Intracellular Habitation of Staphylococcus aureus: Molecular Mechanisms and Prospects for Antimicrobial Therapy
Abstract
:1. Introduction
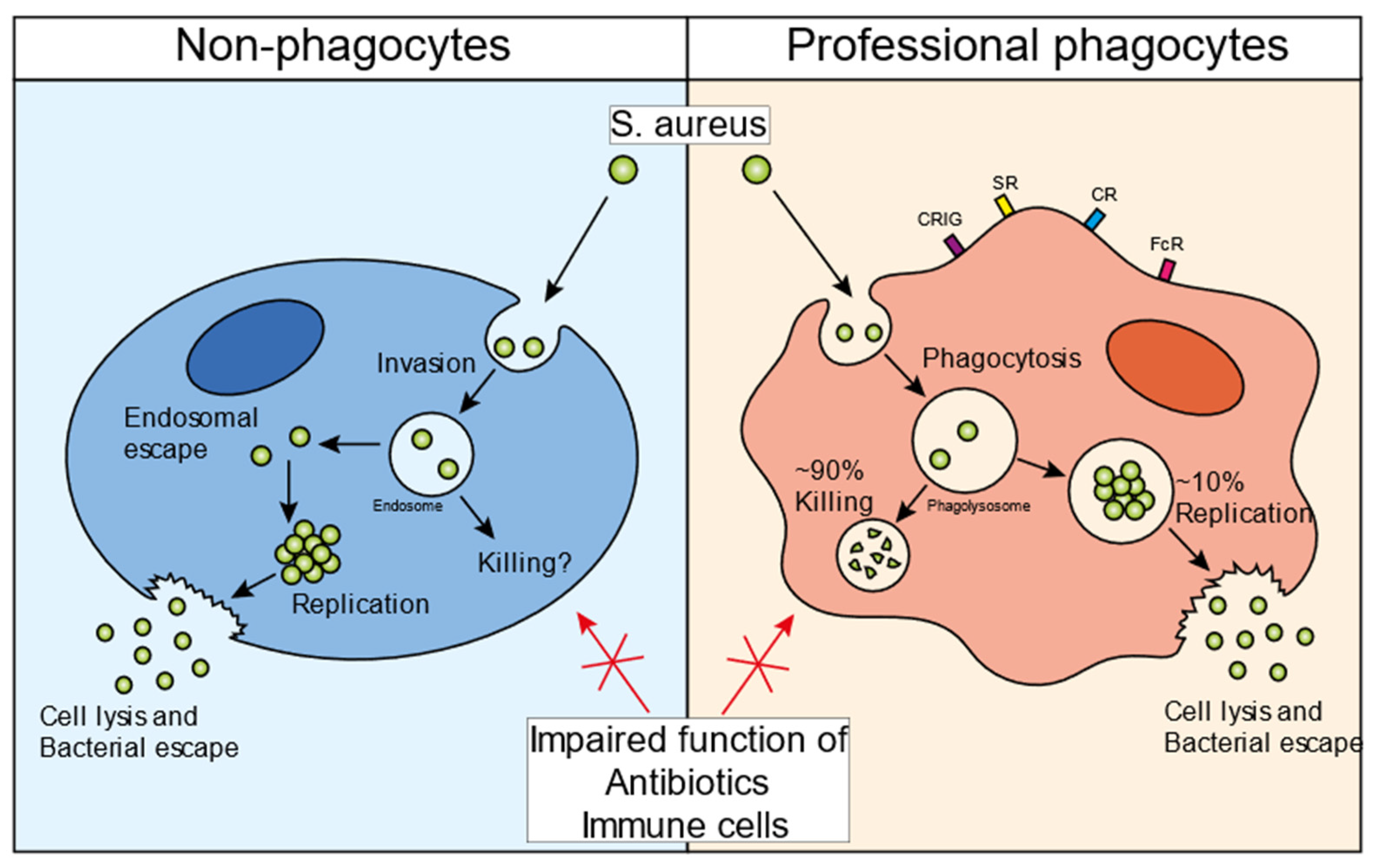
2. The Infectious Cycle of S. aureus in Non-Professional Phagocytes
3. The Infectious Cycle of S. aureus in Professional Phagocytes
4. Intraphagolysosomal Evasion Strategies of S. aureus
5. Bacterial Specificity of the Intracellular Reservoir
6. Intracellular Persistence Expedites Antibiotic Tolerance
7. Treatment Options to Manage Intracellular S. aureus Infections
7.1. Delivery Systems
7.1.1. Intracellular Delivery
7.1.2. S. aureus-Targeted Delivery
7.2. Bio-Conjugated Proteins
7.3. Indirect Killing Mechanisms
7.3.1. Silver Nanoparticles
7.3.2. Cold Atmospheric Plasma
7.3.3. Muramyl Peptides
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Wertheim, H.F.L.; Melles, D.C.; Vos, M.C.; Van Leeuwen, W.; Van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The Role of Nasal Carriage in Staphylococcus aureus Infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus Infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Foster, T.J. Colonization and Infection of the Human Host by Staphylococci: Adhesion, Survival and Immune Evasion. Vet. Dermatol. 2009, 20, 456–470. [Google Scholar] [CrossRef]
- Macneal, W.J.; Frisbee, F.C. One Hundred Patients with Staphylococcus Septicemia Receiving Bacteriophage Service. Am. J. Med. Sci. 1936, 191, 179–195. [Google Scholar] [CrossRef]
- Wyllie, D.H.; Crook, D.W.; Peto, T.E.A. Mortality after Staphylococcus Aureus Bacteraemia in Two Hospitals in Oxfordshire, 1997–2003: Cohort Study. BMJ 2006, 333, 281. [Google Scholar] [CrossRef]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections—United States. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef]
- O’Connell, K.M.G.; Hodgkinson, J.T.; Sore, H.F.; Welch, M.; Salmond, G.P.C.; Spring, D.R. Combating Multidrug-Resistant Bacteria: Current Strategies for the Discovery of Novel Antibacterials. Angew. Chem. Int. Ed. 2013, 52, 10706–10733. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Furuya, E.Y.; Lowy, F.D. Antimicrobial-Resistant Bacteria in the Community Setting. Nat. Rev. Microbiol. 2006, 4, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Voyich, J.M.; Braughton, K.R.; Sturdevant, D.E.; Whitney, A.R.; Saïd-Salim, B.; Porcella, S.F.; Long, R.D.; Dorward, D.W.; Gardner, D.J.; Kreiswirth, B.N.; et al. Insights into Mechanisms Used by Staphylococcus aureus to Avoid Destruction by Human Neutrophils. J. Immunol. 2005, 175, 3907–3919. [Google Scholar] [CrossRef]
- Nannini, E.; Murray, B.E.; Arias, C.A. Resistance or Decreased Susceptibility to Glycopeptides, Daptomycin, and Linezolid in Methicillin-Resistant Staphylococcus aureus. Curr. Opin. Pharmacol. 2010, 10, 516–521. [Google Scholar] [CrossRef]
- Kreisel, K.; Boyd, K.; Langenberg, P.; Roghmann, M.C. Risk Factors for Recurrence in Patients with Staphylococcus aureus Infections Complicated by Bacteremia. Diagn. Microbiol. Infect. Dis. 2006, 55, 179–184. [Google Scholar] [CrossRef]
- Lehar, S.M.; Pillow, T.; Xu, M.; Staben, L.; Kajihara, K.K.; Vandlen, R.; DePalatis, L.; Raab, H.; Hazenbos, W.L.; Hiroshi Morisaki, J.; et al. Novel Antibody-Antibiotic Conjugate Eliminates Intracellular S. Aureus. Nature 2015, 527, 323–328. [Google Scholar] [CrossRef]
- Surewaard, B.G.J.; Deniset, J.F.; Zemp, F.J.; Amrein, M.; Otto, M.; Conly, J.; Omri, A.; Yates, R.M.; Kubes, P. Identification and Treatment of the Staphylococcus aureus Reservoir in Vivo. J. Exp. Med. 2016, 213, 1141–1151. [Google Scholar] [CrossRef]
- Fraunholz, M.; Sinha, B. Intracellular Staphylococcus aureus: Live-in and Let Die. Front. Cell. Infect. Microbiol. 2012, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Pidwill, G.R.; Gibson, J.F.; Cole, J.; Renshaw, S.A.; Foster, S.J. The Role of Macrophages in Staphylococcus aureus Infection. Front. Immunol. 2021, 11, 3506. [Google Scholar] [CrossRef]
- Horn, J.; Stelzner, K.; Rudel, T.; Fraunholz, M. Inside Job: Staphylococcus aureus Host-Pathogen Interactions. Int. J. Med. Microbiol. 2018, 308, 607–624. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Heit, B.; Heinrichs, D.E. Antimicrobial Mechanisms of Macrophages and the Immune Evasion Strategies of Staphylococcus aureus. Pathogens 2015, 4, 826–868. [Google Scholar] [CrossRef]
