Combined Hepatocellular-Cholangiocarcinoma: An Update on Pathology and Diagnostic Approach
Abstract
:1. Introduction
2. Clinical Features
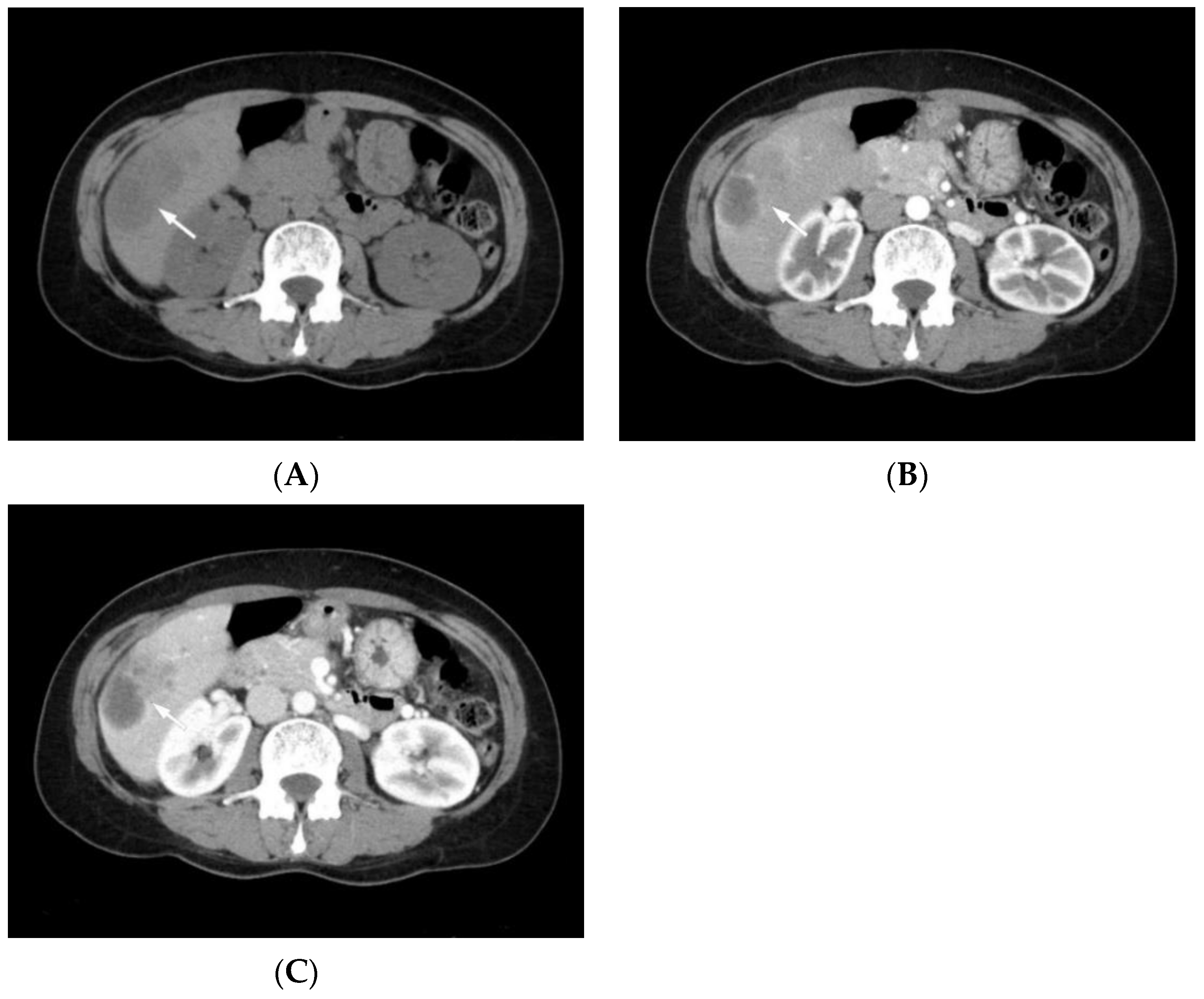
3. Radiological Features
4. Etiology
5. Pathogenesis
6. Pathological Features
6.1. Historical Background in the Classification of cHCC-CCA
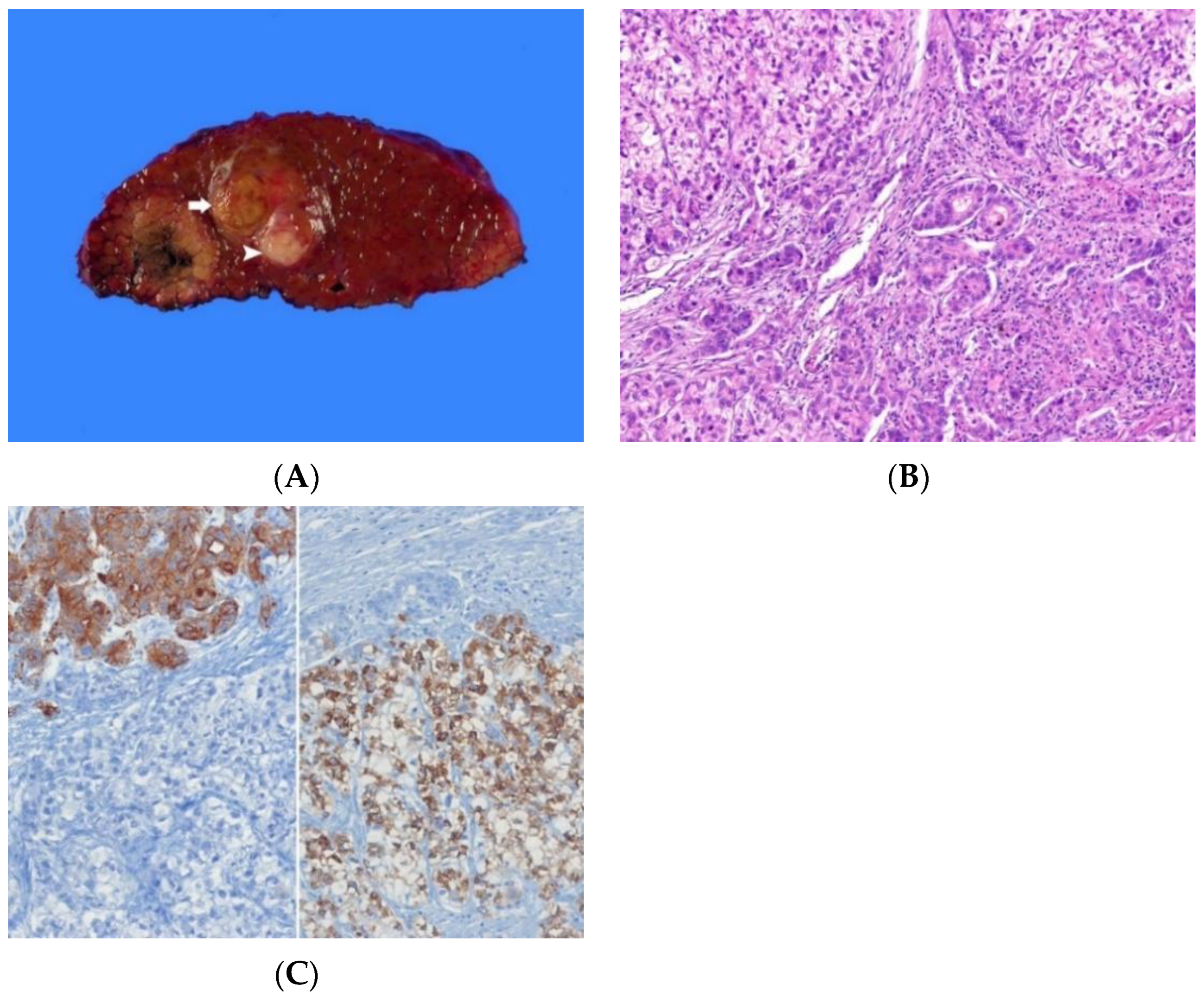
6.2. Macroscopic Appearance
6.3. Histopathology
6.3.1. Combined Hepatocellular-Cholangiocarcinoma
6.3.2. Intermediate Cell Carcinoma
6.3.3. Cholangiolocarcinoma
7. Immunohistochemical Features
8. Molecular Features
9. Diagnostic Approach and Differential Diagnosis
9.1. Specimen Handling
9.1.1. Biopsy Specimen
9.1.2. Partial Hepatectomy Specimen
9.2. Diagnostic Approach
9.3. Differential Diagnosis
10. Future Perspectives
11. Prognosis
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sempoux, C.; Kakar, S.; Kondo, F.; Schirmacher, P. Combined hepatocellular-cholangiocarcinoma and undifferentiated primary liver carcinoma. In WHO Classification of Tumours. Digestive System Tumours, 5th ed.; The WHO Classification of Tumours Editorial Board, Ed.; IARC Press: Lyon, France, 2019; pp. 260–262. [Google Scholar]
- Brunt, E.; Aishima, S.; Clavien, P.A.; Fowler, K.; Goodman, Z.; Gores, G.; Gouw, A.; Kagen, A.; Klimstra, D.; Komuta, M.; et al. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentiation. Hepatology 2018, 68, 113–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, H. Primary carcinoma of the liver. Am. J. Med. Sci. 1903, 126, 1827–1924. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavraka, C.; Rush, H.; Ross, P. Combined hepatocellular cholangiocarcinoma (cHCC-CCA): An update of genetics, molecular biology, and therapeutic interventions. J. Hepatocell. Carcinoma 2018, 6, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schizas, D.; Mastoraki, A.; Routsi, E.; Papapanou, M.; Tsapralis, D.; Vassiliu, P.; Toutouzas, K.; Felekouras, E. Combined hepatocellular-cholangiocarcinoma: An update on epidemiology, classification, diagnosis and management. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Komuta, M.; Yeh, M.M. A review on the update of combined hepatocellular cholangiocarcinoma. Semin. Liver Dis. 2020, 40, 124–130. [Google Scholar] [CrossRef]
- Beaufrère, A.; Calderaro, J.; Paradis, V. Combined hepatocellular-cholangiocarcinoma: An update. J. Hepatol. 2021, 74, 1212–1224. [Google Scholar] [CrossRef]
- Garancini, M.; Goffredo, P.; Pagni, F.; Romano, F.; Roman, S.; Sosa, J.A.; Giardini, V. Combined hepatocellular-cholangiocarcinoma: A population-level analysis of an uncommon primary liver tumor. Liver Transpl. 2014, 20, 952–959. [Google Scholar] [CrossRef]
- Li, R.; Yang, D.; Tang, C.L.; Cai, P.; Ma, K.S.; Ding, S.Y.; Zhang, X.H.; Guo, D.Y.; Yan, X.C. Combined hepatocellular carcinoma and cholangiocarcinoma (biphenotypic) tumors: Clinical characteristics, imaging features of contrast-enhanced ultrasound and computed tomography. BMC Cancer 2016, 16, 158. [Google Scholar] [CrossRef] [Green Version]
- Maeda, T.; Adachi, E.; Kajiyama, K.; Sugimachi, K.; Tsuneyoshi, M. Combined hepatocellular and cholangiocarcinoma: Proposed criteria according to cytokeratin expression and analysis of clinicopathologic features. Hum. Pathol. 1995, 26, 956–964. [Google Scholar] [CrossRef]
- Raevskaya, O.; Appelman, H.; Razumilava, N. A contemporary approach to diagnosis and treatment of combined hepatocellular-cholangiocarcinoma. Curr. Hepatol. Rep. 2020, 19, 478–485. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Deng, M.; Wu, H.Y.; Shi, J.; Mao, L.; Sun, Q.; Tang, M.; Fan, X.S.; Qiu, Y.D.; et al. Clinicopathological, radiologic, and molecular study of 23 combined hepatocellular-cholangiocarcinomas with stem cell features, cholangiolocellular type. Hum. Pathol. 2017, 64, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Shang, X.S.; Wang, W.T.; Hu, X.X.; Zeng, M.S.; Rao, S.X. Different MR features for differentiation of intrahepatic mass-forming cholangiocarcinoma from hepatocellular carcinoma according to tumor size. Br. J. Radiol. 2018, 91, 20180017. [Google Scholar] [CrossRef]
