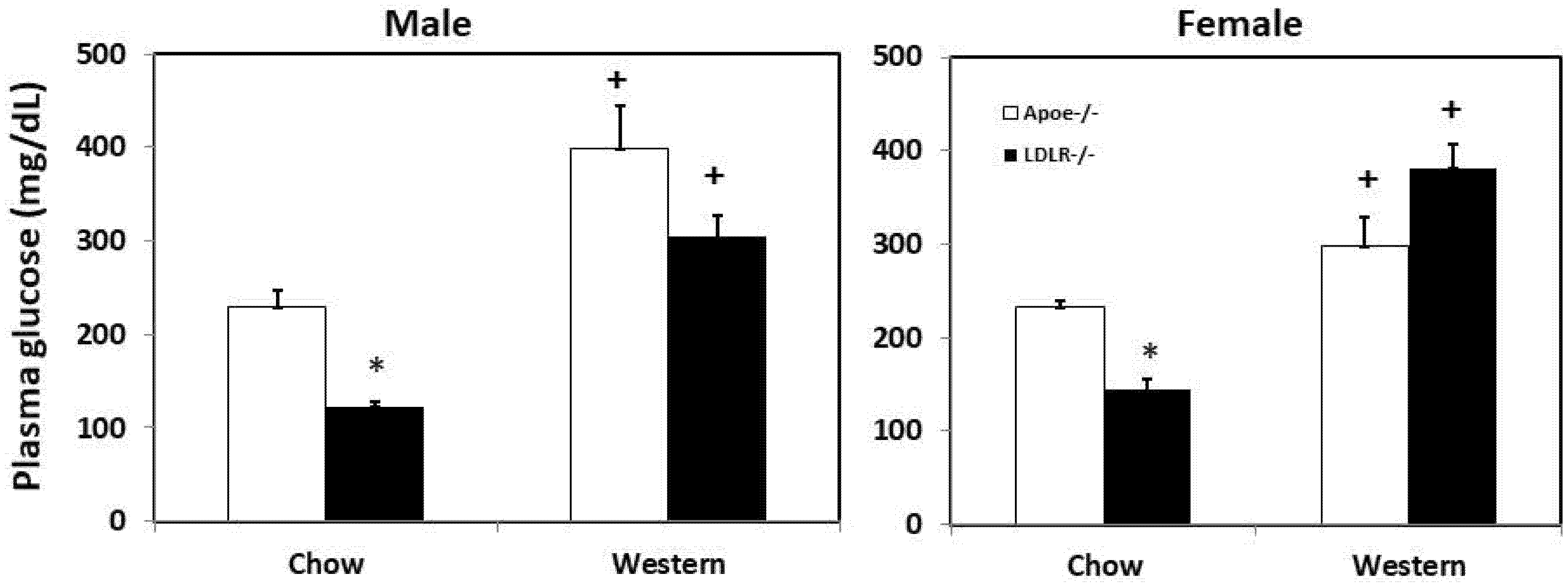
3.2. Fasting Plasma Glucose Levels
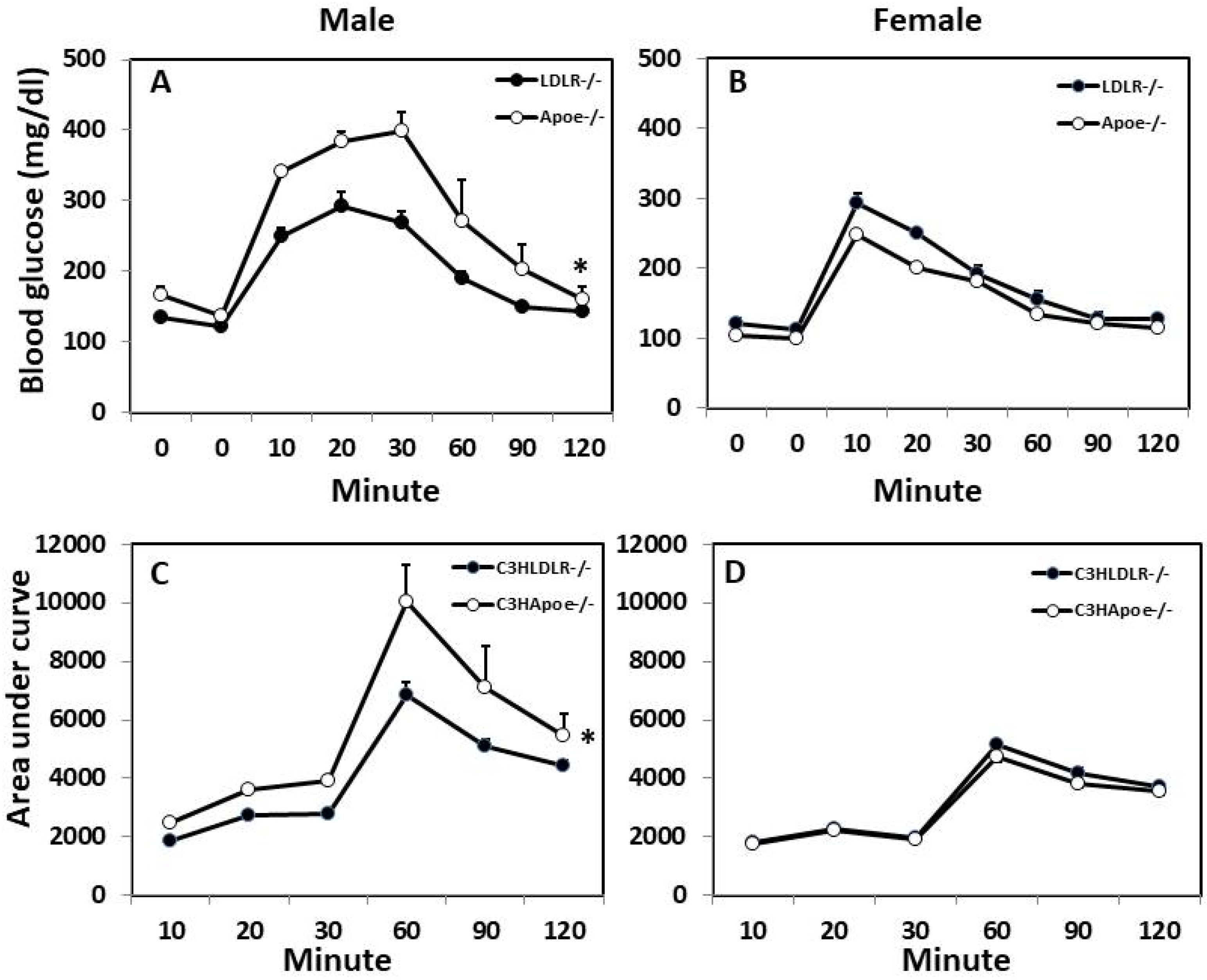
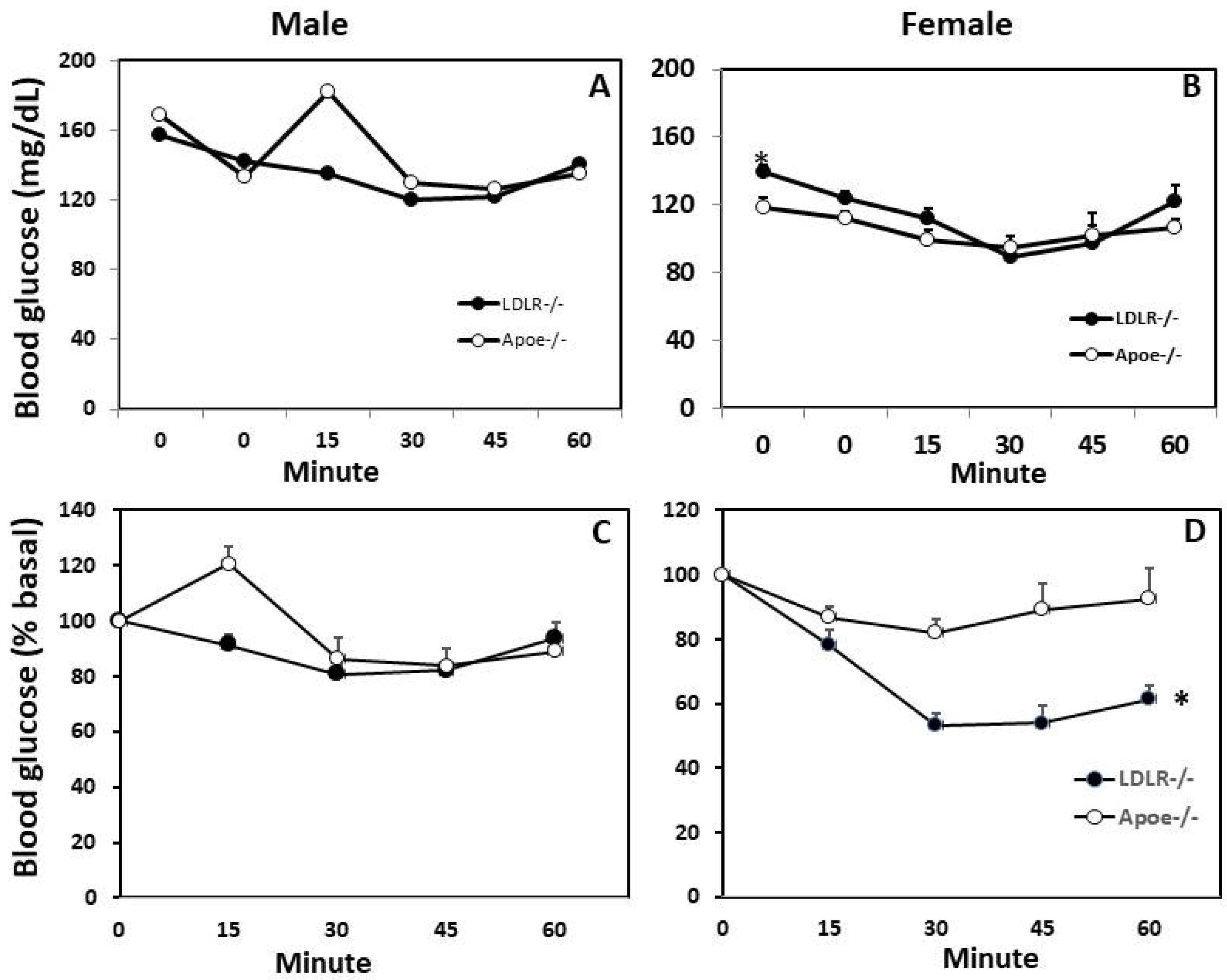
3.3. Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
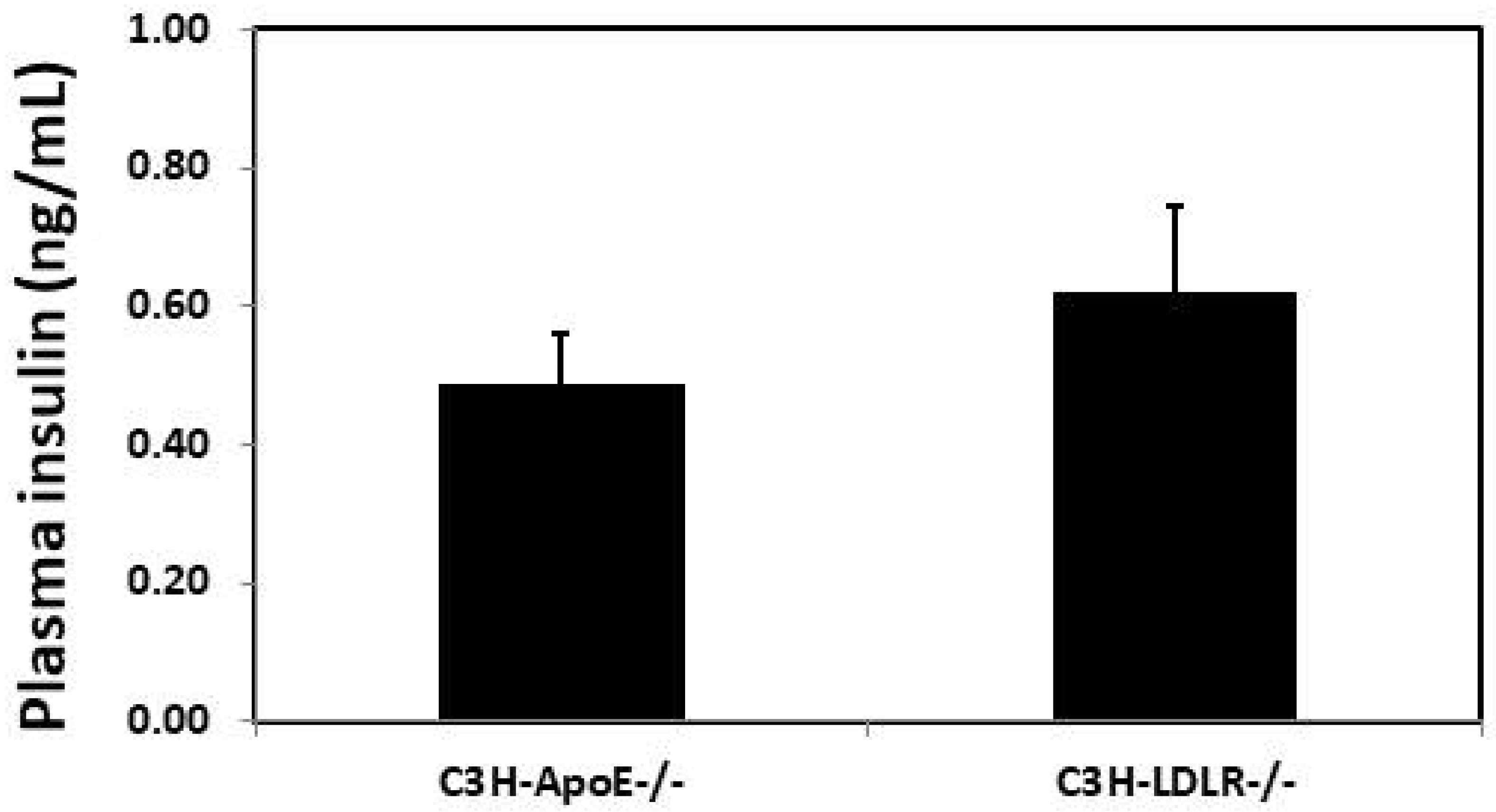
3.4. Plasma insulin Concentration
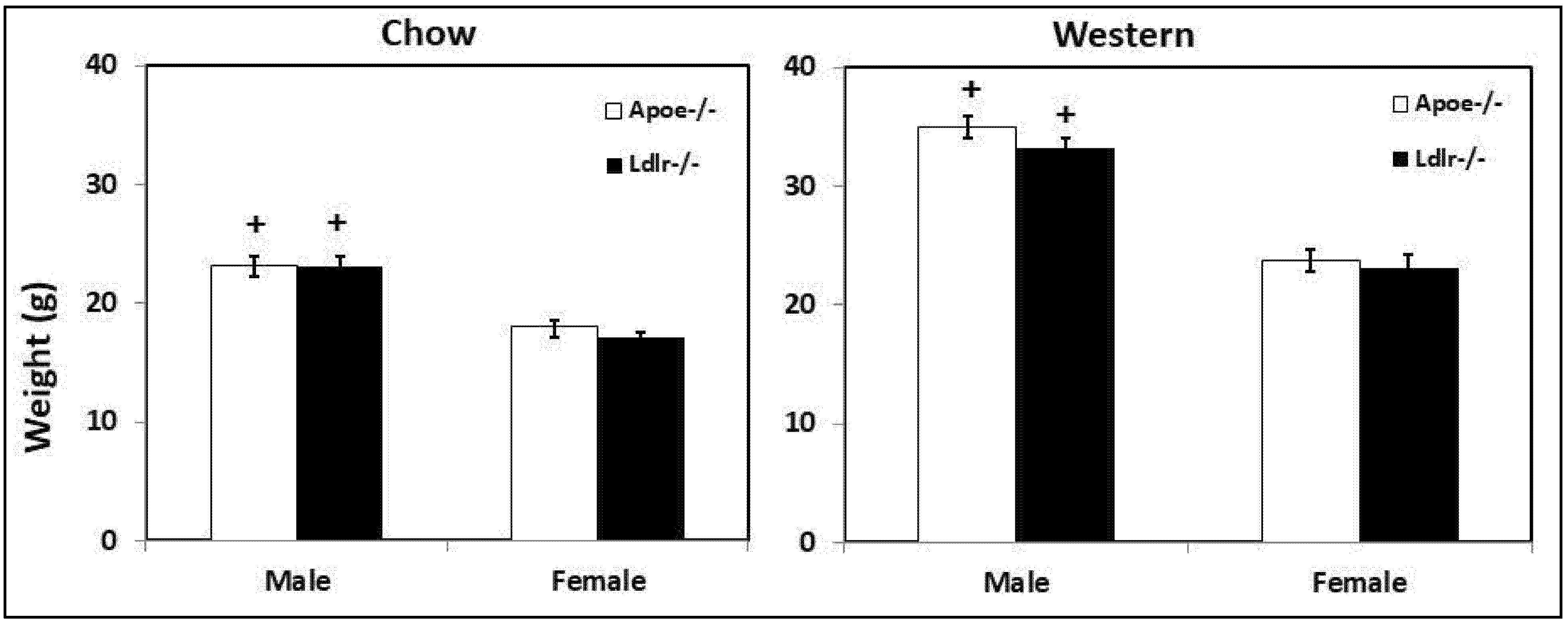
3.5. Body Weight
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update. Circulation 2021, 143, e254–e743. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration; Sarwar, N.; Gao, P.; Seshasai, S.R.K.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. Diabetes Mellitus, Fasting Blood Glucose Concentration, and Risk of Vascular Disease: A Collaborative Meta-Analysis of 102 Prospective Studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Bloomgarden, Z.T. Cardiovascular Disease in Diabetes. Diabetes Care 2008, 31, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Airey, M.; Baxter, H.; Forrester, J.; Kennedy-Martin, T.; Girach, A. Epidemiology of Diabetic Retinopathy and Macular Oedema: A Systematic Review. Eye 2004, 18, 963–983. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; Meigs, J.B.; Sullivan, L.; Fox, C.S.; Nathan, D.M.; RB, S.D. Prediction of Incident Diabetes Mellitus in Middle-Aged Adults: The Framingham Offspring Study. Arch. Intern. Med. 2007, 167, 1068–1074. [Google Scholar] [CrossRef]
- Mykkanen, L.; Kuusisto, J.; Pyorala, K.; Laakso, M. Cardiovascular Disease Risk Factors as Predictors of Type 2 (Non-Insulin-Dependent) Diabetes Mellitus in Elderly Subjects. Diabetologia 1993, 36, 553–559. [Google Scholar] [CrossRef]
- Austin, M.A.; Mykkanen, L.; Kuusisto, J.; Edwards, K.L.; Nelson, C.; Haffner, S.M.; Pyorala, K.; Laakso, M. Prospective Study of Small LDLs as a Risk Factor for Non-Insulin Dependent Diabetes Mellitus in Elderly Men and Women. Circulation 1995, 92, 1770–1778. [Google Scholar] [CrossRef]
