Genetic Basis of the Epidemiological Features and Clinical Significance of Renal Hypouricemia
Abstract
:1. Introduction
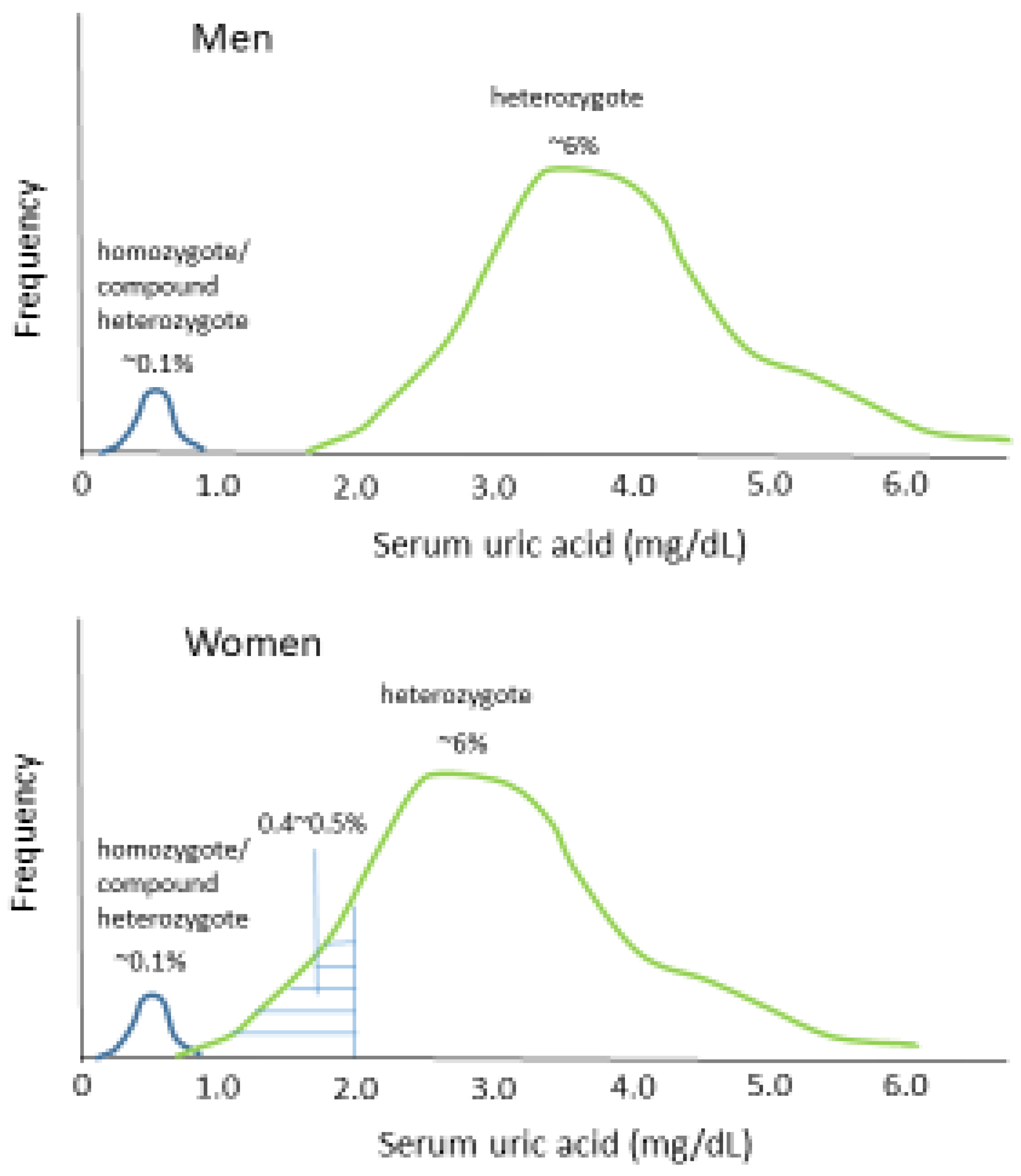
2. Prevalence of Hypouricemia
3. Genetic Basis for the Epidemiological Features of Hypouricemia
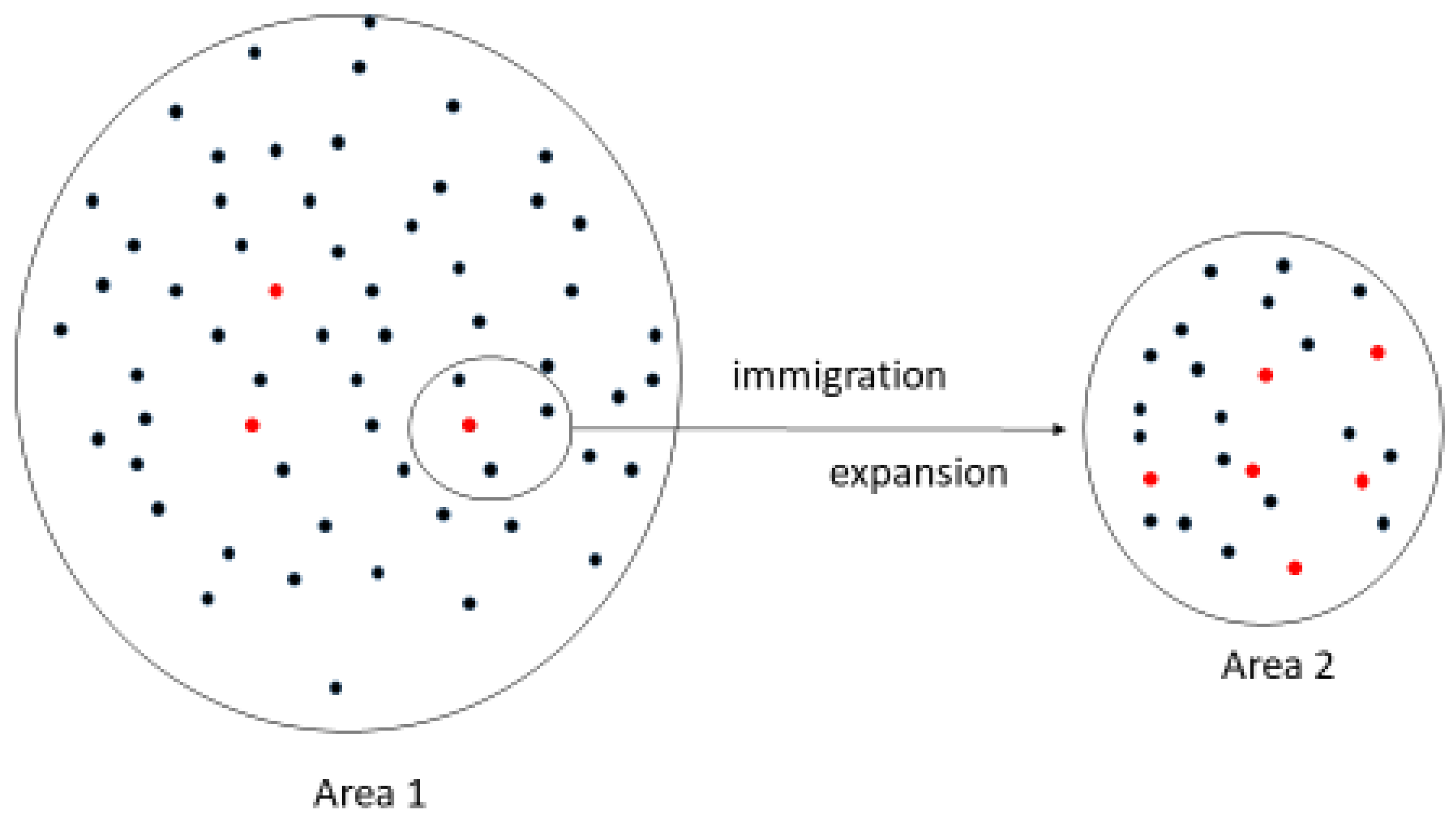
4. Genetic Basis for the Differing Prevalence of Renal Hypouricemia between Different Ethnic Groups
5. Clinical Manifestation of Renal Hypouricemia
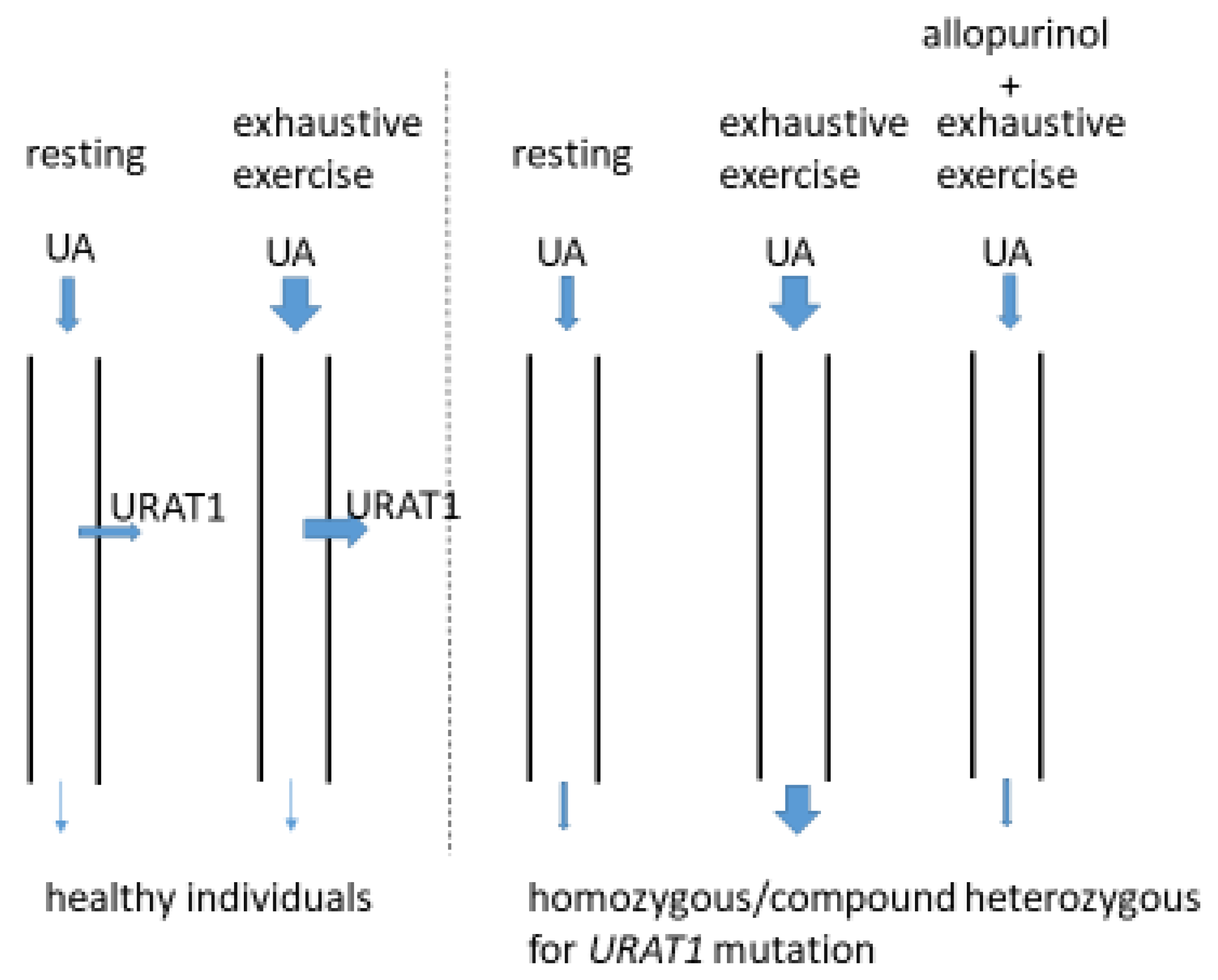
6. Relationships between URAT1 Mutations and Reduced Renal Function
7. Clinical Significance of Heterozygous States for URAT1 Function
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef]
- Kim, S.Y.; Guevara, J.P.; Kim, K.M.; Choi, H.K.; Heitjan, D.F.; Albert, D.A. Hyperuricemia and coronary heart disease: A systematic review and meta-analysis. Arthritis Care Res. 2010, 62, 170–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waring, W.S.; Webb, D.J.; Maxwell, S.R. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J. Cardiovasc. Pharmacol. 2001, 38, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Evans, J.E.; Rock, K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 2003, 425, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Matsuo, H.; Ohtahara, A.; Ogino, K.; Hakoda, M.; Hamada, T.; Hosoyamada, M.; Yamaguchi, S.; Hisatome, I.; Ichida, K.; et al. Clinical practice guideline for renal hypouricemia (1st edition). Hum. Cell 2019, 32, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef]
- Matsuo, H.; Chiba, T.; Nagamori, S.; Nakayama, A.; Domoto, H.; Phetdee, K.; Wiriyasermkul, P.; KikuchI, Y.; Oda, T.; Nishiyama, J.; et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am. J. Hum. Genet. 2008, 83, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Wakasugi, M.; Kazama, J.J.; Narita, I.; Konta, T.; Fujimoto, S.; Iseki, K.; Moriyama, T.; Yamagata, K.; Tsuruya, K.; Asahi, K.; et al. Association between hypouricemia and reduced kidney function: A cross-sectional population-based study in Japan. Am. J. Nephrol. 2015, 41, 138–146. [Google Scholar] [CrossRef]
