Anti-Tumorigenic Effect of a Novel Derivative of 2-Hydroxyoleic Acid and the Endocannabinoid Anandamide on Neuroblastoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of 2-Hydroxyoleoyl Ethanolamide (HU-585)
2.2. Cell Culture
2.3. MTT Test
2.4. Colony-Forming Assay (CFA)
2.5. Cell Migration Assay
2.6. Western Blot Assay
2.7. Senescence-Associated β-Galactosidase Activity Assay
2.8. In Vitro Senolytic Studies
2.9. Murine Xenograft Therapeutic Studies
2.10. Statistical Analysis
3. Results
3.1. Structural Modification of HU-600
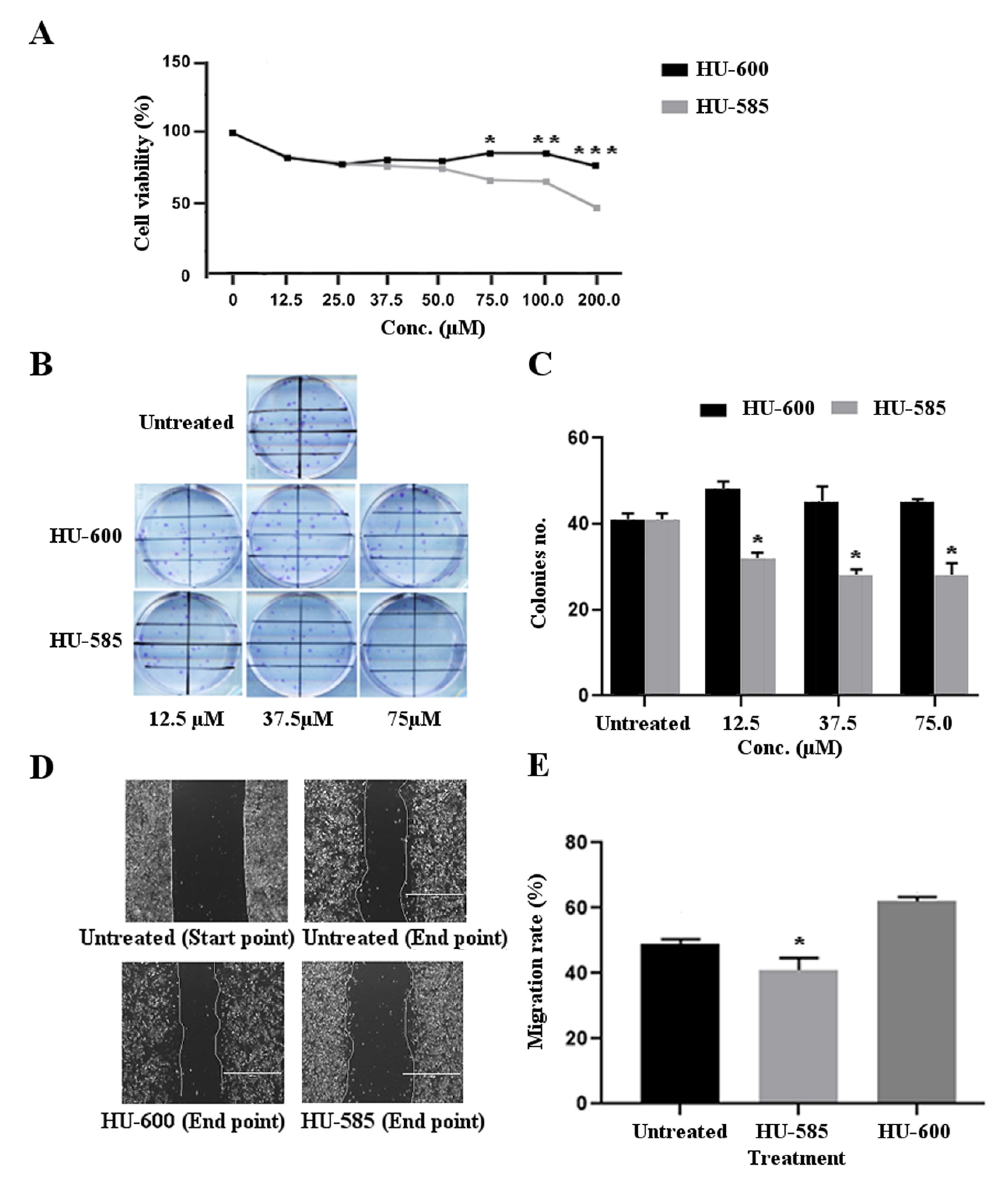
3.2. HU-585 Induces Cell Growth Inhibition, Reduced Colony Formation, and Reduced Migration in the Neuroblastoma Cell Line SK-N-SH In Vitro
3.3. Apoptotic Cell Death and Senescence Following HU-585 Treatment in SK-N-SH Cells
3.4. Combined Treatment of HU-585 with Anti Bcl-2 Compounds ABT-263 or ABT-737 Results in Cell Growth Inhibition In Vitro and in Tumor Growth Delay In Vivo
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis. Primers 2016, 2, 16078. [Google Scholar] [CrossRef] [PubMed]
- Whittle, S.B.; Smith, V.; Doherty, E.; Zhao, S.; McCarty, S.; Zage, P.E. Overview and Recent Advances in the Treatment of Neuroblastoma. Expert Rev. Anticancer Ther. 2017, 17, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Speckhart, B.; Antony, R.; Fernandez, K.S. Long-Term Side Effects of High-Risk Neuroblastoma Survivors in a Referral Center in Central Illinois. JCO 2017, 35, 129. [Google Scholar] [CrossRef]
- Hinz, B.; Ramer, R. Cannabinoids as Anticancer Drugs: Current Status of Preclinical Research. Br. J. Cancer 2022, 126, 1–13. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Shahbazi, F.; Grandi, V.; Banerjee, A.; Trant, J.F. Cannabinoids and Cannabinoid Receptors: The Story so Far. iScience 2020, 23, 101301. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Cannabis, Cannabinoid Receptors, and Endocannabinoid System: Yesterday, Today, and Tomorrow. Acta Pharm. Sin. 2019, 40, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Cort, A.; Müller-Sánchez, C.; Espel, E. Anti-Proliferative and Cytotoxic Effect of Cannabidiol on Human Cancer Cell Lines in Presence of Serum. BMC Res. Notes 2020, 13, 389. [Google Scholar] [CrossRef]
- Hosami, F.; Ghadimkhah, M.H.; Salimi, V.; Ghorbanhosseini, S.S.; Tavakoli-Yaraki, M. The Strengths and Limits of Cannabinoids and Their Receptors in Cancer: Insights into the Role of Tumorigenesis-Underlying Mechanisms and Therapeutic Aspects. Biomed. Pharmacother. 2021, 144, 112279. [Google Scholar] [CrossRef]
- Ramer, R.; Schwarz, R.; Hinz, B. Modulation of the Endocannabinoid System as a Potential Anticancer Strategy. Front. Pharmacol. 2019, 10, 430. [Google Scholar] [CrossRef]