- Brouwers, M.C.G.J.; de Graaf, J.; Simons, N.; Meex, S.; ten Doeschate, S.; van Heertum, S.; Heidemann, B.; Luijten, J.; de Boer, D.; Schaper, N.; et al. Incidence of Type 2 Diabetes in Familial Combined Hyperlipidemia. BMJ Open Diabetes Res. Care 2020, 8, e001107. [Google Scholar] [CrossRef]
- Shi, L.J.; Tang, X.; He, J.; Shi, W. Hyperlipidemia Influences the Accuracy of Glucometer-Measured Blood Glucose Concentrations in Genetically Diverse Mice. Am. J. Med. Sci. 2021, 362, 297–302. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Q.; Choi, W.; Li, J. Mapping and Congenic Dissection of Genetic Loci Contributing to Hyperglycemia and Dyslipidemia in Mice. PLoS ONE 2016, 11, e0148462. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Grainger, A.T.; Manichaikul, A.; Farber, E.; Onengut-Gumuscu, S.; Shi, W. Genetic Linkage of Hyperglycemia and Dyslipidemia in an Intercross between BALB/CJ and SM/J Apoe-Deficient Mouse Strains. BMC Genet. 2015, 16, 133. [Google Scholar] [CrossRef] [PubMed]
- Saleheen, D.; Nazir, A.; Khanum, S.; Haider, S.R.; Frossard, P.M. R1615P: A Novel Mutation in ABCA1 Associated with Low Levels of HDL and Type II Diabetes Mellitus. Int. J. Cardiol. 2006, 110, 259–260. [Google Scholar] [CrossRef]
- Albert, J.S.; Yerges-Armstrong, L.M.; Horenstein, R.B.; Pollin, T.I.; Sreenivasan, U.T.; Chai, S.; Blaner, W.S.; Snitker, S.; O’Connell, J.R.; Gong, D.W.; et al. Null Mutation in Hormone-Sensitive Lipase Gene and Risk of Type 2 Diabetes. N. Engl. J. Med. 2014, 370, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ren, Y.; Luo, R.Z.; Mao, X.; Li, X.; Cao, X.; Guan, L.; Chen, X.; Li, J.; Long, Y.; et al. Novel Mutations of the Lipoprotein Lipase Gene Associated with Hypertriglyceridemia in Members of Type 2 Diabetic Pedigrees. J. Lipid Res. 2007, 48, 1681–1688. [Google Scholar] [CrossRef]