- Kawasoe, S.; Ide, K.; Usui, T.; Kubozono, T.; Yoshifuku, S.; Miyahara, H.; Maenohara, S.; Ohishi, M.; Kawakami, K. Distribution and Characteristics of Hypouricemia within the Japanese General Population: A Cross-Sectional Study. Medicina 2019, 55, 61. [Google Scholar] [CrossRef] [Green Version]
- Koto, R.; Sato, I.; Kuwabara, M.; Seki, T.; Kawakami, K. Temporal trends in the prevalence and characteristics of hypouricaemia: A descriptive study of medical check-up and administrative claims data. Clin. Rheumatol. 2022, 41, 2113–2119. [Google Scholar] [CrossRef]
- Kawamura, Y.; Nakayama, A.; Shimizu, S.; Toyoda, Y.; Nishida, Y.; Hishida, A.; Katsuura-Kamano, S.; Shibuya, K.; Tamura, T.; Kawaguchi, M.; et al. A Proposal for Practical Diagnosis of Renal Hypouricemia: Evidenced from Genetic Studies of Nonfunctional Variants of URAT1/SLC22A12 among 30,685 Japanese Individuals. Biomedicines 2021, 9, 1012. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.S.; Jeong, H.J.; Son, C.N.; Kim, S.H.; Kim, H.J.; Kim, G.H.; Jun, J.B. Distribution of serum uric acid levels and prevalence of hyper- and hypouricemia in a Korean general population of 172,970. Korean J. Intern. Med. 2021, 36 (Suppl. S1), S264–S272. [Google Scholar] [CrossRef] [PubMed]
- Gresser, U.; Gathof, B.; Zöllner, N. Uric acid levels in southern Germany in 1989. A comparison with studies from 1962, 1971, and 1984. Klin. Wochenschr. 1990, 68, 1222–1228. [Google Scholar] [CrossRef]
- Ichida, K.; Hosoyamada, M.; Hisatome, I.; Enomoto, A.; Hikita, M.; Endou, H.; Hosoya, T. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J. Am. Soc. Nephrol 2004, 15, 164–173. [Google Scholar] [CrossRef]
