Abstract
The relationship between inflammation and cancer has attracted attention for a long time. The inflammatory tumor microenvironment consists of inflammatory cells, chemokines, cytokines, and signaling pathways. Among them, inflammatory cytokines play an especially pivotal role in cancer development, prognosis, and treatment. Interleukins, tumor necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β), interferons, and vascular endothelial growth factor (VEGF) are the representative inflammatory cytokines in various cancers, which may promote or inhibit cancer progression. The pro-inflammatory cytokines are associated with advanced cancer stages, resistance to immunotherapy, and poor prognoses, such as in objective response and disease control rates, and progression-free and overall survival. In this review, we selected colorectal, pancreatic, breast, gastric, lung, and prostate cancers, which are well-reported for an association between cancer and inflammatory cytokines. The related cytokines and their effects on each cancer’s development and prognosis were summarized. In addition, the treatment strategies targeting inflammatory cytokines in each carcinoma were also described here. By understanding the biological roles of cancer-related inflammatory cytokines, we may modulate the inflammatory tumor microenvironment for potential cancer treatment.
1. Introduction
Inflammation is an innate immune system that involves the recruitment and activation of immune cells, as well as the function of soluble factors, including cytokines and chemokines. This process has traditionally represented a front line of host defense against harmful stimuli, such as pathogens or irritants, and inflammatory cells are also essential for tissue repair [1]. Beyond this traditional role of inflammation, robust analyses of tumor transcriptomes indicated that inflammation is closely related to tumors by revealing a distinct expression profile of inflammatory cytokines and recruited immune cells in different tumor types [2,3]. In the context of ‘tumor-associated inflammation’, recent extensive studies have demonstrated a reciprocal interaction between inflammation and cancer, providing a comprehensive concept of tumor microenvironment (TME) where tumor cells exist in the network of stromal cells and cells of innate and adaptive immunity. While tumor cells regulate the inflammation state by secreting inflammatory mediators in TME, inflammation also controls cancer development, progression, and response to cancer therapies [4]. Tumor-cell-intrinsic changes during tumorigenesis can elicit inflammation. For instance, the loss of a tumor suppressor, e.g., p53, induces an increased activation of nuclear factor-κB (NF-κB) and an inhibition of DNA repair, which leads to the expression of inflammatory genes and the stimulation of the inflammation pathway [5,6]. Signaling through activated oncogenes can drive the generation of cytokines, chemokines, and various inflammatory factors [7]. In addition, tumor cells in chronic inflammation secrete cytokines (transforming growth factor (TGF)-β and interleukin (IL)-10) and chemokines to prevent dendritic cells (DCs) from the presentation of tumor antigens, and to recruit immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs), regulatory T cells, and M2 macrophages, thereby resulting in the generation of a cancer-promoting inflammation environment by suppressing anti-tumor immune responses [8]. On the other hand, the notion that inflammation plays a significant role in the regulation of cancer is currently well accepted. The immune system either promotes or suppresses all stages of cancer, as well as prognosis and the outcome of cancer treatment. Certain types of cancer are preceded by chronic inflammation, which occurs before tumor initiation and promotes cancer. As shown in colorectal or liver cancer cases, chronic inflammation, such as inflammatory bowel disease or chronic hepatitis, increases the risk of cancer [9]. Reactive oxygen species generated by macrophages during inflammation may cause the accumulation of mutations in normal tissues, and inflammatory cytokines can affect the pro-survival signaling, i.e., the STAT-3 activating pathway, of mutated cells [10,11]. In the course of tumor progression, inflammatory cytokines, including IL-6 and IL-17, facilitate the proliferation of tumor cells, and several cytokines can play an antagonizing role in the immune cells of anti-tumor responses. Inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and IL-1b, can influence the expression of transcription factors that induce epithelial to mesenchymal transitions, which enable the dissemination of tumor cells [12]. However, acute inflammatory responses contribute to anti-tumor immunity in TME. Upon DCs uptaking tumor antigens and becoming mature, they induce inflammatory responses by regulating multiple immune cells, such as M1 macrophages and natural killer cells, via inflammatory cytokines, including interferon (IFN)-γ, IL-1, IL-12, and IL-15, etc. [13].
Here, we focus on the role of inflammatory cytokines in cancer and review the relationships of inflammatory cytokines with various cancer types. In this context, we discuss therapeutic approaches targeting inflammatory cytokines for the development of anti-cancer drugs. Given that the regulation of cancer is tightly engaged with inflammation, specifically inflammatory cytokines, defining the underlying mechanisms will provide insight into advanced strategies for the development of anti-cancer therapies.
2. Inflammatory Cytokines in Cancers
2.1. Colorectal Cancer
Colorectal cancer (CRC) refers to a malignant tumor composed of cancer cells in the large intestine. CRC is largely divided into colon or rectal cancer, depending on where cancer occurs. The incidence risk of CRC is associated with risk factors such as physical inactivity, age, race, or sex [14]. Chronic inflammation is considered to have a strong association with the early stages of tumor onset. CRC commonly occurs via a somatic mutation in a gene that encodes a part of the Wnt signaling pathway; hereditary mutations, such as nonpolyposis colorectal cancer (Lynch syndrome) [15]; or familial adenomatous polyposis [16]. Inherited cases can be prevented or delayed by anti-inflammatory treatment [17,18]. Inflammatory bowel diseases, including Crohn’s disease and ulcerative colitis, increase the risk of CRC related to colitis with poor prognoses [19,20]. Dietary and gut microbiota also affect the progression of chronic enteritis [21,22,23,24,25,26]. Gut microbial distribution changes as CRC progresses, and this change is related to pathological tumor characteristics [27,28]. While certain types of intestinal bacteria may protect the host by promoting an anti-inflammatory immune system, others can induce inflammation or mutation [21,22,23,24,25,26]. Since CRC is closely associated with chronic inflammation, various studies for inflammatory cytokines in CRC have been evaluated.
TNF-α is a well-known tumor-suppressive cytokine that induces apoptosis in specific types of cells. On the other hand, it promotes tumors so that inflammation can proceed to cancer [29,30,31]. Colitis and colitis-associated colon cancer (CAC) proceeded fast in a TNF-α–IL-10-deficient mouse model compared with an IL-10-deficient mouse model. In this study, TNF-α acted as a protective factor against inflammation and a tumor suppressor [32]. When TNF-α plays a tumor promoter role, a TNF-α inhibitor can be an attractive targeted treatment. In a study by Liu et al., a combination therapy of 5-fluorouracil (5-FU) and infliximab (TNF-α inhibitor) showed better outcomes than 5-FU monotherapy [33]. In more than 30% of CRC cases, granulocyte–macrophage colony-stimulating factor (GM-CSF) expression is high. GM-CSF is produced in the hematological part, which may increase anti-cancer immune responses. Overexpression of GM-CSF was strongly associated with increased overall survival rates of CRC patients [34]. Interestingly, when anti-programmed death-1 (PD-1) was used to treat a GM-CSF-silenced mice model, 25% tumor remission was found, while 50% tumor remission was observed from a GM-CSF-secreting mice model [34]. The combination of anti-PD-1 and GM-CSF showed synergetic anti-cancer effects. Another overexpressed inflammatory cytokine in CRC is IL-6. Inhibition of IL-6 or its receptors in a CAC-induced mouse model revealed a decreased tumor burden [11,35]. IL-1β also plays an important role in CRC oncogenesis with increased Toll-IL-1 receptor signaling [36,37]. Furthermore, the IL-1 receptor antagonist inhibited the metastatic process of CRC by suppressing the IL-1α/PI3K/NF-κB pathway [38]. A meta-analysis of serum IL-6 in CRC patients was performed, with a total of 17 studies. IL-6 is mainly produced by T cells, macrophages, and endothelial cells. Elevated serum IL-6 levels correlated with worse overall and disease-free survival rates for CRC [39]. Other inflammatory cytokines such as IL-8, IL-1 receptor antagonist (IL-1RA), and IL-6 were proven to be associated with advanced CRC [40]. The inflammatory cytokines were confirmed to be attractive biomarkers for CRC diagnosis and/or prognosis. Several clinical trials targeting inflammatory cytokines in CRC have been initiated. A phase I/II trial using antibody targeting IL-6 (siltuximab) [41], and a phase III trial of recombinant TNF receptor (etanercept) [42], failed to induce a clinical response. However, in metastatic CRC, MABp1 (IL-1α-targeted antibody) proved to be safe and effective in a phase I study [43]. The IL-1β inhibitor is known to increase the anti-tumor efficacy of 5-FU. In a phase II clinical trial using 5-FU, bevacizumab, and anakinra (IL-1β and α inhibitor) for patients with metastatic CRC, promising activity and a controllable safety profile were shown [44].