- Lacoma, A.; Cano, V.; Moranta, D.; Regueiro, V.; Domínguez-Villanueva, D.; Laabei, M.; González-Nicolau, M.; Ausina, V.; Prat, C.; Bengoechea, J.A. Investigating Intracellular Persistence of Staphylococcus aureus within a Murine Alveolar Macrophage Cell Line. Virulence 2017, 8, 1761–1775. [Google Scholar] [CrossRef] [PubMed]
- Tranchemontagne, Z.R.; Camire, R.B.; O’Donnell, V.J.; Baugh, J.; Burkholder, K.M. Staphylococcus aureus Strain USA300 Perturbs Acquisition of Lysosomal Enzymes and Requires Phagosomal Acidification for Survival inside Macrophages. Infect. Immun. 2015, 84, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Jubrail, J.; Morris, P.; Bewley, M.A.; Stoneham, S.; Johnston, S.A.; Foster, S.J.; Peden, A.A.; Read, R.C.; Marriott, H.M.; Dockrell, D.H. Inability to Sustain Intraphagolysosomal Killing of Staphylococcus aureus Predisposes to Bacterial Persistence in Macrophages. Cell. Microbiol. 2016, 18, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Bayles, K.W.; Wesson, C.A.; Liou, L.E.; Fox, L.K.; Bohach, G.A.; Trumble, W.R. Intracellular Staphylococcus aureus Escapes the Endosome and Induces Apoptosis in Epithelial Cells. Infect. Immun. 1998, 66, 336–342. [Google Scholar] [CrossRef]
- Hess, D.J.; Henry-Stanley, M.J.; Erickson, E.A.; Wells, C.L. Intracellular Survival of Staphylococcus aureus within Cultured Enterocytes. J. Surg. Res. 2003, 114, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Menzies, B.E.; Kourteva, I. Staphylococcus aureus α-Toxin Induces Apoptosis in Endothelial Cells. FEMS Immunol. Med. Microbiol. 2000, 29, 39–45. [Google Scholar] [CrossRef]
- Nair, S.P.; Bischoff, M.; Senn, M.M.; Berger-Bächi, B. The ΣB Regulon Influences Internalization of Staphylococcus aureus by Osteoblasts. Infect. Immun. 2003, 71, 4167–4170. [Google Scholar] [CrossRef]
- Rogers, R.; Tompsett, R. The Survival of Staphylococci within Human Leukocytes. J. Exp. Med. 1952, 28, 470. [Google Scholar] [CrossRef]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, Invasion and Evasion: The Many Functions of the Surface Proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef]
- Moldovan, A.; Fraunholz, M.J. In or out: Phagosomal Escape of Staphylococcus aureus. Cell. Microbiol. 2019, 21, e12997. [Google Scholar] [CrossRef]
- Qazi, S.N.A.; Harrison, S.E.; Self, T.; Williams, P.; Hill, P.J. Real-Time Monitoring of Intracellular Staphylococcus aureus Replication. J. Bacteriol. 2004, 186, 1065–1077. [Google Scholar] [CrossRef]
- Shompole, S.; Henon, K.T.; Liou, L.E.; Dziewanowska, K.; Bohach, G.A.; Bayles, K.W. Biphasic Intracellular Expression of Staphylococcus aureus Virulence Factors and Evidence for Agr-Mediated Diffusion Sensing. Mol. Microbiol. 2003, 49, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Schnaith, A.; Kashkar, H.; Leggio, S.A.; Addicks, K.; Krönke, M.; Krut, O. Staphylococcus aureus Subvert Autophagy for Induction of Caspase-Independent Host Cell Death. J. Biol. Chem. 2007, 282, 2695–2706. [Google Scholar] [CrossRef]
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.H.L.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-Independent Target Gene Control by the Agr Quorum-Sensing System: Insight into the Evolution of Virulence Regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Grosz, M.; Kolter, J.; Paprotka, K.; Winkler, A.C.; Schäfer, D.; Chatterjee, S.S.; Geiger, T.; Wolz, C.; Ohlsen, K.; Otto, M.; et al. Cytoplasmic Replication of Staphylococcus aureus upon Phagosomal Escape Triggered by Phenol-Soluble Modulin α. Cell. Microbiol. 2014, 16, 451–465. [Google Scholar] [CrossRef]
- Surewaard, B.G.J.; Nijland, R.; Spaan, A.N.; Kruijtzer, J.A.W.; de Haas, C.J.C.; van Strijp, J.A.G. Inactivation of Staphylococcal Phenol Soluble Modulins by Serum Lipoprotein Particles. PLoS Pathog. 2012, 8, e1002606. [Google Scholar] [CrossRef]
- Hommes, J.W.; Kratofil, R.M.; Wahlen, S.; de Haas, C.J.C.; Hildebrand, R.B.; Hovingh, G.K.; Otto, M.; van Eck, M.; Hoekstra, M.; Korporaal, S.J.A.; et al. High Density Lipoproteins Mediate in Vivo Protection against Staphylococcal Phenol-Soluble Modulins. Sci. Rep. 2021, 11, 15357. [Google Scholar] [CrossRef] [PubMed]
- Gresham, H.D.; Lowrance, J.H.; Caver, T.E.; Wilson, B.S.; Cheung, A.L.; Lindberg, F.P. Survival of Staphylococcus aureus Inside Neutrophils Contributes to Infection. J. Immunol. 2000, 164, 3713–3722. [Google Scholar] [CrossRef]