- Tan, Y.-X.; Hu, S.-M.; You, Y.-P.; Yang, G.-L.; Wang, W. Replication of Previous Genome-Wide Association Studies of HKDC1, BACE2, SLC16A11 and TMEM163 SNPs in a Gestational Diabetes Mellitus Case-Control Sample from Han Chinese Population. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 983–989. [Google Scholar] [CrossRef]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.M.; Seshasai, S.R.K.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W.; et al. Statins and Risk of Incident Diabetes: A Collaborative Meta-Analysis of Randomised Statin Trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef]
- Swerdlow, D.I.; Preiss, D.; Kuchenbaecker, K.B.; Holmes, M.V.; Engmann, J.E.L.; Shah, T.; Sofat, R.; Stender, S.; Johnson, P.C.D.; Scott, R.A.; et al. HMG-Coenzyme A Reductase Inhibition, Type 2 Diabetes, and Bodyweight: Evidence from Genetic Analysis and Randomised Trials. Lancet 2015, 385, 351–361. [Google Scholar] [CrossRef]
- De Lsf, C.; Am, C.; Ac, S. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors and Incident Type 2 Diabetes: A Systematic Review and Meta-Analysis With Over 96,000 Patient-Years. Diabetes Care 2017, 41, 364–367. [Google Scholar] [CrossRef]
- Climent, E.; Pérez-Calahorra, S.; Marco-Benedí, V.; Plana, N.; Sánchez, R.; Ros, E.; Ascaso, J.F.; Puzo, J.; Almagro, F.; Lahoz, C.; et al. Effect of LDL Cholesterol, Statins and Presence of Mutations on the Prevalence of Type 2 Diabetes in Heterozygous Familial Hypercholesterolemia. Sci. Rep. 2017, 7, 5596. [Google Scholar] [CrossRef]