- Ichida, K.; Hosoyamada, M.; Kamatani, N.; Kamitsuji, S.; Hisatome, I.; Shibasaki, T.; Hosoya, T. Age and origin of the G774A mutation in SLC22A12 causing renal hypouricemia in Japanese. Clin. Genet. 2008, 74, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N.; Mino, Y.; Hosoyamada, M.; Tago, N.; Kokubo, Y.; Endou, H. A high prevalence of renal hypouricemia caused by inactive SLC22A12 in Japanese. Kidney Int. 2004, 66, 935–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, A.; Urano, W.; Yamanaka, M.; Yamanaka, H.; Hosoyamada, M.; Endou, H.; Kamatani, N. A common mutation in an organic anion transporter gene, SLC22A12, is a suppressing factor for the development of gout. Arthritis Rheum. 2005, 52, 2576–2577. [Google Scholar] [CrossRef] [PubMed]
- Tabara, Y.; Kohara, K.; Kawamoto, R.; Hiura, Y.; Nishimura, K.; Morisaki, T.; Kokubo, Y.; Okamura, T.; Tomoike, H.; Iwai, N.; et al. Association of four genetic loci with uric acid levels and reduced renal function: The J-SHIPP Suita study. Am. J. Nephrol. 2010, 32, 279–286. [Google Scholar] [CrossRef]
- Hamajima, N.; Naito, M.; Hishida, A.; Okada, R.; Asai, Y.; Wakai, K. Serum uric acid distribution according to SLC22A12 W258X genotype in a cross-sectional study of a general Japanese population. BMC Med. Genet. 2011, 12, 33. [Google Scholar] [CrossRef] [Green Version]
- Yahyaoui, R.; Esteva, I.; Haro-Mora, J.J.; Almaraz, M.C.; Morcillo, S.; Rojo-Martínez, G.; Martínez, J.; Gómez-Zumaquero, J.M.; González, I.; Hernando, V.; et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J. Clin. Endocrinol. Metab. 2008, 93, 2230–2233. [Google Scholar] [CrossRef] [Green Version]
- Dinour, D.; Gray, N.K.; Ganon, L.; Knox, A.J.; Shalev, H.; Sela, B.A.; Campbell, S.; Sawyer, L.; Shu, X.; Valsamidou, E.; et al. Two novel homozygous SLC2A9 mutations cause renal hypouricemia type 2. Nephrol. Dial. Transplant. 2012, 27, 1035–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anzai, N.; Ichida, K.; Jutabha, P.; Kimura, T.; Babu, E.; Jin, C.J.; Srivastava, S.; Kitamura, K.; Hisatome, I.; Endou, H.; et al. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J. Biol. Chem. 2008, 283, 26834–26838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaito, H.; Ishimori, S.; Nozu, K.; Shima, Y.; Nakanishi, K.; Yoshikawa, N.; Iijima, K. Molecular background of urate transporter genes in patients with exercise-induced acute kidney injury. Am. J. Nephrol. 2013, 38, 316–320. [Google Scholar] [CrossRef]
