Targeting the Complement Cascade for Treatment of Dry Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Methods of Literature Search
3. Risk Factors
3.1. Demographic and Environmental
3.2. Genetic
4. General Pathogenesis of Dry AMD
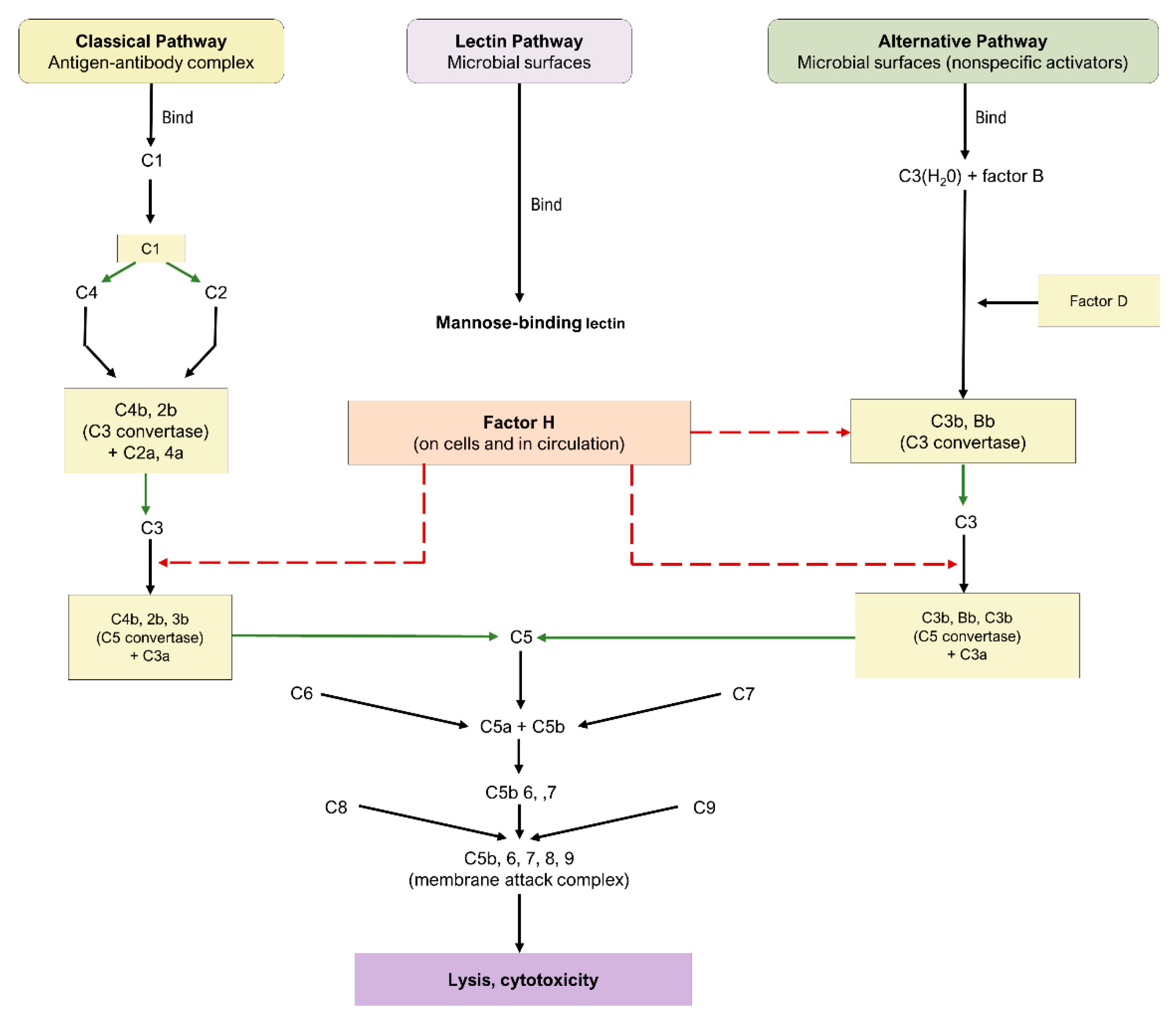
5. Complement Cascade
5.1. Pathways of the Complement Cascade
5.2. Complement Cascade in AMD
6. Current Therapeutic Targets
6.1. C1Q
ANX007
6.2. C3
6.2.1. AMY-106 (Cp40-KKK)
6.2.2. Pegcetacoplan (APL-2)
6.2.3. POT-4 (AL-78898A)
6.2.4. NGM621
6.3. C5
6.3.1. Eculizumab
6.3.2. Avacincaptad Pegol (Zimura® [ARC1905])
6.3.3. Tesidolumab (LFG316)
6.4. Complement Factor B
IONIS-FB-lrx
6.5. Complement Factor D
Lampalizumab (FCFD4514S)
6.6. Complement Factor H
6.6.1. AdCAGfH
6.6.2. GEM103
6.7. Complement Factor I
GT005
6.8. Membrane Attack Complex (MAC)
AAVCAGsCD59 (HMR59)
6.9. Properdin
CLG561
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourne, R.R.; Jonas, J.B.; Bron, A.M.; Cicinelli, M.V.; Das, A.; Flaxman, S.R.; Friedman, D.S.; Keeffe, J.E.; Kempen, J.H.; Leasher, J. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: Magnitude, temporal trends and projections. Br. J. Ophthalmol. 2018, 102, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Park, S.J.; Byun, S.J.; Park, K.H.; Suh, H.S. Incremental economic burden associated with exudative age-related macular degeneration: A population-based study. BMC Health Serv. Res. 2019, 19, 828. [Google Scholar] [CrossRef]
- Sadda, S.R.; Guymer, R.; Holz, F.G.; Schmitz-Valckenberg, S.; Curcio, C.A.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology 2018, 125, 537–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, A.C.; Bressler, N.M.; Bressler, S.B.; Chisholm, I.H.; Coscas, G.; Davis, M.D.; de Jong, P.T.; Klaver, C.C.; Klein, B.E.; Klein, R.; et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv. Ophthalmol. 1995, 39, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Ferris, F.L.; Davis, M.D.; Clemons, T.E.; Lee, L.Y.; Chew, E.Y.; Lindblad, A.S.; Milton, R.C.; Bressler, S.B.; Klein, R. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch. Ophthalmol. 2005, 123, 1570–1574. [Google Scholar] [CrossRef]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Keane, P.A.; Liakopoulos, S.; Ongchin, S.C.; Heussen, F.M.; Msutta, S.; Chang, K.T.; Walsh, A.C.; Sadda, S.R. Quantitative subanalysis of optical coherence tomography after treatment with ranibizumab for neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3115–3120. [Google Scholar] [CrossRef]
- Horner, F.; Lip, P.L.; Mohammed, B.R.; Fusi-Rubiano, W.; Gokhale, E.; Mushtaq, B.; Chavan, R. Comparing Effectiveness of Three Different Anti-VEGF Treatment Regimens for Neovascular Age-Related Macular Degeneration: Two Years’ Real-World Clinical Outcomes. Clin. Ophthalmol. 2021, 15, 1703–1713. [Google Scholar] [CrossRef]
- Arrigo, A.; Saladino, A.; Aragona, E.; Mercuri, S.; Introini, U.; Bandello, F.; Parodi, M.B. Different Outcomes of Anti-VEGF Treatment for Neovascular AMD according to Neovascular Sutypes and Baseline Features: 2-Year Real-Life Clinical Outcomes. BioMed Res. Int. 2021, 2021, 5516981. [Google Scholar] [CrossRef]
- Brown, G.C.; Brown, M.M.; Rapuano, S.; Boyer, D. Cost-Utility Analysis of VEGF Inhibitors for Treating Neovascular Age-Related Macular Degeneration. Am. J. Ophthalmol. 2020, 218, 225–241. [Google Scholar] [CrossRef]
- Stanton, C.M.; Yates, J.R.; den Hollander, A.I.; Seddon, J.M.; Swaroop, A.; Stambolian, D.; Fauser, S.; Hoyng, C.; Yu, Y.; Atsuhiro, K.; et al. Complement factor D in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8828–8834. [Google Scholar] [CrossRef]