2.2. Pancreatic Cancer
Pancreatic cancer is one of the most disastrous cancers and shows a very poor prognosis. Current standards of care for pancreatic cancer are surgical resection with chemotherapy [45]. It shows the lowest 5-year survival rate among cancers between 2007 and 2013 [46]. In most pancreatic cancer cases, it is symptomless until it progresses, and this leads to a poor survival rate. Pancreatic cancer has some relevant risk factors, including cigarette smoking, diabetes mellitus, chronic pancreatitis, and obesity [47,48,49,50,51]. Recently, inflammation has been getting attention because it affects the development and progression of pancreatic cancer. The inflammation process is associated with some carcinogenic processes [52]. Several inflammatory cytokines are known to be related to the oncogenesis of pancreatic cancer.
IL-6 is a pro-inflammatory cytokine that shows diverse functions of cell multiplication, injury, infection, and inflammation [53]. It affects tumor cells to develop pancreatic cancer by controlling vascular endothelial growth factor (VEGF) secretion [54]. Targeting IL-6 was suggested to be one of the therapeutic approaches for pancreatic cancer [55]. IL-8 plays a key role in promoting the angiogenesis of pancreatic cancer. Primary sources for IL-8 are macrophages, platelets, and epithelial cells. IL-8 showed high levels in the serum of pancreatic cancer patients and in the human pancreatic cancer cell line [56,57]. The elevated IL-8 level was related to the low survival rate of pancreatic cancer patients, which has led it to be considered as a marker for prognosis [58]. Interestingly, serum levels of IL-6, IL-8, IL-10, and IL-1RA were significantly increased in pancreatic cancer patients. These cytokine levels were associated with worse survival rates, poor performance status, and/or weight loss [59]. TNF-α is associated with acute and chronic inflammation, autoimmune disease, and inflammation related to cancers [60]. It has two receptors: (i) TNF-receptor 1, which is distributed in all types of cells with a death domain that leads to apoptosis; (ii) TNF-receptor 2, which is only distributed in hematopoietic cells without a death domain. According to the study with a pancreatic-cancer-induced mouse model, TNF-α accelerated tumor growth and metastasis. Furthermore, anti-TNF-α treatment significantly inhibited tumor progression [61]. IL-1β is known to be related to inflammation responses [62], cancer progression [63], and cancer cell invasiveness [64] in pancreatic cancer. In this manner, IL-1β has attracted attention as another therapeutic target for pancreatic cancer. Macrophage migration inhibitory factor (MIF) appears to have a function as a pro-inflammatory cytokine that controls immune and inflammatory responses [65]. MIF is also known to be associated with tumor survival and progression [66,67]. From the phase I clinical study of imalumab (a fully human recombinant antioxidized MIF antibody), the maximum tolerated and biologically active doses have been investigated in pancreatic cancer patients [68]. TGF-β directly inhibits cell proliferation in pancreatic cancer and controls immune response [69]. In a phase I/II study, a TGF-β2-specific inhibitor was used as second-line therapy, and it showed significant improvements in clinical response compared with the current standard of care [70].
2.3. Breast Cancer
Breast cancer is a disease that makes the cells in the breast grow out of control. Breast cancer shows the highest incidence and cause of death in women [60]. It results in 14% of total cancer deaths worldwide [71]. Risk factors for breast cancer include age; genetic mutations, such as BRCA1 and BRCA2; reproductive history, and obesity. The initiation process of breast cancer is not clear; however, inflammation has been suggested as a cause for tumor initiation, progression, angiogenesis, and metastasis [72]. Inflammation is closely related the cancer, in that cell proliferation is mainly derived from inflammatory molecules.
TNF-α promotes the activation, differentiation, survival, or death of cancer cells under specific conditions. It also controls immune and inflammatory responses [73]. TNF-α is rarely detected in healthy women’s serum, while it exists in high levels in breast cancer patients [74,75]. The main cell sources for TNF-α are T cells and macrophages. When 93 breast carcinoma samples were analyzed, 97% of samples were positive for TNF-α. Among them, 61% were considered to be high-grade TNF-α. There was no correlation between TNF-α positivity and relapse-free or overall survival [76]. Anti-TNF-α treatment using a monoclonal antibody (infliximab) against a TNF receptor appears to repress tumor growth, induce tumor degeneration, and inhibit bone metastases in breast cancer-induced mice [77]. TGF-β1 is considered as a prognosis marker for breast cancer. It is mainly produced by T cells and macrophages. Breast cancer patients with high TGF-β1 plasma levels had significantly worse overall and disease-free survival rates [78]. Elevated TGF-β1 levels in metastatic axillary lymph node tissue were associated with metastatic axillary lymph node numbers and tumor size [79]. In breast cancer mouse models, blocking TGF-β signaling was effective in decreasing tumor growth and metastasis [80]. IL-6 was suggested to be another prognostic biomarker of breast cancer. In a study with 87 patients who had hormone-refractory metastatic breast cancer, high levels of IL-6 were notably related to poor survival [81]. IL-12 controls the immunity and inflammatory reactions that mediate cancer progression. It has pro-inflammatory functions via activating cytotoxic immune cells [82]. A phase II clinical study (NCT04095689) using chemotherapy and pembrolizumab plus IL-12 gene therapy with triple-negative breast cancer is ongoing. The combination of chemotherapy and pembrolizumab was proven to enhance the anti-tumor efficacy. In addition, IL-12 gene therapy stimulates the anti-tumor immune response [83]. Gene therapy based on GM-CSF has been proven for its efficacy and safety through clinical trials. In the phase I study, various cancers, including breast cancer, were treated with oncolytic herpes simplex virus expressing GM-CSF. The anti-tumor immune response and tumor necrosis were observed as having a safe profile [84].
2.4. Gastric Cancer
The incidence and mortality rates of gastric cancer have been constantly declining. However, it is still the fifth most common cancer and the fourth leading cause of deaths related to cancer [71]. Among the many factors influencing gastric cancer, chronic atrophic gastritis is most closely related to the occurrence of gastric cancer [85]. Gastric inflammation is commonly caused by Helicobacter pylori and autoimmune gastritis. Gastric inflammation leads to atrophic gastritis, metaplasia, dysplasia, and adenocarcinoma [86,87]. In addition, chronic gastric inflammation increases the risk of gastric cancer. Various cytokines secreted from immune and epithelial cells in chronic inflammation are identified, and they are expected to be potential targets for gastric cancer treatment.