- Stiburkova, B.; Bohatá, J.; Pavelcová, K.; Tasic, V.; Plaseska-Karanfilska, D.; Cho, S.K.; Potočnaková, L.; Šaligová, J. Renal Hypouricemia 1: Rare Disorder as Common Disease in Eastern Slovakia Roma Population. Biomedicines 2021, 9, 1607. [Google Scholar] [CrossRef]
- Claverie-Martin, F.; Trujillo-Suarez, J.; Gonzalez-Acosta, H.; Aparicio, C.; Roldan, M.L.J.; Stiburkova, B.; Ichida, K.; Martín-Gomez, M.A.; Herrero Goñi, M.; Hidalgo-Barquero, M.C.; et al. URAT1 and GLUT9 mutations in Spanish patients with renal hypouricemia. Clin. Chim. Acta 2018, 481, 83–89. [Google Scholar] [CrossRef]
- Cha, D.H.; Gee, H.Y.; Cachau, R.; Choi, J.M.; Park, D.; Jee, S.H.; Ryu, S.; Kim, K.K.; Won, H.H.; Limou, S.; et al. Contribution of SLC22A12 on hypouricemia and its clinical significance for screening purposes. Sci. Rep. 2019, 9, 14360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Wang, K.; Zhou, J.; Wang, C.; Li, X.; Cui, L.; Han, L.; Liu, Z.; Ren, W.; Wang, X.; et al. Amplicon targeted resequencing for SLC2A9 and SLC22A12 identified novel mutations in hypouricemia subjects. Mol. Genet. Genom. Med. 2019, 7, e00722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, S.W.; Chae, J.; Son, H.Y.; Cho, B.; Kim, J.I.; Park, J.H. A population-specific low-frequency variant of SLC22A12 (p.W258*) explains nearby genome-wide association signals for serum uric acid concentrations among Koreans. PLoS ONE 2020, 15, e0231336. [Google Scholar]
- Watanabe, Y.; Naka, I.; Khor, S.S.; Sawai, H.; Hitomi, Y.; Tokunaga, K.; Ohashi, J. Analysis of whole Y-chromosome sequences reveals the Japanese population history in the Jomon period. Sci. Rep. 2019, 9, 8556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiburkova, B.; Gabrikova, D.; Čepek, P.; Šimek, P.; Kristian, P.; Cordoba-Lanus, E.; Claverie-Martin, F. Prevalence of URAT1 allelic variants in the Roma population. Nucleosides Nucleotides Nucleic Acids 2016, 35, 529–535. [Google Scholar] [CrossRef]