- van de Ven, J.P.H.; Nilsson, S.C.; Tan, P.L.; Buitendijk, G.H.S.; Ristau, T.; Mohlin, F.C.; Nabuurs, S.B.; Schoenmaker-Koller, F.E.; Smailhodzic, D.; Campochiaro, P.A.; et al. A functional variant in the CFI gene confers a high risk of age-related macular degeneration. Nat. Genet. 2013, 45, 813–817. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Agodi, A. The association between complement factor H rs1061170 polymorphism and age-related macular degeneration: A comprehensive meta-analysis stratified by stage of disease and ethnicity. Acta Ophthalmol. 2019, 97, e8–e21. [Google Scholar] [CrossRef] [Green Version]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.; den Hollander, A.I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef] [Green Version]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [Green Version]
- Joachim, N.; Mitchell, P.; Kifley, A.; Rochtchina, E.; Hong, T.; Wang, J.J. Incidence and progression of geographic atrophy: Observations from a population-based cohort. Ophthalmology 2013, 120, 2042–2050. [Google Scholar] [CrossRef]
- Kuan, V.; Warwick, A.; Hingorani, A.; Tufail, A.; Cipriani, V.; Burgess, S.; Sofat, R.; Consortium, I.A.G. Association of Smoking, Alcohol Consumption, Blood Pressure, Body Mass Index, and Glycemic Risk Factors With Age-Related Macular Degeneration: A Mendelian Randomization Study. JAMA Ophthalmol. 2021, 139, 1299–1306. [Google Scholar] [CrossRef]
- Seddon, J.M.; Widjajahakim, R.; Rosner, B. Rare and Common Genetic Variants, Smoking, and Body Mass Index: Progression and Earlier Age of Developing Advanced Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 32. [Google Scholar] [CrossRef]
- Yu, Y.; Reynolds, R.; Rosner, B.; Daly, M.J.; Seddon, J.M. Prospective assessment of genetic effects on progression to different stages of age-related macular degeneration using multistate Markov models. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1548–1556. [Google Scholar] [CrossRef]
- Agrón, E.; Mares, J.; Chew, E.Y.; Keenan, T.D.L. Adherence to a Mediterranean Diet and Geographic Atrophy Enlargement Rate: Age-Related Eye Disease Study 2 Report 29. Ophthalmol. Retin. 2022. [Google Scholar] [CrossRef]
- Chapman, N.A.; Jacobs, R.J.; Braakhuis, A.J. Role of diet and food intake in age-related macular degeneration: A systematic review. Clin. Exp. Ophthalmol. 2019, 47, 106–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, J.L.; Khawaja, A.P.; Chan, M.P.; Broadway, D.C.; Peto, T.; Tufail, A.; Luben, R.; Hayat, S.; Bhaniani, A.; Wareham, N.J.; et al. Cross Sectional and Longitudinal Associations between Cardiovascular Risk Factors and Age Related Macular Degeneration in the EPIC-Norfolk Eye Study. PLoS ONE 2015, 10, e0132565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Zhang, H.; Yu, A.; Xie, J. Association between sunlight exposure and risk of age-related macular degeneration: A meta-analysis. BMC Ophthalmol. 2018, 18, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterling, J.K.; Baumann, B.; Foshe, S.; Voigt, A.; Guttha, S.; Alnemri, A.; McCright, S.J.; Li, M.; Zauhar, R.J.; Montezuma, S.R.; et al. Inflammatory adipose activates a nutritional immunity pathway leading to retinal dysfunction. Cell Rep. 2022, 39, 110942. [Google Scholar] [CrossRef] [PubMed]
- Kalu, K.; McMonnies, C.; Arcot, J. Dietary and Non-Dietary Determinants Associated with Changes in Red Blood Cell Omega-3-Essential Fatty Acids and Serum Lutein Status in a Selected Adult Population. Curr. Dev. Nutr. 2022, 6, 295. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Ding, Y.; Liu, Y.; Sun, T.; Fritsche, L.G.; Clemons, T.; Ratnapriya, R.; Klein, M.L.; Cook, R.J.; Liu, Y.; et al. Genome-wide analysis of disease progression in age-related macular degeneration. Hum. Mol. Genet. 2018, 27, 929–940. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Li, A.; Wu, M.; Chen, X.; Xiong, X.; Zhou, Z.; Liu, D. rs10490924 surrounding HTRA1/ARMS2 regulates the susceptibility of age-related macular degeneration. J. Recept. Signal Transduct. 2021, 41, 188–195. [Google Scholar] [CrossRef]
- Micklisch, S.; Lin, Y.; Jacob, S.; Karlstetter, M.; Dannhausen, K.; Dasari, P.; von der Heide, M.; Dahse, H.-M.; Schmölz, L.; Grassmann, F.; et al. Age-related macular degeneration associated polymorphism rs10490924 in ARMS2 results in deficiency of a complement activator. J. Neuroinflamm. 2017, 14, 4. [Google Scholar] [CrossRef] [Green Version]
- Ratnapriya, R.; Acar, İ.E.; Geerlings, M.J.; Branham, K.; Kwong, A.; Saksens, N.T.M.; Pauper, M.; Corominas, J.; Kwicklis, M.; Zipprer, D.; et al. Family-based exome sequencing identifies rare coding variants in age-related macular degeneration. Hum. Mol. Genet. 2020, 29, 2022–2034. [Google Scholar] [CrossRef]
- Hu, M.L.; Quinn, J.; Xue, K. Interactions between Apolipoprotein E Metabolism and Retinal Inflammation in Age-Related Macular Degeneration. Life 2021, 11, 635. [Google Scholar] [CrossRef]
- Liutkeviciene, R.; Vilkeviciute, A.; Kriauciuniene, L.; Banevicius, M.; Budiene, B.; Stanislovaitiene, D.; Zemaitiene, R.; Deltuva, V.P. Association of genetic variants at CETP, AGER, and CYP4F2 locus with the risk of atrophic age-related macular degeneration. Mol. Genet. Genom. Med. 2020, 8, e1357. [Google Scholar] [CrossRef]
- Bhattarai, N.; Hytti, M.; Reinisalo, M.; Kaarniranta, K.; Mysore, Y.; Kauppinen, A. Hydroquinone predisposes for retinal pigment epithelial (RPE) cell degeneration in inflammatory conditions. Immunol. Res. 2022. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Lee, Y.-J.; Hsu, M.-Y.; Wang, M.; Tsou, S.-C.; Chen, C.-C.; Lin, J.-A.; Hsiao, Y.-P.; Lin, H.-W. Protective Effect of Quercetin on Sodium Iodate-Induced Retinal Apoptosis through the Reactive Oxygen Species-Mediated Mitochondrion-Dependent Pathway. Int. J. Mol. Sci. 2021, 22, 4056. [Google Scholar] [CrossRef]
- Soundara Pandi, S.P.; Ratnayaka, J.A.; Lotery, A.J.; Teeling, J.L. Progress in developing rodent models of age-related macular degeneration (AMD). Exp. Eye Res. 2021, 203, 108404. [Google Scholar] [CrossRef]
- Nagata, K.; Hishikawa, D.; Sagara, H.; Saito, M.; Watanabe, S.; Shimizu, T.; Shindou, H. Lysophosphatidylcholine acyltransferase 1 controls mitochondrial reactive oxygen species generation and survival of retinal photoreceptor cells. J. Biol. Chem. 2022, 298, 101958. [Google Scholar] [CrossRef]
- Hsu, M.-Y.; Hsiao, Y.-P.; Lin, Y.-T.; Chen, C.; Lee, C.-M.; Liao, W.-C.; Tsou, S.-C.; Lin, H.-W.; Chang, Y.-Y. Quercetin Alleviates the Accumulation of Superoxide in Sodium Iodate-Induced Retinal Autophagy by Regulating Mitochondrial Reactive Oxygen Species Homeostasis through Enhanced Deacetyl-SOD2 via the Nrf2-PGC-1α-Sirt1 Pathway. Antioxidants 2021, 10, 1125. [Google Scholar] [CrossRef]
- Eells, J.T. Mitochondrial Dysfunction in the Aging Retina. Biology 2019, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Lazzarini, R.; Nicolai, M.; Lucarini, G.; Pirani, V.; Mariotti, C.; Bracci, M.; Mattioli-Belmonte, M. Oxidative stress in retinal pigment epithelium impairs stem cells: A vicious cycle in age-related macular degeneration. Mol. Cell. Biochem. 2022, 477, 67–77. [Google Scholar] [CrossRef]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Ji, J.; Wang, L.; Lv, W.; He, Y.; Li, X.; Feng, G.; Chen, K. A histological study of atherosclerotic characteristics in age-related macular degeneration. Heliyon 2022, 8, e08973. [Google Scholar] [CrossRef] [PubMed]
- van Lookeren Campagne, M.; LeCouter, J.; Yaspan, B.L.; Ye, W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J. Pathol. 2014, 232, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Handa, J.T.; Bowes Rickman, C.; Dick, A.D.; Gorin, M.B.; Miller, J.W.; Toth, C.A.; Ueffing, M.; Zarbin, M.; Farrer, L.A. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat. Commun. 2019, 10, 3347. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kambhampati, S.P.; Bhutto, I.A.; McLeod, D.S.; Lutty, G.A.; Kannan, R.M. Evolution of oxidative stress, inflammation and neovascularization in the choroid and retina in a subretinal lipid induced age-related macular degeneration model. Exp. Eye Res. 2021, 203, 108391. [Google Scholar] [CrossRef]
- Garcia-Garcia, J.; Usategui-Martin, R.; Sanabria, M.R.; Fernandez-Perez, E.; Telleria, J.J.; Coco-Martin, R.M. Pathophysiology of Age-Related Macular Degeneration. Implications for Treatment. Ophthalmic Res. 2022. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Mitchell, P.; Freund, K.B.; Sadda, S.; Holz, F.G.; Brittain, C.; Henry, E.C.; Ferrara, D. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology 2018, 125, 369–390. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Sadda, S.; Staurenghi, G.; Chew, E.Y.; Fleckenstein, M.; Holz, F.G. Geographic Atrophy: Semantic Considerations and Literature Review. Retina 2016, 36, 2250–2264. [Google Scholar] [CrossRef]
- Lorthiois, E.; Anderson, K.; Vulpetti, A.; Rogel, O.; Cumin, F.; Ostermann, N.; Steinbacher, S.; Mac Sweeney, A.; Delgado, O.; Liao, S.-M. Discovery of highly potent and selective small-molecule reversible factor D inhibitors demonstrating alternative complement pathway inhibition in vivo. J. Med. Chem. 2017, 60, 5717–5735. [Google Scholar] [CrossRef]
- Desai, D.; Dugel, P.U. Complement cascade inhibition in geographic atrophy: A review. Eye 2022, 36, 294–302. [Google Scholar] [CrossRef]
- Bora, P.S.; Sohn, J.H.; Cruz, J.M.; Jha, P.; Nishihori, H.; Wang, Y.; Kaliappan, S.; Kaplan, H.J.; Bora, N.S. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J. Immunol. 2005, 174, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Natoli, R.; Fernando, N.; Jiao, H.; Racic, T.; Madigan, M.; Barnett, N.L.; Chu-Tan, J.A.; Valter, K.; Provis, J.; Rutar, M. Retinal Macrophages Synthesize C3 and Activate Complement in AMD and in Models of Focal Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2977–2990. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Sun, X. Complement system and age-related macular degeneration: Drugs and challenges. Drug Des. Dev. Ther. 2019, 13, 2413–2425. [Google Scholar] [CrossRef]
- Lechner, J.; Chen, M.; Hogg, R.E.; Toth, L.; Silvestri, G.; Chakravarthy, U.; Xu, H. Higher plasma levels of complement C3a, C4a and C5a increase the risk of subretinal fibrosis in neovascular age-related macular degeneration. Immun. Ageing 2016, 13, 4. [Google Scholar] [CrossRef] [Green Version]
- Katschke, K.J., Jr.; Xi, H.; Cox, C.; Truong, T.; Malato, Y.; Lee, W.P.; McKenzie, B.; Arceo, R.; Tao, J.; Rangell, L.; et al. Classical and alternative complement activation on photoreceptor outer segments drives monocyte-dependent retinal atrophy. Sci. Rep. 2018, 8, 7348. [Google Scholar] [CrossRef]
- Udsen, M.; Tagmose, C.; Garred, P.; Nissen, M.H.; Faber, C. Complement activation by RPE cells preexposed to TNFα and IFNγ. Exp. Eye Res. 2022, 218, 108982. [Google Scholar] [CrossRef]
- Khandhadia, S.; Cipriani, V.; Yates, J.R.; Lotery, A.J. Age-related macular degeneration and the complement system. Immunobiology 2012, 217, 127–146. [Google Scholar] [CrossRef]
- Nishiguchi, K.M.; Yasuma, T.R.; Tomida, D.; Nakamura, M.; Ishikawa, K.; Kikuchi, M.; Ohmi, Y.; Niwa, T.; Hamajima, N.; Furukawa, K.; et al. C9-R95X polymorphism in patients with neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 508–512. [Google Scholar] [CrossRef] [Green Version]
- Rohrer, B.; Guo, Y.; Kunchithapautham, K.; Gilkeson, G.S. Eliminating complement factor D reduces photoreceptor susceptibility to light-induced damage. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5282–5289. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Dong, N.; Yang, M.; Wang, J.; Feng, X.; Wang, Y. Complement Inhibitors in Age-Related Macular Degeneration: A Potential Therapeutic Option. J. Immunol. Res. 2021, 2021, 9945725. [Google Scholar] [CrossRef]
- Fong, D. Spotlight on ANX007—Inhibition of C1q for GA. In Proceedings of the 2nd Dry AMD Therapeutic Development Summit, Online, 19–20 October 2021. [Google Scholar]
- Jiao, H.; Rutar, M.; Fernando, N.; Yednock, T.; Sankaranarayanan, S.; Aggio-Bruce, R.; Provis, J.; Natoli, R. Subretinal macrophages produce classical complement activator C1q leading to the progression of focal retinal degeneration. Mol. Neurodegener 2018, 13, 45. [Google Scholar] [CrossRef]
- Hughes, S.; Gumas, J.; Lee, R.; Rumano, M.; Berger, N.; Gautam, A.K.; Sfyroera, G.; Chan, A.L.; Gnanaguru, G.; Connor, K.M.; et al. Prolonged intraocular residence and retinal tissue distribution of a fourth-generation compstatin-based C3 inhibitor in non-human primates. Clin. Immunol. 2020, 214, 108391. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Hughes, S.; Gumas, J.; Rumano, M.; Yancopoulou, D.; Mastellos, D.; Lambris, J.D. Prolonged Intraocular residence of a fourth generation compstatin complement C3 inhibitor supports its clinical development for geographic atrophy. In Proceedings of the Retina Society, Virtual, 25 August–22 September 2020. [Google Scholar]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nittala, M.G.; Metlapally, R.; Ip, M.; Chakravarthy, U.; Holz, F.G.; Staurenghi, G.; Waheed, N.; Velaga, S.B.; Lindenberg, S.; Karamat, A.; et al. Association of Pegcetacoplan With Progression of Incomplete Retinal Pigment Epithelium and Outer Retinal Atrophy in Age-Related Macular Degeneration: A Post Hoc Analysis of the FILLY Randomized Clinical Trial. JAMA Ophthalmol. 2022, 140, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Rosenfeld, P.J.; Waheed, N.K.; Singh, R.P.; Ronca, N.; Slakter, J.S.; Staurenghi, G.; Mones, J.; Baumal, C.R.; Saroj, N.; et al. Characterizing New-Onset Exudation in the Randomized Phase 2 FILLY Trial of Complement Inhibitor Pegcetacoplan for Geographic Atrophy. Ophthalmology 2021, 128, 1325–1336. [Google Scholar] [CrossRef]
- Apellis Completes Enrollment in Two Phase 3 Studies of the Targeted C3 Therapy, Pegcetacoplan, in Patients with Geographic Atrophy (GA). Available online: https://investors.apellis.com/news-releases/news-release-details/apellis-completes-enrollment-two-phase-3-studies-targeted-c3 (accessed on 30 May 2022).
- Goldberg, R.; Heier, J.; Wykoff, C.; Staurenghi, G.; Singh, R.P.; Steinle, N.; Boyer, D.; Mones, J.; Holz, F.G.; Bliss, C.; et al. Efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA): 18-month results from the phase 3 OAKS and DERBY studies. In Proceedings of the 2022 ARVO Annual Meeting, Denver, CO, USA, 2 May 2022. [Google Scholar]
- Kaushal, S.; Grossi, F.; Francois, C.; Slakter, J.; Group, A.S. Complement C3 inhibitor POT-4: Clinical Safety of Intravitreal Administration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5010. [Google Scholar]
- Wykoff, C.C.; Hershberger, V.; Eichenbaum, D.; Henry, E.; Younis, H.S.; Chandra, P.; Yuan, N.; Solloway, M.; DePaoli, A. Inhibition of Complement Factor 3 in Geographic Atrophy with NGM621: Phase 1 Dose-Escalation Study Results. Am. J. Ophthalmol. 2022, 235, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Yehoshua, Z.; de Amorim Garcia Filho, C.A.; Nunes, R.P.; Gregori, G.; Penha, F.M.; Moshfeghi, A.A.; Zhang, K.; Sadda, S.; Feuer, W.; Rosenfeld, P.J. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: The COMPLETE study. Ophthalmology 2014, 121, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Filho, C.A.d.A.G.; Yehoshua, Z.; Gregori, G.; Nunes, R.P.; Penha, F.M.; Moshfeghi, A.A.; Zhang, K.; Feuer, W.; Rosenfeld, P.J. Change in Drusen Volume as a Novel Clinical Trial Endpoint for the Study of Complement Inhibition in Age-related Macular Degeneration. Ophthalmic Surg. Lasers Imaging Retin. 2014, 45, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, G.J.; Westby, K.; Csaky, K.G.; Mones, J.; Pearlman, J.A.; Patel, S.S.; Joondeph, B.C.; Randolph, J.; Masonson, H.; Rezaei, K.A. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology 2021, 128, 576–586. [Google Scholar] [CrossRef]
- Sheth, V. Avacincaptad Pegol, A Novel C5 Inhibitor, Demonstrates a Continued Reduction in the Mean Rate of Geographic Atrophy Growth: 18-Month Results from the GATHER1 Clinical Trial. Investig. Ophthalmol. Vis. Sci. 2021, 62, 333. [Google Scholar]
- Jaffe, G.J.; Sahni, J.; Fauser, S.; Geary, R.S.; Schneider, E.; McCaleb, M. Development of IONIS-FB-LRx to Treat Geographic Atrophy Associated with AMD. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4305. [Google Scholar]
- Katschke, K.J., Jr.; Wu, P.; Ganesan, R.; Kelley, R.F.; Mathieu, M.A.; Hass, P.E.; Murray, J.; Kirchhofer, D.; Wiesmann, C.; van Lookeren Campagne, M. Inhibiting alternative pathway complement activation by targeting the factor D exosite. J. Biol. Chem 2012, 287, 12886–12892. [Google Scholar] [CrossRef] [Green Version]
- Yaspan, B.L.; Williams, D.F.; Holz, F.G.; Regillo, C.D.; Li, Z.; Dressen, A.; van Lookeren Campagne, M.; Le, K.N.; Graham, R.R.; Beres, T.; et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci. Transl. Med. 2017, 9, eaaf1443. [Google Scholar] [CrossRef] [Green Version]
- Holz, F.G.; Sadda, S.R.; Busbee, B.; Chew, E.Y.; Mitchell, P.; Tufail, A.; Brittain, C.; Ferrara, D.; Gray, S.; Honigberg, L.; et al. Efficacy and Safety of Lampalizumab for Geographic Atrophy Due to Age-Related Macular Degeneration: Chroma and Spectri Phase 3 Randomized Clinical Trials. JAMA Ophthalmol. 2018, 136, 666–677. [Google Scholar] [CrossRef] [Green Version]
- Heier, J.S.; Pieramici, D.; Chakravarthy, U.; Patel, S.S.; Gupta, S.; Lotery, A.; Lad, E.M.; Silverman, D.; Henry, E.C.; Anderesi, M.; et al. Visual Function Decline Resulting from Geographic Atrophy: Results from the Chroma and Spectri Phase 3 Trials. Ophthalmol. Retin. 2020, 4, 673–688. [Google Scholar] [CrossRef]
- Cashman, S.M.; Gracias, J.; Adhi, M.; Kumar-Singh, R. Adenovirus-mediated delivery of Factor H attenuates complement C3 induced pathology in the murine retina: A potential gene therapy for age-related macular degeneration. J. Gene Med. 2015, 17, 229–243. [Google Scholar] [CrossRef]
- Cabral de Guimaraes, T.A.; Daich Varela, M.; Georgiou, M.; Michaelides, M. Treatments for dry age-related macular degeneration: Therapeutic avenues, clinical trials and future directions. Br J. Ophthalmol. 2022, 106, 297–304. [Google Scholar] [CrossRef]
- Khanani, A.M.; Maturi, R.K.; Bagheri, N.; Bakall, B.; Boyer, D.S.; Couvillion, S.S.; Dhoot, D.S.; Holekamp, N.M.; Jamal, K.N.; Marcus, D.M.; et al. A Phase I, Single Ascending Dose Study of GEM103 (Recombinant Human Complement Factor H) in Patients with Geographic Atrophy. Ophthalmol. Sci. 2022, 2, 100154. [Google Scholar] [CrossRef]
- Gemini Therapeutics Announces Initial Data from Its Ongoing Phase 2a Study of GEM103 in Patients with Geographic Atrophy Secondary to Dry Age-Related Macular Degeneration. 2021. Available online: https://investors.geminitherapeutics.com/news/news-details/2021/Gemini-Therapeutics-Announces-Initial-Data-From-Its-Ongoing-Phase-2a-Study-of-GEM103-in-Patients-With-Geographic-Atrophy-Secondary-to-Dry-Age-Related-Macular-Degeneration/default.aspx (accessed on 1 June 2022).
- Gemini Therapeutics Provides GEM103 Program Update. 2022. Available online: https://investors.geminitherapeutics.com/news/news-details/2022/Gemini-Therapeutics-Provides-GEM103-Program-Update/default.aspx (accessed on 30 May 2022).
- Dreismann, A.K.; McClements, M.E.; Barnard, A.R.; Orhan, E.; Hughes, J.P.; Lachmann, P.J.; MacLaren, R.E. Functional expression of complement factor I following AAV-mediated gene delivery in the retina of mice and human cells. Gene 2021, 28, 265–276. [Google Scholar] [CrossRef]
- Nielsen, J.S. Preliminary Results from a First-in-Human Phase I/II Gene Therapy Trial (FOCUS) of Subretinally Delivered GT005, an Investigational AAV2 Vector, in Patients with Geographic Atrophy Secondary to Age-Related Macular Degeneration. In Proceedings of the ARVO 2022 Annual Meeting, Denver, CO, USA, 1–4 May 2022. [Google Scholar]
- Kumar-Singh, R. The role of complement membrane attack complex in dry and wet AMD—From hypothesis to clinical trials. Exp. Eye Res. 2019, 184, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Cashman, S.M.; Ramo, K.; Kumar-Singh, R. A non membrane-targeted human soluble CD59 attenuates choroidal neovascularization in a model of age related macular degeneration. PLoS ONE 2011, 6, e19078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dugel, P. Clinical Trial Download: Data on a Gene Therapy for Dry and Wet AMD. Retin. Physician 2020, 17, 16–17. [Google Scholar]
- Johnson, L.; Splawski, I.; Baker, L.; Carrion, A.; Nguyen, A.; Twarog, M.; Wang, Y.; Jager, U.; Keating, M.; Dryja, T.P.; et al. Generation and characterization of CLG561: A fully-human, anti-properdin Fab for the treatment of age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1116. [Google Scholar]
- Glazer, L.C.; Williams, J.G.; Gordon, C.M.; Dugel, P.U.; Milton, M.; Valencia, T.; Klein, U.; Kretz, S.; Gedif, K.; Grosskreutz, C.L.; et al. A first in human study of Intravitreal (IVT) CLG561 in Subjects with Advanced Age-Related Macular Degeneration (AMD). Investig. Ophthalmol. Vis. Sci. 2016, 57, 2672. [Google Scholar]
- Abokyi, S.; To, C.-H.; Lam, T.T.; Tse, D.Y. Central Role of Oxidative Stress in Age-Related Macular Degeneration: Evidence from a Review of the Molecular Mechanisms and Animal Models. Oxidative Med. Cell. Longev. 2020, 2020, 7901270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, A.C.; Bok, D. Why the macula? Eye 2018, 32, 858–862. [Google Scholar] [CrossRef] [Green Version]
- Rastoin, O.; Pagès, G.; Dufies, M. Experimental Models in Neovascular Age Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4627. [Google Scholar] [CrossRef]
- Malek, G.; Busik, J.; Grant, M.B.; Choudhary, M. Models of retinal diseases and their applicability in drug discovery. Expert Opin. Drug Discov. 2018, 13, 359–377. [Google Scholar] [CrossRef]
- Belgio, B.; Boschetti, F.; Mantero, S. Towards an In Vitro Retinal Model to Study and Develop New Therapies for Age-Related Macular Degeneration. Bioengineering 2021, 8, 18. [Google Scholar] [CrossRef]
- Lin, J.B.; Serghiou, S.; Miller, J.W.; Vavvas, D.G. Systemic Complement Activation Profiles in Nonexudative Age-Related Macular Degeneration: A Systematic Review. Ophthalmol. Sci. 2022, 2, 100118. [Google Scholar] [CrossRef]
- Yates, J.R.; Sepp, T.; Matharu, B.K.; Khan, J.C.; Thurlby, D.A.; Shahid, H.; Clayton, D.G.; Hayward, C.; Morgan, J.; Wright, A.F.; et al. Complement C3 variant and the risk of age-related macular degeneration. N. Engl. J. Med. 2007, 357, 553–561. [Google Scholar] [CrossRef] [Green Version]
- van Leeuwen, E.M.; Emri, E.; Merle, B.M.J.; Colijn, J.M.; Kersten, E.; Cougnard-Gregoire, A.; Dammeier, S.; Meester-Smoor, M.; Pool, F.M.; de Jong, E.K.; et al. A new perspective on lipid research in age-related macular degeneration. Prog. Retin. Eye Res. 2018, 67, 56–86. [Google Scholar] [CrossRef]
- Xu, Q.; Cao, S.; Rajapakse, S.; Matsubara, J.A. Understanding AMD by analogy: Systematic review of lipid-related common pathogenic mechanisms in AMD, AD, AS and GN. Lipids Health Dis. 2018, 17, 3. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, J.A.; Tian, Y.; Cui, J.Z.; Zeglinski, M.R.; Hiroyasu, S.; Turner, C.T.; Granville, D.J. Retinal Distribution and Extracellular Activity of Granzyme B: A Serine Protease That Degrades Retinal Pigment Epithelial Tight Junctions and Extracellular Matrix Proteins. Front. Immunol. 2020, 11, 574. [Google Scholar] [CrossRef]
- Dörschmann, P.; Klettner, A. Fucoidans as Potential Therapeutics for Age-Related Macular Degeneration-Current Evidence from In Vitro Research. Int. J. Mol. Sci. 2020, 21, 9272. [Google Scholar] [CrossRef]
- Sobolewska, B.; Sabsabi, M.; Ziemssen, F. Importance of Treatment Duration: Unmasking Barriers and Discovering the Reasons for Undertreatment of Anti-VEGF Agents in Neovascular Age-Related Macular Degeneration. Clin. Ophthalmol. 2021, 15, 4317–4326. [Google Scholar] [CrossRef]
- Robin, A.L.; Muir, K.W. Medication adherence in patients with ocular hypertension or glaucoma. Expert Rev. Ophthalmol. 2019, 14, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Cooper, R.C.; Yang, H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release 2019, 306, 29–39. [Google Scholar] [CrossRef]
- Tsai, C.H.; Wang, P.Y.; Lin, I.C.; Huang, H.; Liu, G.S.; Tseng, C.L. Ocular Drug Delivery: Role of Degradable Polymeric Nanocarriers for Ophthalmic Application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, J.R.; Silva, N.C.; Sarmento, B.; Pintado, M. Potential chitosan-coated alginate nanoparticles for ocular delivery of daptomycin. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
| Therapeutic Category or Name | Mechanism | Mode of Administration | Clinical Trial ID, NCT# * | Study Phase (Status) † | Patients, n | Primary Outcome |
|---|---|---|---|---|---|---|
| C1Q ANX007 | ||||||
| Recombinant monoclonal antibody | Intravitreal | NCT04656561 | Phase II (Recruiting) | 240 (Anticipated) | GA growth | |
| C3 | N/A NCT03525600 NCT03525613 NCT04770545 NCT01603043 NCT04465955 | N/A Phase III (Ongoing) Phase III (Ongoing) Phase III (Recruiting) Phase II (Terminated, Adverse effects) Phase II (Ongoing) | ||||
| AMY-106 (Cp40-KKK) | Compstatin analog inhibitor | Intravitreal | N/A | N/A | ||
| Pegcetacoplan (APL-2) | PEGylated peptide inhibitor | Intravitreal | 621 638 1200 (Anticipated) | GA growth GA growth Safety | ||
POT-4 (AL-78898A) | Compstatin analog inhibitor | Intravitreal | 10 | GA growth | ||
NGM621 | Humanized monoclonal antibody | Intravitreal | 320 | GA growth | ||
| C5 Eculizumab Avacincaptad Pegol (Zimura® [ARC1905]) Tesidolumab (LFG316) | Humanized monoclonal antibody PEGylated RNA aptamer Human monoclonal antibody | Intravenous Intravitreal Intravitreal | NCT00935883 NCT04435366 NCT01527500 | Phase II (Completed, Ineffective) Phase III (Ongoing) Phase II (Completed, Ineffective) | 60 448 158 | GA growth/Reduction of drusen volume GA growth GA growth |
| Complement Factor B IONIS-FB-LRx | Anti-sense oligonucleotide | Subcutaneous | NCT03815825 | Phase II (Recruiting) | 330 (Anticipated) | GA growth |
Complement Factor D Lampalizumab (FCFD4514S) | ||||||
Humanized monoclonal antibody | Intravitreal | NCT02247479 NCT02247531 | Phase III (Terminated, Ineffective) Phase III (Terminated, Ineffective) | 906 975 | GA growth GA growth | |
| Complement Factor H AdCAGfH GEM103 | ||||||
Adeno-associated virus | Subretinal gene therapy | N/A | N/A | N/A | N/A | |
| Recombinant human CFH | Intravitreal | NCT04643886 | Phase IIa (Terminated, Effective) | 62 | Safety | |
| Complement Factor I GT005 | ||||||
Adeno-associated virus | Subretinal gene therapy | NCT03846193 NCT04566445 NCT04437368 | Phase I/II (Recruiting) Phase II (Recruiting) Phase II (Recruiting) | 65 (Anticipated) 250 (Anticipated) 75 (Anticipated) | Safety GA growth GA growth | |
| Membrane Attack Complex (MAC) AAVCAGsCD59 (HMR59) | ||||||
Adeno-associated virus | Intravireal gene therapy | NCT04358471 | Phase II (Hiatus) | N/A | GA growth | |
| Properdin CLG561 | ||||||
| Human antibody Fab | Intravitreal | NCT02515942 | Phase II (Completed, Ineffective) | 114 | Safety/IOP change/GA growth |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, P.N.; Patel, P.A.; Land, M.R.; Bakerkhatib-Taha, I.; Ahmed, H.; Sheth, V. Targeting the Complement Cascade for Treatment of Dry Age-Related Macular Degeneration. Biomedicines 2022, 10, 1884. https://doi.org/10.3390/biomedicines10081884
Patel PN, Patel PA, Land MR, Bakerkhatib-Taha I, Ahmed H, Sheth V. Targeting the Complement Cascade for Treatment of Dry Age-Related Macular Degeneration. Biomedicines. 2022; 10(8):1884. https://doi.org/10.3390/biomedicines10081884
Chicago/Turabian StylePatel, Prem N., Parth A. Patel, Matthew R. Land, Ibrahim Bakerkhatib-Taha, Harris Ahmed, and Veeral Sheth. 2022. "Targeting the Complement Cascade for Treatment of Dry Age-Related Macular Degeneration" Biomedicines 10, no. 8: 1884. https://doi.org/10.3390/biomedicines10081884
APA StylePatel, P. N., Patel, P. A., Land, M. R., Bakerkhatib-Taha, I., Ahmed, H., & Sheth, V. (2022). Targeting the Complement Cascade for Treatment of Dry Age-Related Macular Degeneration. Biomedicines, 10(8), 1884. https://doi.org/10.3390/biomedicines10081884