In a clinical study with gastric ulcer patients, IL-17 was proved to be important in the inflammatory response to Helicobacter pylori. Moreover, IL-17 also affects the Helicobacter pylori-associated diseases. IFN-γ showed increased levels in gastric mucosa after Helicobacter pylori infection. IFN-γ upregulates NF- κB signaling so that carcinogenesis occurs [88]. Accordingly, inhibition of IFN-γ can be a key treatment for gastric cancer. IL-6 is a pro-inflammatory cytokine that promotes the growth and progression of gastric cancer. It was identified that IL-6 is overexpressed in the stromal portion of gastric cancer and the elevated IL-6 stimulates the Jak1-STAT3 pathway in gastric cancer via paracrine signaling. This leads to the development of stroma-induced chemoresistance. To overcome the resistance to chemotherapy by targeting IL-6, tocilizumab (anti-IL-6 receptor monoclonal antibody) was used in treatment and it effectively enhanced the anti-tumor effect of chemotherapy in gastric cancer [89]. Several inflammatory cytokines were evaluated to determine whether they may be applied as prognostic biomarkers. Gastric cancer patients with high-IL-17-serum concentrations showed significantly lower 5-year survival rates compared with patients with low IL-17 rates [90]. The expression of IL-22 receptors in gastric cancer appears to be associated with lymphatic invasion and poor prognosis [91]. Furthermore, high levels of IL-6 were also related to poor prognosis with recurrence and the overall survival rates of gastric cancer patients [92]. In a clinical trial, gene therapy using GM-CSF has been proven useful for its efficacy and safety against gastric cancer [84]. Currently, PD-1/programmed death ligand-1 (PD-L1) immune checkpoint inhibitors (ICIs) are often selected for cancer treatment. ICIs inhibit the immunosuppressive mechanisms of tumor cells. ICIs utilize host autoimmune functions for antitumor activity while anti-cancer agents attack the cancer cells directly. However, unfortunately, only a few selected cancer patients responded to this immunotherapy due to different PD-1/PD-L1 expression levels. Infiltrated macrophage and PD-L1 expression in gastric cancer showed high correlation. IL-6 and TNF-α from macrophages induce PD-L1 via the NF-κB and STAT3 signaling pathways. Elevated PD-L1 levels in gastric cancer cells promote the proliferation of gastric cancer cells [93]. IL-6, TNF-α, and PD-L1 may be attractive targets for gastric cancer treatment.
2.5. Lung Cancer
Lung cancer remains a major public health issue worldwide with both high incidence and mortality rates [71]. Most lung cancers do not cause any typical symptoms, such as cough, chest pain, shortness of breath, and hoarseness, at an early stage. Delayed diagnosis may lead to poor survival rates in lung cancer patients [94]. According to the American Cancer Society’s report, the 5-year relative survival rates of patients with a distant metastasis of lung cancer are 8% and 3% for non-small cell lung cancer (NSCLC) and small-cell lung cancer (SCLC), respectively [14]. Understanding the specific inflammatory cytokines from the lung cancer will help for early diagnosis and novel treatment strategies.
From previous pre-clinical studies, the correlation between inflammatory cytokines and lung cancer progression has been suggested. TNF-α acts as either a tumor suppressor or promoter in lung cancer. As a tumor suppressor, TNF-α played an essential role in CD8 T cell-mediated lung cancer cell elimination in vivo. The tumor regression from a lung cancer mouse model using CD8 T cell epitope was dependent on TNF-α levels [95]. On the other hand, as a tumor promoter, TNF-α attenuated Fas-induced A549 human lung carcinoma cell death. This anti-cancer cell death effect was affected by NF-κB, phoshatidylinositole-3 kinase (PI3-K), and mitogen-activated protein kinase (MAPK) pathways, in addition to increased anti-apoptotic protein and FLICE-like inhibitory protein long (FLIPL) [96]. In addition, TNF-α was proved to stimulate tumor growth and metastasis in lung cancer-bearing mice. The tumor promoting effect of TNF-α was dependent on the activity of NF-κB, which induces anti-apoptotic proteins, such as B-cell lymphoma-extra large (Bcl-XL), cellular inhibitor of apoptosis protein (cIAP)1, and cIAP2 [97]. In these cases, the anti-TNF-α drugs were suggested to reduce inflammation-induced tumor progression. IL-4 receptors were also expressed in lung cancer, and when the IL-4 receptor-targeted agent was administered, high cytotoxicity was observed in lung cancer cells and lung cancer-induced mice. IL-4 cytotoxin was proposed as a novel therapeutic approach for lung cancer [98]. VEGF is another overexpressed inflammatory cytokine in lung cancer. A combination of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) and anti-VEGF enhanced the anti-tumor efficacy to overcome the EGFR TKI resistance in lung cancer-induced mice [99]. The VEGF inhibitors bevacizumab and ramucirumab have been approved for NSCLC treatment in combination with chemotherapy [100]. Evidence from clinical studies also proved the correlation between inflammatory cytokines and lung cancer prognosis. Song et al. enrolled 48 NSCLC patients and 40 healthy controls to evaluate the relationship between inflammatory cytokines and NSCLC. The levels of TNF-α, IL-6, IL-8, and VEGF in serum were significantly higher in NSCLC patients compared with the healthy control group. Furthermore, TNF-α, IL-8, and VEGF levels were increased in accordance with the advanced stages of NSCLC [101]. Brenner’s team conducted a case–control study with 807 lung cancer patients and 807 smoking-matched controls. Lung cancer patients showed increased IL-6 and IL-8 levels in the serum, and the association was stronger among former and current smokers. In the study, it was also found that IL-6 and IL-8 levels are increased many years before the diagnosis of lung cancer [102]. From another clinical study, IL-10 and TGF-β1 from NSCLC patients showed significantly higher serum levels than both the healthy control and benign tumor groups. Down-regulated DNA methyltransferases increased forkhead box protein P3 (Foxp3) genes, and consequently these Foxp3+ T cells induced the immunosuppressive cytokines IL-10 and TGF-β1. The immunosuppressive microenvironment led to the tumor progression of NSCLC [103]. Inflammatory cytokines were also applied to predict the ICI treatment response from NSCLC patients. IL-10 and IL-10 receptors were found in lung cancer tissue from NSCLC patients and lung cancer cell line cultures. A positive correlation was discovered between IL-10 level and tumor size, which resulted in poor prognosis. IL-10 counteracted IFN-γ effects on the PD-1/PD-L1 pathway, which induced tumor resistance to ICIs. In this manner, IL-10 may be used to predict ICI treatment success forecasts in NSCLC before ICI treatment. Patients with lower IL-10 will show better ICI response and prognosis [104]. From another recent study, 125 NSCLC patients who received a PD-1/PD-L1 inhibitor were enrolled to investigate whether baseline serum IL-6 levels may predict ICI treatment efficacy. The subjects with low IL-6 (<13.1 pg/mL) showed significantly higher objective response and disease control rates than those with high IL-6 levels. In addition, the median progression-free and overall survival rates were significantly longer in the low-IL-6 group compared with the high-IL-6 group. In this study, the IL-6 levels in serum were suggested as a potential biomarker to predict the efficacy of ICI treatment in NSCLC [105].
2.6. Prostate Cancer
Prostate cancer is one of the most challenging cancer types among men. Cancer progression and therapeutic resistance often lead to high mortality rates [106]. Prostate cancer ranks as the second leading cause of cancer-related deaths among American men [107]. Well-known risk factors for prostate cancer include older age [108], black race [109,110], BRCA mutations [111,112], and family history [113]. Interest in the linkages between inflammation and prostate cancer has increased. Chronic inflammatory disease, such as prostatitis, was proved to increase the risk of prostate cancer [114,115]. On the other hand, some negative associations between chronic inflammation and prostate cancer have also been reported [116,117]. Knowledge of the role of inflammatory cytokines in prostate cancer progression may provide an optimized targeted-therapy strategy.