- Gresham, D.; Morar, B.; Underhill, P.A.; Passarino, G.; Lin, A.A.; Wise, C.; Angelicheva, D.; Calafell, F.; Oefner, P.J.; Shen, P.; et al. Origins and divergence of the Roma (gypsies). Am. J. Hum. Genet. 2001, 69, 1314–1331. [Google Scholar] [CrossRef] [Green Version]
- Morar, B.; Gresham, D.; Angelicheva, D.; Tournev, I.; Gooding, R.; Guergueltcheva, V.; Schmidt, C.; Abicht, A.; Lochmuller, H.; Tordai, A.; et al. Mutation history of the roma/gypsies. Am. J. Hum. Genet. 2004, 75, 596–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamjav, H.; Zalán, A.; Béres, J.; Nagy, M.; Chang, Y.M. Genetic structure of the paternal lineage of the Roma people. Am. J. Phys. Anthropol. 2011, 145, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Uchida, S.; Shibata, S.; Tomioka, N.H.; Matsumoto, K.; Hosoyamada, M. Xanthine Oxidoreductase Inhibitors Suppress the Onset of Exercise-Induced AKI in High HPRT Activity Urat1-Uox Double Knockout Mice. J. Am. Soc. Nephrol. 2022, 33, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Hosoyamada, M. Hypothetical Mechanism of Exercise-Induced Acute Kidney Injury Associated with Renal Hypouricemia. Biomedicines 2021, 9, 1847. [Google Scholar] [CrossRef]
- Yeun, J.Y.; Hasbargen, J.A. Renal hypouricemia: Prevention of exercise-induced acute renal failure and a review of the literature. Am. J. Kidney Dis. 1995, 25, 937–946. [Google Scholar] [CrossRef]
- Bhasin, B.; Stiburkova, B.; De Castro-Pretelt, M.; Beck, N.; Bodurtha, J.N.; Atta, M.G. Hereditary renal hypouricemia: A new role for allopurinol? Am. J. Med. 2014, 127, e3–e4. [Google Scholar] [CrossRef]
- George, J.; Carr, E.; Davies, J.; Belch, J.J.; Struthers, A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 2006, 114, 2508–2516. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, I. Acute renal failure with severe loin pain and patchy renal ischemia after anaerobic exercise in patients with or without renal hypouricemia. Nephron 2002, 91, 559–570. [Google Scholar] [CrossRef]
- Ettinger, B.; Tang, A.; Citron, J.T.; Livermore, B.; Williams, T. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N. Engl. J. Med. 1986, 315, 1386–1389. [Google Scholar] [CrossRef]
- Kanda, E.; Muneyuki, T.; Kanno, Y.; Suwa, K.; Nakajima, K. Uric acid level has a U-shaped association with loss of kidney function in healthy people: A prospective cohort study. PLoS ONE 2015, 10, e0118031. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Furuhashi, M.; Tanaka, M.; Numata, K.; Hisasue, T.; Hanawa, N.; Koyama, M.; Osanami, A.; Higashiura, Y.; Inyaku, M.; et al. U-shaped relationship between serum uric acid level and decline in renal function during a 10-year period in female subjects: BOREAS-CKD2. Hypertens. Res. 2021, 44, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Sakano, T.; Igarashi, T.; Itami, N.; Ogawa, T. ARF Assocoated with Renal Hypouricemia Research Group. Exercise-induced acute renal failure associated with renal hypouricaemia: Results of a questionnaire-based survey in Japan. Nephrol. Dial. Transplant. 2004, 19, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.N.; Tan, P.K.; Hyndman, D.; Liu, S.; Iverson, C.; Nanavati, P.; Hagerty, D.T.; Manhard, K.; Shen, Z.; Girardet, J.-L.; et al. Lesinurad, a novel, oral compound for gout, acts to decrease serum uric acid through inhibition of urate transporters in the kidney. Arthritis Res. Ther. 2016, 18, 214. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Niño, M.D.; Zheng-Lin, B.; Valiño-Rivas, L.; Sanz, A.B.; Ramos, A.M.; Luño, J.; Goicoechea, M.; Ortiz, A. Lesinurad: What the nephrologist should know. Clin. Kidney J. 2017, 10, 679–687. [Google Scholar] [CrossRef]