- Koziel, J.; Maciag-Gudowska, A.; Mikolajczyk, T.; Bzowska, M.; Sturdevant, D.E.; Whitney, A.R.; Shaw, L.N.; DeLeo, F.R.; Potempa, J. Phagocytosis of Staphylococcus aureus by Macrophages Exerts Cytoprotective Effects Manifested by the Upregulation of Antiapoptotic Factors. PLoS ONE 2009, 4, e5210. [Google Scholar] [CrossRef]
- Spaan, A.N.; Surewaard, B.G.J.; Nijland, R.; van Strijp, J.A.G. Neutrophils Versus Staphylococcus aureus: A Biological Tug of War. Annu. Rev. Microbiol. 2013, 67, 629–650. [Google Scholar] [CrossRef]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and Tissue Specialization. Annu. Rev. Immunol. 2015, 33, 643–675; 0324141122. [Google Scholar] [CrossRef]
- Rogers, D.E. Studies on Bacteriemia I. Mechanisms Relating to the Persistence of Bacteriemia in Rabbits Following the Intravenous Injection of Staphylococci. J. Exp. Med. 1956, 103, 713–742. [Google Scholar] [CrossRef]
- Thwaites, G.E.; Gant, V. Are Bloodstream Leukocytes Trojan Horses for the Metastasis of Staphylococcus aureus? Nat. Rev. Microbiol. 2011, 9, 215–222. [Google Scholar] [CrossRef]
- Krezalek, M.A.; Hyoju, S.; Zaborin, A.; Okafor, E.; Chandrasekar, L.; Bindokas, V.; Guyton, K.; Montgomery, C.P.; Daum, R.S.; Zaborina, O.; et al. Can Methicillin-Resistant Staphylococcus aureus Silently Travel from the Gut to the Wound and Cause Postoperative Infection? Modeling the “Trojan Horse Hypothesis”. Ann. Surg. 2018, 267, 749–758. [Google Scholar] [CrossRef]
- Venditti, M.; Falcone, M.; Micozzi, A.; Carfagna, P.; Taglietti, F.; Serra, P.F.; Martino, P. Staphylococcus aureus Bacteremia in Patients with Hematologic Malignancies: A Retrospective Case-Control Study. Haematologica 2003, 88, 923–930. [Google Scholar]
- Krenkel, O.; Tacke, F. Liver Macrophages in Tissue Homeostasis and Disease. Nat. Rev. Immunol. 2017, 17, 306–321. [Google Scholar] [CrossRef]
- Jenne, C.N.; Kubes, P. Immune Surveillance by the Liver. Nat. Immunol. 2013, 14, 996–1006. [Google Scholar] [CrossRef]
- Pollitt, E.J.G.; Szkuta, P.T.; Burns, N.; Foster, S.J. Staphylococcus aureus Infection Dynamics. PLoS Pathog. 2018, 14, e1007112. [Google Scholar] [CrossRef]
- Surewaard, B.G.J.; Thanabalasuriar, A.; Zeng, Z.; Tkaczyk, C.; Cohen, T.S.; Bardoel, B.W.; Jorch, S.K.; Deppermann, C.; Bubeck Wardenburg, J.; Davis, R.P.; et al. α-Toxin Induces Platelet Aggregation and Liver Injury during Staphylococcus aureus Sepsis. Cell Host Microbe 2018, 24, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Jorch, S.K.; Surewaard, B.G.J.; Hossain, M.; Peiseler, M.; Deppermann, C.; Deng, J.; Bogoslowski, A.; van der Wal, F.; Omri, A.; Hickey, M.J.; et al. Peritoneal GATA6+ Macrophages Function as a Portal for Staphylococcus aureus Dissemination. J. Clin. Investig. 2019, 129, 4643–4656. [Google Scholar] [CrossRef]
- Kitur, K.; Parker, D.; Nieto, P.; Ahn, D.S.; Cohen, T.S.; Chung, S.; Wachtel, S.; Bueno, S.; Prince, A. Toxin-Induced Necroptosis Is a Major Mechanism of Staphylococcus aureus Lung Damage. PLoS Pathog. 2015, 11, e1004820. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Cosío, G.; Grinstein, S. Antimicrobial Mechanisms of Phagocytes and Bacterial Evasion Strategies. Nat. Rev. Microbiol. 2009, 7, 355–366. [Google Scholar] [CrossRef]
- Kahl, B.C.; Goulian, M.; Van Wamel, W.; Herrmann, M.; Simon, S.M.; Kaplan, G.; Peters, G.; Cheung, A.L. Staphylococcus aureus RN6390 Replicates and Induces Apoptosis in a Pulmonary Epithelial Cell Line. Infect. Immun. 2000, 68, 5385–5392. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Heit, B.; Heinrichs, D.E. Intracellular Replication of Staphylococcus aureus in Mature Phagolysosomes in Macrophages Precedes Host Cell Death, and Bacterial Escape and Dissemination. Cell. Microbiol. 2016, 18, 514–535. [Google Scholar] [CrossRef]
- Jarry, T.M.; Cheung, A.L. Staphylococcus aureus Escapes More Efficiently from the Phagosome of a Cystic Fibrosis Bronchial Epithelial Cell Line than from Its Normal Counterpart. Infect. Immun. 2006, 74, 2568–2577. [Google Scholar] [CrossRef]
- Lâm, T.T.; Giese, B.; Chikkaballi, D.; Kühn, A.; Wolber, W.; Pané-Farré, J.; Schäfer, D.; Engelmann, S.; Fraunholz, M.; Sinha, B. Phagolysosomal Integrity Is Generally Maintained after Staphylococcus aureus Invasion of Nonprofessional Phagocytes but Is Modulated by Strain 6850. Infect. Immun. 2010, 78, 3392–3393. [Google Scholar] [CrossRef]
- Lewis, M.L.; Surewaard, B.G.J. Neutrophil Evasion Strategies by Streptococcus Pneumoniae and Staphylococcus aureus. Cell Tissue Res. 2018, 371, 489–503. [Google Scholar] [CrossRef]
- Karavolos, M.H.; Horsburgh, M.; Ingham, E.; Foster, S.J. Role and Regulation of the Superoxide Dismutases of Staphylococcus aureus. Microbiology 2003, 149, 2749–2758. [Google Scholar] [CrossRef]
- Das, D.; Saha, S.S.; Bishayi, B. Intracellular Survival of Staphylococcus aureus: Correlating Production of Catalase and Superoxide Dismutase with Levels of Inflammatory Cytokines. Inflamm. Res. 2008, 57, 340–349. [Google Scholar] [CrossRef]
- Cosgrove, K.; Coutts, G.; Jonsson, I.M.; Tarkowski, A.; Kokai-Kun, J.F.; Mond, J.J.; Foster, S.J. Catalase (KatA) and Alkyl Hydroperoxide Reductase (AhpC) Have Compensatory Roles in Peroxide Stress Resistance and Are Required for Survival, Persistence, and Nasal Colonization in Staphylococcus aureus. J. Bacteriol. 2007, 189, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Mashruwala, A.A.; Boyd, J.M. The Staphylococcus aureus SrrAB Regulatory System Modulates Hydrogen Peroxide Resistance Factors, Which Imparts Protection to Aconitase during Aerobic Growth. PLoS ONE 2017, 12, e0170283. [Google Scholar] [CrossRef]
- Pandey, S.; Sahukhal, G.S.; Elasri, M.O. The MsaABCR Operon Regulates the Response to Oxidative Stress in Staphylococcus aureus. J. Bacteriol. 2019, 201, e00417-19. [Google Scholar] [CrossRef]
- Nobre, L.S.; Gonçalves, V.L.; Saraiva, L.M. Flavohemoglobin of Staphylococcus aureus. Methods Enzymol. 2008, 436, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Grayczyk, J.P.; Alonzo, F., III. Staphylococcus aureus Lipoic Acid Synthesis Limits Macrophage Reactive Oxygen and Nitrogen Species Production to Promote Survival during Infection. Infect. Immun. 2019, 87, e00344-19. [Google Scholar] [CrossRef]
- De Jong, N.W.M.; Ramyar, K.X.; Guerra, F.E.; Nijland, R.; Fevre, C.; Voyich, J.M.; McCarthy, A.J.; Garcia, B.L.; Van Kessel, K.P.M.; Van Strijp, J.A.G.; et al. Immune Evasion by a Staphylococcal Inhibitor of Myeloperoxidase. Proc. Natl. Acad. Sci. USA 2017, 114, 9439–9444. [Google Scholar] [CrossRef] [PubMed]
- Leliefeld, P.H.C.; Pillay, J.; Vrisekoop, N.; Heeres, M.; Tak, T.; Kox, M.; Rooijakkers, S.H.M.; Kuijpers, T.W.; Pickkers, P.; Leenen, L.P.H.; et al. Differential Antibacterial Control by Neutrophil Subsets. Blood Adv. 2018, 2, 1344–1354. [Google Scholar] [CrossRef]
- Villanueva, M.; García, B.; Valle, J.; Rapún, B.; Ruiz De Los Mozos, I.; Solano, C.; Martí, M.; Penadés, J.R.; Toledo-Arana, A.; Lasa, I. Sensory Deprivation in Staphylococcus aureus. Nat. Commun. 2018, 9, 523. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Kuiack, R.C.; McGavin, M.J.; Heinrichs, D.E. Staphylococcus aureus Uses the GraXRS Regulatory System To Sense and Adapt to the Acidified Phagolysosome in Macrophages. MBio 2018, 9, e01143-18. [Google Scholar] [CrossRef]
- Chan, P.F.; Foster, S.J.; Ingham, E.; Clements, M.O. The Staphylococcus aureus Alternative Sigma Factor σ(B) Controls the Environmental Stress Response but Not Starvation Survival or Pathogenicity in a Mouse Abscess Model. J. Bacteriol. 1998, 180, 6082–6089. [Google Scholar] [CrossRef]
- Olivier, A.C.; Lemaire, S.; Van Bambeke, F.; Tulkens, P.M.; Oldfield, E. Role of RsbU and Staphyloxanthin in Phagocytosis and Intracellular Growth of Staphylococcus aureus in Human Macrophages and Endothelial Cells. J. Infect. Dis. 2009, 200, 1367–1370. [Google Scholar] [CrossRef]
- Bera, A.; Biswas, R.; Herbert, S.; Götz, F. The Presence of Peptidoglycan O-Acetyltransferase in Various Staphylococcal Species Correlates with Lysozyme Resistance and Pathogenicity. Infect. Immun. 2006, 74, 4598–4604. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Park, B.G.; Wolf, A.J.; Brikos, C.; Goodridge, H.S.; Becker, C.A.; Reyes, C.N.; Miao, E.A.; Aderem, A.; Götz, F.; et al. Staphylococcus aureus Evades Lysozyme-Based Peptidoglycan Digestion That Links Phagocytosis, Inflammasome Activation, and IL-1β Secretion. Cell Host Microbe 2010, 7, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Kristian, S.A.; Dürr, M.; Van Strijp, J.A.G.; Neumeister, B.; Peschel, A. MprF-Mediated Lysinylation of Phospholipids in Staphylococcus aureus Leads to Protection against Oxygen-Independent Neutrophil Killing. Infect. Immun. 2003, 71, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Jack, R.W.; Otto, M.; Collins, L.V.; Staubitz, P.; Nicholson, G.; Kalbacher, H.; Nieuwenhuizen, W.F.; Jung, G.; Tarkowski, A.; et al. Staphylococcus aureus Resistance to Human Defensins and Evasion of Neutrophil Killing via the Novel Virulence Factor MprF Is Based on Modification of Membrane Lipids with L-Lysine. J. Exp. Med. 2001, 193, 1067–1076. [Google Scholar] [CrossRef]
- Peschel, A.; Otto, M.; Jack, R.W.; Kalbacher, H.; Jung, G.; Götz, F. Inactivation of the Dlt Operon in Staphylococcus aureus Confers Sensitivity to Defensins, Protegrins, and Other Antimicrobial Peptides. J. Biol. Chem. 1999, 274, 8405–8410. [Google Scholar] [CrossRef]
- Li, M.; Cha, D.J.; Lai, Y.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M. The Antimicrobial Peptide-Sensing System Aps of Staphylococcus aureus. Mol. Microbiol. 2007, 66, 1136–1147. [Google Scholar] [CrossRef]
- Kubica, M.; Guzik, K.; Koziel, J.; Zarebski, M.; Richter, W.; Gajkowska, B.; Golda, A.; Maciag-Gudowska, A.; Brix, K.; Shaw, L.; et al. A Potential New Pathway for Staphylococcus aureus Dissemination: The Silent Survival of S. Aureus Phagocytosed by Human Monocyte-Derived Macrophages. PLoS ONE 2008, 3, e1409. [Google Scholar] [CrossRef]
- Surewaard, B.; de Haas, C.; Vervoort, F.; Rigby, K.; DeLeo, F.; Otto, M.; van Strijp, J.; Nijland, R. Staphylococcal Alpha-Phenol Soluble Modulins Contribute to Neutrophil Lysis after Phagocytosis. Cell Microbiol. 2013, 15, 1427–1437. [Google Scholar] [CrossRef]
- Geiger, T.; Francois, P.; Liebeke, M.; Fraunholz, M.; Goerke, C.; Krismer, B.; Schrenzel, J.; Lalk, M.; Wolz, C. The Stringent Response of Staphylococcus aureus and Its Impact on Survival after Phagocytosis through the Induction of Intracellular PSMs Expression. PLoS Pathog. 2012, 8, e1003016. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Watson, D.W.; Surewaard, B.G.J.; Kubes, P.; Heinrichs, D.E. The Surreptitious Survival of the Emerging Pathogen Staphylococcus Lugdunensis within Macrophages as an Immune Evasion Strategy. Cell. Microbiol. 2018, 20, e12869. [Google Scholar] [CrossRef] [PubMed]
- Boldock, E.; Surewaard, B.G.J.; Shamarina, D.; Na, M.; Fei, Y.; Ali, A.; Williams, A.; Pollitt, E.J.G.; Szkuta, P.; Morris, P.; et al. Human Skin Commensals Augment Staphylococcus aureus Pathogenesis. Nat. Microbiol. 2018, 3, 881–890. [Google Scholar] [CrossRef]
- Gibson, J.F.; Pidwill, G.R.; Carnell, O.T.; Surewaard, B.G.J.; Shamarina, D.; Sutton, J.A.F.; Jeffery, C.; Derré-Bobillot, A.; Archambaud, C.; Siggins, M.K.; et al. Commensal Bacteria Augment Staphylococcus aureus Infection by Inactivation of Phagocyte-Derived Reactive Oxygen Species. PLoS Pathog. 2021, 17, e1009880. [Google Scholar] [CrossRef]
- Davis, J.M.; Ramakrishnan, L. The Role of the Granuloma in Expansion and Dissemination of Early Tuberculous Infection. Cell 2009, 136, 37–49. [Google Scholar] [CrossRef]
- Drevets, D.A. Dissemination of Listeria Monocytogenes by Infected Phagocytes. Infect. Immun. 1999, 67, 3512–3517. [Google Scholar] [CrossRef]
- Peters, N.C.; Egen, J.G.; Secundino, N.; Debrabant, A.; Kimblin, N.; Kamhawi, S.; Lawyer, P.; Fay, M.P.; Germain, R.N.; Sacks, D. In Vivo Imaging Reveals an Essential Role for Neutrophils in Leishmaniasis Transmitted by Sand Flies. Science 2008, 321, 970–975. [Google Scholar] [CrossRef]
- Shi, M.; Li, S.S.; Zheng, C.; Jones, G.J.; Kim, K.S.; Zhou, H.; Kubes, P.; Mody, C.H. Real-Time Imaging of Trapping and Urease-Dependent Transmigration of Cryptococcus Neoformans in Mouse Brain. J. Clin. Investig. 2010, 120, 1683–1693. [Google Scholar] [CrossRef]
- Sedlyarov, V.; Eichner, R.; Girardi, E.; Essletzbichler, P.; Goldmann, U.; Nunes-Hasler, P.; Srndic, I.; Moskovskich, A.; Heinz, L.X.; Kartnig, F.; et al. The Bicarbonate Transporter SLC4A7 Plays a Key Role in Macrophage Phagosome Acidification. Cell Host Microbe 2018, 23, 766–774. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Fowler, V.G.; Yeaman, M.R.; Perdreau-Remington, F.; Kreiswirth, B.N.; Bayer, A.S. Phenotypic and Genotypic Characteristics of Persistent Methicillin-Resistant Staphylococcus aureus Bacteremia In Vitro and in an Experimental Endocarditis Model. J. Infect. Dis. 2009, 199, 201–208. [Google Scholar] [CrossRef]
- Bishr, A.S.; Abdelaziz, S.M.; Yahia, I.S.; Yassien, M.A.; Hassouna, N.A.; Aboshanab, K.M. Association of Macrolide Resistance Genotypes and Synergistic Antibiotic Combinations for Combating Macrolide-Resistant MRSA Recovered from Hospitalized Patients. Biology 2021, 10, 624. [Google Scholar] [CrossRef]
- Klevens, R.M.; Morrison, M.A.; Nadle, J.; Petit, S.; Gershman, K.; Ray, S.; Harrison, L.H.; Lynfield, R.; Dumyati, G.; Townes, J.M.; et al. Invasive Methicillin-Resistant Staphylococcus aureus Infections in the United States. J. Am. Med. Assoc. 2007, 298, 1763–1771. [Google Scholar] [CrossRef]
- Holmes, B.; Quie, P.G.; Windhorst, D.B.; Pollara, B.; Good, R.A. Protection of Phagocytized Bacteria from the Killing Action of Antibiotics. Nature 1966, 210, 1131–1132. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Kim, J.Y.; Park, D.W.; Sohn, J.W.; Kim, M.J. Predictors of Persistent Methicillin-Resistant Staphylococcus aureus Bacteraemia in Patients Treated with Vancomycin. J. Antimicrob. Chemother. 2010, 65, 1015–1018. [Google Scholar] [CrossRef]
- Yancey, R.J.; Sanchez, M.S.; Ford, C.W. Activity of Antibiotics against Staphylococcus aureus within Polymorphonuclear Neutrophils. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 107–113. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Barcia-Macay, M.; Lemaire, S.; Tulkens, P.M. Cellular Pharmacodynamics and Pharmacokinetics of Antibiotics: Current Views and Perspectives. Curr. Opin. Drug Discov. Dev. 2006, 9, 218–230. [Google Scholar]
- Barcia-Macay, M.; Seral, C.; Mingeot-Leclercq, M.P.; Tulkens, P.M.; Van Bambeke, F. Pharmacodynamic Evaluation of the Intracellular Activities of Antibiotics against Staphylococcus aureus in a Model of THP-1 Macrophages. Antimicrob. Agents Chemother. 2006, 50, 841–851. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Shah, P.M. Intracellular Activity of Vancomycin and Ly333328, a New Semisynthetic Glycopeptide, against Methicillin-Resistant Staphylococcus aureus. Infection 1998, 26, 165–167. [Google Scholar] [CrossRef]
- Sabath, L.D.; Wallace, S.J.; Gerstein, D.A. Suppression of Intrinsic Resistance to Methicillin and Other Penicillins in Staphylococcus aureus. Antimicrob. Agents Chemother. 1972, 2, 350–355. [Google Scholar] [CrossRef]
- Krut, O.; Sommer, H.; Krönke, M. Antibiotic-Induced Persistence of Cytotoxic Staphylococcus aureus in Non-Phagocytic Cells. J. Antimicrob. Chemother. 2004, 53, 167–173. [Google Scholar] [CrossRef]
- Sandberg, A.; Lemaire, S.; Van Bambeke, F.; Tulkens, P.M.; Hughes, D.; Von Eiff, C.; Frimodt-Møller, N. Intra- and Extracellular Activities of Dicloxacillin and Linezolid against a Clinical Staphylococcus aureus Strain with a Small-Colony-Variant Phenotype in an In Vitro Model of THP-1 Macrophages and an In Vivo Mouse Peritonitis Model. Antimicrob. Agents Chemother. 2011, 55, 1443–1452. [Google Scholar] [CrossRef]
- Kriegeskorte, A.; Grubmüller, S.; Huber, C.; Kahl, B.C.; von Eiff, C.; Proctor, R.A.; Peters, G.; Eisenreich, W.; Becker, K. Staphylococcus aureus Small Colony Variants Show Common Metabolic Features in Central Metabolism Irrespective of the Underlying Auxotrophism. Front. Cell. Infect. Microbiol. 2014, 4, 141. [Google Scholar] [CrossRef]
- Kahl, B.; Herrmann, M.; Everding, A.S.; Koch, H.G.; Becker, K.; Harms, E.; Proctor, R.A.; Peters, G. Persistent Infection with Small Colony Variant Strains of Staphylococcus aureus in Patients with Cystic Fibrosis. J. Infect. Dis. 1998, 177, 1023–1029. [Google Scholar] [CrossRef]
- Sendi, P.; Rohrbach, M.; Graber, P.; Frei, R.; Ochsner, P.E.; Zimmerli, W. Staphylococcus aureus Small Colony Variants in Prosthetic Joint Infection. Clin. Infect. Dis. 2006, 43, 961–967. [Google Scholar] [CrossRef]
- Cho, H.; Uehara, T.; Bernhardt, T.G. Beta-Lactam Antibiotics Induce a Lethal Malfunctioning of the Bacterial Cell Wall Synthesis Machinery. Cell 2014, 159, 1300–1311. [Google Scholar] [CrossRef]
- Davis, B.D.; Chen, L.; Tai, P.C. Misread Protein Creates Membrane Channels: An Essential Step in the Bactericidal Action of Aminoglycosides. Proc. Natl. Acad. Sci. USA 1986, 83, 6164–6168. [Google Scholar] [CrossRef]
- Malik, M.; Zhao, X.; Drlica, K. Lethal Fragmentation of Bacterial Chromosomes Mediated by DNA Gyrase and Quinolones. Mol. Microbiol. 2006, 61, 810–825. [Google Scholar] [CrossRef]
- Sendi, P.; Proctor, R.A. Staphylococcus aureus as an Intracellular Pathogen: The Role of Small Colony Variants. Trends Microbiol. 2009, 17, 54–58. [Google Scholar] [CrossRef]
- Edwards, A.M. Phenotype Switching Is a Natural Consequence of Staphylococcus aureus Replication. J. Bacteriol. 2012, 194, 5404–5412. [Google Scholar] [CrossRef]
- Rowe, S.E.; Wagner, N.J.; Li, L.; Beam, J.E.; Wilkinson, A.D.; Radlinski, L.C.; Zhang, Q.; Miao, E.A.; Conlon, B.P. Reactive Oxygen Species Induce Antibiotic Tolerance during Systemic Staphylococcus aureus Infection. Nat. Microbiol. 2020, 5, 282–290. [Google Scholar] [CrossRef]
- Beam, J.E.; Wagner, N.J.; Shook, J.C.; Bahnson, E.S.M.; Fowler, V.G.J.; Rowe, S.E.; Conlon, B.P. Macrophage-Produced Peroxynitrite Induces Antibiotic Tolerance and Supersedes Intrinsic Mechanisms of Persister Formation. Infect. Immun. 2021, 89, e00286-21. [Google Scholar] [CrossRef]
- Jacobs, R.F.; Wilson, C.B. Activity of Antibiotics in Chronic Granulomatous Disease Leukocytes. Pediatr. Res. 1983, 17, 916–919. [Google Scholar] [CrossRef]
- Hulme, J. Application of Nanomaterials in the Prevention, Detection, and Treatment of Methicillin-Resistant Staphylococcus aureus (MRSA). Pharmaceutics 2022, 14, 805. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Kendall, S.; Good, L. Targeting the Hard to Reach: Challenges and Novel Strategies in the Treatment of Intracellular Bacterial Infections. Br. J. Pharmacol. 2017, 174, 2225–2236. [Google Scholar] [CrossRef]
- Vanamala, K.; Tatiparti, K.; Bhise, K.; Sau, S.; Scheetz, M.H.; Rybak, M.J.; Andes, D.; Iyer, A.K. Novel Approaches for the Treatment of Methicillin-Resistant Staphylococcus aureus: Using Nanoparticles to Overcome Multidrug Resistance. Drug Discov. Today 2021, 26, 31–43. [Google Scholar] [CrossRef]
- Pinto-Alphandary, H.; Balland, O.; Laurent, M.; Andremont, A.; Puisieux, F.; Couvreur, P. Intracellular Visualization of Ampicillin-Loaded Nanoparticles in Peritoneal Macrophages Infected in Vitro with Salmonella Typhimurium. Pharm. Res. 1994, 11, 38–46. [Google Scholar] [CrossRef]
- Forestier, F.; Gerrier, P.; Chaumard, C.; Quero, A.M.; Couvreur, P.; Labarre, C. Effect of Nanoparticle-Bound Ampicillin on the Survival of Listeria Monocytogenes in Mouse Peritoneal Macrophages. J. Antimicrob. Chemother. 1992, 30, 173–179. [Google Scholar] [CrossRef]
- Pornpattananangkul, D.; Zhang, L.; Olson, S.; Aryal, S.; Obonyo, M.; Vecchio, K.; Huang, C.-M.; Zhang, L. Bacterial Toxin-Triggered Drug Release from Gold Nanoparticle-Stabilized Liposomes for the Treatment of Bacterial Infection. J. Am. Chem. Soc. 2011, 133, 4132–4139. [Google Scholar] [CrossRef]
- Maji, R.; Omolo, C.A.; Agrawal, N.; Maduray, K.; Hassan, D.; Mokhtar, C.; Mackhraj, I.; Govender, T. PH-Responsive Lipid-Dendrimer Hybrid Nanoparticles: An Approach to Target and Eliminate Intracellular Pathogens. Mol. Pharm. 2019, 16, 4594–4609. [Google Scholar] [CrossRef]
- Omolo, C.A.; Megrab, N.A.; Kalhapure, R.S.; Agrawal, N.; Jadhav, M.; Mocktar, C.; Rambharose, S.; Maduray, K.; Nkambule, B.; Govender, T. Liposomes with PH Responsive ‘on and off’ Switches for Targeted and Intracellular Delivery of Antibiotics. J. Liposome Res. 2021, 31, 45–63. [Google Scholar] [CrossRef]
- Senior, J.H. Fate and Behavior of Liposomes in Vivo: A Review of Controlling Factors. Crit. Rev. Ther. Drug Carrier Syst. 1987, 3, 123–193. [Google Scholar]
- Li, Y.; Liu, Y.; Ren, Y.; Su, L.; Li, A.; An, Y.; Rotello, V.; Zhang, Z.; Wang, Y.; Liu, Y.; et al. Coating of a Novel Antimicrobial Nanoparticle with a Macrophage Membrane for the Selective Entry into Infected Macrophages and Killing of Intracellular Staphylococci. Adv. Funct. Mater. 2020, 30, 2004942. [Google Scholar] [CrossRef]
- Peck, M.; Rothenberg, M.E.; Deng, R.; Lewin-Koh, N.; She, G.; Kamath, A.V.; Carrasco-Tringuero, M.; Saad, O.; Castro, A.; Teufel, L.; et al. A Phase 1, Randomized, Single-Ascending-Dose Study To Investigate the Safety, Tolerability, and Pharmacokinetics of DSTA4637S, an Anti-Staphylococcus aureus Thiomab Antibody-Antibiotic Conjugate, in Healthy Volunteers. Antimicrob. Agents Chemother. 2019, 63, e02588-18. [Google Scholar] [CrossRef]
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.G.; Kim, D.; Mann, A.P.; Mölder, T.; Teesalu, T.; et al. Antibiotic-Loaded Nanoparticles Targeted to the Site of Infection Enhance Antibacterial Efficacy. Nat. Biomed. Eng. 2018, 2, 95–103. [Google Scholar] [CrossRef]
- Röhrig, C.; Huemer, M.; Lorgé, D.; Luterbacher, S.; Phothaworn, P.; Schefer, C.; Sobieraj, A.M.; Zinsli, L.V.; Mairpady Shambat, S.; Leimer, N.; et al. Targeting Hidden Pathogens: Cell-Penetrating Enzybiotics Eradicate Intracellular Drug-Resistant Staphylococcus aureus. MBio 2020, 11, e00209-20. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, S.; Oves, M.; Khan, A.U. Obliteration of Bacterial Growth and Biofilm through ROS Generation by Facilely Synthesized Green Silver Nanoparticles. PLoS ONE 2017, 12, e0181363. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, S.; Elsherif, W.M.; Hamed, R. Effect of Silver Nanoparticles on Vancomycin Resistant Staphylococcus aureus Infection in Critically Ill Patients. Pathog. Glob. Health 2021, 115, 315–324. [Google Scholar] [CrossRef]
- Duchesne, C.; Frescaline, N.; Blaise, O.; Lataillade, J.-J.; Banzet, S.; Dussurget, O.; Rousseau, A. Cold Atmospheric Plasma Promotes Killing of Staphylococcus aureus by Macrophages. mSphere 2021, 6, e00217-21. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lim, D.J.; Lee, M.Y.; Lee, W.J.; Chang, S.E.; Won, C.H. Prospective, Comparative Clinical Pilot Study of Cold Atmospheric Plasma Device in the Treatment of Atopic Dermatitis. Sci. Rep. 2021, 11, 14461. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V.; Khaitov, R.M. Strategies for Using Muramyl Peptides—Modulators of Innate Immunity of Bacterial Origin—in Medicine. Front. Immunol. 2021, 12, 607178. [Google Scholar] [CrossRef]
- Ahn, K.B.; Jeon, J.H.; Baik, J.E.; Park, O.-J.; Kang, S.-S.; Yun, C.-H.; Park, J.-H.; Han, S.H. Muramyl Dipeptide Potentiates Staphylococcal Lipoteichoic Acid Induction of Cyclooxygenase-2 Expression in Macrophages. Microbes Infect. 2014, 16, 153–160. [Google Scholar] [CrossRef]
- Bougarn, S.; Cunha, P.; Harmache, A.; Fromageau, A.; Gilbert, F.B.; Rainard, P. Muramyl Dipeptide Synergizes with Staphylococcus aureus Lipoteichoic Acid to Recruit Neutrophils in the Mammary Gland and to Stimulate Mammary Epithelial Cells. Clin. Vaccine Immunol. 2010, 17, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
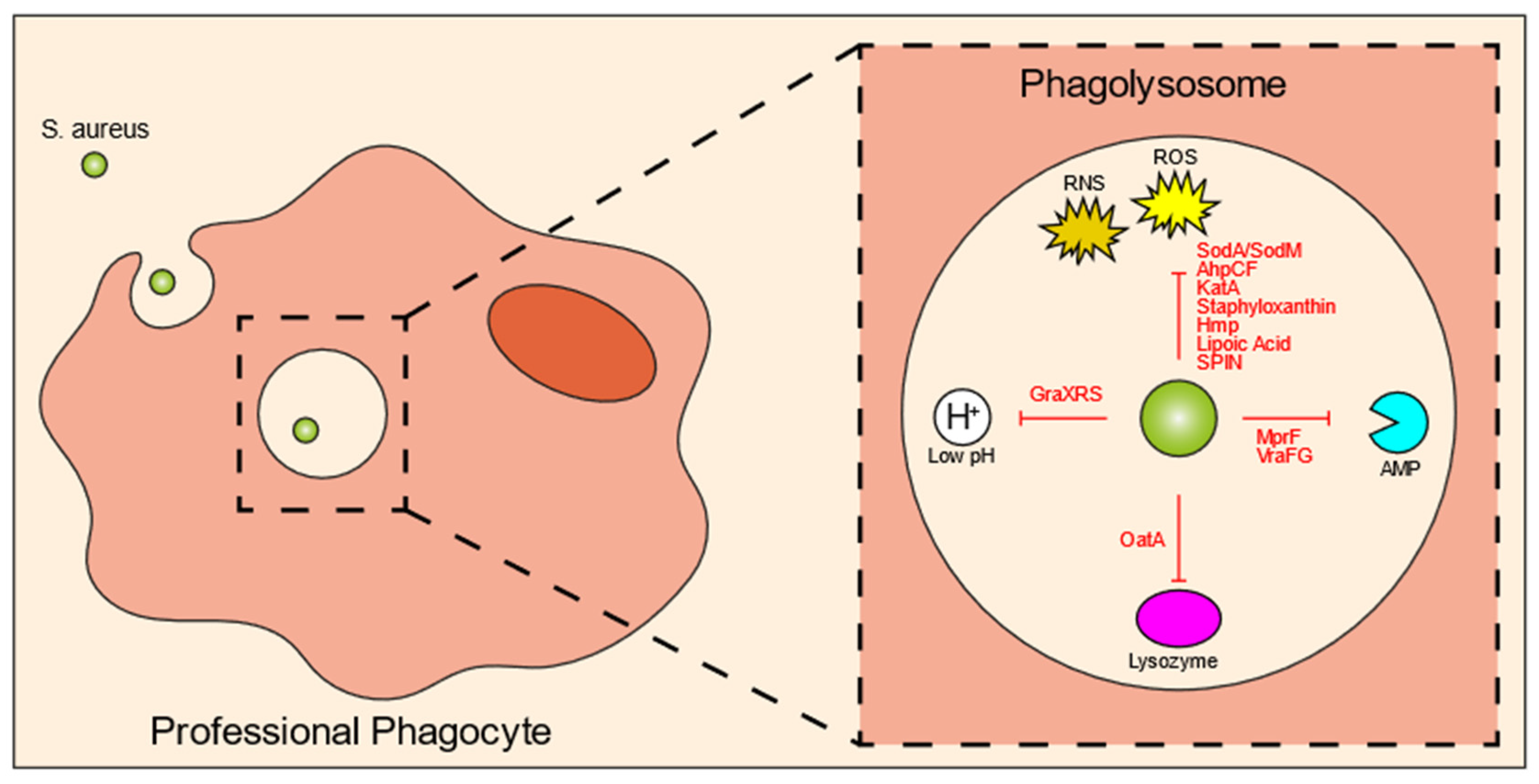

| Intracellular Killing Mechanisms | S. aureus Evasion Molecule | Mechanism/Explanation | References |
|---|---|---|---|
| ROS | SodA and SodM | Incapacitate superoxide radicals | Karavolos et al., 2003; Das, Saha and Bishayi 2008 [57,58] |
| AhpCF | Resists peroxides | Cosgrove et al., 2007; Mashruwala and Boyd 2017 [59,60] | |
| KatA | Resists H2O2 | Cosgrove et al., 2007; Mashruwala and Boyd 2017 [59,60] | |
| Staphyloxanthin | Antioxidant | Pandey, Sahukhal and Elasri 2019 [61] | |
| Hmp | Resistance to nitric oxide | Nobre, Gonçalves and Saraiva 2008 [62] | |
| lipoic acid | Restricts ROS and RNS production | Grayczyk et al., 2019 [63] | |
| SPIN | Inhibits MPO | de Jong et al., 2017 [64] | |
| Acidification | GraXRS | Senses low pH and allows resistance to acidic environment | Flannagan et al., 2018 [67] |
| Enzymes | OatA | Modifies lysozymal target | Bera et al., 2006; Shimada et al., 2010 [70,71] |
| Antimicrobial peptides | MprF | Resistance to defensins and protegrins | Kristian et al., 2003; Peschel et al., 2001; Peschel et al., 1999 [72,73,74] |
| VraFG | Promotes resistance to cationic AMPs | Li et al., 2007 [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hommes, J.W.; Surewaard, B.G.J. Intracellular Habitation of Staphylococcus aureus: Molecular Mechanisms and Prospects for Antimicrobial Therapy. Biomedicines 2022, 10, 1804. https://doi.org/10.3390/biomedicines10081804
Hommes JW, Surewaard BGJ. Intracellular Habitation of Staphylococcus aureus: Molecular Mechanisms and Prospects for Antimicrobial Therapy. Biomedicines. 2022; 10(8):1804. https://doi.org/10.3390/biomedicines10081804
Chicago/Turabian StyleHommes, Josefien W., and Bas G. J. Surewaard. 2022. "Intracellular Habitation of Staphylococcus aureus: Molecular Mechanisms and Prospects for Antimicrobial Therapy" Biomedicines 10, no. 8: 1804. https://doi.org/10.3390/biomedicines10081804
APA StyleHommes, J. W., & Surewaard, B. G. J. (2022). Intracellular Habitation of Staphylococcus aureus: Molecular Mechanisms and Prospects for Antimicrobial Therapy. Biomedicines, 10(8), 1804. https://doi.org/10.3390/biomedicines10081804