- Besseling, J.; Kastelein, J.J.P.; Defesche, J.C.; Hutten, B.A.; Hovingh, G.K. Association between Familial Hypercholesterolemia and Prevalence of Type 2 Diabetes Mellitus. JAMA 2015, 313, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, N.J.; Shih, D.M.; Sun, V.Z.; Wang, X.; Lusis, A.J. Determinants of Atherosclerosis Susceptibility in the C3H and C57BL/6 Mouse Model: Evidence for Involvement of Endothelial Cells but Not Blood Cells or Cholesterol Metabolism. Circ. Res. 2000, 86, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, X.; Wang, N.J.; McBride, W.H.; Lusis, A.J. Effect of Macrophage-Derived Apolipoprotein E on Established Atherosclerosis in Apolipoprotein E-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, X.; Wong, J.; Hedrick, C.C.; Wong, H.; Castellani, L.W.; Lusis, A.J. Effect of Macrophage-Derived Apolipoprotein E on Hyperlipidemia and Atherosclerosis of LDLR-Deficient Mice. Biochem. Biophys. Res. Commun. 2004, 317, 223–229. [Google Scholar] [CrossRef]
- Tian, J.; Pei, H.; Sanders, J.M.; Angle, J.F.; Sarembock, I.J.; Matsumoto, A.H.; Helm, G.A.; Shi, W. Hyperlipidemia Is a Major Determinant of Neointimal Formation in LDL Receptor-Deficient Mice. Biochem. Biophys. Res. Commun. 2006, 345, 1004–1009. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Chai, W.; Chen, M.H.; Liu, Z.; Shi, W. Hyperglycemia in Apolipoprotein E-Deficient Mouse Strains with Different Atherosclerosis Susceptibility. Cardiovasc. Diabetol. 2011, 10, 117. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Chen, M.H.; Liu, Z.; Shi, W. Variation in Type 2 Diabetes-Related Phenotypes among Apolipoprotein E-Deficient Mouse Strains. PLoS ONE 2015, 10, e0120935. [Google Scholar] [CrossRef]
