Abstract
Numerous studies have highlighted the role of the gastrointestinal system in Parkinson disease pathogenesis. It is likely triggered by proinflammatory markers produced by specific gut bacteria. This review’s aim is to identify gut bacterial biomarkers of Parkinson disease. A comprehensive search for original research papers on gut microbiota composition in Parkinson disease was conducted using the PubMed, Embase, and Scopus databases. Research papers on intestinal permeability, nasal and oral microbiomes, and interventional studies were excluded. The yielded results were categorized into four groups: Parkinson disease vs. healthy controls; disease severity; non-motor symptoms; and clinical phenotypes. This review was conducted in accordance with the PRISMA 2020 statement. A total of 51 studies met the eligibility criteria. In the Parkinson disease vs. healthy controls group, 22 bacteria were deemed potentially important. In the disease severity category, two bacteria were distinguished. In the non-motor symptoms and clinical phenotypes categories, no distinct pathogen was identified. The studies in this review report bacteria of varying taxonomic levels, which prevents the authors from reaching a clear conclusion. Future research should follow a unified methodology in order to identify potential biomarkers for Parkinson disease.
1. Introduction
Parkinson disease (PD), a neurodegenerative disorder characterized by gradual dopaminergic neuronal loss in the substantia nigra (SN), is most recognizable for its hallmark motor manifestation: tremor, rigidity, and bradykinesia. However, PD also involves a wide range of less distinctive, but quite common non-motor symptoms (NMS) [1,2,3]. They are often neglected during patient evaluation, since movement impairment and its eventual progression tend to be the most pronounced aspects of PD [4]. Nevertheless, the non-motor manifestation of this disease has been able to provide some valuable insight into its possible pathogenesis. Hyposmia and constipation are particularly notable, as they may precede motor symptom onset by even 20 years [5]. This observation suggests a peripheral origin of PD. Additionally, the discovery of Lewy bodies (abnormal deposits of alpha-synuclein) in the intestinal submucosal and myenteric plexuses of PD patients explicitly highlights the role of the intestine in PD etiology [6,7,8]. Dual-hit theory, proposed by Hawkes, Tredici, and Braak, implicates two places in the body as sites of PD origin—the olfactory nerves and intestinal plexuses [9]. Moreover, it suggests that neural damage initiated in the gut spreads to the central nervous system (CNS) via the vagus nerve [9], and it is only after neurodegeneration reaches the substantia nigra, the motor phase of PD begins [5].
A recent paper by Horsager et al. proposed a new and updated model of PD pathogenesis [10]. It categorizes PD into two subtypes: the body—first phenotype, and the brain—first phenotype [10]. Clinical [11,12] and neuropathological [13,14] evidence suggests that, in a subset of PD patients, neurodegeneration originates and firstly propagates through the CNS, with the autonomic nervous system being affected at a later stage of this disease [10]. These findings are inconsistent with the above-mentioned dual-hit hypothesis, hence the proposal of distinguishing a separate phenotype of PD—the brain-first subtype [9]. Nevertheless, PD patients categorized into the body-first subtype exhibit symptoms in an order compatible with the dual-hit hypothesis, and it is this particular group that pertains to this systematic review [9].
To date, the exact trigger of intestinal neurodegeneration remains elusive. However, the process of alpha synuclein aggregation, and thus Lewy body formation, in the intestine correlates with increased intestinal permeability, which was demonstrated by a study comparing PD subjects to healthy controls [15]. This dysfunction in intestinal barrier integrity is most likely initiated by proinflammatory factors produced by certain bacteria residing in the gut [15]. A recent study found a significant correlation between the consumption of narrow spectrum penicillin and a higher prevalence of PD [16]. This further supports the theory that gut dysbiosis may trigger intestinal neurodegeneration, as it is well-documented that antibiotic exposure has a selective and long-lasting effect on gut microbiome composition [17]. However, the question of whether a specific taxonomic group is potentially responsible for PD development remains unresolved.
Aside from further exploring PD pathogenesis, this review will hopefully advance research into potential gut biomarkers for PD risk group assessment. Currently, a diagnosis of PD is typically made upon its motor manifestation, which occurs when an estimate of 30–60% of neurons in the substantia nigra (SN) have already been damaged [18,19]. Detecting precise PD risk biomarkers would lead to quicker diagnoses, ideally before these patients even begin to experience the motor phase of this disease. This would hopefully pave the way for earlier medical supervision and a more holistic, prevention-based approach towards individuals with prodromal PD. Additionally, implicating specific gut bacteria would help corroborate the possible effectiveness of interventional fecal microbiota transplantation (FMT) as a method of treatment in PD. If it were to be approved, it would be the first line of therapy directed against the precise cause of idiopathic PD, as currently available treatment is limited to alleviating symptoms without curbing this disease’s progression [20].
2. Materials and Methods
In this review, original research papers investigating gut microbiota composition in Parkinson disease were analyzed. A systematic literature search was performed according to the 2020 PRISMA updated guideline statement [21]. The review was registered in PROSPERO (ID CRD42022337225). The process was overseen by two authors (J.M.N., M.K.). The PubMed, Embase, and Scopus databases were searched on 13 June 2022, for original research articles involving human subjects. The terms “(Parkinson disease AND gut AND (bacteria OR microbiome OR microbiota)” were used for PubMed and Embase, and “(Parkinson disease AND gut AND (bacteria OR microbiome OR microbiota) AND (LIMIT-TO (SUBJAREA, “MEDI”)) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (EXCLUDE (EXACTKEYWORD, “Nonhuman”) OR (EXCLUDE (EXACTKEYWORD, “Animals”)) for Scopus. Only full-text articles published in English from 2000 to 1 June 2022 were included in the final analysis.
The yielded studies were categorized into four main groups: (1) PD subjects versus healthy controls (HC), (2) markers of disease severity, (3) markers of NMS in PD, and (4) markers of PD clinical phenotypes. A further analysis of these individual groups was later conducted. It firstly involved identifying all of the bacteria evaluated in each study in each of the above-mentioned groups. Subsequently, it was determined whether these studies had established a decrease or increase of the specified bacteria for PD subjects. An overall conclusion was then reached based on an assessment of coinciding outcomes.
3. Results
Our search using the above-mentioned terms revealed 1674 results in the Scopus database, 990 results in the Embase database, and 551 results in the PubMed database. After an automatic (EndNote) and manual (J.M.N., M.K.) removal of duplicates, a total of 2441 papers were identified that met the search criteria. In the next step, the available abstracts were read to identify original research papers on changes in the gastrointestinal microbiota of PD patients. We excluded original papers on intestinal permeability, nasal and oral microbiomes, and interventional studies (e.g., treatment with probiotics, fecal matter transplantation). A PRISMA flow diagram of the search procedure is available as Figure 1. We identified a total of 51 original papers on 15 June 2022.
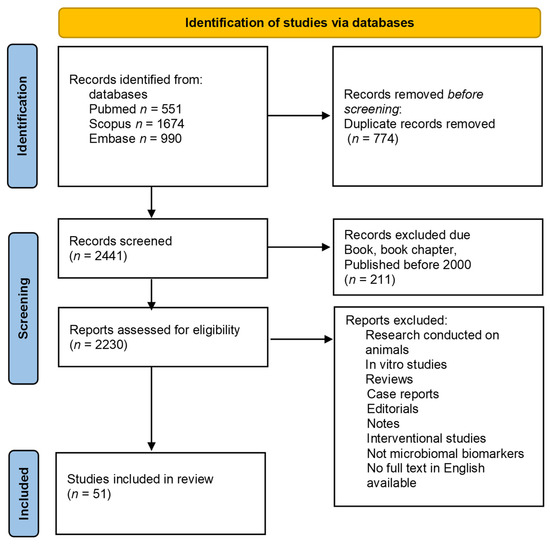
Figure 1.
PRISMA flowchart in accordance with the PRISMA 2020 statement. (n—number).
The yielded studies were then categorized into the previously mentioned groups, which revealed the following results: 38 studies in the PD subjects vs. HC group, 26 studies in the PD severity group, 17 in the NMS group, and 9 in the PD clinical phenotype group. Some studies matched into more than one of these categories.
3.1. Parkinson Disease Patients and Healthy Controls
Most studies investigating gut microbiota in PD chose to compare PD subjects to healthy controls. This is the preferred methodology when attempting to identify specific PD biomarkers, as it limits external, interfering factors. The full results of the previously mentioned analysis can be found as Table S1, and a summarized version can be found below as Table 1. After careful examination of Table S1, 22 bacteria (regardless of their taxonomic level) were deemed potentially significant, with five or more papers implicating the same bacteria, at the same taxonomic level, with the same outcome for PD subjects. Fourteen of the 22 identified bacteria were increased in PD: Akkermansia genus (12 studies), Verrucomicrobiaceae family (8 studies), Rikenellaceae family (8 studies), Lactobacillus genus (8 studies), Lactobacillaceae family (7 studies), Bifidobacterium genus (7 studies), Bifidobacteriaceae family (7 studies), Proteobacteria phylum (6 studies), Alistipes genus (6 studies), Actinobacteria phylum (6 studies), Verrucomicrobia phylum (6 studies), Enterobacteriaceae family (5 studies), Streptococcus genus (five studies), and Ruminococcaceae family (5 studies). The remaining eight of the 22 bacteria were found to be decreased in PD. These include: Roseburia genus (11 studies), Lachnospiraceae family (10 studies), Faecalibacterium genus (9 studies), Prevotellaceae family (6 studies), Prevotella genus (5 studies), Blautia genus (5 studies), Bacteroidetes phylum (5 studies), and Fusicatenibacter genus (5 studies). An additional group of bacteria, the Desulfovibrionaceae family, was considered potentially valuable, even though it did not meet the previously mentioned criteria. The decision to distinguish this bacterial family was based on a singular study dedicating its investigation to this specific bacterial group [22]. This study was able to reach a clear and concise conclusion of the Desulfovibrionaceae family’s significance as a potential PD biomarker.

Table 1.
Parkinson disease patients and healthy controls: research papers and their conclusions—summarized version ([↑/↓]-increase/decrease of abundance in comparison patients with Parkinson’s Disease to Healthy Control, PD—patients with Parkinson’s Disease, HC—healthy controls).
3.2. Parkinson Disease Severity
Disease severity and/or disease duration may alter the gut microbiome in PD patients. It has been hypothesized that, throughout the course of this disease, certain bacteria may gradually change in abundance concomitantly with PD symptom progression. The identified studies on PD severity and their conclusions can be found below in Table 2. However, several differing interpretations of disease severity were found between studies, hence the discrepancy among their methodological approaches: 17 papers used the Hoehn & Yahr scale, 22 papers used UPDRS, 10 papers used Levodopa Equivalent Dose (LED), and 12 of them used disease duration for PD severity evaluations. Some studies applied more than one of the above-mentioned methods. In this category, the maximum number of coinciding outcomes attributed to a specific bacterium was three, a number held only by two bacteria: the genus Blautia and the genus Bilophila, reported separately in three papers. Both of them could potentially be of value as PD severity biomarkers. Nevertheless, further evidence on these bacteria is essential in order to reach any preliminary conclusions on their merit. Additionally, the Desulfovibrionaceae family was deemed potentially significant as a PD severity biomarker, even though it was not particularly distinctive in the conducted analysis (Table 2). Similarly to the PD vs. HC category, the decision to distinguish this bacterial family was based on a singular study dedicating its investigation to this specific bacterial group [22].

Table 2.
Parkinson disease severity: research papers and their conclusions (H&Y—Hoehn and Yahr Scale, UPRDS—Unified Parkinson’s Disease Rating Scale, LED—Levodopa Equivalent Dose, UPRDS-III—Unified Parkinson’s Disease Rating Scale 3rd part, DD— disease duration, PD—patient with Parkinson’s disease, HC—healthy control, DC—diseased control).
3.3. Non-Motor Symptoms
NMS of PD may be particularly burdensome for certain patients. The discovery of distinct NMS biomarkers could be a useful tool when targeting specific non-motor symptoms for treatment. In addition to this, identifying NMS biomarkers could provide new insight into PD pathogenesis. The following non-motor symptoms were examined in the identified studies: nine papers chose to focus on gastrointestinal (GI) symptoms (most commonly constipation; with the use of i.a. the Bristol and Wexner scales); four focused on cognitive decline (MoCA and MMSE scales were mostly used); one paper analyzed difficulties in daily living (using the PDQ-39 scale), body mass index (BMI), depression, and chronic pain; and one paper explicitly analyzed weight loss (WL). The results are summarized in Table 3. After careful examination of Table 3, the authors were not able to identify a single potential biomarker, as the number of papers on individual NMS was not sufficient in order to reach a clear, concise conclusion. Additionally, some studies generated contradictory outcomes. Akkermansia was found to be elevated in constipated PD patients in two studies [49,58].

Table 3.
Non-motor symptoms of Parkinson disease: research papers and their conclusions (PD—patients with Parkinson’s disease, HC—healthy controls, C—constipation, WS—Wexner score, NMSQ—Non-Motor Symptoms Questionnaire, PDQ39—The Parkinson’s Disease Questionnaire, MoCA—Montreal Cognitive Assessment, MMSE—Mini-mental state examination, NMS—non-motor symptoms, IBS—Irritable bowel syndrome).
3.4. Clinical Phenotype
The pathophysiology of individual PD clinical subtypes is yet to be resolved. However, some studies have been able to identify a link between distinct bacteria and PD motor phenotype. The results of the previously mentioned analysis of studies investigating clinical phenotypes can be found in Table 4. The following clinical phenotypes were evaluated in the selected studies: one study examined both early-onset PD and late-onset PD; one study distinguished non-tremor PD; four focused on tremor-dominant PD; two studies focused on postural instability and gait disorders (PIGD); one examined dyskinetic PD; and finally one study analyzed hypokinetic-rigid PD. After careful examination of Table 4, the authors were not able to identify any potential biomarkers, as the number of papers on individual clinical phenotypes of PD was not sufficient to reach a clear, concise conclusion.

Table 4.
Clinical phenotypes of Parkinson disease: research papers and their conclusions (TD—tremor-dominant phenotype of Parkinson’s disease, NTD—non-tremor dominant phenotypes of Parkinson’s disease, PD—patient with Parkinson’s disease, HC—healthy control, PIGD—Postural Instability and Gait Disorder).
4. Discussion
Microbiological research on PD pathophysiology is rapidly gaining momentum. In contrast to other neurodegenerative diseases involving the CNS, early involvement of the peripheral nervous system is a unique, yet somewhat puzzling, element of PD [8,9,67]. The role that bacteria have in triggering intestinal inflammation is currently being extensively investigated, with research proposing a handful of mechanisms. These include the following: (1) a disruption of the mucus layer in the intestine (Akkermansia muciniphila, Bifidobacterium, Desulfovibrionaceae), (2) a disruption in short-chained fatty acid (SCFA) production, (3) an increased production of proinflammatory cytokines (TNF, IL-1, IL-17, IFN-gamma, and IL-6), and (4) lipopolysaccharide (LPS) production in the intestine [22,68,69]. Microbial metabolites and their pathophysiological link to PD are an especially important and constantly growing area of research. It has been established that stool and serum bacterial metabolites, along with inflammatory cytokines, have an influence on glia maturation and functioning [70]. Substances such as LPS or SCFA play vital roles in the complex process that is neuroinflammation [69,71,72]. Mucosal layer disturbances caused by bacteria and their byproducts, in conjunction with intestinal barrier integrity dysfunction, may lead to an increased enteric nervous system exposure to high amounts of toxins [15]. Elevated levels of fecal calprotectin (a marker of intestinal inflammation), fecal alpha-1-antitrypsin, and fecal zonulin (both markers of intestinal permeability) in PD patients further support this hypothesis [73].
Identifying a distinct microbe involved in PD pathogenesis would be groundbreaking, as it could initiate a shift from the current symptomatic treatment of PD to more causative and targeted therapies. One of the main limitations of this review is that the currently available studies apply differing approaches towards microbiome analysis, both in methodology and nomenclature. Regarding methodology, the studies yielded in this review used either 16s rRNA or 16s rDNA for microbiological analyses, with some additionally analyzing bacterial metabolites. As for nomenclature, different taxonomic levels of bacteria were examined, which led to some difficulties when searching for coinciding results among studies. It cannot be presumed, for example, that an increase of a specific species reported in one study is equivalent to an increase of the whole taxonomic family to which this species belongs, and vice versa.
Microbiota studies are often difficult to interpret due to a great diversity of obtained results. Numerous external factors such as diet, circadian rhythm, concomitant diseases, and medication have an enormous influence on gut microbiota [74,75]. The results of our literature review are partially in line with a recent meta-analysis conducted by Toh et al. [76]. The authors analyzed raw 16s rRNA sequences from 10 studies, which included a total of 969 PD patients and 734 controls. They established that factors, such as race (Caucasian vs. non-Caucasian) and geographical location, influence intestinal microbiota composition. They reported elevated levels of Akkermansia and Hungatella and reduced levels of Roseburia and Faecalibacterium in PD subjects. Interestingly, the authors determined that a reduced abundance of Roseburia and unclassified Lachnospiracea was identified in both Caucasian and non-Caucasian cohorts. The results of this important study suggest that a specific pathogens may be involved in PD pathogenesis, irrespective of race.
A reduced abundance of the Roseburia species in PD, which has been reported in our review as well as in a recent metanalysis, is of particular interest. This species is considered “a biomarker of good health” [1]. Its decreased levels were described not only in PD, but also in both depressive and bipolar disorders, and in Alzheimer disease [77,78]. These commensal bacteria play an important role in the production of colonic butyrate, which is considered to be one of the most important SCFAs [79]. Lower levels of Roseburia and certain serum SCFAs in PD subjects are most likely correlated with one another.
A study explicitly focusing on the Desulfovibrionaceae family is particularly noteworthy, as it clearly establishes the importance of the Desulfovibrionaceae in PD pathogenesis [22]. According to Murros et al., members of this bacterial family adhere to the intestinal wall whilst producing LPS and hydrogen sulfide, a chemical considered neurotoxic in high concentrations. It has been proposed that hydrogen sulfide may trigger alpha-synuclein aggregation, leading to intestinal neurodegeneration. Bacteria from the Desulfovibrionaceae family, when present in disproportionate amounts, disrupt butyrate production [22]. This unique set of features, combined with the corroborating results of the above-mentioned study, explicitly implicate the involvement of the Desulfovibrionaceae in PD pathogenesis.
Another important aspect of microbial PD research is the heterogeneity of the disease itself. It has been postulated that PD should be considered a syndrome rather than a homogeneous disease, due to its varied manifestation among PD patients [80]. Although it is possible that patients with different PD phenotypes share specific microbiome signatures, the results from the studies investigating distinct PD subtype pathogens are incompatible with one another [33,34,37,41,43,58,66].
Finally, it cannot be presumed that the microbial trigger potentially involved in PD pathogenesis is present throughout the whole course of the disease. It may reside in the intestine only for a limited amount of time, perhaps even years before motor symptom onset. If this were to be the case, the microbial culprit would be undetectable at the time of stool sample collection. Moreover, constipation, a common non-motor symptom of PD, may have a significant impact on the diverse results achieved in the conducted research [48]. It has been well-documented that constipation greatly influences gut microbiota in non-PD subjects [80,81]. It is therefore crucial that constipation be considered an important modifying factor when analyzing gut bacteria composition. The question remains whether changes in gut microbiota are the cause or the consequence of altered peristaltic movements in PD subjects [82].
5. Conclusions
We conclude that future research on gut microbiota in PD subjects should be comprised of large, international data sets, preferably with patients of varying ethnic backgrounds and residing in different geographical locations. This would subdue the interference of local factors, such as diet. Nevertheless, recent studies have already been able to reach some interesting and valuable preliminary conclusions, which may be helpful in determining future directions of research in this field. The findings deemed most significant in this review were elevated levels of the Akkermansia genus, the Verrucomicrobiaceae family, the Rikenellaceae family, and the Lactobacillus genus in PD subjects. These bacteria could potentially be used as PD biomarkers. In addition to this, reduced levels of the Roseburia genus and the Lachnospiraceae family in PD subjects were also noteworthy; we encourage that they be further investigated in the future for their potentiality as biomarkers. Each of the above-mentioned bacteria, which have been highlighted by previous studies of gut microbiota in PD, could possibly serve as a target for prospective microbiota-modifying therapies, such as probiotic treatment or fecal matter transplantation. Moreover, it is our recommendation that detailed clinical characteristics of PD patients be recorded, as this step is crucial for distinguishing distinctive bacterial fingerprints. Unified nomenclatures of microbial taxonomic levels should be adopted between studies, as it would allow for more precise and transparent conclusions when comparing their outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines10092057/s1, Table S1. Parkinson disease patients and healthy controls: research papers and their conclusions—full version (PD—patients with Parkinson’s disease, HC—healthy controls) [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,60,64,83].
Author Contributions
Conceptualization, M.F., J.M.N. and M.K.; methodology, M.F., J.M.N. and M.K.; formal analysis, M.K., J.M.N. and M.F.; investigation, M.K. and J.M.N.; data curation, M.F., J.M.N. and M.K.; writing—original draft preparation, M.K., J.M.N. and M.F.; writing—review and editing, M.F., J.M.N., M.K., D.K. and A.F.; visualization, M.K.; supervision, M.F., D.K. and A.F.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used in this study are available in Table S1 in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dulovic, M.; Vos, M. Sleep dysfunction in Parkinson’s disease: Novel molecular mechanism and implications for therapy. Mov. Disord. 2018, 33, 1558–1559. [Google Scholar] [CrossRef] [PubMed]
- Tarakad, A.; Jankovic, J. Anosmia and Ageusia in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 133, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Visanji, N.P.; Liu, L.W.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef]
- Hermanowicz, N.; Jones, S.A.; Hauser, R.A. Impact of non-motor symptoms in Parkinson’s disease: A PMDAlliance survey. Neuropsychiatr. Dis. Treat. 2019, 15, 2205–2212. [Google Scholar] [CrossRef]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. A timeline for Parkinson’s disease. Parkinsonism. Relat. Disord. 2010, 16, 79–84. [Google Scholar] [CrossRef]
- Qualman, S.J.; Haupt, H.M.; Yang, P.; Hamilton, S.R. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology 1984, 87, 848–856. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Takahashi, H.; Takeda, S.; Ohama, E.; Ikuta, F. Parkinson’s disease: The presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 1988, 76, 217–221. [Google Scholar] [CrossRef]
- Beach, T.G.; Adler, C.H.; Sue, L.I.; Vedders, L.; Lue, L.; White Iii, C.L.; Akiyama, H.; Caviness, J.N.; Shill, H.A.; Sabbagh, M.N.; et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010, 119, 689–702. [Google Scholar] [CrossRef]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. Parkinson’s disease: A dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007, 33, 599–614. [Google Scholar] [CrossRef]
- Horsager, J.; Knudsen, K.; Sommerauer, M. Clinical and imaging evidence of brain-first and body-first Parkinson’s disease. Neurobiol. Dis. 2022, 164, 105626. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, X.H.; Guedes Soares, C. Experimental study on the response of multi-layered protective structure subjected to underwater contact explosions. Int. J. Impact Eng. 2017, 100, 23–34. [Google Scholar] [CrossRef]
- Sixel-Döring, F.; Zimmermann, J.; Wegener, A.; Mollenhauer, B.; Trenkwalder, C. The Evolution of REM Sleep Behavior Disorder in Early Parkinson Disease. Sleep 2016, 39, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Is Braak staging valid for all types of Parkinson’s disease? J. Neural Transm. 2019, 126, 423–431. [Google Scholar] [CrossRef]
- Parkkinen, L.; Pirttilä, T.; Alafuzoff, I. Applicability of current staging/categorization of α-synuclein pathology and their clinical relevance. Acta Neuropathol. 2008, 115, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef]
- Ternak, G.; Kuti, D.; Kovacs, K.J. Dysbiosis in Parkinson’s disease might be triggered by certain antibiotics. Med. Hypotheses 2020, 137, 109564. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2015, 6, 1543. [Google Scholar] [CrossRef]
- Ma, S.Y.; Roytta, M.; Rinne, J.O.; Collan, Y.; Rinne, U.K. Correlation between neuromorphometry in the substantia nigra and clinical features in Parkinson’s disease using disector counts. J. Neurol. Sci. 1997, 151, 83–87. [Google Scholar] [CrossRef]
- Greffard, S.; Verny, M.; Bonnet, A.M.; Beinis, J.Y.; Gallinari, C.; Meaume, S.; Piette, F.; Hauw, J.J.; Duyckaerts, C. Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch. Neurol. 2006, 63, 584–588. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Murros, K.E.; Huynh, V.A.; Takala, T.M.; Saris, P.E.J. Desulfovibrio Bacteria Are Associated With Parkinson’s Disease. Front. Cell. Infect. Microbiol. 2021, 11, 652617. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, P.; Chen, Z.; Sui, X.; Xie, X.; Zhang, J. Alteration of the fecal microbiota in North-Eastern Han Chinese population with sporadic Parkinson’s disease. Neurosci. Lett. 2019, 707, 134297. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, G.; Li, Z.; Wu, B.; Luo, E.; Qiu, X.; Guo, J.; Xia, Z.; Zheng, C.; Su, Q.; et al. Altered Actinobacteria and Firmicutes Phylum Associated Epitopes in Patients With Parkinson’s Disease. Front. Immunol. 2021, 12, 632482. [Google Scholar] [CrossRef]
- Kenna, J.E.; Chua, E.G.; Bakeberg, M.; Tay, A.; McGregor, S.; Gorecki, A.; Horne, M.; Marshall, B.; Mastaglia, F.L.; Anderton, R.S. Changes in the Gut Microbiome and Predicted Functional Metabolic Effects in an Australian Parkinson’s Disease Cohort. Front. Neurosci. 2021, 15, 756951. [Google Scholar] [CrossRef]
- Li, C.; Cui, L.; Yang, Y.; Miao, J.; Zhao, X.; Zhang, J.; Cui, G.; Zhang, Y. Gut microbiota differs between parkinson’s disease patients and healthy controls in northeast China. Front. Mol. Neurosci. 2019, 12, 171. [Google Scholar] [CrossRef]
- Kim, C.H.; Jung, J.; Lee, Y.U.; Kim, K.H.; Kang, S.; Kang, G.H.; Chu, H.; Kim, S.Y.; Lee, S. Comparison of Metabolites and Gut Microbes between Patients with Parkinson’s Disease and Healthy Individuals—A Pilot Clinical Observational Study (STROBE Compliant). Healthcare 2022, 10, 302. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Hu, X.; Wang, T.; Liang, S.; Duan, Y.; Jin, F.; Qin, B. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Science China. Life Sci. 2017, 60, 1223–1233. [Google Scholar] [CrossRef]
- Lin, A.; Zheng, W.; He, Y.; Tang, W.; Wei, X.; He, R.; Huang, W.; Su, Y.; Huang, Y.; Zhou, H.; et al. Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat. Disord. 2018, 53, 82–88. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Huang, P.; Li, B.; Du, J.; He, Y.; Su, B.; Xu, L.M.; Wang, L.; et al. Gut metagenomics-derived genes as potential biomarkers of Parkinson’s disease. Brain 2020, 143, 2474–2489. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.A.; Saltykova, I.V.; Zhukova, I.A.; Alifirova, V.M.; Zhukova, N.G.; Dorofeeva, Y.B.; Tyakht, A.V.; Kovarsky, B.A.; Alekseev, D.G.; Kostryukova, E.S.; et al. Analysis of gut microbiota in patients with parkinson’s disease. Bull. Exp. Biol. Med. 2017, 162, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Pietrucci, D.; Cerroni, R.; Unida, V.; Farcomeni, A.; Pierantozzi, M.; Mercuri, N.B.; Biocca, S.; Stefani, A.; Desideri, A. Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Parkinsonism Relat. Disord. 2019, 65, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chen, C.C.; Chiang, H.L.; Liou, J.M.; Chang, C.M.; Lu, T.P.; Chuang, E.Y.; Tai, Y.C.; Cheng, C.; Lin, H.Y.; et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflamm. 2019, 16, 129. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, F.; Cao, J.; Ding, W.; Yan, S.; Shi, W.; Wen, S.; Yao, L. Alterations of gut microbiota and metabolome with Parkinson’s disease. Microb. Pathog. 2021, 160, 105187. [Google Scholar] [CrossRef]
- Zhang, F.; Yue, L.; Fang, X.; Wang, G.; Li, C.; Sun, X.; Jia, X.; Yang, J.; Song, J.; Zhang, Y.; et al. Altered gut microbiota in Parkinson’s disease patients/healthy spouses and its association with clinical features. Parkinsonism Relat. Disord. 2020, 81, 84–88. [Google Scholar] [CrossRef]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Vascellari, S.; Palmas, V.; Melis, M.; Pisanu, S.; Cusano, R.; Uva, P.; Perra, D.; Madau, V.; Sarchioto, M.; Oppo, V.; et al. Gut microbiota and metabolome alterations associated with Parkinson’s disease. mSystems 2020, 5, e00561-20. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.D.; Xiao, Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef]
- Ren, T.; Gao, Y.; Qiu, Y.; Jiang, S.; Zhang, Q.; Zhang, J.; Wang, L.; Zhang, Y.; Wang, L.; Nie, K. Gut Microbiota Altered in Mild Cognitive Impairment Compared With Normal Cognition in Sporadic Parkinson’s Disease. Front. Neurol. 2020, 11, 137. [Google Scholar] [CrossRef]
- Weis, S.; Schwiertz, A.; Unger, M.M.; Becker, A.; Faßbender, K.; Ratering, S.; Kohl, M.; Schnell, S.; Schäfer, K.H.; Egert, M. Effect of Parkinson’s disease and related medications on the composition of the fecal bacterial microbiota. npj Parkinson’s Dis. 2019, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Wallen, Z.D.; Appah, M.; Dean, M.N.; Sesler, C.L.; Factor, S.A.; Molho, E.; Zabetian, C.P.; Standaert, D.G.; Payami, H. Characterizing dysbiosis of gut microbiome in PD: Evidence for overabundance of opportunistic pathogens. npj Parkinson’s Dis. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Aho, V.T.E.; Pereira, P.A.B.; Voutilainen, S.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. Gut microbiota in Parkinson’s disease: Temporal stability and relations to disease progression. EBioMedicine 2019, 44, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Baldini, F.; Hertel, J.; Sandt, E.; Thinnes, C.C.; Neuberger-Castillo, L.; Pavelka, L.; Betsou, F.; Krüger, R.; Thiele, I. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. 2020, 18, 62. [Google Scholar] [CrossRef]
- Barichella, M.; Severgnini, M.; Cilia, R.; Cassani, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cancello, R.; Ceccarani, C.; Faierman, S.; et al. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 2019, 34, 396–405. [Google Scholar] [CrossRef]
- Bedarf, J.R.; Hildebrand, F.; Coelho, L.P.; Sunagawa, S.; Bahram, M.; Goeser, F.; Bork, P.; Wüllner, U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017, 9, 39. [Google Scholar] [CrossRef]
- Chen, S.J.; Chen, C.C.; Liao, H.Y.; Lin, Y.T.; Wu, Y.W.; Liou, J.M.; Wu, M.S.; Kuo, C.H.; Lin, C.H. Association of Fecal and Plasma Levels of Short-Chain Fatty Acids with Gut Microbiota and Clinical Severity in Patients with Parkinson Disease. Neurology 2022, 98, E848–E858. [Google Scholar] [CrossRef]
- Chen, W.; Bi, Z.; Zhu, Q.; Gao, H.; Fan, Y.; Zhang, C.; Liu, X.; Ye, M. An analysis of the characteristics of the intestinal flora in patients with Parkinson s disease complicated with constipation. Am. J. Transl. Res. 2021, 13, 13710–13722. [Google Scholar]
- Cirstea, M.S.; Yu, A.C.; Golz, E.; Sundvick, K.; Kliger, D.; Radisavljevic, N.; Foulger, L.H.; Mackenzie, M.; Huan, T.; Finlay, B.B.; et al. Microbiota Composition and Metabolism Are Associated With Gut Function in Parkinson’s Disease. Mov. Disord. 2020, 35, 1208–1217. [Google Scholar] [CrossRef]
- Cosma-Grigorov, A.; Meixner, H.; Mrochen, A.; Wirtz, S.; Winkler, J.; Marxreiter, F. Changes in Gastrointestinal Microbiome Composition in PD: A Pivotal Role of Covariates. Front. Neurol. 2020, 11, 1041. [Google Scholar] [CrossRef]
- Del Chierico, F.; Grassini, P.; Quagliariello, A.; Torti, M.; Russo, A.; Reddel, S.; Stocchi, F. The impact of intestinal microbiota on weight loss in Parkinson’s disease patients: A pilot study. Future Microbiol. 2020, 15, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P.; et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Lubomski, M.; Xu, X.; Holmes, A.J.; Muller, S.; Yang, J.Y.H.; Davis, R.L.; Sue, C.M. Nutritional Intake and Gut Microbiome Composition Predict Parkinson’s Disease. Front. Aging Neurosci. 2022, 14, 881872. [Google Scholar] [CrossRef]
- Lubomski, M.; Xu, X.; Holmes, A.J.; Muller, S.; Yang, J.Y.H.; Davis, R.L.; Sue, C.M. The Gut Microbiome in Parkinson’s Disease: A Longitudinal Study of the Impacts on Disease Progression and the Use of Device-Assisted Therapies. Front. Aging Neurosci. 2022, 14, 875261. [Google Scholar] [CrossRef]
- Jin, M.; Li, J.; Liu, F.; Lyu, N.; Wang, K.; Wang, L.; Liang, S.; Tao, H.; Zhu, B.; Alkasir, R. Analysis of the Gut Microflora in Patients With Parkinson’s Disease. Front. Neurosci. 2019, 13, 1184. [Google Scholar] [CrossRef]
- Minato, T.; Maeda, T.; Fujisawa, Y.; Tsuji, H.; Nomoto, K.; Ohno, K.; Hirayama, M. Progression of Parkinson’s disease is associated with gut dysbiosis: Two-year follow-up study. PLoS ONE 2017, 12, e0187307. [Google Scholar] [CrossRef]
- Khedr, E.M.; Ali, A.M.; Deaf, E.; Hassan, H.M.; Alaa, A.; Gamea, A. Gut microbiota in Parkinson’s disease patients: Hospital-based study. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 153. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishiwaki, H.; Ito, M.; Iwaoka, K.; Takahashi, K.; Suzuki, Y.; Taguchi, K.; Yamahara, K.; Tsuboi, Y.; Kashihara, K.; et al. Altered gut microbiota in Parkinson’s disease patients with motor complications. Parkinsonism Relat. Disord. 2022, 95, 11–17. [Google Scholar] [CrossRef]
- Rosario, D.; Bidkhori, G.; Lee, S.; Bedarf, J.; Hildebrand, F.; Le Chatelier, E.; Uhlen, M.; Ehrlich, S.D.; Proctor, G.; Wüllner, U.; et al. Systematic analysis of gut microbiome reveals the role of bacterial folate and homocysteine metabolism in Parkinson’s disease. Cell Rep. 2021, 34, 108807. [Google Scholar] [CrossRef]
- Cilia, R.; Piatti, M.; Cereda, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cassani, E.; Bonvegna, S.; Ferrarese, C.; Zecchinelli, A.L.; et al. Does gut microbiota influence the course of Parkinson’s disease? A 3-Year prospective exploratory study in de novo patients. J. Parkinson’s Dis. 2021, 11, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, H.; Ito, M.; Hamaguchi, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Yoshida, T.; Hanada, H.; Takeuchi, I.; et al. Short chain fatty acids-producing and mucin-degrading intestinal bacteria predict the progression of early Parkinson’s disease. npj Parkinson’s Dis. 2022, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, X.; Qian, Y.; Xu, S.; Mo, C.; Yan, Z.; Yang, X.; Xiao, Q. Plasma branched-chain and aromatic amino acids correlate with the gut microbiota and severity of Parkinson’s disease. npj Parkinson’s Dis. 2022, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Mertsalmi, T.H.; Aho, V.T.E.; Pereira, P.A.B.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. More than constipation–bowel symptoms in Parkinson’s disease and their connection to gut microbiota. Eur. J. Neurol. 2017, 24, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.C.; Shih, L.C.; Wu, P.H.; Hsieh, Y.C.; Lee, C.H.; Lin, S.H.; Wang, H. Exploring the Causal Effect of Constipation on Parkinson’s Disease Through Mediation Analysis of Microbial Data. Front. Cell. Infect. Microbiol. 2022, 12, 871710. [Google Scholar] [CrossRef]
- Vascellari, S.; Melis, M.; Palmas, V.; Pisanu, S.; Serra, A.; Perra, D.; Santoru, M.L.; Oppo, V.; Cusano, R.; Uva, P.; et al. Clinical phenotypes of Parkinson’s disease associate with distinct gut microbiota and metabolome enterotypes. Biomolecules 2021, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; de Vos, R.A.; Bohl, J.; Del Tredici, K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett. 2006, 396, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Luo, Y.; Ray Chaudhuri, K.; Reynolds, R.; Tan, E.-K.; Pettersson, S. The role of gut dysbiosis in Parkinson’s disease: Mechanistic insights and therapeutic options. Brain 2021, 144, 2571–2593. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.F.; Shen, Y.Q. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s Disease. Ageing Res. Rev. 2018, 45, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Spiegel, J.; Dillmann, U.; Grundmann, D.; Bürmann, J.; Faßbender, K.; Schäfer, K.-H.; Unger, M.M. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat. Disord. 2018, 50, 104–107. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Matenchuk, B.A.; Mandhane, P.J.; Kozyrskyj, A.L. Sleep, circadian rhythm, and gut microbiota. Sleep Med. Rev. 2020, 53, 101340. [Google Scholar] [CrossRef]
- Toh, T.S.; Chong, C.W.; Lim, S.-Y.; Bowman, J.; Cirstea, M.; Lin, C.-H.; Chen, C.-C.; Appel-Cresswell, S.; Finlay, B.B.; Tan, A.H. Gut microbiome in Parkinson’s disease: New insights from meta-analysis. Parkinsonism Relat. Disord. 2022, 94, 1–9. [Google Scholar] [CrossRef]
- Knuesel, T.; Mohajeri, M.H. The Role of the Gut Microbiota in the Development and Progression of Major Depressive and Bipolar Disorder. Nutrients 2021, 14, 37. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Hendriksen, H.M.A.; de Leeuw, F.A.; Doorduijn, A.S.; van Leeuwenstijn, M.; Teunissen, C.E.; Barkhof, F.; Scheltens, P.; Kraaij, R.; van Duijn, C.M.; et al. Gut Microbiota Composition Is Related to AD Pathology. Front. Immunol. 2021, 12, 794519. [Google Scholar] [CrossRef]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef]
- Bousdouni, P.; Kandyliari, A.; Koutelidakis, A.E. Probiotics and Phytochemicals: Role on Gut Microbiota and Efficacy on Irritable Bowel Syndrome, Functional Dyspepsia, and Functional Constipation. Gastrointest. Disord. 2022, 4, 30–48. [Google Scholar] [CrossRef]
- Hofman, D.; Kudla, U.; Miqdady, M.; Nguyen, T.V.H.; Moran-Ramos, S.; Vandenplas, Y. Faecal Microbiota in Infants and Young Children with Functional Gastrointestinal Disorders: A Systematic Review. Nutrients 2022, 14, 974. [Google Scholar] [CrossRef] [PubMed]
- Nandwana, V.; Nandwana, N.K.; Das, Y.; Saito, M.; Panda, T.; Das, S.; Almaguel, F.; Hosmane, N.S.; Das, B.C. The Role of Microbiome in Brain Development and Neurodegenerative Diseases. Molecules 2022, 27, 3402. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.H.; Chong, C.W.; Lim, S.Y.; Yap, I.K.S.; Teh, C.S.J.; Loke, M.F.; Song, S.L.; Tan, J.Y.; Ang, B.H.; Tan, Y.Q.; et al. Gut Microbial Ecosystem in Parkinson Disease: New Clinicobiological Insights from Multi-Omics. Ann. Neurol. 2021, 89, 546–559. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).