IL-30 overexpression by prostate cancer stem-like cells (PCSLC) promoted tumor onset and progression in vivo. IL-30 also played a critical role in PCSLC spread to lymph nodes and bone marrow by increasing the CXCR5/CXCL13 axis and lung metastasis through the CXCR4/CXCL12 axis. In this study, suppressing PCSLC by the proper targeting of upstream drivers was suggested as a potential treatment against prostate cancer progression and recurrence [118]. Interestingly, TGF-β1 shows double-faced functions in prostate cancer progression. At early stages, it acts as a cancer growth inhibitor, while at advanced stages, it promotes cancer development [119,120]. Park et al. reported that TGF-β1 activates IL-6 in human prostate cancer cells via synergistic signaling pathways, which are Smad2, p38-NF-κB, JNK, and Ras. IL-6 accelerates cancer cell proliferation and survival, which influence the progression and metastasis of prostate cancer. In addition, elevated IL-6 may contribute to the conversion of TGF-β1′s role as a prostate cancer promoter. Anti-IL-6 neutralizing antibody or antisense IL-6 effectively inactivated IL-6 signaling, leading to TGF-β-mediated apoptosis [121]. Various clinical trials also showed the association between inflammatory cytokines and prostate cancer prognosis. First, IL-6 levels in serum are increased in patients with prostate cancer, and it significantly correlated with cancer prognosis. A clinical study from Nakashima’s team measured IL-6 levels in serum samples from stages B, C, and D prostate cancer patients. In this study, high serum IL-6 levels were associated with the advanced stages of prostate cancer and poor survival rate [122]. IL-6 has been reported to increase erythrocyte sedimentation rate [123], which was proved to be a prognostic factor in the survival of advanced prostate cancer patients [124]. Another study from Michalaki et al. reported elevated IL-6 and TNF-α serum levels in prostate cancer patients compared with healthy controls. These increased inflammatory cytokines were correlated with advanced stages, metastasis, and poor overall survival in patients with prostate cancer [125]. TNF-α was also suggested to play an important role in the development of cachexia from prostate cancer patients. The patients with high-TNF-α serum levels showed higher performance status and mortality rates than the patients with undetectable TNF-α serum levels [126]. IL-17 is another inflammatory cytokine overexpressed in prostate cancer. Steiner et al. performed a screening of inflammatory cytokines from normal, benign hyperplastic, and malignant prostate tissues. IL-17 was rarely expressed in the normal prostate, whereas its expression was increased in benign hyperplastic and malignant prostates. In addition, a significant correlation was monitored between IL-17 level and both IL-6 and IL-8 levels in malignant prostate specimens [127].
3. Conclusions
In this review, representative inflammatory cytokines in colorectal, pancreatic, breast, gastric, lung, and prostate cancers were discussed. From the preclinical studies using cancer cell lines and cancer-induced animal models, some potential treatment strategies targeting inflammatory cytokines were suggested (Table 1). In addition, from the clinical trials, the associations between inflammatory cytokines and cancer prognosis were evaluated (Table 2 and Figure 1). Taken all together, by understanding the biological roles of inflammatory cytokines toward the cancers, we may modulate the inflammatory tumor microenvironment to find ideal cancer-targeted treatment.

Table 1.
The suggested treatment strategies from preclinical studies according to inflammatory cytokines in cancers.

Table 2.
The association between inflammatory cytokines in cancers and prognosis from clinical studies.
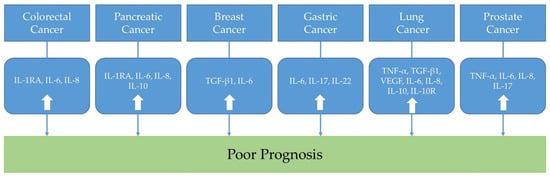
Figure 1.
The association between inflammatory cytokines in cancers and prognosis. (IL: interleukin; IL-1RA: IL-1 receptor antagonist; TNF-α: tumor necrosis factor-α; TGF: transforming growth factor; VEGF: vascular endothelial growth factor; IL-10R: interleukin-10 receptor).
Author Contributions
Conceptualization, H.-M.L. and J.-E.C.; writing—original draft preparation, H.-M.L., H.-J.L. and J.-E.C.; writing—review and editing, H.-M.L., H.-J.L. and J.-E.C.; visualization, H.-M.L. and J.-E.C.; supervision, J.-E.C.; funding acquisition, J.-E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1F1A1065569).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| TME | tumor microenvironment |
| NF-κB | nuclear factor-κB |
| TGF | transforming growth factor |
| IL | interleukin |
| DC | dendritic cell |
| MDSC | myeloid-derived suppressor cell |
| TNF | tumor necrosis factor |
| IFN | interferon |
| CRC | colorectal cancer |
| CAC | colitis-associated colon cancer |
| 5-FU | 5-fluorouracil |
| GM-CSF | granulocyte–macrophage colony-stimulating factor |
| IL-1RA | IL-1 receptor antagonist |
| VEGF | vascular endothelial growth factor |
| MIF | migration-inhibitory factor |
| PD-1 | programmed death-1 |
| PD-L1 | programmed death ligand-1 |
| ICI | immune checkpoint inhibitor |
| NSCLC | non-small-cell lung cancer |
| SCLC | small-cell lung cancer |
| PI3-K | phoshatidylinositole-3 kinase |
| MAPK | mitogen-activated protein kinase |
| FLIPL | FLICE-like inhibitory protein long |
| Bcl-XL | B-cell lymphoma-extra large |
| cIAP | cellular inhibitor of apoptosis protein |
| EGFR | epidermal growth factor receptor |
| TKI | tyrosine kinase inhibitor |
| Foxp3 | forkhead box protein P3 |
| PCSLC | prostate cancer stem-like cells |
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e24. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Kim, H.-J.; Choi, M.S.; Chang, J.-E. Crosstalk between Autophagy and Inflammatory Processes in Cancer. Life 2021, 11, 903. [Google Scholar] [CrossRef] [PubMed]
- Schwitalla, S.; Ziegler, P.K.; Horst, D.; Becker, V.; Kerle, I.; Begus-Nahrmann, Y.; Lechel, A.; Rudolph, K.L.; Langer, R.; Slotta-Huspenina, J.; et al. Loss of p53 in Enterocytes Generates an Inflammatory Microenvironment Enabling Invasion and Lymph Node Metastasis of Carcinogen-Induced Colorectal Tumors. Cancer Cell 2013, 23, 93–106. [Google Scholar] [CrossRef]
- Andriani, G.A.; Almeida, V.P.; Faggioli, F.; Mauro, M.; Tsai, W.L.; Santambrogio, L.; Maslov, A.; Gadina, M.; Campisi, J.; Vijg, J.; et al. Whole Chromosome Instability induces senescence and promotes SASP. Sci. Rep. 2016, 6, 35218. [Google Scholar] [CrossRef]
- Kortlever, R.M.; Sodir, N.M.; Wilson, C.H.; Burkhart, D.L.; Pellegrinet, L.; Brown Swigart, L.; Littlewood, T.D.; Evan, G.I. Myc Cooperates with Ras by Programming Inflammation and Immune Suppression. Cell 2017, 171, 1301–1315.e14. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Trinchieri, G. Cancer and Inflammation: An Old Intuition with Rapidly Evolving New Concepts. Annu. Rev. Immunol. 2012, 30, 677–706. [Google Scholar] [CrossRef] [PubMed]
- Canli, Ö.; Nicolas, A.M.; Gupta, J.; Finkelmeier, F.; Goncharova, O.; Pesic, M.; Neumann, T.; Horst, D.; Löwer, M.; Sahin, U.; et al. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell 2017, 32, 869–883.e5. [Google Scholar] [CrossRef] [Green Version]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.-Y.Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 Are Required for Survival of Intestinal Epithelial Cells and Development of Colitis-Associated Cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and inflammation: Inseparable actors of cancer progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef]
- Shalapour, S.; Karin, M. Pas de Deux: Control of Anti-tumor Immunity by Cancer-Associated Inflammation. Immunity 2019, 51, 15–26. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society’s Report. 2020.
