The Role of miR-29s in Human Cancers—An Update
Abstract
1. Introduction
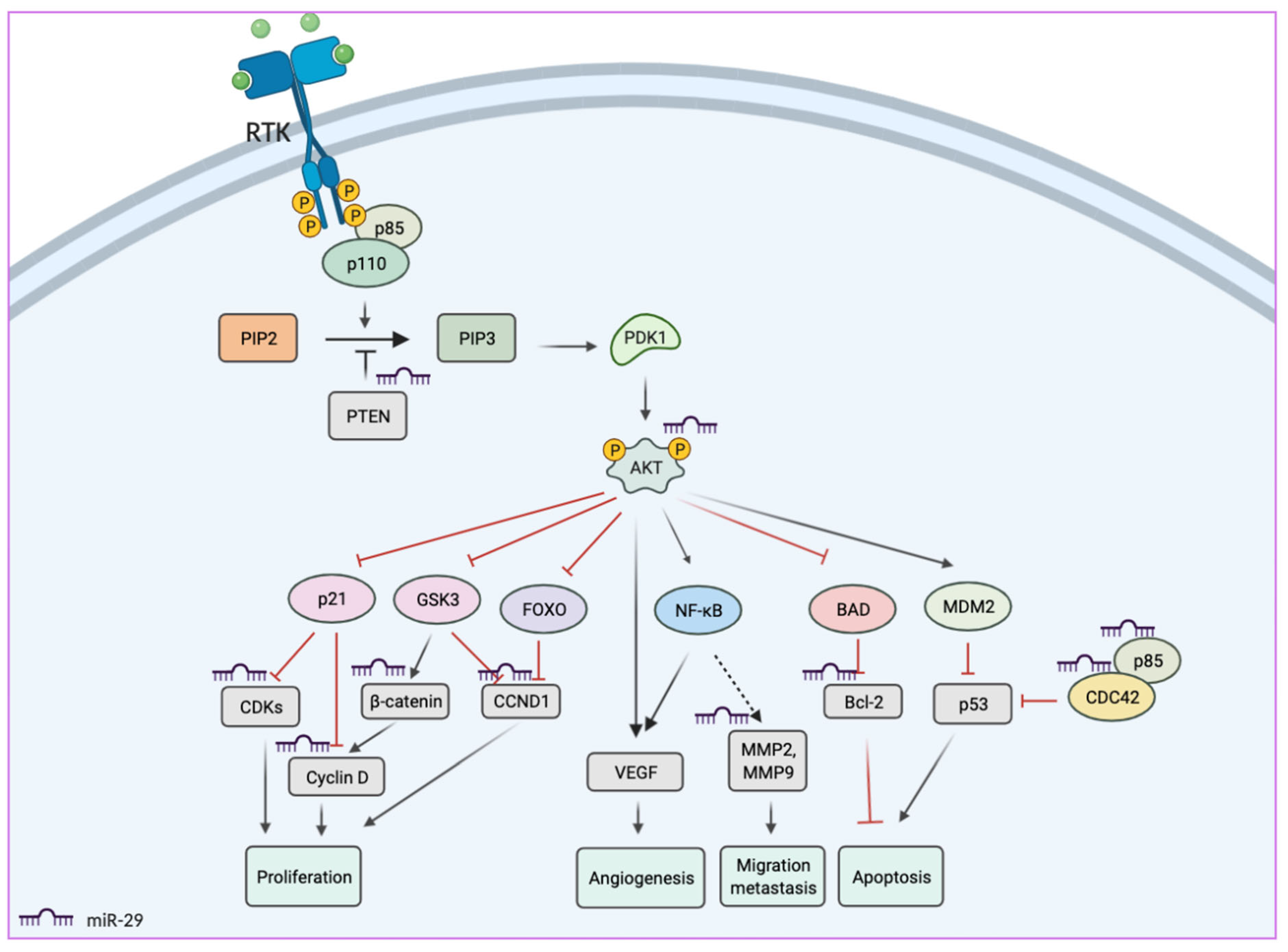
2. miR-29 Functions in Cancers
3. MiR-29s as Biomarkers
3.1. MiR-29s as Biomarkers in Colorectal Cancer
3.2. miR-29s as Biomarkers in Bladder Cancer
3.3. miR-29s as Biomarkers in Hepatocellular Carcinoma
3.4. miR-29s as Biomarkers in Pancreatic Cancer
3.5. miR-29s as Diagnostic Biomarkers in Lung Cancer
3.6. miR-29s as Biomarkers in Leukemia and Lymphoma
3.7. miR-29s as Biomarkers in Kidney Cancer
3.8. miR-29s as Biomarkers in Breast Cancer
| Samples | Sample Size & Methods | Outcome | Results | Ref. |
|---|---|---|---|---|
| Blood samples |
| MiR-29a was significantly down-regulated in the blood of patients with Luminal A-like breast tumors compared to healthy controls. | Combined miR-29a, miR-181a and miR-652 (AUC: 0.80, sensitivity: 77% and specificity: 74%) | [129] |
| Serum sample |
| MiR-2 was significantly higher in breast cancer patients compared to healthy controls. | MiR-29c AUC: 0.724 (95% CI 0.638–0.810) | [46] |
| Serum |
| MiR-29a was significantly elevated in the serum of breast cancer patients (p < 0.05). | MiR-29a was elevated more than 5-folds by SOLiD sequencing. | [130] |
| Tissue samples |
| MiR-29a was significantly upregulated in breast cancer as compared with their respective healthy controls (p < 0.001). | MiR-29a (AUC:0.969, Sensitivity: 93.3%, specificity: 91.1%) | [131] |
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Kwon, J.J.; Factora, T.D.; Dey, S.; Kota, J. A Systematic Review of miR-29 in Cancer. Mol. Ther. -Oncolytics 2018, 12, 173–194. [Google Scholar] [CrossRef]
- Chang, T.-C.; Yu, D.; Lee, Y.-S.; Wentzel, E.A.; Arking, D.E.; West, K.M.; Dang, C.V.; Thomas-Tikhonenko, A.; Mendell, J.T. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat. Genet. 2008, 40, 43–50. [Google Scholar] [CrossRef]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A Mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef]
- Hwang, H.-W.; Wentzel, E.A.; Mendell, J.T. A hexanucleotide element directs MicroRNA nuclear import. Science 2007, 315, 97–100. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, J.; Wang, G.-K.; Zhang, J.-T.; Huang, S.; Qin, Y.-W.; Jing, Q. Uracils at nucleotide position 9–11 are required for the rapid turnover of miR-29 family. Nucleic Acids Res. 2011, 39, 4387–4395. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Ma, L.-M.; Guo, Y.-H.; Zhang, Y.-C.; Zhou, H.; Shao, P.; Chen, Y.-Q.; Qu, L.-H. Deep sequencing of human nuclear and cytoplasmic small RNAs reveals an unexpectedly complex subcellular distribution of miRNAs and tRNA 3′ trailers. PLoS ONE 2010, 5, e10563. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Hua, X.; Tang, H.; Chen, R.; Yang, T.; Das, S.; Xiao, J. CRISPR/Cas9-Mediated miR-29b Editing as a Treatment of Different Types of Muscle Atrophy in Mice. Mol. Ther. 2020, 28, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Sassi, Y.; Avramopoulos, P.; Ramanujam, D.P.; Grüter, L.; Werfel, S.; Giosele, S.; Brunner, A.-D.; Esfandyari, D.; Papadopoulou, A.-S.; De Strooper, B.; et al. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat. Commun. 2017, 8, 1614. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Urban, G.-W.; Bettermann, K.; Vucur, M.; Zimmermann, H.W.; Schmidt, S.; Janssen, J.; Koppe, C.; Knolle, P.; Castoldi, M.; et al. Micro-RNA Profiling Reveals a Role for miR-29 in Human and Murine Liver Fibrosis. Hepatology 2011, 53, 209–218. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Lian, X.; Zhu, Z.; Chen, X.; Pei, W.; Li, S.; Abbas, A.; Wang, Y.; Tian, L. MicroRNA-29b mediates lung mesenchymal-epithelial transition and prevents lung fibrosis in the silicosis model. Mol. Ther. -Nucleic Acids 2019, 14, 20–31. [Google Scholar] [CrossRef]