- Abdel Razek, A.A.K.; El-Serougy, L.G.; Saleh, G.A.; Shabana, W.; Abd El-Wahab, R. Liver imaging reporting and data system version 2018: What radiologists need to know. J. Comput. Assist. Tomogr. 2020, 44, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, H.; Joo, I.; Lee, J.M. Combined hepatocellular-cholangiocarcinoma: Changes in the 2019 World Health Organization histological classification system and potential impact on imaging-based diagnosis. Korean J. Radiol. 2020, 21, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Potretzke, T.A.; Tan, B.R.; Doyle, M.B.; Brunt, E.M.; Heiken, J.P.; Fowler, K.J. Imaging features of biphenotypic primary liver carcinoma (hepatocholangiocarcinoma) and the potential to mimic hepatocellular carcinoma: LI-RADS analysis of CT and MRI features in 61 cases. AJR Am. J. Roentgenol. 2016, 207, 25–31. [Google Scholar] [CrossRef]
- Wells, M.L.; Venkatesh, S.K.; Chandan, V.S.; Fidler, J.L.; Fletcher, J.G.; Johnson, G.B.; Hough, D.M.; Roberts, L.R. Biphenotypic hepatic tumors: Imaging findings and review of literature. Abdom. Imaging 2015, 40, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Venanzio Setola, S.; Sandomenico, F.; Luisa Barretta, M.; Belli, A.; Palaia, R.; Tatangelo, F.; Grassi, R.; Izzo, F.; et al. Major and ancillary features according to LI-RADS in the assessment of combined hepatocellular- cholangiocarcinoma. Radiol Oncol. 2020, 54, 149–158. [Google Scholar] [CrossRef]
- American College of Radiology. CT/MR Liver Imaging Reporting and Data System Version 2018. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LIRADS-v2018 (accessed on 10 April 2022).