- Shen, Z.; Gillen, M.; Miner, J.N.; Bucci, G.; Wilson, D.M.; Hall, J.W. Pharmacokinetics, pharmacodynamics, and tolerability of verinurad, a selective uric acid reabsorption inhibitor, in healthy Japanese and non-Asian male subjects. Drug. Des. Dev. Ther. 2017, 11, 2077–2086. [Google Scholar] [CrossRef] [Green Version]
- Fitz-Patrick, D.; Roberson, K.; Niwa, K.; Fujimura, T.; Mori, K.; Hall, J.; Yan, X.; Shen, Z.; Liu, S.; Ito, Y.; et al. Safety and efficacy of verinurad, a selective URAT1 inhibitor, for the treatment of patients with gout and/or asymptomatic hyperuricemia in the United States and Japan: Findings from two phase II trials. Mod. Rheumatol. 2019, 29, 1042–1052. [Google Scholar] [CrossRef] [Green Version]
- Hosoya, T.; Fushimi, M.; Okui, D.; Sasaki, T.; Ohashi, T. Open-label study of long-term administration of dotinurad in Japanese hyperuricemic patients with or without gout. Clin. Exp. Nephrol. 2020, 24 (Suppl. S1), 80–91. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, T.; Ashizawa, N.; Matsumoto, K.; Saito, R.; Motoki, K.; Sakai, M.; Chikamatsu, N.; Hagihara, C.; Hashiba, M.; Iwanaga, T. Pharmacological Evaluation of Dotinurad.; a Selective Urate Reabsorption Inhibitor. J. Pharmacol. Exp. Ther. 2019, 371, 162–170. [Google Scholar] [CrossRef]
- Yamamoto, T.; Moriwaki, Y.; Takahashi, S.; Hada, T.; Higashino, K. Renal excretion of purine bases. Effects of probenecid, benzbromarone and pyrazinamide. Nephron 1988, 48, 116–120. [Google Scholar] [CrossRef]
- Okabayashi, Y.; Yamamoto, I.; Komatsuzaki, Y.; Niikura, T.; Yamakawa, T.; Katsumata, H.; Kawabe, M.; Katsuma, A.; Nakada, Y.; Kobayashi, A.; et al. Rare case of nephrocalcinosis in the distal tubules caused by hereditary renal hypouricaemia 3 months after kidney transplantation. Nephrology 2016, 21 (Suppl. S1), 67–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichida, K.; Matsuo, H.; Takada, T.; Nakayama, A.; Murakami, K.; Shimizu, T.; Yamanashi, Y.; Kasuga, H.; Nakashima, H.; Nakamura, T.; et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 2012, 3, 764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, H.; Takada, T.; Ichida, K.; Nakamura, T.; Nakayama, A.; Ikebuchi, Y.; Ito, K.; Kusanagi, Y.; Chiba, T.; Tadokoro, S.; et al. Common Defects of ABCG2, a High-Capacity Urate Exporter, Cause Gout: A Function-Based Genetic Analysis in a Japanese Population. Sci. Transl. Med. 2009, 1, 5ra11. [Google Scholar] [CrossRef] [PubMed]
| Reports | Number of Alleles Examined | Number of W258X Alleles | Percentage of W258X Alleles | Area (Japan) |
|---|---|---|---|---|
| Iwai, N et al. [16] | 3750 | 89 | 2.37 | Suita |
| Taniguchi, A et al. [17] | 1960 | 45 | 2.3 | Tokyo |
| Tabara, Y et al. [18] | 10330 | 263 | 2.55 | Suita + Ehime |
| Hamajima, N et al. [19] | 9586 | 235 | 2.34 | Hamamatsu |
| Men (n = 108) | Women (n = 932) | |||||||
|---|---|---|---|---|---|---|---|---|
| SUA (mg/dL) | Number of Deficient URAT1 Alleles | Number of Deficient URAT1 Alleles | ||||||
| 0 | 1 | 2 | Total | 0 | 1 | 2 | Total | |
| 0.0–1.0 | 2 | 4 | 11 | 17 | 0 | 6 | 13 | 19 |
| 1.1–2.0 | 1 | 3 | 0 | 4 | 20 | 37 | 0 | 57 |
| 2.1–3.0 | 29 | 58 | 0 | 87 | 570 | 286 | 0 | 856 |
| Men (n = 13,607) | Women (n = 17,078) | |||
|---|---|---|---|---|
| SUA (mg/dL) | Number | Frequency (%) | Number | Frequency (%) |
| 0.0–1.0 | 20 | 0.15 | 23 | 0.13 |
| 1.1–2.0 | 4 | 0.03 | 70 | 0.41 |
| 2.1–3.0 | 107 | 0.79 | 1093 | 6.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakoda, M.; Ichida, K. Genetic Basis of the Epidemiological Features and Clinical Significance of Renal Hypouricemia. Biomedicines 2022, 10, 1696. https://doi.org/10.3390/biomedicines10071696
Hakoda M, Ichida K. Genetic Basis of the Epidemiological Features and Clinical Significance of Renal Hypouricemia. Biomedicines. 2022; 10(7):1696. https://doi.org/10.3390/biomedicines10071696
Chicago/Turabian StyleHakoda, Masayuki, and Kimiyoshi Ichida. 2022. "Genetic Basis of the Epidemiological Features and Clinical Significance of Renal Hypouricemia" Biomedicines 10, no. 7: 1696. https://doi.org/10.3390/biomedicines10071696