- Qin, W.; Chung, A.C.; Huang, X.R.; Meng, X.-M.; Hui, D.; Yu, C.-M.; Sung, J.J.Y.; Lan, H.Y. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J. Am. Soc. Nephrol. 2011, 22, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, R.; Goto, Y.; Kojima, S.; Enokida, H.; Chiyomaru, T.; Kinoshita, T.; Sakamoto, S.; Fuse, M.; Nakagawa, M.; Naya, Y.; et al. Tumor-suppressive microRNA-29s inhibit cancer cell migration and invasion via targeting LAMC1 in prostate cancer. Int. J. Oncol. 2014, 45, 401–410. [Google Scholar] [CrossRef]
- Wang, T.; Hou, J.; Jian, S.; Luo, Q.; Wei, J.; Li, Z.; Wang, X.; Bai, P.; Duan, B.; Xing, J.; et al. miR-29b negatively regulates MMP2 to impact gastric cancer development by suppress gastric cancer cell migration and tumor growth. J. Cancer 2018, 9, 3776–3786. [Google Scholar] [CrossRef]
- Duhachek-Muggy, S.; Zolkiewska, A. ADAM12-L is a direct target of the miR-29 and miR-200 families in breast cancer. BMC Cancer 2015, 15, 93. [Google Scholar] [CrossRef]
- Kogure, T.; Costinean, S.; Yan, I.; Braconi, C.; Croce, C.; Patel, T. Hepatic miR-29ab1 expression modulates chronic hepatic injury. J. Cell. Mol. Med. 2012, 16, 2647–2654. [Google Scholar] [CrossRef]
- Liang, C.; Bu, S.; Fan, X. Suppressive effect of microRNA-29b on hepatic stellate cell activation and its crosstalk with TGF-β1/Smad3. Cell Biochem. Funct. 2016, 34, 326–333. [Google Scholar] [CrossRef]
- van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Friedman, R.C.; Marquez, R.T.; Keck, K.; Kong, B.; Icardi, M.S.; Brown, K.E.; Burge, C.B.; Schmidt, W.N.; Wang, Y.; et al. Hepatitis C Virus Infection and Hepatic Stellate Cell Activation Downregulate miR-29: miR-29 Overexpression Reduces Hepatitis C Viral Abundance in Culture. J. Infect. Dis. 2011, 203, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.L.; Yu, G.; Latimer, P.A.; Stack, C.; Robinson, K.; Dalby, C.M.; Kaminski, N.; van Rooij, E. MicroRNA mimicry blocks pulmonary fibrosis. EMBO Mol. Med. 2014, 6, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Garzon, R.; Cimmino, A.; Liu, Z.; Zanesi, N.; Callegari, E.; Liu, S.; Alder, H.; Costinean, S.; Fernandez-Cymering, C.; et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. USA 2007, 104, 15805–15810. [Google Scholar] [CrossRef]
- Cui, H.; Wang, L.; Gong, P.; Zhao, C.; Zhang, S.; Zhang, K.; Zhou, R.; Zhao, Z.; Fan, H. Deregulation between miR-29b/c and DNMT3A is associated with epigenetic silencing of the CDH1 gene, affecting cell migration and invasion in gastric cancer. PLoS ONE 2015, 10, e0123926. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, W.; Wu, Z.; Liu, Y.; Shi, Y.; Gong, J.; Shen, W.; Liu, C. miR-29c-3p regulates DNMT3B and LATS1 methylation to inhibit tumor progression in hepatocellular carcinoma. Cell Death Dis. 2019, 10, 48. [Google Scholar] [CrossRef]
- Gondaliya, P.; Dasare, A.P.; Jash, K.; Tekade, R.K.; Srivastava, A.; Kalia, K. miR-29b attenuates histone deacetylase-4 mediated podocyte dysfunction and renal fibrosis in diabetic nephropathy. J. Diabetes Metab. Disord. 2019, 19, 13–27. [Google Scholar] [CrossRef]
- Amodio, N.; Stamato, M.A.; Gullà, A.M.; Morelli, E.; Romeo, E.; Raimondi, L.; Pitari, M.R.; Ferrandino, I.; Misso, G.; Caraglia, M.; et al. Therapeutic targeting of miR-29b/HDAC4 epigenetic loop in multiple myeloma. Mol. Cancer Ther. 2016, 15, 1364–1375. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, B.; Xu, X.; Sessa, W.C. Ten-eleven translocation (Tet) and thymine DNA glycosylase (TDG), components of the demethylation pathway, are direct targets of miRNA-29a. Biochem. Biophys. Res. Commun. 2013, 437, 368–373. [Google Scholar] [CrossRef]