- Yuan, Z.; Pei, H.; Roberts, D.J.; Zhang, Z.; Rowlan, J.S.; Matsumoto, A.H.; Shi, W. Quantitative Trait Locus Analysis of Neointimal Formation in an Intercross between C57BL/6 and C3H/HeJ Apolipoprotein E-Deficient Mice. Circ. Genet. 2009, 2, 220–228. [Google Scholar] [CrossRef][Green Version]
- Clee, S.M.; Attie, A.D. The Genetic Landscape of Type 2 Diabetes in Mice. Endocr. Rev. 2007, 28, 48–83. [Google Scholar] [CrossRef]
- Schreyer, S.A.; Vick, C.; Lystig, T.C.; Mystkowski, P.; LeBoeuf, R.C. LDL Receptor but Not Apolipoprotein E Deficiency Increases Diet-Induced Obesity and Diabetes in Mice. Am. J. Physiol. Metab. 2002, 282, E207–E214. [Google Scholar] [CrossRef]
- Phillips, J.W.; Barringhaus, K.G.; Sanders, J.M.; Yang, Z.; Chen, M.; Hesselbacher, S.; Czarnik, A.C.; Ley, K.; Nadler, J.; Sarembock, I.J. Rosiglitazone Reduces the Accelerated Neointima Formation after Arterial Injury in a Mouse Injury Model of Type 2 Diabetes. Circulation 2003, 108, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Reardon, C.A. Diet and Murine Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.T.; Grainger, A.T.; Manichaikul, A.; Shi, W. Genetic Linkage of Oxidative Stress with Cardiometabolic Traits in an Intercross Derived from Hyperlipidemic Mouse Strains. Atherosclerosis 2019, 293, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory Links Between High Fat Diets and Diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef] [PubMed]
- Tudorache, I.F.; Trusca, V.G.; Gafencu, A.V. Apolipoprotein E—A Multifunctional Protein with Implications in Various Pathologies as a Result of Its Structural Features. Comput. Struct. Biotechnol. J. 2017, 15, 359–365. [Google Scholar] [CrossRef]
- Tanaka, T.; Oyama, T.; Sugie, S.; Shimizu, M. Different Susceptibilities between Apoe- and Ldlr-Deficient Mice to Inflammation-Associated Colorectal Carcinogenesis. Int. J. Mol. Sci. 2016, 17, 1806. [Google Scholar] [CrossRef]