- Rowan, A.J.; Lamlum, H.; Ilyas, M.; Wheeler, J.; Straub, J.; Papadopoulou, A.; Bicknell, D.; Bodmer, W.F.; Tomlinson, I.P.M. APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits”. Proc. Natl. Acad. Sci. USA 2000, 97, 3352–3357. [Google Scholar] [CrossRef]
- Groden, J.; Thliveris, A.; Samowitz, W.; Carlson, M.; Gelbert, L.; Albertsen, H.; Joslyn, G.; Stevens, J.; Spirio, L.; Robertson, M.; et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991, 66, 589–600. [Google Scholar] [CrossRef]
- Labayle, D.; Fischer, D.; Vielh, P.; Drouhin, F.; Pariente, A.; Bories, C.; Duhamel, O.; Trousset, M.; Attali, P. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology 1991, 101, 635–639. [Google Scholar] [CrossRef]
- Burn, J.; Gerdes, A.M.; MacRae, F.; Mecklin, J.P.; Moeslein, G.; Olschwang, S.; Eccles, D.; Evans, D.G.; Maher, E.R.; Bertario, L.; et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: An analysis from the CAPP2 randomised controlled trial. Lancet 2011, 378, 2081–2087. [Google Scholar] [CrossRef]
- Feagins, L.A.; Souza, R.F.; Spechler, S.J. Carcinogenesis in IBD: Potential targets for the prevention of colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 297–305. [Google Scholar] [CrossRef]
- Lakatos, P.L.; Lakatos, L. Risk for colorectal cancer in ulcerative colitis: Changes, causes and management strategies. World J. Gastroenterol. 2008, 14, 3937–3947. [Google Scholar] [CrossRef]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef]
- Viaud, S.; Daillère, R.; Boneca, I.G.; Lepage, P.; Pittet, M.J.; Ghiringhelli, F.; Trinchieri, G.; Goldszmid, R.; Zitvoge, L. Harnessing the intestinal microbiome for optimal therapeutic immunomodulation. Cancer Res. 2014, 74, 4217–4221. [Google Scholar] [CrossRef] [PubMed]
- Gagliani, N.; Hu, B.; Huber, S.; Elinav, E.; Flavell, R.A. The fire within: Microbes inflame tumors. Cell 2014, 157, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Asquith, M.; Powrie, F. An innately dangerous balancing act: Intestinal homeostasis, inflammation, and colitis-associated cancer. J. Exp. Med. 2010, 207, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Feng, Q.; Liang, S.; Jia, H.; Stadlmayr, A.; Tang, L.; Lan, Z.; Zhang, D.; Xia, H.; Xu, X.; Jie, Z.; et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat. Commun. 2015, 6, 6528. [Google Scholar] [CrossRef]
- Dejea, C.M.; Wick, E.C.; Hechenbleikner, E.M.; White, J.R.; Mark Welch, J.L.; Rossettid, B.J.; Peterson, S.N.; Snesrud, E.C.; Borisy, G.G.; Lazarev, M.; et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 18321–18326. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Popivanova, B.K.; Kitamura, K.; Wu, Y.; Kondo, T.; Kagaya, T.; Kaneko, S.; Oshima, M.; Fujii, C.; Mukaida, N. Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Invest. 2008, 118, 560–570. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, J.; Rychahou, P.G.; Qiu, S.; Evers, B.M.; Zhou, B.P. Stabilization of Snail by NF-κB Is Required for Inflammation-Induced Cell Migration and Invasion. Cancer Cell 2009, 15, 416–428. [Google Scholar] [CrossRef] [Green Version]
- Hale, L.P.; Greer, P.K. A novel murine model of inflammatory bowel disease and inflammation-associated colon cancer with ulcerative colitis-like features. PLoS ONE 2012, 7, e41797. [Google Scholar] [CrossRef]
- Liu, F.; Ai, F.; Tian, L.; Liu, S.; Zhao, L.; Wang, X. Infliximab enhances the therapeutic effects of 5-fluorouracil resulting in tumor regression in colon cancer. Onco. Targets. Ther. 2016, 9, 5999–6008. [Google Scholar] [CrossRef] [PubMed]
- Urdinguio, R.G.; Fernandez, A.F.; Moncada-Pazos, A.; Huidobro, C.; Rodriguez, R.M.; Ferrero, C.; Martinez-Camblor, P.; Obaya, A.J.; Bernal, T.; Parra-Blanco, A.; et al. Immune-dependent and independent antitumor activity of GM-CSF aberrantly expressed by mouse and human colorectal tumors. Cancer Res. 2013, 73, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Bollrath, J.; Phesse, T.J.; von Burstin, V.A.; Putoczki, T.; Bennecke, M.; Bateman, T.; Nebelsiek, T.; Lundgren-May, T.; Canli, Ö.; Schwitalla, S.; et al. gp130-Mediated Stat3 Activation in Enterocytes Regulates Cell Survival and Cell-Cycle Progression during Colitis-Associated Tumorigenesis. Cancer Cell 2009, 15, 91–102. [Google Scholar] [CrossRef]
- Xiao, H.; Gulen, M.F.; Qin, J.; Yao, J.; Bulek, K.; Kish, D.; Altuntas, C.Z.; Wald, D.; Ma, C.; Zhou, H.; et al. The Toll-Interleukin-1 Receptor Member SIGIRR Regulates Colonic Epithelial Homeostasis, Inflammation, and Tumorigenesis. Immunity 2007, 26, 461–475. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Han, G.C.; Wang, R.X.; Xiao, H.; Hou, C.M.; Guo, R.F.; Dou, Y.; Shen, B.F.; Li, Y.; et al. Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal Immunol. 2014, 7, 1106–1115. [Google Scholar] [CrossRef]
- Ma, J.; Sun, X.; Guo, T.; Su, H.; Chen, Q.; Gong, Z.; Qi, J.; Zhao, X. Interleukin-1 receptor antagonist inhibits angiogenesis via blockage IL-Iα/PI3K/NF-κB pathway in human colon cancer cell. Cancer Manag. Res. 2017, 9, 481–493. [Google Scholar] [CrossRef]
- Xu, J.; Ye, Y.; Zhang, H.; Szmitkowski, M.; Mäkinen, M.J.; Li, P.; Xia, D.; Yang, J.; Wu, Y.; Wu, H. Diagnostic and prognostic value of serum interleukin-6 in colorectal cancer. Medicine 2016, 95, 1–10. [Google Scholar] [CrossRef]
- Kantola, T.; Klintrup, K.; Väyrynen, J.P.; Vornanen, J.; Bloigu, R.; Karhu, T.; Herzig, K.H.; Näpänkangas, J.; Mäkelä, J.; Karttunen, T.J.; et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br. J. Cancer 2012, 107, 1729–1736. [Google Scholar] [CrossRef]
- Angevin, E.; Tabernero, J.; Elez, E.; Cohen, S.J.; Bahleda, R.; Van Laethem, J.L.; Ottensmeier, C.; Lopez-Martin, J.A.; Clive, S.; Joly, F.; et al. A Phase I/II, multiple-dose, dose-escalation study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with advanced solid tumors. Clin. Cancer Res. 2014, 20, 2192–2204. [Google Scholar] [CrossRef] [Green Version]
- Jatoi, A.; Dakhil, S.R.; Nguyen, P.L.; Sloan, J.A.; Kugler, J.W.; Rowland, K.M.; Soori, G.S.; Wender, D.B.; Fitch, T.R.; Novotny, P.J.; et al. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: Results from NOOC1 from the North Central Cancer Treatment Group. Cancer 2007, 110, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Hui, D.; Bruera, E.; Janku, F.; Naing, A.; Falchook, G.S.; Piha-Paul, S.; Wheler, J.J.; Fu, S.; Tsimberidou, A.M.; et al. MABp1, a first-in-class true human antibody targeting interleukin-1α in refractory cancers: An open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014, 15, 656–666. [Google Scholar] [CrossRef]