- Mott, J.L.; Kobayashi, S.; Bronk, S.F.; Gores, G.J. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 2007, 26, 6133–6140. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, L.; Zhang, J.; Wang, Y.; Liang, H.; Fan, G.; Jiang, Z.; Zhang, C.-Y.; Chen, X.; Zhou, G. MiR-29b suppresses the proliferation and migration of osteosarcoma cells by targeting CDK6. Protein Cell 2016, 7, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Ren, L.; Sun, C.; Yu, L.; Bian, X.; Zhou, X.; Wen, Y.; Hua, D.; Zhao, S.; Luo, W.; et al. miR-29a/b/c function as invasion suppressors for gliomas by targeting CDC42 and predict the prognosis of patients. Br. J. Cancer 2017, 117, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhu, Y.; Gao, J.; Fu, J.; Liu, C.; Liu, Y.; Song, C.; Zhu, S.; Leng, Y.; Wang, G.; et al. MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br. J. Cancer 2014, 110, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, C.; Lu, Z.; Guo, L.; Ge, Q. Analysis of serum genome-wide microRNAs for breast cancer detection. Clin. Chim. Acta 2012, 413, 1058–1065. [Google Scholar] [CrossRef]
- Du, L.; Jiang, X.; Duan, W.; Wang, R.; Wang, L.; Zheng, G.; Yan, K.; Wang, L.; Li, J.; Zhang, X.; et al. Cell-free microRNA expression signatures in urine serve as novel noninvasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Oncotarget 2017, 8, 40832–40842. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, C.; Chen, X.; Wang, J.; Chen, S.; Tang, S.; Yu, T. Identification of circulating miRNAs as novel prognostic biomarkers for bladder cancer. Math. Biosci. Eng. 2019, 17, 834–844. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Fiskus, W.; Lin, J.; Lwin, T.; Rao, R.; Zhang, Y.; Chan, J.C.; Fu, K.; Marquez, V.E.; et al. Coordinated silencing of MYC-Mediated miR-29 by HDAC3 and EZH2 as a therapeutic target of histone modification in aggressive B-Cell lymphomas. Cancer Cell 2012, 22, 506–523. [Google Scholar] [CrossRef]
- Wang, H.; Garzon, R.; Sun, H.; Ladner, K.J.; Singh, R.; Dahlman, J.; Cheng, A.; Hall, B.M.; Qualman, S.J.; Chandler, D.S.; et al. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell 2008, 14, 369–381. [Google Scholar] [CrossRef]
- Eyholzer, M.; Schmid, S.; Wilkens, L.; Mueller, B.U.; Pabst, T. The tumour-suppressive miR-29a/b1 cluster is regulated by CEBPA and blocked in human AML. Br. J. Cancer 2010, 103, 275–284. [Google Scholar] [CrossRef]
- Baylin, S.B. DNA methylation and gene silencing in cancer. Nat. Clin. Pract. Oncol. 2005, 2, S4–S11. [Google Scholar] [CrossRef]
- Latif, F.; Tory, K.; Gnarra, J.; Yao, M.; Duh, F.-M.; Orcutt, M.L.; Stackhouse, T.; Kuzmin, I.; Modi, W.; Geil, L.; et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 1993, 260, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Horii, T.; Kimura, M.; Ochiya, T.; Tajima, S.; Hatada, I. miR-29 represses the activities of DNA methyltransferases and DNA demethylases. Int. J. Mol. Sci. 2013, 14, 14647–14658. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, Z.-N.; Cheng, X.-H.; Zhang, Y.-F.; Dai, X.; Bao, G.-M.; Zhou, L.-B. MiR-29c suppresses cell invasion and migration by directly targeting CDK6 in gastric carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7920–7928. [Google Scholar] [PubMed]
- Zhao, Z.; Wang, L.; Song, W.; Cui, H.; Chen, G.; Qiao, F.; Hu, J.; Zhou, R.; Fan, H. Reduced miR-29a-3p expression is linked to the cell proliferation and cell migration in gastric cancer. World J. Surg. Oncol. 2015, 13, 101. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Huang, S.; Wan, X.; Luo, H.; Wu, D. MiRNA-29c regulates cell growth and invasion by targeting CDK6 in bladder cancer. Am. J. Transl. Res. 2015, 7, 1382. [Google Scholar]
- Zhang, M.; Guo, W.; Qian, J.; Wang, B. Negative regulation of CDC42 expression and cell cycle progression by miR-29a in breast cancer. Open Med. 2016, 11, 78–82. [Google Scholar] [CrossRef]
- Gong, J.-N.; Yu, J.; Lin, H.-S.; Zhang, X.-H.; Yin, X.-L.; Xiao, Z.; Wang, F.; Wang, X.-S.; Su, R.; Shen, C.; et al. The role, mechanism and potentially therapeutic application of microRNA-29 family in acute myeloid leukemia. Cell Death Differ. 2014, 21, 100–112. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, C.; Liu, L.; Zhou, J. A key role of microRNA-29b in suppression of osteosarcoma cell proliferation and migration via modulation of VEGF. Int. J. Clin. Exp. Pathol. 2014, 7, 5701–5708. [Google Scholar]