- Tonini, C.L.; Campagnaro, B.P.; Louro, L.P.S.; Pereira, T.M.C.; Vasquez, E.C.; Meyrelles, S.S. Effects of Aging and Hypercholesterolemia on Oxidative Stress and DNA Damage in Bone Marrow Mononuclear Cells in Apolipoprotein E-Deficient Mice. Int. J. Mol. Sci. 2013, 14, 3325–3342. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Lin, C.-H.; Huang, T.-H.; Chuang, S.-Y. In Vivo Rodent Models of Type 2 Diabetes and Their Usefulness for Evaluating Flavonoid Bioactivity. Nutrients 2019, 11, 530. [Google Scholar] [CrossRef]
- Chobot, A.; Górowska-Kowolik, K.; Sokołowska, M.; Jarosz-Chobot, P. Obesity and Diabetes—Not Only a Simple Link between Two Epidemics. Diabetes Metab. Res. Rev. 2018, 34, e3042. [Google Scholar] [CrossRef]
- Bennett, B.J.; Davis, R.C.; Civelek, M.; Orozco, L.; Wu, J.; Qi, H.; Pan, C.; Packard, R.R.; Eskin, E.; Yan, M.; et al. Genetic Architecture of Atherosclerosis in Mice: A Systems Genetics Analysis of Common Inbred Strains. PLoS Genet. 2015, 11, e1005711. [Google Scholar] [CrossRef]
- De Brito, G.; Lupinacci, F.C.; Beraldo, F.H.; Santos, T.G.; Roffé, M.; Lopes, M.H.; de Lima, V.C.; Martins, V.R.; Hajj, G.N. Loss of Prion Protein Is Associated with the Development of Insulin Resistance and Obesity. Biochem. J. 2017, 474, 2981–2991. [Google Scholar] [CrossRef] [PubMed]
- Vercalsteren, E.; Vranckx, C.; Frederix, L.; Lox, M.; Lijnen, H.R.; Scroyen, I.; Hemmeryckx, B. Advanced-Age C57BL/6JRj Mice Do Not Develop Obesity upon Western-Type Diet Exposure. Adipocyte 2019, 8, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Rankinen, T.; Sarzynski, M.A.; Ghosh, S.; Bouchard, C. Are There Genetic Paths Common to