- Isambert, N.; Hervieu, A.; Rébé, C.; Hennequin, A.; Borg, C.; Zanetta, S.; Chevriaux, A.; Richard, C.; Derangère, V.; Limagne, E.; et al. Fluorouracil and bevacizumab plus anakinra for patients with metastatic colorectal cancer refractory to standard therapies (IRAFU): A single-arm phase 2 study. Oncoimmunology 2018, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Andersen, D.K.; Korc, M.; Petersen, G.M.; Eibl, G.; Li, D.; Rickels, M.R.; Chari, S.T.; Abbruzzese, J.L. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes 2017, 66, 1103–1110. [Google Scholar] [CrossRef]
- Bosetti, C.; Rosato, V.; Li, D.; Silverman, D.; Petersen, G.M.; Bracci, P.M.; Neale, R.E.; Muscat, J.; Anderson, K.; Gallinger, S.; et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: An analysis from the International Pancreatic Cancer Case-Control Consortium. Ann. Oncol. 2014, 25, 2065–2072. [Google Scholar] [CrossRef]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef]
- Pang, Y.; Kartsonaki, C.; Guo, Y.; Bragg, F.; Yang, L.; Bian, Z.; Chen, Y.; Iona, A.; Millwood, I.Y.; Lv, J.; et al. Diabetes, plasma glucose and incidence of pancreatic cancer: A prospective study of 0.5 million Chinese adults and a meta-analysis of 22 cohort studies. Int. J. Cancer 2017, 140, 1781–1788. [Google Scholar] [CrossRef]
- Koyanagi, Y.N.; Matsuo, K.; Ito, H.; Tamakoshi, A.; Sugawara, Y.; Hidaka, A.; Wada, K.; Oze, I.; Kitamura, Y.; Liu, R.; et al. Body-mass index and pancreatic cancer incidence: A pooled analysis of nine population-based cohort studies with more than 340,000 Japanese subjects. J. Epidemiol. 2018, 28, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Zitvogel, L.; Pietrocola, F.; Kroemer, G. Nutrition, inflammation and cancer. Nat. Immunol. 2017, 18, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Rincon, M. Interleukin-6: From an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 2012, 33, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.F.; Wang, S.X.; Zhang, F.R.; Peng, L.; Wang, S.X.; Xiao, Y.; Zhang, M. Interleukin-1α, 6 regulate the secretion of vascular endothelial growth factor A, C in pancreatic cancer. Hepatobiliary Pancreat. Dis. Int. 2005, 4, 460–463. [Google Scholar] [PubMed]
- Roshani, R.; McCarthy, F.; Hagemann, T. Inflammatory cytokines in human pancreatic cancer. Cancer Lett. 2014, 345, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Ochi, N.; Sawai, H.; Yasuda, A.; Takahashi, H.; Funahashi, H.; Takeyama, H.; Tong, Z.; Guha, S. CXCL8/IL-8 and CXCL12/SDF-lα co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int. J. Cancer 2009, 124, 853–861. [Google Scholar] [CrossRef]
- Wigmore, S.J.; Fearon, K.C.; Sangster, K.; Maingay, J.P.; Garden, O.J.; Ross, J.A. Cytokine regulation of constitutive production of interleukin-8 and -6 by human pancreatic cancer cell lines and serum cytokine concentrations in patients with pancreatic cancer. Int. J. Oncol. 2002, 21, 881–886. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, M.; Yu, G.Z.; Qin, X.R.; Jin, G.; Chen, P.; Zhu, M.H. Interleukin-8, a promising predictor for prognosis of pancreatic cancer. World J. Gastroenterol. 2012, 18, 1123–1129. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Tucker, S.L.; Li, D.; Abbruzzese, J.L.; Kurzrock, R. Cytokines in pancreatic carcinoma: Correlation with phenotypic characteristics and prognosis. Cancer 2004, 101, 2727–2736. [Google Scholar] [CrossRef]
- Chu, W.M. Tumor necrosis factor. Cancer Lett. 2013, 328, 222–225. [Google Scholar] [CrossRef]
- Egberts, J.H.; Cloosters, V.; Noack, A.; Schniewind, B.; Thon, L.; Klose, S.; Kettler, B.; Von Forstner, C.; Kneitz, C.; Tepel, J.; et al. Anti-tumor necrosis factor therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2008, 68, 1443–1450. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.N.; Dotan, S.; Elkabets, M.; White, M.R.; Reich, E.; Carmi, Y.; Song, X.; Dvozkin, T.; Krelin, Y.; Voronov, E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006, 25, 387–408. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.; Basso, D.; Fogar, P.; Mazza, S.; Navaglia, F.; Zambon, C.F.; Falda, A.; Pedrazzoli, S.; Ancona, E.; Plebani, M. Pancreatic cancer cells invasiveness is mainly affected by interleukin-1β not by transforming growth factor-β1. Int. J. Biol. Markers 2005, 20, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Calandra, T.; Roger, T. Macrophage migration inhibitory factor: A regulator of innate immunity. Nat. Rev. Immunol. 2003, 3, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.P.; Deuster, O.; Balzer-Geldsetzer, M.; Meyer, B.; Dodel, R.; Bacher, M. The role of macrophage inhibitory factor in tumorigenesis and central nervous system tumors. Cancer 2009, 115, 2031–2040. [Google Scholar] [CrossRef]
- Hagemann, T.; Robinson, S.C.; Thompson, R.G.; Charles, K.; Kulbe, H.; Balkwill, F.R. Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol. Cancer Ther. 2007, 6, 1993–2002. [Google Scholar] [CrossRef]
- Mahalingam, D.; Patel, M.R.; Sachdev, J.C.; Hart, L.L.; Halama, N.; Ramanathan, R.K.; Sarantopoulos, J.; Völkel, D.; Youssef, A.; de Jong, F.A.; et al. Phase I study of imalumab (BAX69), a fully human recombinant antioxidized macrophage migration inhibitory factor antibody in advanced solid tumours. Br. J. Clin. Pharmacol. 2020, 86, 1836–1848. [Google Scholar] [CrossRef]
- Alvarez, C.; Bass, B.L. Role of Transforming Growth Factor-Beta in Growth and Injury Response of the Pancreatic Duct Epithelium in Vitro. J. Gastrointest. Surg. 1999, 3, 178–184. [Google Scholar] [CrossRef]
- Oettle, H.; Seufferlein, T.; Luger, T.; Schmid, R.M.; von Wichert, G.; Endlicher, E.; Garbe, C.; Kaehler, K.K.; Enk, A.; Schneider, A.; et al. Final results of a phase I/II study in patients with pancreatic cancer, malignant melanoma, and colorectal carcinoma with trabedersen. J. Clin. Oncol. 2012, 30, 4034. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Desplat-Jégo, S.; Burkly, L.; Putterman, C. Targeting TNF and its family members in autoimmune/inflammatory disease. Mediators Inflamm. 2014, 2014, 628748. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.M.; Peng, J.; Andridge, R.R.; Lindgren, M.E.; Povoski, S.P.; Lipari, A.M.; Agnese, D.M.; Farrar, W.B.; Yee, L.D.; Carson, W.E.; et al. Inflammatory cytokines and comorbidity development in breast cancer survivors versus noncancer controls: Evidence for accelerated aging? J. Clin. Oncol. 2017, 35, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Elsadek, A.E.; Al-Shokary, A.H.; Abdelghani, W.E.; Kamal, N.M.; Ibrahim, A.O.; El-Shorbagy, H.H.; Suliman, H.A.; Barseem, N.F.; Abdel Maksoud, Y.H.; Azab, S.M.; et al. Serum Levels of Interleukin-6 and Tumor Necrosis Factor Alpha in Children with Attention-Deficit Hyperactivity Disorder. J. Pediatr. Neurosci. 2020, 15, 402–408. [Google Scholar] [CrossRef]