- Chen, L.; Xiao, H.; Wang, Z.-H.; Huang, Y.; Liu, Z.-P.; Ren, H.; Song, H. miR-29a suppresses growth and invasion of gastric cancer cells in vitro by targeting VEGF-A. BMB Rep. 2014, 47, 39–44. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, Y.; Gao, J.; Yan, Z.; Li, Z.; Zou, X.; Li, Y.; Wang, J.; Guo, Y. miR-29b-3p regulated osteoblast differentiation via regulating IGF-1 secretion of mechanically stimulated osteocytes. Cell. Mol. Biol. Lett. 2019, 24, 11. [Google Scholar] [CrossRef]
- Hu, H.-H.; Chen, D.-Q.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Vaziri, N.D.; Zhao, Y.-Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Komers, R.; Carew, R.; Winbanks, C.E.; Xu, B.; Herman-Edelstein, M.; Koh, P.; Thomas, M.; Jandeleit-Dahm, K.; Gregorevic, P.; et al. Suppression of microRNA-29 expression by TGF-β1 promotes collagen expression and renal fibrosis. J. Am. Soc. Nephrol. 2012, 23, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Cushing, L.; Kuang, P.P.; Qian, J.; Shao, F.; Wu, J.; Little, F.; Thannickal, V.J.; Cardoso, W.V.; Lü, J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011, 45, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Koshizuka, K.; Kikkawa, N.; Hanazawa, T.; Yamada, Y.; Okato, A.; Arai, T.; Katada, K.; Okamoto, Y.; Seki, N. Inhibition of integrin β1-mediated oncogenic signalling by the antitumor microRNA-29 family in head and neck squamous cell carcinoma. Oncotarget 2017, 9, 3663–3676. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Tong, B.-D.; Wu, Y.-J.; Xiong, W. MicroRNA-29 mediates TGFβ1-induced extracellular matrix synthesis by targeting wnt/β-catenin pathway in human orbital fibroblasts. Int. J. Clin. Exp. Pathol. 2014, 7, 7571–7577. [Google Scholar]
- Wang, Y.; Liu, C.; Luo, M.; Zhang, Z.; Gong, J.; Li, J.; You, L.; Dong, L.; Su, R.; Lin, H. Chemotherapy-induced miRNA-29c/catenin-δ signaling suppresses metastasis in gastric cancer. Cancer Res. 2015, 75, 1332–1344. [Google Scholar] [CrossRef]
- Jiang, J.; Yu, C.; Chen, M.; Zhang, H.; Tian, S.; Sun, C. Reduction of miR-29c enhances pancreatic cancer cell migration and stem cell-like phenotype. Oncotarget 2015, 6, 2767. [Google Scholar] [CrossRef]
- Tréhoux, S.; Lahdaoui, F.; Delpu, Y.; Renaud, F.; Leteurtre, E.; Torrisani, J.; Jonckheere, N.; Van Seuningen, I. Micro-RNAs miR-29a and miR-330-5p function as tumor suppressors by targeting the MUC1 mucin in pancreatic cancer cells. Biochim. Et Biophys. Acta 2015, 1853, 2392–2403. [Google Scholar] [CrossRef]
- Luo, D.-J.; Li, L.-J.; Huo, H.-F.; Liu, X.-Q.; Cui, H.-W.; Jiang, D.-M. MicroRNA-29b sensitizes osteosarcoma cells to doxorubicin by targeting matrix metalloproteinase 9 (MMP-9) in osteosarcoma. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1434–1442. [Google Scholar]
- Bargaje, R.; Gupta, S.; Sarkeshik, A.; Park, R.; Xu, T.; Sarkar, M.; Halimani, M.; Roy, S.S.; Yates, J.; Pillai, B. Identification of novel targets for miR-29a using miRNA proteomics. PLoS ONE 2012, 7, e43243. [Google Scholar] [CrossRef]
- Park, S.-Y.; Lee, J.H.; Ha, M.; Nam, J.-W.; Kim, V.N. miR-29 miRNAs activate p53 by targeting p85α and CDC42. Nat. Struct. Mol. Biol. 2009, 16, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Ren, J.B.; Liu, M.; Yu, L. Targeting miR-29 induces apoptosis of osteosarcoma MG-63 cells via regulation of TGF-β1/PUMA signal. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3552–3560. [Google Scholar] [PubMed]
- Yu, J.; Wang, Z.; Kinzler, K.W.; Vogelstein, B.; Zhang, L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 1931–1936. [Google Scholar] [CrossRef]
- Pei, Y.-F.; Lei, Y.; Liu, X.-Q. MiR-29a promotes cell proliferation and EMT in breast cancer by targeting ten eleven translocation 1. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Geng, P.; Shi, L.; Wang, Q.; Wang, P. miR-29 promotes osteosarcoma cell proliferation and migration by targeting PTEN. Oncol. Lett. 2019, 17, 883–890. [Google Scholar] [CrossRef]
- Pekarsky, Y.; Croce, C.M. Is miR-29 an oncogene or tumor suppressor in CLL? Oncotarget 2010, 1, 224. [Google Scholar] [CrossRef]