Obesity, Cardiovascular Disease Outcomes, and Cardiovascular Risk Factors? Circ. Res. 2015, 116, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Oriá, R.B.; Vieira, C.M.G.; Pinkerton, R.C.; de Castro Costa, C.M.; Lopes, M.B.; Hussaini, I.; Shi, W.; Brito, G.A.C.; Lima, A.A.M.; Guerrant, R.L. Apolipoprotein E Knockout Mice Have Accentuated Malnutrition with Mucosal Disruption and Blunted Insulin-like Growth Factor I Responses to Refeeding. Nutr. Res. N. Y. N 2006, 26, 427–435. [Google Scholar] [CrossRef]
- Fitzgibbons, T.P.; Kelly, M.; Kim, J.K.; Czech, M.P. Resistance to Diet Induced Obesity in the Apolipoprotein E Deficient Mouse Is Associated with an Attenuated Transcriptional Response in Visceral Fat. bioRxiv 2018. [Google Scholar] [CrossRef]
- Ngai, Y.F.; Quong, W.L.; Glier, M.B.; Glavas, M.M.; Babich, S.L.; Innis, S.M.; Kieffer, T.J.; Gibson, W.T. Ldlr−/− Mice Display Decreased Susceptibility to Western-Type Diet-Induced Obesity Due to Increased Thermogenesis. Endocrinology 2010, 151, 5226–5236. [Google Scholar] [CrossRef]
- Ishibashi, S.; Perrey, S.; Chen, Z.; Osuga, J.I.; Shimada, M.; Ohashi, K.; Harada, K.; Yazaki, Y.; Yamada, N. Role of the Low Density Lipoprotein (LDL) Receptor Pathway in the Metabolism of Chylomicron Remnants. A Quantitative Study in Knockout Mice Lacking the LDL Receptor, Apolipoprotein E, or Both. J. Biol. Chem. 1996, 271, 22422–22427. [Google Scholar] [CrossRef]
- Twisk, J.; Gillian-Daniel, D.L.; Tebon, A.; Wang, L.; Barrett, P.H.R.; Attie, A.D. The Role of the LDL Receptor in Apolipoprotein B Secretion. J. Clin. Investig. 2000, 105, 521–532. [Google Scholar] [CrossRef]
- Getz, G.S.; Reardon, C.A. Do the Apoe−/− and Ldlr−/−mice Yield the Same Insight on Atherogenesis? Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1734–1741. [Google Scholar] [CrossRef]