- Zou, X.; Luo, Y.; Morelli, J.N.; Hu, X.; Shen, Y.; Hu, D. Differentiation of hepatocellular carcinoma from intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma in high-risk patients matched to MR field strength: Diagnostic performance of LI-RADS version 2018. Abdom. Radiol. 2021, 46, 3168–3178. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, S.; Zhao, L.; Zhang, X.; Li, M.; Ding, J.; Yan, K.; Jing, X. CEUS and CT/MRI LI-RADS in association with serum biomarkers for differentiation of combined hepatocellular-cholangiocarcinoma from hepatocellular carcinoma. Front. Oncol. 2022, 12, 897090. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Weber, S.; Tickoo, S.K.; Koea, J.B.; Obiekwe, S.; Fong, Y.; DeMatteo, R.P.; Blumgart, L.H.; Klimstra, D. Combined hepatocellular and cholangiocarcinoma: Demographic, clinical, and prognostic factors. Cancer 2002, 94, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, Y.; Aishima, S.; Kuroda, Y.; Iguchi, T.; Taguchi, K.; Asayama, Y.; Taketomi, A.; Kinukawa, N.; Honda, H.; Tsuneyoshi, M. Biliary phenotype of hepatocellular carcinoma after preoperative transcatheter arterial chemoembolization. J. Gastroenterol. Hepatol. 2008, 23, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Zen, C.; Zen, Y.; Mitry, R.R.; Corbeil, D.; Karbanová, J.; O’Grady, J.; Karani, J.; Kane, P.; Heaton, N.; Portmann, B.C.; et al. Mixed phenotype hepatocellular carcinoma after transarterial chemoembolization and liver transplantation. Liver Transpl. 2011, 17, 943–954. [Google Scholar] [CrossRef]
- Kojiro, M. Pathology of Hepatocellular Carcinoma; Blackwell Publishing Ltd.: Malden, MA, USA, 2006; pp. 105–115. [Google Scholar]
- Theise, N.D.; Yao, J.L.; Harada, K.; Hytiroglou, P.; Portmann, B.; Thung, S.N.; Tsui, W.; Ohta, H.; Nakanuma, Y. Hepatic ‘stem cell’ malignancies in adults: Four cases. Histopathology 2003, 43, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Qian, M.; Chen, I.H.; Finkelstein, D.; Onar-Thomas, A.; Johnson, M.; Calabrese, C.; Bahrami, A.; López-Terrada, D.H.; Yang, J.J.; et al. acquisition of cholangiocarcinoma traits during advanced hepatocellular carcinoma development in mice. Am. J. Pathol. 2018, 188, 656–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, R.A.; Lisa, J.R. Combined liver cell and bile duct carcinoma. Am. J. Pathol. 1949, 25, 647–655. [Google Scholar]
- Edmondson, H.A.; Steiner, P.E. Primary carcinoma of the liver: A study of 100 cases among 48,900 necropsies. Cancer 1954, 7, 462–503. [Google Scholar] [CrossRef]
- Goodman, Z.D.; Ishak, K.G.; Langloss, J.M.; Sesterhenn, I.A.; Rabin, L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer 1985, 55, 124–135. [Google Scholar] [CrossRef]
- Wittekind, C.; Fischer, H.P.; Ponchon, T. Combined hepatocellular-cholangiocarcinoma. In WHO Classification of Tumours of Digestive System, 3rd ed.; Hamilton, S.R., Aaltonen, L.A., Eds.; IARC Press: Lyon, France, 2000; p. 181. [Google Scholar]
- Theise, N.D.; Nakashima, O.; Park, Y.N.; Nakanuma, Y. Combined hepatocellular-cholangiocarcinoma. In WHO Classification of Tumours of Digestive System, 4th ed.; Bosman, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; IARC Press: Lyon, France, 2010; pp. 225–227. [Google Scholar]
- Akiba, J.; Nakashima, O.; Hattori, S.; Tanikawa, K.; Takenaka, M.; Nakayama, M.; Kondo, R.; Nomura, Y.; Koura, K.; Ueda, K.; et al. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am. J. Surg. Pathol. 2013, 37, 496–505. [Google Scholar] [CrossRef]
- Sasaki, M.; Sato, H.; Kakuda, Y.; Sato, Y.; Choi, J.H.; Nakanuma, Y. Clinicopathological significance of ‘subtypes with stem-cell feature’ in combined hepatocellular- cholangiocarcinoma. Liver Int. 2015, 35, 1024–1035. [Google Scholar] [CrossRef]
- Torbenson, M.; Zen, Y.; Yeh, M.M. Combined hepatocellular-cholangiocarcinoma. In Tumors of the Liver. AFIP Atlas of Tumor Pathology; Series 4, Fascicle 27; American Registry of Pathology: Washington, DC, USA, 2018; pp. 265–278. [Google Scholar]
- Torbenson, M.; Zen, Y.; Yeh, M.M. Hepatocellular carcinoma. In Tumors of the Liver. AFIP Atlas of Tumor Pathology; Series 4, Fascicle 27; American Registry of Pathology: Washington, DC, USA, 2018; pp. 39–88. [Google Scholar]
- Torbenson, M.; Zen, Y.; Yeh, M.M. Intrahepatic cholangiocarcinoma and precursor lesions. In Tumors of the Liver. AFIP Atlas of Tumor Pathology; Series 4, Fascicle 27; American Registry of Pathology: Washington, DC, USA, 2018; pp. 201–254. [Google Scholar]