- Peng, Q.; Feng, Z.; Shen, Y.; Zhu, J.; Zou, L.; Shen, Y.; Zhu, Y. Integrated analyses of microRNA-29 family and the related combination biomarkers demonstrate their widespread influence on risk, recurrence, metastasis and survival outcome in colorectal cancer. Cancer Cell Int. 2019, 19, 181. [Google Scholar] [CrossRef]
- Basati, G.; Razavi, A.E.; Pakzad, I.; Malayeri, F.A. Circulating levels of the miRNAs, miR-194, and miR-29b, as clinically useful biomarkers for colorectal cancer. Tumor Biol. 2016, 37, 1781–1788. [Google Scholar] [CrossRef]
- Li, L.; Guo, Y.; Chen, Y.; Wang, J.; Zhen, L.; Guo, X.; Liu, J.; Jing, C. The diagnostic efficacy and biological effects of microRNA-29b for colon cancer. Technol. Cancer Res. Treat. 2016, 15, 772–779. [Google Scholar] [CrossRef]
- Yamada, A.; Horimatsu, T.; Okugawa, Y.; Nishida, N.; Honjo, H.; Ida, H.; Kou, T.; Kusaka, T.; Sasaki, Y.; Yagi, M.; et al. Serum miR-21, miR-29a, and miR-125b are promising biomarkers for the early detection of colorectal neoplasia. Clin. Cancer Res. 2015, 21, 4234–4242. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, A.; Li, J.; Fu, J.; Wang, G.; Yang, Y.; Cui, L.; Sun, J. Fecal miR-29a and miR-224 as the noninvasive biomarkers for colorectal cancer. Cancer Biomark. 2016, 16, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Ramzy, I.; Hasaballah, M.; Marzaban, R.; Shaker, O.; Soliman, Z.A. Evaluation of microRNAs-29a, 92a and 145 in colorectal carcinoma as candidate diagnostic markers: An Egyptian pilot study. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-G.; Gu, J. Serum microRNA-29a is a promising novel marker for early detection of colorectal liver metastasis. Cancer Epidemiol. 2012, 36, e61–e67. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Yamamoto, H.; Uemura, M.; Nishimura, J.; Hata, T.; Takemasa, I.; Ikenaga, M.; Ikeda, M.; Murata, K.; Mizushima, T.; et al. MicroRNA-29b is a Novel Prognostic Marker in Colorectal Cancer. Ann. Surg. Oncol. 2015, 22, 1410–1418. [Google Scholar] [CrossRef]
- Aharonov, R.; Weissmann-Brenner, A.; Kushnir, M.; Yanai, G.L.; Gibori, H.; Purim, O.; Kundel, Y.; Morgenstern, S.; Halperin, M.; Niv, Y.; et al. Tumor microRNA-29a expression and the risk of recurrence in stage II colon cancer. Int. J. Oncol. 2012, 40, 2097–2103. [Google Scholar] [CrossRef]
- Wang, G.; Kwan, B.C.-H.; Lai, F.M.-M.; Chow, K.-M.; Li, P.K.-T.; Szeto, C.-C. Urinary miR-21, miR-29, and miR-93: Novel biomarkers of fibrosis. Am. J. Nephrol. 2012, 36, 412–418. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, Q.; Cheng, W.; Zhang, Z.; Wang, J.; Ge, J. Effect of miR-29b-1* and miR-29c knockdown on cell growth of the bladder cancer cell line T24. J. Int. Med Res. 2013, 41, 1803–1810. [Google Scholar] [CrossRef]
- Fan, Y.; Song, X.; Du, H.; Luo, C.; Wang, X.; Yang, X.; Wang, Y.; Wu, X. Down-regulation of miR-29c in human bladder cancer and the inhibition of proliferation in T24 cell via PI3K-AKT pathway. Med Oncol. 2014, 31, 65. [Google Scholar] [CrossRef]
- Dyrskjøt, L.; Ostenfeld, M.S.; Bramsen, J.B.; Silahtaroglu, A.N.; Lamy, P.; Ramanathan, R.; Fristrup, N.; Jensen, J.L.; Andersen, C.L.; Zieger, K.; et al. Genomic Profiling of MicroRNAs in Bladder Cancer: miR-129 Is Associated with Poor Outcome and Promotes Cell Death In vitro. Cancer Res. 2009, 69, 4851. [Google Scholar] [CrossRef]
- Rosenberg, E.; Baniel, J.; Spector, Y.; Faerman, A.; Meiri, E.; Aharonov, R.; Margel, D.; Goren, Y.; Nativ, O. Predicting progression of bladder urothelial carcinoma using microRNA expression. Br. J. Urol. 2013, 112, 1027–1034. [Google Scholar] [CrossRef]