- Huang, L.; Ramirez, J.C.; Frampton, G.A.; Golden, L.E.; Quinn, M.A.; Pae, H.Y.; Horvat, D.; Liang, L.; DeMorrow, S. Anandamide Exerts Its Antiproliferative Actions on Cholangiocarcinoma by Activation of the GPR55 Receptor. Lab. Investig. 2011, 91, 1007–1017. [Google Scholar] [CrossRef]
- Pagano, E.; Borrelli, F. Targeting Cannabinoid Receptors in Gastrointestinal Cancers for Therapeutic Uses: Current Status and Future Perspectives. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-S.; Huang, C.-J.; Cheng, H.-H.; Chou, C.-T.; Lee, H.-Y.; Wang, J.-L.; Chen, I.-S.; Liu, S.-I.; Lu, Y.-C.; Chang, H.-T.; et al. Anandamide-Induced Ca2+ Elevation Leading to P38 MAPK Phosphorylation and Subsequent Cell Death via Apoptosis in Human Osteosarcoma Cells. Toxicology 2007, 231, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wu, T.-T.; Jiang, P.-C.; Li, Z.-Q.; Chen, X.-J.; Fu, K.; Wang, W.; Gong, R. Anti-carcinogenic Activity of Anandamide on Human Glioma In Vitro and In Vivo. Mol. Med. Rep. 2016, 13, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- Massalha, W.; Markovits, M.; Pichinuk, E.; Feinstein-Rotkopf, Y.; Tarshish, M.; Mishra, K.; Llado, V.; Weil, M.; Escriba, P.V.; Kakhlon, O. Minerval (2-Hydroxyoleic Acid) Causes Cancer Cell Selective Toxicity by Uncoupling Oxidative Phosphorylation and Compromising Bioenergetic Compensation Capacity. Biosci. Rep. 2019, 39, BSR20181661. [Google Scholar] [CrossRef]
- Terés, S.; Lladó, V.; Higuera, M.; Barceló-Coblijn, G.; Martin, M.L.; Noguera-Salvà, M.A.; Marcilla-Etxenike, A.; García-Verdugo, J.M.; Soriano-Navarro, M.; Saus, C.; et al. 2-Hydroxyoleate, a Nontoxic Membrane Binding Anticancer Drug, Induces Glioma Cell Differentiation and Autophagy. Proc. Natl. Acad. Sci. USA 2012, 109, 8489. [Google Scholar] [CrossRef]
- Torgersen, M.L.; Klokk, T.I.; Kavaliauskiene, S.; Klose, C.; Simons, K.; Skotland, T.; Sandvig, K. The Anti-Tumor Drug 2-Hydroxyoleic Acid (Minerval) Stimulates Signaling and Retrograde Transport. Oncotarget 2016, 7, 86871–86888. [Google Scholar] [CrossRef]
- Martin, M.L.; Barceló-Coblijn, G.; de Almeida, R.F.M.; Noguera-Salvà, M.A.; Terés, S.; Higuera, M.; Liebisch, G.; Schmitz, G.; Busquets, X.; Escribá, P.V. The Role of Membrane Fatty Acid Remodeling in the Antitumor Mechanism of Action of 2-Hydroxyoleic Acid. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 1405–1413. [Google Scholar] [CrossRef]
- Fisher, T.; Golan, H.; Schiby, G.; Prichen, S.; Smoum, R.; Moshe, I.; Peshes-Yaloz, N.; Castiel, A.; Waldman, D.; Gallily, R.; et al. In Vitro and in Vivo Efficacy of Non-Psychoactive Cannabidiol in Neuroblastoma. Curr. Oncol. 2016, 23, 15–22. [Google Scholar] [CrossRef]
- Adam, W.; Lazarus, M.; Schmerder, A.; Humpf, H.-U.; Saha-Möller, C.R.; Schreier, P. Synthesis of Optically Active α-Hydroxy Acids by Kinetic Resolution Through Lipase-Catalyzed Enantioselective Acetylation. Eur. J. Org. Chem. 1998, 1998, 2013–2018. [Google Scholar] [CrossRef]
- Jang, E.-J.; Choi, W.R.; Kim, S.-Y.; Hong, S.-S.; Rhee, I.; Lee, S.-J.; Choi, S.W.; Choi, H.-G.; Lim, S.-J. 2-Hydroxyoleic Acid-Inserted Liposomes as a Multifunctional Carrier of Anticancer Drugs. Drug Deliv. 2017, 24, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Escribá, P.V. Membrane-Lipid Therapy: A Historical Perspective of Membrane-Targeted Therapies—From Lipid Bilayer Structure to the Pathophysiological Regulation of Cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.D.; Vandenberg, C.J.; Scott, C.L.; Wei, A.H.; Cory, S.; Huang, D.C.S.; Roberts, A.W. In Vivo Efficacy of the Bcl-2 Antagonist ABT-737 against Aggressive Myc-Driven Lymphomas. Proc. Natl. Acad. Sci. USA 2008, 105, 17961–17966. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, W.; He, Q.; Wu, Y.; Lu, Z.; Sun, J.; Liu, Z.; Shao, Y.; Wang, A. Oleic Acid Induces Apoptosis and Autophagy in the Treatment of Tongue Squamous Cell Carcinomas. Sci. Rep. 2017, 7, 11277. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Alemany, R.; Casas, J.; Kitajka, K.; Lanier, S.M.; Escriba, P.V. Influence of the Membrane Lipid Structure on Signal Processing via G Protein-Coupled Receptors. Mol. Pharmacol. 2005, 68, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Węder, K.; Mach, M.; Hąc-Wydro, K.; Wydro, P. Studies on the Interactions of Anticancer Drug-Minerval—with Membrane Lipids in Binary and Ternary Langmuir Monolayers. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 2329–2336. [Google Scholar] [CrossRef]
- Piotto, S.; Concilio, S.; Bianchino, E.; Iannelli, P.; López, D.J.; Terés, S.; Ibarguren, M.; Barceló-Coblijn, G.; Martin, M.L.; Guardiola-Serrano, F.; et al. Differential Effect of 2-Hydroxyoleic Acid Enantiomers on Protein (Sphingomyelin Synthase) and Lipid (Membrane) Targets. Biochim. Biophys. Acta 2014, 1838, 1628–1637. [Google Scholar] [CrossRef]
- Ibarguren, M.; López, D.J.; Encinar, J.A.; González-Ros, J.M.; Busquets, X.; Escribá, P.V. Partitioning of Liquid-Ordered/Liquid-Disordered Membrane Microdomains Induced by the Fluidifying Effect of 2-Hydroxylated Fatty Acid Derivatives. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 2553–2563. [Google Scholar] [CrossRef] [PubMed]
- Barceló-Coblijn, G.; Martin, M.L.; de Almeida, R.F.; Noguera-Salvà, M.A.; Marcilla-Etxenike, A.; Guardiola-Serrano, F.; Lüth, A.; Kleuser, B.; Halver, J.E.; Escribá, P.V. Sphingomyelin and Sphingomyelin Synthase (SMS) in the Malignant Transformation of Glioma Cells and in 2-Hydroxyoleic Acid Therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 19569–19574. [Google Scholar] [CrossRef] [PubMed]
- Patsos, H.A.; Greenhough, A.; Hicks, D.J.; Al Kharusi, M.; Collard, T.J.; Lane, J.D.; Paraskeva, C.; Williams, A.C. The Endogenous Cannabinoid, Anandamide, Induces COX-2-Dependent Cell Death in Apoptosis-Resistant Colon Cancer Cells. Int. J. Oncol. 2010, 37, 187–193. [Google Scholar] [CrossRef]
- Orellana-Serradell, O.; Poblete, C.E.; Sanchez, C.; Castellón, E.A.; Gallegos, I.; Huidobro, C.; Llanos, M.N.; Contreras, H.R. Proapoptotic Effect of Endocannabinoids in Prostate Cancer Cells. Oncol. Rep. 2015, 33, 1599–1608. [Google Scholar] [CrossRef]
- Brown, I.; Cascio, M.G.; Wahle, K.W.J.; Smoum, R.; Mechoulam, R.; Ross, R.A.; Pertwee, R.G.; Heys, S.D. Cannabinoid Receptor-Dependent and -Independent Anti-Proliferative Effects of Omega-3 Ethanolamides in Androgen Receptor-Positive and -Negative Prostate Cancer Cell Lines. Carcinogenesis 2010, 31, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid Composition of the Cancer Cell Membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Park, M.J.; Ye, S.-K.; Kim, C.-W.; Kim, Y.-N. Elevated Levels of Cholesterol-Rich Lipid Rafts in Cancer Cells Are Correlated with Apoptosis Sensitivity Induced by Cholesterol-Depleting Agents. Am. J. Pathol. 2006, 168, 1107–1118. [Google Scholar] [CrossRef]
- Guerra, F.S.; da Sampaio, L.; Konig, S.; Bonamino, M.; Rossi, M.I.D.; Costa, M.L.; Fernandes, P.; Mermelstein, C. Membrane Cholesterol Depletion Reduces Breast Tumor Cell Migration by a Mechanism That Involves Non-Canonical Wnt Signaling and IL-10 Secretion. Transl. Med. Commun. 2016, 1, 3. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zhou, D.; Zheng, G. Targeting Anti-Apoptotic BCL-2 Family Proteins for Cancer Treatment. Future Med. Chem. 2020, 12, 563–565. [Google Scholar] [CrossRef]
- Walensky, L.D. BCL-2 in the Crosshairs: Tipping the Balance of Life and Death. Cell Death Differ. 2006, 13, 1339–1350. [Google Scholar] [CrossRef]
- Llado, V.; Gutierrez, A.; Martínez, J.; Casas, J.; Terés, S.; Higuera, M.; Galmés, A.; Saus, C.; Besalduch, J.; Busquets, X.; et al. Minerval Induces Apoptosis in Jurkat and Other Cancer Cells. J. Cell. Mol. Med. 2010, 14, 659–670. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Fairbrother, W.J.; Leverson, J.D.; Souers, A.J. From Basic Apoptosis Discoveries to Advanced Selective BCL-2 Family Inhibitors. Nat. Rev. Drug Discov. 2017, 16, 273–284. [Google Scholar] [CrossRef]
- Edison, N.; Curtz, Y.; Paland, N.; Mamriev, D.; Chorubczyk, N.; Haviv-Reingewertz, T.; Kfir, N.; Morgenstern, D.; Kupervaser, M.; Kagan, J.; et al. Degradation of Bcl-2 by XIAP and ARTS Promotes Apoptosis. Cell Rep. 2017, 21, 442–454. [Google Scholar] [CrossRef]
- Kirsch, D.G.; Doseff, A.; Chau, B.N.; Lim, D.-S.; de Souza-Pinto, N.C.; Hansford, R.; Kastan, M.B.; Lazebnik, Y.A.; Hardwick, J.M. Caspase-3-Dependent Cleavage of Bcl-2 Promotes Release of Cytochrome c. J. Biol. Chem. 1999, 274, 21155–21161. [Google Scholar] [CrossRef] [PubMed]
- Lestini, B.J.; Goldsmith, K.C.; Fluchel, M.N.; Liu, X.; Chen, N.L.; Goyal, B.; Pawel, B.R.; Hogarty, M.D. Mcl1 Downregulation Sensitizes Neuroblastoma to Cytotoxic Chemotherapy and Small Molecule Bcl2-Family Antagonists. Cancer Biol. Ther. 2009, 8, 1587–1595. [Google Scholar] [CrossRef]
- Place, A.E.; Goldsmith, K.; Bourquin, J.-P.; Loh, M.L.; Gore, L.; Morgenstern, D.A.; Sanzgiri, Y.; Hoffman, D.; Zhou, Y.; Ross, J.A.; et al. Accelerating Drug Development in Pediatric Cancer: A Novel Phase I Study Design of Venetoclax in Relapsed/Refractory Malignancies. Future Oncol. 2018, 14, 2115–2129. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; van Deursen, J.M. Senescence and Apoptosis: Dueling or Complementary Cell Fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef]
- Wyld, L.; Bellantuono, I.; Tchkonia, T.; Morgan, J.; Turner, O.; Foss, F.; George, J.; Danson, S.; Kirkland, J.L. Senescence and Cancer: A Review of Clinical Implications of Senescence and Senotherapies. Cancers 2020, 12, 2134. [Google Scholar] [CrossRef]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef]
- Goldman, J.W.; Shi, P.; Reck, M.; Paz-Ares, L.; Koustenis, A.; Hurt, K.C. Treatment Rationale and Study Design for the JUNIPER Study: A Randomized Phase III Study of Abemaciclib with Best Supportive Care Versus Erlotinib with Best Supportive Care in Patients with Stage IV Non-Small-Cell Lung Cancer with a Detectable KRAS Mutation Whose Disease Has Progressed After Platinum-Based Chemotherapy. Clin. Lung Cancer 2016, 17, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Rader, J.; Russell, M.R.; Hart, L.S.; Nakazawa, M.S.; Belcastro, L.T.; Martinez, D.; Li, Y.; Carpenter, E.L.; Attiyeh, E.F.; Diskin, S.J.; et al. Dual CDK4/CDK6 Inhibition Induces Cell-Cycle Arrest and Senescence in Neuroblastoma. Clin. Cancer Res. 2013, 19, 6173–6182. [Google Scholar] [CrossRef]
- Wang, L.; Leite de Oliveira, R.; Wang, C.; Fernandes Neto, J.M.; Mainardi, S.; Evers, B.; Lieftink, C.; Morris, B.; Jochems, F.; Willemsen, L.; et al. High-Throughput Functional Genetic and Compound Screens Identify Targets for Senescence Induction in Cancer. Cell Rep. 2017, 21, 773–783. [Google Scholar] [CrossRef]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Däbritz, J.H.M.; Zhao, Z.; Yu, Y.; Dörr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-Associated Reprogramming Promotes Cancer Stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef]
- Llanos, S.; Serrano, M. Senescence and Cancer: In the Name of Immunosuppression. Cancer Cell 2016, 30, 507–508. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Canino, C.; Mori, F.; Cambria, A.; Diamantini, A.; Germoni, S.; Alessandrini, G.; Borsellino, G.; Galati, R.; Battistini, L.; Blandino, R.; et al. SASP Mediates Chemoresistance and Tumor-Initiating-Activity of Mesothelioma Cells. Oncogene 2012, 31, 3148–3163. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Kauser, K.; Campisi, J.; Beauséjour, C.M. Secretion of Vascular Endothelial Growth Factor by Primary Human Fibroblasts at Senescence. J. Biol. Chem. 2006, 281, 29568–29574. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the P53 Tumor Suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, S.; Coppe, J.-P.; Krtolica, A.; Campisi, J. Stromal-Epithelial Interactions in Aging and Cancer: Senescent Fibroblasts Alter Epithelial Cell Differentiation. J. Cell Sci. 2005, 118, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of Apoptosis by the BCL-2 Protein Family: Implications for Physiology and Therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Paez-Ribes, M.; González-Gualda, E.; Doherty, G.J.; Muñoz-Espín, D. Targeting Senescent Cells in Translational Medicine. EMBO Mol. Med. 2019, 11, e10234. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golan, H.; Mechoulam, R.; Smoum, R.; Cohen-Zada, E.; Pri-Chen, S.; Wiener, S.; Grinberg, I.; Bar-Lev, D.D.; Haj, C.G.; Fisher, T.; et al. Anti-Tumorigenic Effect of a Novel Derivative of 2-Hydroxyoleic Acid and the Endocannabinoid Anandamide on Neuroblastoma Cells. Biomedicines 2022, 10, 1552. https://doi.org/10.3390/biomedicines10071552
Golan H, Mechoulam R, Smoum R, Cohen-Zada E, Pri-Chen S, Wiener S, Grinberg I, Bar-Lev DD, Haj CG, Fisher T, et al. Anti-Tumorigenic Effect of a Novel Derivative of 2-Hydroxyoleic Acid and the Endocannabinoid Anandamide on Neuroblastoma Cells. Biomedicines. 2022; 10(7):1552. https://doi.org/10.3390/biomedicines10071552
Chicago/Turabian StyleGolan, Hana, Raphael Mechoulam, Reem Smoum, Efrat Cohen-Zada, Sara Pri-Chen, Sapir Wiener, Igor Grinberg, Dekel D. Bar-Lev, Christeeneh G. Haj, Tamar Fisher, and et al. 2022. "Anti-Tumorigenic Effect of a Novel Derivative of 2-Hydroxyoleic Acid and the Endocannabinoid Anandamide on Neuroblastoma Cells" Biomedicines 10, no. 7: 1552. https://doi.org/10.3390/biomedicines10071552
APA StyleGolan, H., Mechoulam, R., Smoum, R., Cohen-Zada, E., Pri-Chen, S., Wiener, S., Grinberg, I., Bar-Lev, D. D., Haj, C. G., Fisher, T., & Toren, A. (2022). Anti-Tumorigenic Effect of a Novel Derivative of 2-Hydroxyoleic Acid and the Endocannabinoid Anandamide on Neuroblastoma Cells. Biomedicines, 10(7), 1552. https://doi.org/10.3390/biomedicines10071552