- Lytle, K.A.; Jump, D.B. Is Western Diet-Induced Nonalcoholic Steatohepatitis in Ldlr-/- Mice Reversible? PLoS ONE 2016, 11, e0146942. [Google Scholar] [CrossRef]
- Li, J.; Lu, Z.; Wang, Q.; Su, Z.; Bao, Y.; Shi, W. Characterization of Bglu3, a Mouse Fasting Glucose Locus, and Identification of Apcs as an Underlying Candidate Gene. Physiol. Genom. 2012, 44, 345–351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, W.; Chen, M.H.; Shi, W. Influence of Phthalates on Glucose Homeostasis and Atherosclerosis in Hyperlipidemic Mice. BMC Endocr. Disord. 2015, 15, 13. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Li, J.; Bao, K.; Chen, M.-H.; Liu, Z. Ldlr-Deficient Mice with an Atherosclerosis-Resistant Background Develop Severe Hyperglycemia and Type 2 Diabetes on a Western-Type Diet. Biomedicines 2022, 10, 1429. https://doi.org/10.3390/biomedicines10061429
Shi W, Li J, Bao K, Chen M-H, Liu Z. Ldlr-Deficient Mice with an Atherosclerosis-Resistant Background Develop Severe Hyperglycemia and Type 2 Diabetes on a Western-Type Diet. Biomedicines. 2022; 10(6):1429. https://doi.org/10.3390/biomedicines10061429
Chicago/Turabian StyleShi, Weibin, Jing Li, Kelly Bao, Mei-Hua Chen, and Zhenqi Liu. 2022. "Ldlr-Deficient Mice with an Atherosclerosis-Resistant Background Develop Severe Hyperglycemia and Type 2 Diabetes on a Western-Type Diet" Biomedicines 10, no. 6: 1429. https://doi.org/10.3390/biomedicines10061429
APA StyleShi, W., Li, J., Bao, K., Chen, M.-H., & Liu, Z. (2022). Ldlr-Deficient Mice with an Atherosclerosis-Resistant Background Develop Severe Hyperglycemia and Type 2 Diabetes on a Western-Type Diet. Biomedicines, 10(6), 1429. https://doi.org/10.3390/biomedicines10061429