- Xu, X.-D.; Wu, X.-H.; Fan, Y.-R.; Tan, B.; Quan, Z.; Luo, C.-L. Exosome-derived microRNA-29c induces apoptosis of BIU-87 cells by down regulating BCL-2 and MCL-1. Asian Pac. J. Cancer Prev. 2014, 15, 3471–3476. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Lurje, I.; Czigany, Z.; Bednarsch, J.; Roderburg, C.; Isfort, P.; Neumann, U.P.; Lurje, G. Treatment Strategies for Hepatocellular Carcinoma—A Multidisciplinary Approach. Int. J. Mol. Sci. 2019, 20, 1465. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Waljee, A.K.; Patel, N.; Chen, E.Y.; Tiro, J.; Marrero, J.A.; Yopp, A.C. Therapeutic delays lead to worse survival among patients with hepatocellular carcinoma. J. Natl. Compr. Cancer Netw. 2013, 11, 1101–1108. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Chang, Y.-H.; Li, C.-J.; Huang, Y.-H.; Tsai, M.-C.; Chu, P.-Y.; Lin, H.-Y. New Insights into the Role of miR-29a in Hepatocellular Carcinoma: Implications in Mechanisms and Theragnostics. J. Pers. Med. 2021, 11, 219. [Google Scholar] [CrossRef]
- Kong, G.; Zhang, J.; Zhang, S.; Shan, C.; Ye, L.; Zhang, X. Upregulated microRNA-29a by hepatitis B virus X protein enhances hepatoma cell migration by targeting PTEN in cell culture model. PLoS ONE 2011, 6, e19518. [Google Scholar] [CrossRef]
- Yu, L.-X.; Zhang, B.-L.; Yang, Y.; Wang, M.-C.; Lei, G.-L.; Gao, Y.; Liu, H.; Xiao, C.-H.; Xu, J.-J.; Qin, H.; et al. Exosomal microRNAs as potential biomarkers for cancer cell migration and prognosis in hepatocellular carcinoma patient-derived cell models. Oncol. Rep. 2019, 41, 257–269. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, Z. Diagnostic value of a microRNA signature panel in exosomes for patients with hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 1478. [Google Scholar]
- Zhou, Y.; Wang, X.; Sun, L.; Zhou, L.; Ma, T.C.; Song, L.; Wu, J.G.; Li, J.L.; Ho, W.Z. Toll-like receptor 3-activated macrophages confer anti-HCV activity to hepatocytes through exosomes. FASEB J. 2016, 30, 4132–4140. [Google Scholar] [CrossRef]
- Zhu, H.-T.; Hasan, A.M.E.; Liu, R.-B.; Zhang, Z.-C.; Zhang, X.; Wang, J.; Wang, H.-Y.; Wang, F.; Shao, J.-Y. Serum microRNA profiles as prognostic biomarkers for HBV-positive hepatocellular carcinoma. Oncotarget 2016, 7, 45637. [Google Scholar] [CrossRef]
- Jampoka, K.; Muangpaisarn, P.; Khongnomnan, K.; Treeprasertsuk, S.; Tangkijvanich, P.; Payungporn, S. Serum miR-29a and miR-122 as potential biomarkers for non-alcoholic fatty liver disease (NAFLD). MicroRNA 2018, 7, 215–222. [Google Scholar] [CrossRef]
- Zhu, H.-T.; Dong, Q.-Z.; Sheng, Y.-Y.; Wei, J.-W.; Wang, G.; Zhou, H.-J.; Ren, N.; Jia, H.-L.; Ye, Q.-H.; Qin, L.-X. MicroRNA-29a-5p is a novel predictor for early recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection. PLoS ONE 2012, 7, e52393. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Wang, S.; Liu, Z.; Xiu, M. MiR-29a suppresses cell proliferation by targeting SIRT1 in hepatocellular carcinoma. Cancer Biomark. 2018, 22, 151–159. [Google Scholar] [CrossRef]
- Zhu, X.-C.; Dong, Q.-Z.; Zhang, X.-F.; Deng, B.; Jia, H.-L.; Ye, Q.-H.; Qin, L.-X.; Wu, X.-Z. microRNA-29a suppresses cell proliferation by targeting SPARC in hepatocellular carcinoma. Int. J. Mol. Med. 2012, 30, 1321–1326. [Google Scholar] [CrossRef]
- Vila-Navarro, E.; Vila-Casadesús, M.; Moreira, L.; Duran-Sanchon, S.; Sinha, R.; Ginés, À.; Fernández-Esparrach, G.; Miquel, R.; Cuatrecasas, M.; Castells, A. MicroRNAs for detection of pancreatic neoplasia: Biomarker discovery by next-generation sequencing and validation in 2 independent cohorts. Ann. Surg. 2017, 265, 1226. [Google Scholar] [CrossRef]
- Dobre, M.; Herlea, V.; Vlăduţ, C.; Ciocîrlan, M.; Balaban, V.; Constantinescu, G.; Diculescu, M.; Milanesi, E. Dysregulation of miRNAs Targeting the IGF-1R Pathway in Pancreatic Ductal Adenocarcinoma. Cells 2021, 10, 1856. [Google Scholar] [CrossRef]
- Ganepola, G.A.; Rutledge, J.R.; Suman, P.; Yiengpruksawan, A.; Chang, D.H. Novel blood-based microRNA biomarker panel for early diagnosis of pancreatic cancer. World J. Gastrointest. Oncol. 2014, 6, 22–33. [Google Scholar] [CrossRef]
- Humeau, M.; Vignolle-Vidoni, A.; Sicard, F.; Martins, F.; Bournet, B.; Buscail, L.; Torrisani, J.; Cordelier, P. Salivary MicroRNA in Pancreatic Cancer Patients. PLoS ONE 2015, 10, e0130996. [Google Scholar] [CrossRef]