- De Vito, C.; Sarker, D.; Ross, P.; Heaton, N.; Quaglia, A. Histological heterogeneity in primary and metastatic classic combined hepatocellular-cholangiocarcinoma: A case series. Virchows Arch. 2017, 471, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Robrechts, C.; De Vos, R.; Van den Heuvel, M.; Van Cutsem, E.; Van Damme, B.; Desmet, V.; Roskams, T. Primary liver tumour of intermediate (hepatocyte-bile duct cell) phenotype: A progenitor cell tumour? Liver 1998, 18, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, C.; Han, K.H.; Choi, J.; Kim, Y.B.; Kim, J.K.; Park, Y.N. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J. Hepatol. 2004, 40, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Steiner, P.E.; Higginson, J. Cholangiolocellular carcinoma of the liver. Cancer 1959, 12, 753–759. [Google Scholar] [CrossRef]
- Balitzer, D.; Joseph, N.M.; Ferrell, L.; Shafizadeh, N.; Jain, D.; Zhang, X.; Yeh, M.; di Tommaso, L.; Kakar, S. Immunohistochemical and molecular features of cholangiolocellular carcinoma are similar to well-differentiated intrahepatic cholangiocarcinoma. Mod. Pathol. 2019, 32, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Nakanuma, Y.; Klimstra, D.S.; Komuta, M.; Zen, Y. Intrahepatic cholangiocarcinoma. In WHO Classification of Tumours. Digestive System Tumours, 5th ed.; The WHO Classification of Tumours Editorial Board, Ed.; IARC Press: Lyon, France, 2019; pp. 254–259. [Google Scholar]
- Brunt, E.M.; Paradis, V.; Sempoux, C.; Theise, N.D. Biphenotypic (hepatobiliary) primary liver carcinomas: The work in progress. Hepat. Oncol. 2015, 2, 255–273. [Google Scholar] [CrossRef] [Green Version]
- Sempoux, C.; Paradis, V.; Saxena, R. Variant differentiation patterns in primary liver carcinoma. Semin. Diagn. Pathol. 2017, 34, 176–182. [Google Scholar] [CrossRef]
- El Jabbour, T.; Lagana, S.M.; Lee, H. Update on hepatocellular carcinoma: Pathologists’ review. World J. Gastroenterol. 2019, 25, 1653–1665. [Google Scholar] [CrossRef]
- Sciarra, A.; Park, Y.N.; Sempoux, C. Updates in the diagnosis of combined hepatocellular-cholangiocarcinoma. Hum. Pathol. 2020, 96, 48–55. [Google Scholar] [CrossRef]
- Kim, H.; Choi, G.H.; Na, D.C.; Ahn, E.Y.; Kim, G.I.; Lee, J.E.; Cho, J.Y.; Yoo, J.E.; Choi, J.S.; Park, Y.N. Human hepatocellular carcinomas with “Stemness”-related marker expression: Keratin 19 expression and a poor prognosis. Hepatology 2011, 54, 1707–1717. [Google Scholar] [CrossRef]
- Yokomichi, N.; Nishida, N.; Umeda, Y.; Taniguchi, F.; Yasui, K.; Toshima, T.; Mori, Y.; Nyuya, A.; Tanaka, T.; Yamada, T.; et al. heterogeneity of epigenetic and epithelial mesenchymal transition marks in hepatocellular carcinoma with keratin 19 proficiency. Liver Cancer 2019, 8, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Chen, L.; Zhang, C.; Fujita, M.; Li, R.; Yan, S.M.; Ong, C.K.; Liao, X.; Gao, Q.; Sasagawa, S.; et al. genomic and transcriptomic profiling of combined hepatocellular and intrahepatic cholangiocarcinoma reveals distinct molecular subtypes. Cancer Cell 2019, 35, 932–947. [Google Scholar] [CrossRef] [PubMed]
- Malvi, D.; de Biase, D.; Fittipaldi, S.; Grillini, M.; Visani, M.; Pession, A.; D’Errico, A.; Vasuri, F. Immunomorphology and molecular biology of mixed primary liver cancers: Is Nestin a marker of intermediate-cell carcinoma? Histopathology 2020, 76, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Sato, Y.; Nakanuma, Y. Is nestin a diagnostic marker for combined hepatocellular-cholangiocarcinoma? Histopathology 2022, 80, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Coulouarn, C.; Cavard, C.; Rubbia-Brandt, L.; Audebourg, A.; Dumont, F.; Jacques, S.; Just, P.A.; Clément, B.; Gilgenkrantz, H.; Perret, C.; et al. Combined hepatocellular-cholangiocarcinomas exhibit progenitor features and activation of Wnt and TGFβ signaling pathways. Carcinogenesis 2012, 33, 1791–1796. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, A.; Furuta, M.; Shiraishi, Y.; Gotoh, K.; Kawakami, Y.; Arihiro, K.; Nakamura, T.; Ueno, M.; Ariizumi, S.; Nguyen, H.H.; et al. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat. Commun. 2015, 6, 6120. [Google Scholar] [CrossRef]
- Moeini, A.; Sia, D.; Zhang, Z.; Camprecios, G.; Stueck, A.; Dong, H.; Montal, R.; Torrens, L.; Martinez-Quetglas, I.; Fiel, M.I.; et al. Mixed hepatocellular cholangiocarcinoma tumors: Cholangiolocellular carcinoma is a distinct molecular entity. J. Hepatol. 2017, 66, 952–961. [Google Scholar] [CrossRef] [Green Version]
- Joseph, N.M.; Tsokos, C.G.; Umetsu, S.E.; Shain, A.H.; Kelley, R.K.; Onodera, C.; Bowman, S.; Talevich, E.; Ferrell, L.D.; Kakar, S.; et al. Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. J. Pathol. 2019, 248, 164–178. [Google Scholar] [CrossRef]
- Komuta, M. Histological heterogeneity of primary liver cancers: Clinical relevance, diagnostic pitfalls and the pathologist’s role. Cancers 2021, 13, 2871. [Google Scholar] [CrossRef]
- Gigante, E.; Ronot, M.; Bertin, C.; Ciolina, M.; Bouattour, M.; Dondero, F.; Cauchy, F.; Soubrane, O.; Vilgrain, V.; Paradis, V. Combining imaging and tumour biopsy improves the diagnosis of combined hepatocellular-cholangiocarcinoma. Liver Int. 2019, 39, 2386–2396. [Google Scholar] [CrossRef]
- Torbenson, M.S. Liver. In Surgical Pathology Dissection. An Illustrated Guide, 2nd ed.; Westra, W.H., Hruban, R.H., Phelps, T.H., Isacson, C., Eds.; Springer: New York, NY, USA, 2003; pp. 76–81. [Google Scholar]
- Torbenson, M.S. Masses of the liver. In Mills and Sternberg’s Diagnostic Surgical Pathology, 7th ed.; Longacre, T.A., Greenson, J.K., Hornick, J.L., Reuter, V.E., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2022; pp. 1890–1949. [Google Scholar]
- Saraf, S.A.; Tan, L.L.Y.; Leow, W.Q. A diagnostic approach to combined hepatocellular- cholangocarcinomas (cHCC-CCA). Am. J. Clin. Case Rep. 2021, 1, AJCCR-01-1020. [Google Scholar]
- Westerhoff, M.; Lamps, L.W.; Kakar, S. Hepatectomy specimen handling. In Diagnostic Pathology. Hepatobiliary and Pancreas, 3rd ed.; Elsevier: Philadelphia, PA, USA, 2022; pp. 348–349. [Google Scholar]
- Torbenson, M.S. Diagnostic approach to well-differentiated hepatocellular carcinoma. Diagn. Histopathol. 2021, 28, 69–78. [Google Scholar] [CrossRef]
- Suriawinata, A.A.; Thung, S.N. Liver Pathology. An Atlas and Concise Guide; Demos Medicla Publishing: New York, NY, USA, 2011; pp. 1–15. [Google Scholar]
- Torbenson, M.S.; Ng, I.O.L.; Park, Y.N.; Roncalli, M.; Sakamato, M. Hepatocellular carcinoma. In WHO Classification of Tumours. Digestive System Tumours, 5th ed.; The WHO Classification of Tumours Editorial Board, Ed.; IARC Press: Lyon, France, 2019; pp. 229–239. [Google Scholar]
- Westerhoff, M.; Lamps, L.W.; Kakar, S. Combined hepatocellular-cholangiocarcinoma. In Diagnostic Pathology. Hepatobiliary and Pancreas, 3rd ed.; Elsevier: Philadelphia, PA, USA, 2022; pp. 294–297. [Google Scholar]
- Lee, S.H.; Luong, T.V. Combined hepatocellular-cholangiocarcinoma: A rare biphenotypic primary liver cancer. Diagn. Histopathol. 2021, 28, 116–118. [Google Scholar] [CrossRef]
- Yeh, M.M. Pathology of combined hepatocellular-cholangiocarcinoma. J. Gastroenterol. Hepatol. 2010, 25, 1485–1492. [Google Scholar] [CrossRef]
- Cui, K.; Ou, Y.; Shen, Y.; Li, S.; Sun, Z. Clinical value of circulating tumor cells for the diagnosis and prognosis of hepatocellular carcinoma (HCC): A systematic review and meta-analysis. Medicine 2020, 99, e22242. [Google Scholar] [CrossRef] [PubMed]
- Trevisan França de Lima, L.; Broszczak, D.; Zhang, X.; Bridle, K.; Crawford, D.; Punyadeera, C. The use of minimally invasive biomarkers for the diagnosis and prognosis of hepatocellular carcinoma. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188451. [Google Scholar] [CrossRef]
- Baj, J.; Bryliński, Ł.; Woliński, F.; Granat, M.; Kostelecka, K.; Duda, P.; Flieger, J.; Teresiński, G.; Buszewicz, G.; Furtak-Niczyporuk, M.; et al. Biomarkers and genetic markers of hepatocellular carcinoma and cholangiocarcinoma-What do we already know. Cancers 2022, 14, 1493. [Google Scholar] [CrossRef]
- Cutolo, C.; Dell’Aversana, F.; Fusco, R.; Grazzini, G.; Chiti, G.; Simonetti, I.; Bruno, F.; Palumbo, P.; Pierpaoli, L.; Valeri, T.; et al. Combined hepatocellular-cholangiocarcinoma: What the multidisciplinary team should know. Diagnostics 2022, 12, 890. [Google Scholar] [CrossRef]
- Calderaro, J.; Seraphin, T.P.; Luedde, T.; Simon, T.G. Artificial intelligence for the prevention and clinical management of hepatocellular carcinoma. J. Hepatol. 2022, 76, 1348–1361. [Google Scholar] [CrossRef]
- Schmauch, B.; Romagnoni, A.; Pronier, E.; Saillard, C.; Maillé, P.; Calderaro, J.; Kamoun, A.; Sefta, M.; Toldo, S.; Zaslavskiy, M.; et al. A deep learning model to predict RNA-Seq expression of tumours from whole slide images. Nat. Commun. 2020, 11, 3877. [Google Scholar] [CrossRef] [PubMed]
- Saillard, C.; Schmauch, B.; Laifa, O.; Moarii, M.; Toldo, S.; Zaslavskiy, M.; Pronier, E.; Laurent, A.; Amaddeo, G.; Regnault, H.; et al. Predicting survival after hepatocellular carcinoma resection using deep learning on histological slides. Hepatology 2020, 72, 2000–2013. [Google Scholar] [CrossRef] [PubMed]
- Aloia, T.; Pawlik, T.M.; Taouli, B.; Rubbia-Brandt, L.; Vauthey, J.N. Intrahepatic bile ducts. In AJCC Caner Staging Manual, 8th ed.; Amin, M.B., Ed.; American Joint Committee on Cancer, Springer: Chicago, IL, USA, 2017; pp. 295–302. [Google Scholar]
- Tickoo, S.K.; Zee, S.Y.; Obiekwe, S.; Xiao, H.; Koea, J.; Robiou, C.; Blumgart, L.H.; Jarnagin, W.; Ladanyi, M.; Klimstra, D.S. Combined hepatocellular-cholangiocarcinoma: A histopathologic, immunohistochemical, and in situ hybridization study. Am. J. Surg. Pathol. 2002, 26, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Ariizumi, S.; Kotera, Y.; Katagiri, S.; Nakano, M.; Yamamoto, M. Combined hepatocellular-cholangiocarcinoma had poor outcomes after hepatectomy regardless of Allen and Lisa class or the predominance of intrahepatic cholangiocarcinoma cells within the tumor. Ann. Surg. Oncol. 2012, 19, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Lee, K.W.; Heo, J.S.; Kim, S.J.; Choi, S.H.; Kim, Y.I.; Joh, J.W. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg. Today 2006, 36, 892–897. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, B.H.; Qiu, S.J.; Ren, Z.G.; Zhou, J.; Chen, X.H.; Zhou, Y.; Fan, J. Combined hepatocellular carcinoma and cholangiocarcinoma: Clinical features, treatment modalities, and prognosis. Ann. Surg. Oncol. 2012, 19, 2869–2876. [Google Scholar] [CrossRef]
- Wachtel, M.S.; Zhang, Y.; Xu, T.; Chiriva-Internati, M.; Frezza, E.E. Combined hepatocellular cholangiocarcinomas; analysis of a large database. Clin. Med. Pathol. 2008, 1, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Yap, A.Q.; Chen, C.L.; Yong, C.C.; Kuo, F.Y.; Wang, S.H.; Lin, C.C.; Liu, Y.W.; Lin, T.L.; Li, W.F.; Millan, C.A.; et al. Clinicopathological factors impact the survival outcome following the resection of combined hepatocellular carcinoma and cholangiocarcinoma. Surg. Oncol. 2013, 22, 55–60. [Google Scholar] [CrossRef]





| 2000 WHO Classification (3rd Edition) | 2010 WHO Classification (4th Edition) | 2019 WHO Classification (5th Edition) | |
|---|---|---|---|
| Tumor category | Malignant epithelial tumors | Malignancies of mixed or uncertain origin | Malignant biliary tumors |
| Tumor entities or subtypes | cHCC-CCA | cHCC-CCA, classical type | cHCC-CCA (b) |
| cHCC-CCA with stem cell features (a), typical subtype | |||
| cHCC-CCA with stem cell features, intermediate-cell type | Intermediate cell carcinoma (c) | ||
| cHCC-CCA with stem cell features, cholangiolocellular type | Cholangiolocarcinoma (d) |
| HCC | CCA | cHCC-CCA | Stem/Progenitor Cell | ICC | CLC | |
|---|---|---|---|---|---|---|
| Tumor cell morphology | Polygonal tumor cells with round nuclei and abundant eosinophilic cytoplasm | Small to medium-sized, cuboidal or columnar cells with palely eosinophilic or vacuolated cytoplasm | Tumor cell morphology showing both unequivocal hepatocytic and cholangiocytic differentiation | Small uniform cells with hyperchromatic nuclei, scant cytoplasm, and a high nuclear/ cytoplasmic ratio | Tumor cells are smaller than normal hepatocytes, but larger than stem/progenitor cells; monotonous intermediate features between hepatocytes and cholangiocytes | Tumor cells resembling cholangioles (or canals of Hering); usually much smaller than normal hepatocytes and relatively less cytoplasm |
| Architecture | Trabecular, solid, pseudoglandular pattern | Glandular or tubular pattern with a variable-sized lumen, solid, cord-like, or micropapillary pattern | Two components are either close to each other or intermingled; the transition between them can be poorly defined or sharp | Small nests | Trabeculae, cords, solid nests, or strands | Tubular, cord-like, anastomosing pattern (antler-like pattern) or thin, malignant ductular-like structure |
| Bile | Present | Absent | Present | Absent | Absent | Absent |
| Mucin | Absent | Present | Present | Absent | Absent | Absent |
| Other histologic features | Steatosis, Mallory-Denk bodies, hyaline bodies, pale bodies | Frequently abundant fibrous stroma | Transitional area between HCC and CCA components shows mixed features with intermediate morphology | Most often found at interface between a nest of carcinoma and the adjoining tumoral desmoplastic stroma | Marked desmoplastic or acellular hyalinized stroma | Densely hyalinized stroma; may show trabecular and replacing growth at its interface with the surrounding nontumorous liver |
| Marker | Staining Pattern | Approximate Positivity | Comment | |
|---|---|---|---|---|
| Hepatocytic differentiation | Arginase-1 | Nuclear & cytoplasmic | 90% of HCC | Better than HepPar-1 in poorly differentiated HCC |
| HepPar-1 | Cytoplasmic | 90% of HCC | Better than Arginase-1 in well differentiated HCC | |
| Glypican-3 | Cytoplasmic | 70–90% of HCC | Poorly differentiated HCCs are more likely to be positive | |
| Polyclonal CEA | Canalicular | 60–80% of HCC | Poorly differentiated HCCs are frequently negative | |
| CD10 | Canalicular | 60–80% of HCC | Poorly differentiated HCCs are frequently negative | |
| Alpha-fetoprotein | Cytoplasmic | 30% of HCC | Well differentiated HCCs are frequently negative | |
| Albumin mRNA in situ hybridization | Cytoplasmic | >95% of HCC | ||
| Cholangiocytic differentiation | Cytokeratin 7 | Cytoplasmic | 90% of CCA | |
| Cytokeratin 19 | Cytoplasmic | 80–90% of CCA | ||
| EpCAM (MOC31) | Membrane | 80–90% of CCA | ||
| CA19-9 | Cytoplasmic | 60% of CCA | ||
| Albumin mRNA in situ hybridization | Cytoplasmic | 50–90% of CCA | 90% of small duct type; 50% of large duct type | |
| Stem/progenitor cells | CK19 | Cytoplasmic | ||
| EpCAM (MOC31) | Membrane | |||
| CD56 (NCAM) | Cytoplasm | |||
| CD117 (KIT) | Cytoplasmic | |||
| CD133 | Cytoplasm | |||
| SALL4 | Nuclear |
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor Classification | Hepatocellular Carcinoma | cHCC-CCA | ICC | CLC | Intrahepatic Cholangiocarcinoma | |||||
| HCC | HCC with SPCF/P | HCC with Cholangiocyte IHC Expression | cHCC-CCA | cHCC-CCA with SPCF/P | iCCA with Hepatocyte IHC Expression | iCCA with SPCF/P | iCCA | |||
| Histological features | Typical HCC | Typical HCC with SPCF | Typical HCC | Typical HCC & CCA | Typical HCC & CCA with SPCF | Typical intermediate cell features | Typical CLC features (>80% of tumor cells) | Typical CCA | Typical CCA with SPCF | Typical CCA |
| Immuno- histochemical features | Hepatocytic markers (+) | Hepatocytic markers (+) & stem/progenitor cell makers (+) | Hepatocytic markers (+) & cholangiocytic markers (+) | Hepatocytic markers (+) & cholangiocytic markers (+) | Hepatocytic markers (+), cholangiocytic markers (+) & stem/progenitor cell markers (+) | Hepatocytic markers (+) & cholangiocytic markers (+) | Cholangiocytic markers (+) & CD56 (NCAM) (+), CD117 (KIT) (+) | Cholangiocytic markers (+) & hepatocytic markers (+) | Cholangiocytic markers (+) & stem/progenitor cell makers (+) | Cholangiocytic markers (+) |
| Hepatocellular Carcinoma | Intrahepatic Cholangiocarcinoma | cHCC-CCA |
|---|---|---|
| TP53 mutations (60%) TERT gene promoter mutations (50–60%) CTNNB1 mutations (40%) | KRAS mutations (20% of large duct type; ~0% of small duct type) TP53 mutations (30% of large duct type) IDH1 mutations (15% of small duct type; ~0% of large duct type) FGRF2 translocation (10% of small duct type; ~0% of large duct type) ARID1A mutations BAP1 mutations PBRM1 mutations | TP53 mutations (80%) TERT promoter mutations (80%) KRAS mutations (55%) CTNNB1 mutations (20%) AXIN1 mutations (20%) IDH1 mutations KMT2D mutations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.H.; Ro, J.Y. Combined Hepatocellular-Cholangiocarcinoma: An Update on Pathology and Diagnostic Approach. Biomedicines 2022, 10, 1826. https://doi.org/10.3390/biomedicines10081826
Choi JH, Ro JY. Combined Hepatocellular-Cholangiocarcinoma: An Update on Pathology and Diagnostic Approach. Biomedicines. 2022; 10(8):1826. https://doi.org/10.3390/biomedicines10081826
Chicago/Turabian StyleChoi, Joon Hyuk, and Jae Y. Ro. 2022. "Combined Hepatocellular-Cholangiocarcinoma: An Update on Pathology and Diagnostic Approach" Biomedicines 10, no. 8: 1826. https://doi.org/10.3390/biomedicines10081826
APA StyleChoi, J. H., & Ro, J. Y. (2022). Combined Hepatocellular-Cholangiocarcinoma: An Update on Pathology and Diagnostic Approach. Biomedicines, 10(8), 1826. https://doi.org/10.3390/biomedicines10081826