- Leek, R.D.; Landers, R.; Fox, S.B.; Ng, F.; Harris, A.L.; Lewis, C.E. Association of tumour necrosis factor alpha and its receptors with thymidine phosphorylase expression in invasive breast carcinoma. Br. J. Cancer 1998, 77, 2246–2251. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Wakabayashi, H.; Matsumine, A.; Sudo, A.; Uchida, A. TNF inhibitor suppresses bone metastasis in a breast cancer cell line. Biochem. Biophys. Res. Commun. 2011, 407, 525–530. [Google Scholar] [CrossRef]
- Grau, A.M.; Wen, W.; Ramroopsingh, D.S.; Gao, Y.T.; Zi, J.; Cai, Q.; Shu, X.O.; Zheng, W. Circulating transforming growth factor-β-1 and breast cancer prognosis: Results from the Shanghai Breast Cancer Study. Breast Cancer Res. Treat. 2008, 112, 335–341. [Google Scholar] [CrossRef]
- Ivanović, V.; Dedović-Tanić, N.; Milovanović, Z.; Lukić, S.; Nikolić, S.; Baltić, V.; Stojiljković, B.; Budišin, N.; Savovski, K.; Demajo, M.; et al. Quantification of transforming growth factor beta 1 levels in metastatic axillary lymph node tissue extracts from breast cancer patients: A new specimen source. Anal. Quant. Cytol. Histol. 2009, 31, 288–295. [Google Scholar]
- Liu, J.; Liao, S.; Diop-Frimpong, B.; Chen, W.; Goel, S.; Naxerova, K.; Ancukiewicz, M.; Boucher, Y.; Jain, R.K.; Xu, L. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc. Natl. Acad. Sci. USA 2012, 109, 16618–16623. [Google Scholar] [CrossRef]
- Bachelot, T.; Ray-Coquard, I.; Menetrier-Caux, C.; Rastkha, M.; Duc, A.; Blay, J.Y. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br. J. Cancer 2003, 88, 1721–1726. [Google Scholar] [CrossRef]
- Balasubbramanian, D.; Goodlett, B.L.; Mitchell, B.M. Is IL-12 pro-inflammatory or anti-inflammatory? Depends on the blood pressure. Cardiovasc. Res. 2019, 115, 998–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, T.; Chen, L.; Wei, X. Inflammatory cytokines in cancer: Comprehensive understanding and clinical progress in gene therapy. Cells 2021, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.C.C.; Coffin, R.S.; Davis, C.J.; Graham, N.J.; Groves, N.; Guest, P.J.; Harrington, K.J.; James, N.D.; Love, C.A.; McNeish, I.; et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 2006, 12, 6737–6747. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Rowley, D.A.; Schreiber, H. Inflammation as a tumor promoter in cancer induction. Semin. Cancer Biol. 2004, 14, 433–439. [Google Scholar] [CrossRef]
- Correa, P. Helicobacter pylori and gastric carcinogenesis. Am. J. Surg. Pathol. 1995, 19 (Suppl. S1), S37–S43. [Google Scholar]
- Fox, J.G.; Wang, T.C. Review series Inflammation, atrophy, and gastric cancer. J. Clin. Invest. 2007, 117, 60–69. [Google Scholar] [CrossRef]
- Xu, Y.H.; Li, Z.L.; Qiu, S.F. IFN-γ Induces Gastric Cancer Cell Proliferation and Metastasis Through Upregulation of Integrin β3-Mediated NF-κB Signaling. Transl. Oncol. 2018, 11, 182–192. [Google Scholar] [CrossRef]
- Ham, I.H.; Oh, H.J.; Jin, H.; Bae, C.A.; Jeon, S.M.; Choi, K.S.; Son, S.Y.; Han, S.U.; Brekken, R.A.; Lee, D.; et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol. Cancer 2019, 18, 1–14. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Chen, J.; Liu, Y.; Zhao, X.; Tan, B.; Ai, J.; Zhang, Z.; Song, J.; Shan, B. Prevalence of Th17 and Treg cells in gastric cancer patients and its correlation with clinical parameters. Oncol. Rep. 2013, 30, 1215–1222. [Google Scholar] [CrossRef]
- Fukui, H.; Zhang, X.; Sun, C.; Hara, K.; Kikuchi, S.; Yamasaki, T.; Kondo, T.; Tomita, T.; Oshima, T.; Watari, J.; et al. IL-22 produced by cancer-associated fibroblasts promotes gastric cancer cell invasion via STAT3 and ERK signaling. Br. J. Cancer 2014, 111, 763–771. [Google Scholar] [CrossRef]
- Szczepanik, A.M.; Scislo, L.; Scully, T.; Walewska, E.; Siedlar, M.; Kolodziejczyk, P.; Lenart, M.; Rutkowska, M.; Galas, A.; Czupryna, A.; et al. IL-6 serum levels predict postoperative morbidity in gastric cancer patients. Gastric Cancer 2011, 14, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Zhang, H.; Zhou, Z.; Chen, M.; Wang, Q. Tumor-associated macrophages induce PD-L1 expression in gastric cancer cells through IL-6 and TNF-ɑ signaling. Exp. Cell Res. 2020, 396, 112315. [Google Scholar] [CrossRef]
- Chang, J.E.; Lee, D.S.; Ban, S.W.; Oh, J.; Jung, M.Y.; Kim, S.H.; Park, S.J.; Persaud, K.; Jheon, S. Analysis of volatile organic compounds in exhaled breath for lung cancer diagnosis using a sensor system. Sens. Actuators B Chem. 2018, 255, 800–807. [Google Scholar] [CrossRef]
- Prévost-Blondel, A.; Roth, E.; Rosenthal, F.M.; Pircher, H. Crucial Role of TNF-α in CD8 T Cell-Mediated Elimination of 3LL-A9 Lewis Lung Carcinoma Cells In Vivo. J. Immunol. 2000, 164, 3645–3651. [Google Scholar] [CrossRef]
- Kastamoulas, M.; Chondrogiannis, G.; Kanavaros, P.; Vartholomatos, G.; Bai, M.; Briasoulis, E.; Arvanitis, D.; Galani, V. Cytokine effects on cell survival and death of A549 lung carcinoma cells. Cytokine 2013, 61, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.L.; Maeda, S.; Hsu, L.C.; Yagita, H.; Karin, M. Inhibition of NF-κB in cancer cells converts inflammation- induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer Cell 2004, 6, 297–305. [Google Scholar] [CrossRef]
- Kawakami, M.; Kawakami, K.; Stepensky, V.A.; Maki, R.A.; Robin, H.; Muller, W.; Husain, S.R.; Puri, R.K. Interleukin 4 receptor on human lung cancer: A molecular target for cytotoxin therapy. Clin. Cancer Res. 2002, 8, 3503–3511. [Google Scholar]
- Li, H.; Takayama, K.; Wang, S.; Shiraishi, Y.; Gotanda, K.; Harada, T.; Furuyama, K.; Iwama, E.; Ieiri, I.; Okamoto, I.; et al. Addition of bevacizumab enhances antitumor activity of erlotinib against non-small cell lung cancer xenografts depending on VEGF expression. Cancer Chemother. Pharmacol. 2014, 74, 1297–1305. [Google Scholar] [CrossRef]
- Frezzetti, D.; Gallo, M.; Maiello, M.R.; D’Alessio, A.; Esposito, C.; Chicchinelli, N.; Normanno, N.; De Luca, A. VEGF as a potential target in lung cancer. Expert Opin. Ther. Targets 2017, 21, 959–966. [Google Scholar] [CrossRef]
- Song, X.Y.; Zhou, S.J.; Xiao, N.; Li, Y.S.; Zhen, D.Z.; Su, C.Y.; Liu, Z.D. Research on the relationship between serum levels of inflammatory cytokines and non-small cell lung cancer. Asian Pacific J. Cancer Prev. 2013, 14, 4765–4768. [Google Scholar] [CrossRef]