- Lorber, G.; Benenson, S.; Rosenberg, S.; Gofrit, O.N.; Pode, D. A single dose of 240 mg gentamicin during transrectal prostate biopsy significantly reduces septic complications. Urology 2013, 82, 998–1003. [Google Scholar] [CrossRef]
- Hsieh, T.-Y.; Wang, S.-C.; Kao, Y.-L.; Chen, W.-J. Adding gentamicin to fluoroquinolone-based antimicrobial prophylaxis reduces transrectal ultrasound-guided prostate biopsy-related infection rate. Urol. Sci. 2016, 27, 91–95. [Google Scholar] [CrossRef]
- Makena, M.R.; Gatla, H.; Verlekar, D.; Sukhavasi, S.; Pandey, M.K.; Pramanik, K.C. Wnt/β-Catenin signaling: The culprit in pancreatic carcinogenesis and therapeutic resistance. Int. J. Mol. Sci. 2019, 20, 4242. [Google Scholar] [CrossRef]
- Nagano, H.; Tomimaru, Y.; Eguchi, H.; Hama, N.; Wada, H.; Kawamoto, K.; Kobayashi, S.; Mori, M.; Doki, Y. MicroRNA-29a induces resistance to gemcitabine through the Wnt/β-catenin signaling pathway in pancreatic cancer cells. Int. J. Oncol. 2013, 43, 1066–1072. [Google Scholar] [CrossRef]
- Sun, X.-J.; Liu, B.-Y.; Yan, S.; Jiang, T.-H.; Cheng, H.-Q.; Jiang, H.-S.; Cao, Y.; Mao, A.-W. MicroRNA-29a promotes pancreatic cancer growth by inhibiting tristetraprolin. Cell. Physiol. Biochem. 2015, 37, 707–718. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Z.; Wang, Q. Emerging therapies for small cell lung cancer. J. Hematol. Oncol. 2019, 12, 45. [Google Scholar] [CrossRef]
- Heegaard, N.H.H.; Schetter, A.J.; Welsh, J.A.; Yoneda, M.; Bowman, E.D.; Harris, C.C. Circulating micro-RNA expression profiles in early stage nonsmall cell lung cancer. Int. J. Cancer 2012, 130, 1378–1386. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Q.; Zhang, M.; Su, W.; Wang, Z.; Li, Y.; Zhang, J.; Etherton-Beer, C.; Yang, S.; Chen, G. Serum microRNA signature is capable of early diagnosis for non-small cell lung cancer. Int. J. Biol. Sci. 2019, 15, 1712–1722. [Google Scholar] [CrossRef]
- He, Q.; Fang, Y.; Lu, F.; Pan, J.; Wang, L.; Gong, W.; Fei, F.; Cui, J.; Zhong, J.; Hu, R.; et al. Analysis of differential expression profile of miRNA in peripheral blood of patients with lung cancer. J. Clin. Lab. Anal. 2019, 33, e23003. [Google Scholar] [CrossRef]
- Liu, X.; Lv, X.; Yang, Q.; Jin, H.; Zhou, W.; Fan, Q. MicroRNA-29a functions as a tumor suppressor and increases cisplatin sensitivity by targeting NRAS in lung cancer. Technol. Cancer Res. Treat. 2018, 17, 1533033818758905. [Google Scholar] [CrossRef]
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA 2012, 109, E2110–E2116. [Google Scholar] [CrossRef]
- Liu, L.; Bi, N.; Wu, L.; Ding, X.; Men, Y.; Zhou, W.; Li, L.; Zhang, W.; Shi, S.; Song, Y.; et al. MicroRNA-29c functions as a tumor suppressor by targeting VEGFA in lung adenocarcinoma. Mol. Cancer 2017, 16, 50. [Google Scholar] [CrossRef]
- Fang, C.; Zhu, D.-X.; Dong, H.-J.; Zhou, Z.-J.; Wang, Y.-H.; Liu, L.; Fan, L.; Miao, K.-R.; Liu, P.; Xu, W.; et al. Serum microRNAs are promising novel biomarkers for diffuse large B cell lymphoma. Ann. Hematol. 2012, 91, 553–559. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.-S.; Yang, G.-H.; Zhai, P.-F.; Xiao, Z.; Xia, L.-Y.; Chen, L.-R.; Wang, Y.; Bi, L.-X.; Liu, N.; et al. miR-29a and miR-142-3p downregulation and diagnostic implication in human acute myeloid leukemia. Mol. Biol. Rep. 2012, 39, 2713–2722. [Google Scholar] [CrossRef]
- Visone, R.; Rassenti, L.Z.; Veronese, A.; Taccioli, C.; Costinean, S.; Aguda, B.D.; Volinia, S.; Ferracin, M.; Palatini, J.; Balatti, V.; et al. Karyotype-specific microRNA signature in chronic lymphocytic leukemia. Blood 2009, 114, 3872–3879. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, Y.; Kuai, W.; Sun, X.; Chen, H.; Hong, Z. Prognostic value of miR-29a expression in pediatric acute myeloid leukemia. Clin. Biochem. 2013, 46, 49–53. [Google Scholar] [CrossRef]
- Yamada, Y.; Sugawara, S.; Arai, T.; Kojima, S.; Kato, M.; Okato, A.; Yamazaki, K.; Naya, Y.; Ichikawa, T.; Seki, N. Molecular pathogenesis of renal cell carcinoma: Impact of the anti-tumor miR-29 family on gene regulation. Int. J. Urol. 2018, 25, 953–965. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, J.; Lei, Z.; Wan, L.; Zhu, X.; Ye, F.; Tong, Y. Expression and functional role of miR-29b in renal cell carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 14161. [Google Scholar]
- Wu, Z.; Huang, X.; Huang, X.; Zou, Q.; Guo, Y. The inhibitory role of Mir-29 in growth of breast cancer cells. J. Exp. Clin. Cancer Res. 2013, 32, 98. [Google Scholar] [CrossRef]
- Sung, H.; Rosenberg, P.S.; Chen, W.-Q.; Hartman, M.; Lim, W.-Y.; Chia, K.S.; Mang, O.W.-K.; Chiang, C.-J.; Kang, D.; Ngan, R.K.-C.; et al. Female breast cancer incidence among asian and western populations: More similar than expected. J. Natl. Cancer Inst. 2015, 107, djv107. [Google Scholar] [CrossRef]
- Jang, G.B.; Kim, J.Y.; Cho, S.D.; Park, K.S.; Jung, J.Y.; Lee, H.Y.; Hong, I.S.; Nam, J.S. Blockade of Wnt/β-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci. Rep. 2015, 5, srep12465. [Google Scholar] [CrossRef]
- Rostas, J.W.; Pruitt, H.C.; Metge, B.J.; Mitra, A.; Bailey, S.K.; Bae, S.; Singh, K.P.; Devine, D.J.; Dyess, D.L.; Richards, W.O.; et al. microRNA-29 negatively regulates EMT regulator N-myc interactor in breast cancer. Mol. Cancer 2014, 13, 200. [Google Scholar] [CrossRef]
- Shinden, Y.; Iguchi, T.; Akiyoshi, S.; Ueo, H.; Ueda, M.; Hirata, H.; Sakimura, S.; Uchi, R.; Takano, Y.; Eguchi, H.; et al. miR-29b is an indicator of prognosis in breast cancer patients. Mol. Clin. Oncol. 2015, 3, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Muluhngwi, P.; Alizadeh-Rad, N.; Vittitow, S.L.; Kalbfleisch, T.S.; Klinge, C.M. The miR-29 transcriptome in endocrine-sensitive and resistant breast cancer cells. Sci. Rep. 2017, 7, 5205. [Google Scholar] [CrossRef]
- Milevskiy, M.J.; Sandhu, G.K.; Wronski, A.; Korbie, D.; Brewster, B.L.; Shewan, A.; Edwards, S.L.; French, J.D.; Brown, M.A. MiR-29b-1-5p is altered in BRCA1 mutant tumours and is a biomarker in basal-like breast cancer. Oncotarget 2018, 9, 33577. [Google Scholar] [CrossRef]
- Chou, J.; Lin, J.H.; Brenot, A.; Kim, J.-W.; Provot, S.; Werb, Z. GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. Nat. Cell Biol. 2013, 15, 201–213. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, F.; Li, W.; Xu, C.; Li, L.; Chen, L.; Liu, Y.; Sun, P. miR-29a suppresses MCF-7 cell growth by downregulating tumor necrosis factor receptor 1. Tumor Biol. 2017, 39, 1010428317692264. [Google Scholar] [CrossRef]
- Li, H.; Luo, J.; Xu, B.; Luo, K.; Hou, J. MicroRNA-29a inhibits cell migration and invasion by targeting Roundabout 1 in breast cancer cells. Mol. Med. Rep. 2015, 12, 3121–3126. [Google Scholar] [CrossRef]
- Shen, H.; Li, L.; Yang, S.; Wang, D.; Zhong, S.; Zhao, J.; Tang, J. MicroRNA-29a contributes to drug-resistance of breast cancer cells to adriamycin through PTEN/AKT/GSK3β signaling pathway. Gene 2016, 593, 84–90. [Google Scholar] [CrossRef]
- Cochrane, D.R.; Jacobsen, B.; Connaghan, K.D.; Howe, E.N.; Bain, D.L.; Richer, J.K. Progestin regulated miRNAs that mediate progesterone receptor action in breast cancer. Mol. Cell. Endocrinol. 2012, 355, 15–24. [Google Scholar] [CrossRef]
- McDermott, A.M.; Miller, N.; Wall, D.; Martyn, L.M.; Ball, G.; Sweeney, K.J.; Kerin, M. Identification and Validation of Oncologic miRNA Biomarkers for Luminal A-like Breast Cancer. PLoS ONE 2014, 9, e87032. [Google Scholar] [CrossRef]
- Wu, Q.; Lu, Z.; Li, H.; Lu, J.; Guo, L.; Ge, Q. Next-generation sequencing of microRNAs for breast cancer detection. J. Biomed. Biotechnol. 2011, 2011, 597145. [Google Scholar] [CrossRef]
- Raeisi, F.; Mahmoudi, E.; Dehghani-Samani, M.; Hosseini, S.S.E.; Ghahfarrokhi, A.M.; Arshi, A.; Forghanparast, K.; Ghazanfari, S. Differential expression profile of miR-27b, miR-29a, and miR-155 in chronic lymphocytic leukemia and breast cancer patients. Mol. Ther. -Oncolytics 2020, 16, 230–237. [Google Scholar] [CrossRef] [PubMed]
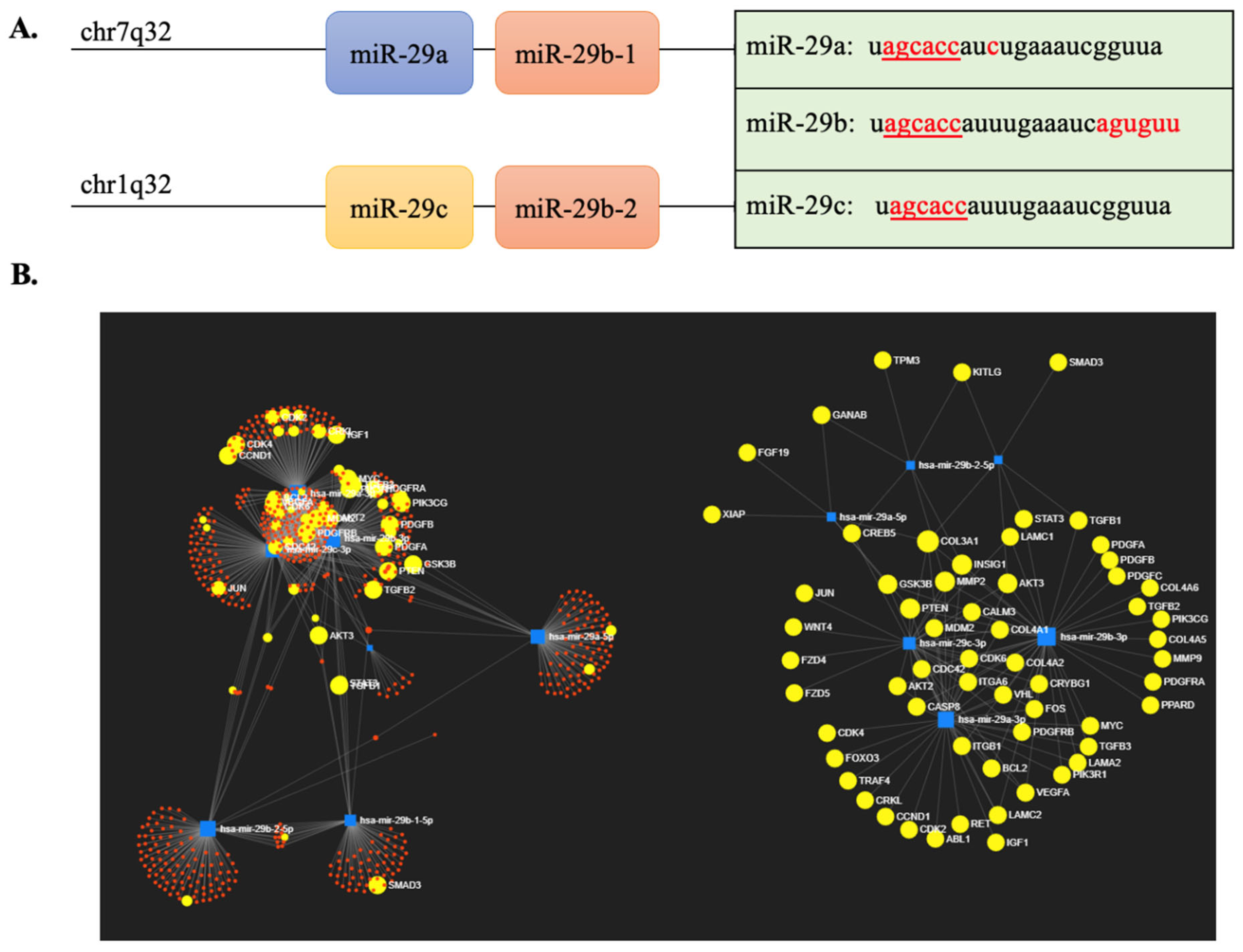

| Sample | Sample Size | Outcome | