The Emerging Role of Branched-Chain Amino Acids in Liver Diseases
Abstract
:1. Introduction
2. Circulation of BCAAs
2.1. BCAA Transport and Metabolism
2.2. BCAAs’ Signaling and Its Benefits
3. Circulatory BCAAs Level as an Indicator of a Dysmetabolic State
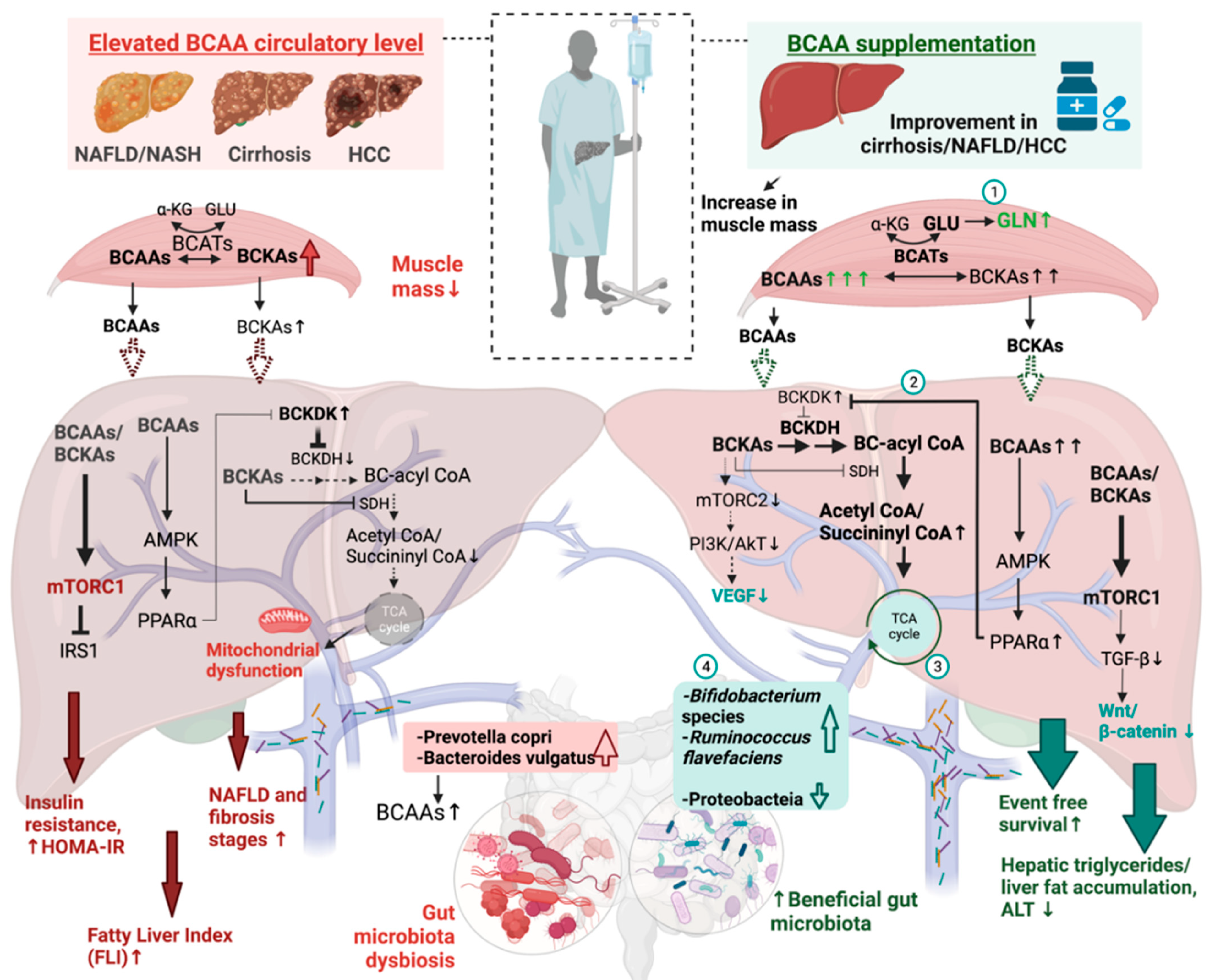
3.1. High Circulatory BCAAs Level in NAFLD Patients
3.2. Rising BCAA Levels in HCC Patients
4. BCAA as a Treatment for Liver Diseases
4.1. BCAA as a Therapeutic Treatment in Humans
| Type of Studies * | Interventions | Patients/Control | Sample Size | Duration | Outcome #/Outcome Measures * | Ref. |
|---|---|---|---|---|---|---|
| Multicenter RCT | VAL, LEU, ILE | Advanced liver cirrhosis | 232 | 6 months |
| [70] |
| Double-blinded RCT | VAL, LEU, ILE | Advanced cirrhosis | 174 | 12 months |
| [71] |
| N/A | VAL, LEU, ILE /AAA | Cirrhosis | 104 | >6 months |
| [13] |
| N/A | VAL, LEU, ILE | Cirrhosis | 211 | ≥6 months |
| [73] |
| Single-blinded RCT | AXA1665 (Leu: Ile: Val) | Child–Pugh A and B Cirrhosis | 16 | 15 days |
| [75] |
| Single-blinded, Multicenter RCT | AXA1125 (VAL, LEU, ILE, ARG, GLN) | Patients with NAFLD with and without T2D | 102 | 16 weeks |
| [16] |
| RCT | VAL, LEU, ILE | HCC | 51 | 12 months |
| [72] |
| Ongoing clinical studies | ||||||
| Triple-blinded RCT, Phase II | AXA1125 (VA, LEU, ILE, ARG, GLN) | NASH with fibrosis | 273 | 48 weeks | Improvement in steatohepatitis, resolution of NASH/ fibrosis | [76] |
| RCT | VAL, LEU, ILE | Cirrhosis | 60 | 3 months | Muscle mass, insulin-resistant | [77] |
4.2. BCAA as a Prophylactic Treatment of Liver Diseases in Animals
5. BCAA Promotes Hepatic Health through Modulation of Gut Microbiota
6. Conclusions
7. Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Marcellin, P.; Kutala, B.K. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018, 38 (Suppl. 1), 2–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paik, J.M.; Golabi, P.; Younossi, Y.; Mishra, A.; Younossi, Z.M. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology 2020, 72. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Lim, J.K.; Patton, H.; El-Serag, H.B. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020, 158, 1822–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, A.; Alt, Y.; Koch, S.; Nelles, C.; Düber, C.; Lang, H.; Otto, G.; Zimmermann, T.; Marquardt, J.U.; Galle, P.R.; et al. Treatment and survival of non-alcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer 2015, 15, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, S.; Yokosuka, O.; Imazeki, F.; Tagawa, M.; Omata, M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: A prospective study of 251 patients. Hepatology 1995, 21, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, K.; Hyogo, H.; Nakajima, T.; Ono, M.; Kawaguchi, T.; Honda, K.; Eguchi, Y.; Nozaki, Y.; Kawanaka, M.; Tanaka, S.; et al. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease and alcoholic liver disease: Multicenter survey. J. Gastroenterol. 2015, 51, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Functional amino acids in nutrition and health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef] [Green Version]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Lischka, J.; Schanzer, A.; Hojreh, A.; Ssalamah, A.B.; Item, C.B.; de Gier, C.; Walleczek, N.; Metz, T.F.; Jakober, I.; Greber-Platzer, S.; et al. A branched-chain amino acid-based metabolic score can predict liver fat in children and adolescents with severe obesity. Pediatr. Obes. 2020, 16, e12739. [Google Scholar] [CrossRef]
- Laferrère, B.; Reilly, D.; Arias, S.; Swerdlow, N.; Gorroochurn, P.; Bawa, B.; Bose, M.; Teixeira, J.; Stevens, R.D.; Wenner, B.R.; et al. Differential Metabolic Impact of Gastric Bypass Surgery Versus Dietary Intervention in Obese Diabetic Subjects Despite Identical Weight Loss. Sci. Transl. Med. 2011, 3, 80re2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukushima, K.; Harada, S.; Takeuchi, A.; Kurihara, A.; Iida, M.; Fukai, K.; Kuwabara, K.; Kato, S.; Matsumoto, M.; Hirata, A.; et al. Association between dyslipidemia and plasma levels of branched-chain amino acids in the Japanese population without diabetes mellitus. J. Clin. Lipidol. 2019, 13, 932–939.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Muto, Y.; Moriwaki, H.; Yamato, M. Effect of long-term oral supplementation with branched-chain amino acid granules on the prognosis of liver cirrhosis. Gastroenterol. Jpn. 1989, 24, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Hu, W.; Fu, Z.; Sun, L.; Zhou, Y.; Gong, Y.; Yang, T.; Zhou, H. The positive association of branched-chain amino acids and metabolic dyslipidemia in Chinese Han population. Lipids Heal. Dis. 2016, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hoyles, L.; Fernández-Real, J.-M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef]
- Harrison, S.A.; Baum, S.J.; Gunn, N.T.; Younes, Z.H.; Kohli, A.; Patil, R.; Koziel, M.J.; Chera, H.; Zhao, J.; Chakravarthy, M.V. Safety, Tolerability, and Biologic Activity of AXA1125 and AXA1957 in Subjects With Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2021, 116, 2399–2409. [Google Scholar] [CrossRef]
- Cha, J.H.; Bae, S.H.; Kim, H.L.; Park, N.R.; Choi, E.S.; Jung, E.S.; Choi, J.Y.; Yoon, S.K. Branched-Chain Amino Acids Ameliorate Fibrosis and Suppress Tumor Growth in a Rat Model of Hepatocellular Carcinoma with Liver Cirrhosis. PLoS ONE 2013, 8, e77899. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- HoleČek, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tajiri, K.; Shimizu, Y. Branched-chain amino acids in liver diseases. Transl. Gastroenterol. Hepatol. 2018, 3, 47. [Google Scholar] [CrossRef] [Green Version]
- Bifari, F.; Nisoli, E. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: A pharmacological point of view. J. Cereb. Blood Flow Metab. 2016, 174, 1366–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernstrom, J.D. Branched-Chain Amino Acids and Brain Function. J. Nutr. 2005, 135, 1539S–1546S. [Google Scholar] [CrossRef]
- Biswas, D.; Duffley, L.; Pulinilkunnil, T. Role of branched-chain amino acid–catabolizing enzymes in intertissue signaling, metabolic remodeling, and energy homeostasis. FASEB J. 2019, 33, 8711–8731. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Reid, T.M.; Bronson, S.; Vary, T.C.; Hajnal, A.; Lynch, C.J.; Hutson, S.M. Disruption of BCATm in Mice Leads to Increased Energy Expenditure Associated with the Activation of a Futile Protein Turnover Cycle. Cell Metab. 2007, 6, 181–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, E.J.; Wankell, M.; Palamuthusingam, P.; McFarlane, C.; Hebbard, L. Targeting the PI3K/Akt/mTOR Pathway in Hepatocellular Carcinoma. Biomedicines 2021, 9, 1639. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, E.; Eliasson, J.; Karlsson, H.; Köhnke, R. Branched-Chain Amino Acids Activate Key Enzymes in Protein Synthesis after Physical Exercise. J. Nutr. 2006, 136 (Suppl. 1), 269S–273S. [Google Scholar] [CrossRef]
- Moberg, M.; Apró, W.; Ekblom, B.; van Hall, G.; Holmberg, H.-C.; Blomstrand, E. Activation of mTORC1 by leucine is potentiated by branched-chain amino acids and even more so by essential amino acids following resistance exercise. Am. J. Physiol. Physiol. 2016, 310, C874–C884. [Google Scholar] [CrossRef] [Green Version]
- Holz, M.K.; Ballif, B.A.; Gygi, S.P.; Blenis, J. mTOR and S6K1 Mediate Assembly of the Translation Preinitiation Complex through Dynamic Protein Interchange and Ordered Phosphorylation Events. Cell 2005, 123, 569–580. [Google Scholar] [CrossRef] [Green Version]
- Apró, W.; Blomstrand, E. Influence of supplementation with branched-chain amino acids in combination with resistance exercise on p70S6 kinase phosphorylation in resting and exercising human skeletal muscle. Acta Physiol. 2010, 200, 237–248. [Google Scholar] [CrossRef]
- Ferreira, M.P.; Li, R.; Cooke, M.; Kreider, R.B.; Willoughby, D.S. Periexercise coingestion of branched-chain amino acids and carbohydrate in men does not preferentially augment resistance exercise–induced increases in phosphatidylinositol 3 kinase/protein kinase B–mammalian target of rapamycin pathway markers indicative of muscle protein synthesis. Nutr. Res. 2014, 34, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Frigerio, F.; Watanabe, M.; Picard, F.; Joaquin, M.; Sticker, M.; Fumagalli, S.; Allegrini, P.R.; Kozma, S.C.; Auwerx, J.; et al. Erratum: Corrigendum: Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004, 431, 485. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, A.G.; O’Neill, E. PI3K/Akt-mediated regulation of p53 in cancer. Biochem. Soc. Trans. 2014, 42, 798–803. [Google Scholar] [CrossRef]
- Bakan, I.; Laplante, M. Connecting mTORC1 signaling to SREBP-1 activation. Curr. Opin. Lipidol. 2012, 23, 226–234. [Google Scholar] [CrossRef]
- Jiao, J.; Han, S.-F.; Zhang, W.; Xu, J.-Y.; Tong, X.; Yin, X.-B.; Yuan, L.-X.; Qin, L.-Q. Chronic leucine supplementation improves lipid metabolism in C57BL/6J mice fed with a high-fat/cholesterol diet. Food Nutr. Res. 2016, 60, 31304. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Zhang, F.; Sun, D.; Wang, X.; Zhang, X.; Zhang, J.; Yan, F.; Huang, C.; Xie, H.; Lin, C.; et al. Branched-Chain Amino Acids Exacerbate Obesity-Related Hepatic Glucose and Lipid Metabolic Disorders via Attenuating Akt2 Signaling. Diabetes 2020, 69, 1164–1177. [Google Scholar] [CrossRef]
- Hagiwara, A.; Nishiyama, M.; Ishizaki, S. Branched-chain amino acids prevent insulin-induced hepatic tumor cell proliferation by inducing apoptosis through mTORC1 and mTORC2-dependent mechanisms. J. Cell. Physiol. 2011, 227, 2097–2105. [Google Scholar] [CrossRef]
- Guo, X.; Huang, C.; Lian, K.; Wang, S.; Zhao, H.; Yan, F.; Zhang, X.; Zhang, J.; Xie, H.; An, R.; et al. BCKA down-regulates mTORC2-Akt signal and enhances apoptosis susceptibility in cardiomyocytes. Biochem. Biophys. Res. Commun. 2016, 480, 106–113. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Dormond, O.; Contreras, A.; Meijer, E.; Datta, D.; Flynn, E.; Pal, S.; Briscoe, D.M. CD40-Induced Signaling in Human Endothelial Cells Results in mTORC2- and Akt-Dependent Expression of Vascular Endothelial Growth Factor In Vitro and In Vivo. J. Immunol. 2008, 181, 8088–8095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafei, M.A.; Forshaw, T.; Davis, J.; Flemban, A.; Qualtrough, D.; Dean, S.; Perks, C.; Dong, M.; Newman, R.; Conway, M.E. BCATc modulates crosstalk between the PI3K/Akt and the Ras/ERK pathway regulating proliferation in triple negative breast cancer. Oncotarget 2020, 11, 1971–1987. [Google Scholar] [CrossRef] [PubMed]
- Takegoshi, K.; Honda, M.; Okada, H.; Takabatake, R.; Nagata, N.; Campbell, J.S.; Nishikawa, M.; Shimakami, T.; Shirasaki, T.; Sakai, Y.; et al. Branched-chain amino acids prevent hepatic fibrosis and development of hepatocellular carcinoma in a non-alcoholic steatohepatitis mouse model. Oncotarget 2017, 8, 18191–18205. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.E.; Friedman, S.L. Mechanisms of hepatic fibrogenesis. Best Pr. Res. Clin. Gastroenterol. 2011, 25, 195–206. [Google Scholar] [CrossRef]
- Shang, S.; Hua, F.; Hu, Z.-W. The regulation of β-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 2017, 8, 33972–33989. [Google Scholar] [CrossRef] [Green Version]
- Arakawa, M.; Masaki, T.; Nishimura, J.; Seike, M.; Yoshimatsu, H. The effects of branched-chain amino acid granules on the accumulation of tissue triglycerides and uncoupling proteins in diet-induced obese mice. Endocr. J. 2011, 58, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Berg, E.H.V.D.; Flores-Guerrero, J.L.; Gruppen, E.G.; de Borst, M.H.; Wolak-Dinsmore, J.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Non-Alcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: Role of Circulating Branched-Chain Amino Acids. Nutrients 2019, 11, 705. [Google Scholar] [CrossRef] [Green Version]
- Borggreve, S.E.; Hillege, H.L.; Wolffenbuttel, B.H.R.; de Jong, P.E.; Bakker, S.J.L.; van der Steege, G.; van Tol, A.; Dullaart, R.P.F.; PREVEND Study Group. The effect of cholesteryl ester transfer protein -629C->A promoter polymorphism on high-density lipoprotein cholesterol is dependent on serum triglycerides. J. Clin. Endocrinol. Metab. 2005, 90, 4198–4204. [Google Scholar] [CrossRef] [Green Version]
- Chashmniam, S.; Ghafourpour, M.; Farimani, A.R.; Gholami, A.; Ghoochani, B.F.N.M. Metabolomic Biomarkers in the Diagnosis of Non-Alcoholic Fatty Liver Disease. Zahedan J. Res. Med Sci. 2019, 19. [Google Scholar] [CrossRef] [Green Version]
- Bhupathiraju, S.N.; Guasch-Ferré, M.; Gadgil, M.D.; Newgard, C.B.; Bain, J.R.; Muehlbauer, M.J.; Ilkayeva, O.R.; Scholtens, D.M.; Hu, F.B.; Kanaya, A.M.; et al. Dietary Patterns among Asian Indians Living in the United States Have Distinct Metabolomic Profiles That Are Associated with Cardiometabolic Risk. J. Nutr. 2018, 148, 1150–1159. [Google Scholar] [CrossRef]
- Männistö, V.T.; Simonen, M.; Hyysalo, J.; Soininen, P.; Kangas, A.; Kaminska, D.; Matte, A.K.; Venesmaa, S.; Käkelä, P.; Kärjä, V.; et al. Ketone body production is differentially altered in steatosis and non-alcoholic steatohepatitis in obese humans. Liver Int. 2014, 35, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobias, D.K.; Clish, C.; Mora, S.; Li, J.; Liang, L.; Hu, F.B.; Manson, J.E.; Zhang, C. Dietary Intakes and Circulating Concentrations of Branched-Chain Amino Acids in Relation to Incident Type 2 Diabetes Risk Among High-Risk Women with a History of Gestational Diabetes Mellitus. Clin. Chem. 2018, 64, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, R.J.O.; Rizo-Roca, D.; Chibalin, A.V.; Chorell, E.; Furrer, R.; Katayama, S.; Harada, J.; Karlsson, H.K.R.; Handschin, C.; Moritz, T.; et al. Branched-chain amino acid metabolism is regulated by ERRα in primary human myotubes and is further impaired by glucose loading in type 2 diabetes. Diabetologia 2021, 64, 2077–2091. [Google Scholar] [CrossRef]
- Gaggini, M.; Carli, F.; Bugianesi, E.; Gastaldelli, A.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Feldman, A.; Eder, S.; Felder, T.; Paulweber, B.; Zandanell, S.; Stechemesser, L.; Schranz, M.; Strebinger, G.; Huber-Schönauer, U.; Niederseer, D.; et al. Clinical and metabolic characterization of obese subjects without non-alcoholic fatty liver: A targeted metabolomics approach. Diabetes Metab. 2018, 45, 132–139. [Google Scholar] [CrossRef]
- Galarregui, C.; Cantero, I.; Marin-Alejandre, B.A.; Monreal, J.I.; Elorz, M.; Benito-Boillos, A.; Herrero, J.I.; de la O, V.; Ruiz-Canela, M.; Hermsdorff, H.H.M.; et al. Dietary intake of specific amino acids and liver status in subjects with nonalcoholic fatty liver disease: Fatty liver in obesity (FLiO) study. Eur. J. Nutr. 2020, 60, 1769–1780. [Google Scholar] [CrossRef]
- Hasegawa, T.; Iino, C.; Endo, T.; Mikami, K.; Kimura, M.; Sawada, N.; Nakaji, S.; Fukuda, S. Changed Amino Acids in NAFLD and Liver Fibrosis: A Large Cross-Sectional Study without Influence of Insulin Resistance. Nutrients 2020, 12, 1450. [Google Scholar] [CrossRef]
- Weickert, M.O.; Roden, M.; Isken, F.; Hoffmann, D.; Nowotny, P.; Osterhoff, M.; Blaut, M.; Alpert, C.; Gögebakan, O.; Bumke-Vogt, C.; et al. Effects of supplemented isoenergetic diets differing in cereal fiber and protein content on insulin sensitivity in overweight humans. Am. J. Clin. Nutr. 2011, 94, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Grzych, G.; Vonghia, L.; Bout, M.-A.; Weyler, J.; Verrijken, A.; Dirinck, E.; Curt, M.J.C.; Van Gaal, L.; Paumelle, R.; Francque, S.; et al. Plasma BCAA Changes in Patients With NAFLD Are Sex Dependent. J. Clin. Endocrinol. Metab. 2020, 105, 2311–2321. [Google Scholar] [CrossRef]
- Lake, A.D.; Novak, P.; Shipkova, P.; Aranibar, N.; Robertson, D.G.; Reily, M.D.; Lehman-McKeeman, L.D.; Vaillancourt, R.R.; Cherrington, N.J. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids 2015, 47, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Sánchez, M.S.; Vázquez, C.; Peiró, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic. Biol. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, M.R.N.; Luo, Y.; Di Poto, C.; Varghese, R.S.; Ferrarini, A.; Zhang, C.; Sarhan, N.I.; Soliman, H.; Tadesse, M.G.; Ziada, D.H.; et al. GC-MS Based Plasma Metabolomics for Identification of Candidate Biomarkers for Hepatocellular Carcinoma in Egyptian Cohort. PLoS ONE 2015, 10, e0127299. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kato, M.; Marui, K.; Murakami, T.; Onishi, K.; Adachi, T.; Matsuoka, J.; Ueki, H.; Yoshino, T.; Tsuruta, M.; et al. Easy clinical predictor for low BCAA to tyrosine ratio in chronic liver disease patients with hepatocellular carcinoma: Usefulness of ALBI score as nutritional prognostic marker. Cancer Med. 2021, 10, 3584–3592. [Google Scholar] [CrossRef]
- Tada, T.; Kumada, T.; Toyoda, H.; Kiriyama, S.; Tanikawa, M.; Hisanaga, Y.; Kanamori, A.; Kitabatake, S.; Yama, T. Impact of the branched-chain amino acid to tyrosine ratio and branched-chain amino acid granule therapy in patients with hepatocellular carcinoma: A propensity score analysis. J. Gastroenterol. Hepatol. 2015, 30, 1412–1419. [Google Scholar] [CrossRef]
- Buchard, B.; Teilhet, C.; Samarakoon, N.A.; Massoulier, S.; Joubert-Zakeyh, J.; Blouin, C.; Reynes, C.; Sabatier, R.; Biesse-Martin, A.-S.; Vasson, M.-P.; et al. Two Metabolomics Phenotypes of Human Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease According to Fibrosis Severity. Metabolites 2021, 11, 54. [Google Scholar] [CrossRef]
- Ericksen, R.E.; Lim, S.L.; McDonnell, E.; Shuen, W.H.; Vadiveloo, M.; White, P.J.; Ding, Z.; Kwok, R.; Lee, P.; Radda, G.K.; et al. Loss of BCAA Catabolism during Carcinogenesis Enhances mTORC1 Activity and Promotes Tumor Development and Progression. Cell Metab. 2019, 29, 1151–1165.e6. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, Y.; Lian, K.; Shentu, X.; Fang, J.; Shao, J.; Chen, M.; Wang, Y.; Zhou, M.; Sun, H. BCAA Catabolic Defect Alters Glucose Metabolism in Lean Mice. Front. Physiol. 2019, 10, 1140. [Google Scholar] [CrossRef] [Green Version]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study Of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef]
- Gil Park, J.; Tak, W.Y.; Park, S.Y.; Kweon, Y.O.; Chung, W.J.; Jang, B.K.; Bae, S.H.; Lee, H.J.; Jang, J.Y.; Suk, K.T.; et al. Effects of Branched-Chain Amino Acid (BCAA) Supplementation on the Progression of Advanced Liver Disease: A Korean Nationwide, Multicenter, Prospective, Observational, Cohort Study. Nutrients 2020, 12, 1429. [Google Scholar] [CrossRef]
- Marchesini, G.; Bianchi, G.; Merli, M.; Amodio, P.; Panella, C.; Loguercio, C.; Fanelli, F.R.; Abbiati, R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: A double-blind, randomized trial. Gastroenterology 2003, 124, 1792–1801. [Google Scholar] [CrossRef]
- Nojiri, S.; Fujiwara, K.; Shinkai, N.; Iio, E.; Joh, T. Effects of branched-chain amino acid supplementation after radiofrequency ablation for hepatocellular carcinoma: A randomized trial. Nutrition 2016, 33, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Hayaishi, S.; Chung, H.; Kudo, M.; Ishikawa, E.; Takita, M.; Ueda, T.; Kitai, S.; Inoue, T.; Yada, N.; Hagiwara, S.; et al. Oral Branched-Chain Amino Acid Granules Reduce the Incidence of Hepatocellular Carcinoma and Improve Event-Free Survival in Patients with Liver Cirrhosis. Dig. Dis. 2011, 29, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, R.G.; Fiorello, F.; Politi, F.; D’Amico, G.; Pagliaro, L. Hepatocellular carcinoma. Am. J. Dig. Dis. 1991, 36, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, M.V.; Neutel, J.; Confer, S.; Zhao, P.; Tatsuta, N.; Rebello, S.; Comb, W.C.; Hamill, M.; Tramontin, T.; Carroll, S.; et al. Safety, Tolerability, and Physiological Effects of AXA1665, a Novel Composition of Amino Acids, in Subjects With Child–Pugh A and B Cirrhosis. Clin. Transl. Gastroenterol. 2020, 11, e00222. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials Database: NCT04880187. Available online: https://clinicaltrials.gov/ct2/show/NCT04880187 (accessed on 30 January 2022).
- ClinicalTrials Database: NCT04246918. Available online: https://clinicaltrials.gov/ct2/show/NCT04246918 (accessed on 30 January 2022).
- Luo, L.; Sun, W.; Zhu, W.; Li, S.; Zhang, W.; Xu, X.; Fang, D.; Grahn, T.H.M.; Jiang, L.; Zheng, Y. BCAT1 decreases the sensitivity of cancer cells to cisplatin by regulating mTOR-mediated autophagy via branched-chain amino acid metabolism. Cell Death Dis. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Honda, T.; Ishigami, M.; Luo, F.; Lingyun, M.; Ishizu, Y.; Kuzuya, T.; Hayashi, K.; Nakano, I.; Ishikawa, T.; Feng, G.-G.; et al. Branched-chain amino acids alleviate hepatic steatosis and liver injury in choline-deficient high-fat diet induced NASH mice. Metabolism 2017, 69, 177–187. [Google Scholar] [CrossRef]
- Iwao, M.; Gotoh, K.; Arakawa, M.; Endo, M.; Honda, K.; Seike, M.; Murakami, K.; Shibata, H. Supplementation of branched-chain amino acids decreases fat accumulation in the liver through intestinal microbiota-mediated production of acetic acid. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Muyyarikkandy, M.S.; McLeod, M.; Maguire, M.; Mahar, R.; Kattapuram, N.; Zhang, C.; Surugihalli, C.; Muralidaran, V.; Vavilikolanu, K.; Mathews, C.E.; et al. Branched chain amino acids and carbohydrate restriction exacerbate ketogenesis and hepatic mitochondrial oxidative dysfunction during NAFLD. FASEB J. 2020, 34, 14832–14849. [Google Scholar] [CrossRef]
- Nishimura, J.; Masaki, T.; Arakawa, M.; Seike, M.; Yoshimatsu, H. Isoleucine Prevents the Accumulation of Tissue Triglycerides and Upregulates the Expression of PPARα and Uncoupling Protein in Diet-Induced Obese Mice. J. Nutr. 2010, 140, 496–500. [Google Scholar] [CrossRef] [Green Version]
- Burri, L.; Thoresen, G.H.; Berge, R.K. The Role of PPARαActivation in Liver and Muscle. PPAR Res. 2010, 2010, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regnier, M.; Polizzi, A.; Smati, S.; Lukowicz, C.; Fougerat, A.; Lippi, Y.; Fouché, E.; Lasserre, F.; Naylies, C.; Bétoulières, C.; et al. Hepatocyte-specific deletion of Pparα promotes NAFLD in the context of obesity. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, H.-S.; Lee, B.-K.; Kwon, Y.-J.; Lee, J.-W. Relationship between Muscle Mass and Non-Alcoholic Fatty Liver Disease. Biology 2021, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Song, X.; Chen, Y.; Chen, X.; Yu, C. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Hepatol. Int. 2019, 14, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Tejavath, A.S.; Mathur, A.; Nathiya, D.; Singh, P.; Raj, P.; Suman, S.; Mundada, P.R.; Atif, S.; Rai, R.R.; Tomar, B.S. Impact of Branched Chain Amino Acid on Muscle Mass, Muscle Strength, Physical Performance, Combined Survival, and Maintenance of Liver Function Changes in Laboratory and Prognostic Markers on Sarcopenic Patients With Liver Cirrhosis (BCAAS Study): A Randomized Clinical Trial. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Holecek, M.; Siman, P.; Vodenicarovova, M.; Kandar, R. Alterations in protein and amino acid metabolism in rats fed a branched-chain amino acid- or leucine-enriched diet during postprandial and postabsorptive states. Nutr. Metab. 2016, 13, 12. [Google Scholar] [CrossRef] [Green Version]
- Cruzat, V.; Macedo Rogero, M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [Green Version]
- Honda, Y.; Kessoku, T.; Sumida, Y.; Kobayashi, T.; Kato, T.; Ogawa, Y.; Tomeno, W.; Imajo, K.; Fujita, K.; Yoneda, M.; et al. Efficacy of glutathione for the treatment of nonalcoholic fatty liver disease: An open-label, single-arm, multicenter, pilot study. BMC Gastroenterol. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Oh, J.; Lee, J.; Cho, M.; Kim, H.; Chun, J.; Lee, J.; Yoon, Y.; Kang, W. Characterization of Gut Microbiome in Korean Patients with Metabolic Associated Fatty Liver Disease. Nutrients 2021, 13, 1013. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, S.; Zou, D.; Dong, D.; He, X.; Liu, N.; Liu, W.; Huang, L. Metabolic shifts and structural changes in the gut microbiota upon branched-chain amino acid supplementation in middle-aged mice. Amino Acids 2016, 48, 2731–2745. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Kamikado, K.; Suda, W.; Takii, H.; Mikami, Y.; Suganuma, N.; Hattori, M.; Koga, Y. A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci. Rep. 2017, 7, 43522. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Lonardo, A.; Targher, G. Glucagon-Like Peptide-1 Receptor Agonists for Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: An Updated Meta-Analysis of Randomized Controlled Trials. Metabolites 2021, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Moran-Ramos, S.; Macias-Kauffer, L.; López-Contreras, B.E.; Villamil-Ramírez, H.; Ocampo-Medina, E.; León-Mimila, P.; del Rio-Navarro, B.E.; Granados-Portillo, O.; Ibarra-Gonzalez, I.; Vela-Amieva, M.; et al. A higher bacterial inward BCAA transport driven by Faecalibacterium prausnitzii is associated with lower serum levels of BCAA in early adolescents. Mol. Med. 2021, 27, 1–12. [Google Scholar] [CrossRef]
- Munukka, E.; Rintala, A.; Toivonen, R.; Nylund, M.; Yang, B.; Takanen, A.; Hänninen, A.; Vuopio, J.; Huovinen, P.; Jalkanen, S.; et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J. 2017, 11, 1667–1679. [Google Scholar] [CrossRef] [Green Version]
- Iino, C.; Endo, T.; Mikami, K.; Hasegawa, T.; Kimura, M.; Sawada, N.; Nakaji, S.; Fukuda, S. Significant decrease in Faecalibacterium among gut microbiota in nonalcoholic fatty liver disease: A large BMI- and sex-matched population study. Hepatol. Int. 2019, 13, 748–756. [Google Scholar] [CrossRef]
- Yoshida, N.; Yamashita, T.; Osone, T.; Hosooka, T.; Shinohara, M.; Kitahama, S.; Sasaki, K.; Sasaki, D.; Yoneshiro, T.; Suzuki, T.; et al. Bacteroides spp. promotes branched-chain amino acid catabolism in brown fat and inhibits obesity. iScience 2021, 24. [Google Scholar] [CrossRef]
- Le Roy, T.; Llopis, M.; Lepage, P.; Bruneau, A.; Rabot, S.; Bevilacqua, C.; Martin, P.; Philippe, C.; Walker, F.; Bado, A.; et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2012, 62, 1787–1794. [Google Scholar] [CrossRef]
- Caussy, C.; Tripathi, A.; Humphrey, G.; Bassirian, S.; Singh, S.; Faulkner, C.; Bettencourt, R.; Rizo, E.; Richards, L.; Xu, Z.Z.; et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat. Commun. 2019, 10, 1406. [Google Scholar] [CrossRef]
- Dong, T.S.; Katzka, W.; Lagishetty, V.; Luu, K.; Hauer, M.; Pisegna, J.; Jacobs, J.P. A Microbial Signature Identifies Advanced Fibrosis in Patients with Chronic Liver Disease Mainly Due to NAFLD. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, E.K.K.; Felicianna; Xu, J.-H.; Zhan, Q.; Zeng, Z.; El-Nezami, H. The Emerging Role of Branched-Chain Amino Acids in Liver Diseases. Biomedicines 2022, 10, 1444. https://doi.org/10.3390/biomedicines10061444
Lo EKK, Felicianna, Xu J-H, Zhan Q, Zeng Z, El-Nezami H. The Emerging Role of Branched-Chain Amino Acids in Liver Diseases. Biomedicines. 2022; 10(6):1444. https://doi.org/10.3390/biomedicines10061444
Chicago/Turabian StyleLo, Emily Kwun Kwan, Felicianna, Jing-Hang Xu, Qiao Zhan, Zheng Zeng, and Hani El-Nezami. 2022. "The Emerging Role of Branched-Chain Amino Acids in Liver Diseases" Biomedicines 10, no. 6: 1444. https://doi.org/10.3390/biomedicines10061444
APA StyleLo, E. K. K., Felicianna, Xu, J.-H., Zhan, Q., Zeng, Z., & El-Nezami, H. (2022). The Emerging Role of Branched-Chain Amino Acids in Liver Diseases. Biomedicines, 10(6), 1444. https://doi.org/10.3390/biomedicines10061444