- Brenner, D.R.; Fanidi, A.; Grankvist, K.; Muller, D.C.; Brennan, P.; Manjer, J.; Byrnes, G.; Hodge, A.; Severi, G.; Giles, G.G.; et al. Inflammatory Cytokines and Lung Cancer Risk in 3 Prospective Studies. Am. J. Epidemiol. 2017, 185, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Zhang, S.; Xu, J.; Liu, G.; Zhang, L.; Xie, E.; Gao, L.; Li, D.; Sun, R.; Wang, F.; et al. Non-small-cell lung cancer-induced immunosuppression by increased human regulatory T cells via Foxp3 promoter demethylation. Cancer Immunol. Immunother. 2016, 65, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Vahl, J.M.; Friedrich, J.; Mittler, S.; Trump, S.; Heim, L.; Kachler, K.; Balabko, L.; Fuhrich, N.; Geppert, C.I.; Trufa, D.I.; et al. Interleukin-10-regulated tumour tolerance in non-small cell lung cancer. Br. J. Cancer 2017, 117, 1644–1655. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.H.; Park, C.K.; Chung, C.; Oh, I.J.; Kim, Y.C.; Park, D.; Kim, J.; Kwon, G.C.; Kwon, I.; Sun, P.; et al. Baseline serum interleukin-6 levels predict the response of patients with advanced non-small cell lung cancer to pd-1/pd-l1 inhibitors. Immune Netw. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Archer, M.; Dogra, N.; Kyprianou, N. Inflammation as a driver of prostate cancer metastasis and therapeutic resistance. Cancers 2020, 12, 2984. [Google Scholar] [CrossRef]
- Zhou, K.; Arslanturk, S.; Craig, D.B.; Heath, E.; Draghici, S. Discovery of primary prostate cancer biomarkers using cross cancer learning. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Spickett, I.; Robertson, J. Prostate Cancer: The Ongoing Challenge. Prim. Heal. Care 2010, 20, 16–21. [Google Scholar] [CrossRef]
- Mahal, B.A.; Berman, R.A.; Taplin, M.-E.; Huang, F.W. Prostate Cancer–Specific Mortality Across Gleason Scores in Black vs. Nonblack Men. JAMA 2018, 320, 2479–2481. [Google Scholar] [CrossRef]
- Mahal, B.A.; Alshalalfa, M.; Spratt, D.E.; Davicioni, E.; Zhao, S.G.; Feng, F.Y.; Rebbeck, T.R.; Nguyen, P.L.; Huang, F.W. Prostate Cancer Genomic-risk Differences Between African-American and White Men Across Gleason Scores. Eur. Urol. 2019, 75, 1038–1040. [Google Scholar] [CrossRef]
- Messina, C.; Cattrini, C.; Soldato, D.; Vallome, G.; Caffo, O.; Castro, E.; Olmos, D.; Boccardo, F.; Zanardi, E. BRCA Mutations in Prostate Cancer: Prognostic and Predictive Implications. J. Oncol. 2020, 2020, 4986365. [Google Scholar] [CrossRef]
- Shah, S.; Rachmat, R.; Enyioma, S.; Ghose, A.; Revythis, A.; Boussios, S. Brca mutations in prostate cancer: Assessment, implications and treatment considerations. Int. J. Mol. Sci. 2021, 22, 12628. [Google Scholar] [CrossRef] [PubMed]
- Albright, F.; Stephenson, R.A.; Agarwal, N.; Teerlink, C.C.; Lowrance, W.T.; Farnham, J.M.; Albright, L.A.C. Prostate cancer risk prediction based on complete prostate cancer family history. Prostate 2015, 75, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, J.; Zhang, Y.; Zhu, H.; Liu, J.; Pumill, C. The role of prostatitis in prostate cancer: Meta-analysis. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Perletti, G.; Monti, E.; Magri, V.; Cai, T.; Cleves, A.; Trinchieri, A.; Montanari, E. The association between prostatitis and prostate cancer. Syst. Rev. Meta-Analysis. Arch. Ital. Di Urol. E Androl. 2017, 89, 259–265. [Google Scholar] [CrossRef]
- Karakiewicz, P.I.; Benayoun, S.; Bégin, L.R.; Duclos, A.; Valiquette, L.; McCormack, M.; Bénard, F.; Saad, F.; Perrotte, P. Chronic inflammation is negatively associated with prostate cancer and high-grade prostatic intraepithelial neoplasia on needle biopsy. Int. J. Clin. Pract. 2007, 61, 425–430. [Google Scholar] [CrossRef]
- Yli-Hemminki, T.H.; Laurila, M.; Auvinen, A.; Määttänen, L.; Huhtala, H.; Tammela, T.L.J.; Kujala, P.M. Histological inflammation and risk of subsequent prostate cancer among men with initially elevated serum prostate-specific antigen (PSA) concentration in the Finnish prostate cancer screening trial. BJU Int. 2013, 112, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, C.; Ciummo, S.L.; Cipollone, G.; Caputo, S.; Bellone, M.; Carlo, E. Di Interleukin-30/il27p28 shapes prostate cancer stem-like cell behavior and is critical for tumor onset and metastasization. Cancer Res. 2018, 78, 2654–2668. [Google Scholar] [CrossRef]
- Stearns, M.E.; Garcia, F.U.; Fudge, K.; Rhim, J.; Wang, M. Role of interleukin 10 and transforming growth factor β1 in the angiogenesis and metastasis of human prostate primary tumor lines from orthotopic implants in severe combined immunodeficiency mice. Clin. Cancer Res. 1999, 5, 711–720. [Google Scholar]
- Barrack, E.R. TGF in prostate cancer: A growth inhibitor that can enhance tumorigenicity. Prostate 1997, 31, 61–70. [Google Scholar] [CrossRef]
- Park, J.I.; Lee, M.G.; Cho, K.; Park, B.J.; Chae, K.S.; Byun, D.S.; Ryu, B.K.; Park, Y.K.; Chi, S.G. Transforming growth factor-β1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-κB, JNK, and Ras signaling pathways. Oncogene 2003, 22, 4314–4332. [Google Scholar] [CrossRef]
- Nakashima, J.; Tachibana, M.; Horiguchi, Y.; Oya, M.; Ohigashi, T.; Asakura, H.; Murai, M. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin. Cancer Res. 2000, 6, 2702–2706. [Google Scholar] [PubMed]
- Ljungberg, B.; Grankvist, K.; Rasmuson, T. Serum interleukin-6 in relation to acute-phase reactants and survival in patients with renal cell carcinoma. Eur. J. Cancer 1997, 33, 1794–1798. [Google Scholar] [CrossRef]
- Jan-ErikJohansson; Swen-OlofAndersson; LarsHolmberg; ReinholdBergström Prognostic Factors in Progression-Free Survival and Corrected Survival in Patients with Advanced Prostatic Cancer: Results from a Randomized Study Comprising 150 Patients Treated with Orchiectomy or Estrogens. J. Urol. 1991, 146, 1327–1332. [CrossRef]
- Michalaki, V.; Syrigos, K.; Charles, P.; Waxman, J. Serum levels of IL-6 and TNF-α correlate with clinicopathological features and patient survival in patients with prostate cancer. Br. J. Cancer 2004, 90, 2312–2316. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, J.; Tachibana, M.; Ueno, M.; Miyajima, A.; Baba, S.; Murai, M. Association between tumor necrosis factor in serum and cachexia in patients with prostate cancer. Clin. Cancer Res. 1998, 4, 1743–1748. [Google Scholar]
- Steiner, G.E.; Newman, M.E.; Paikl, D.; Stix, U.; Memaran-Dagda, N.; Lee, C.; Marberger, M.J. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate 2003, 56, 171–182. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).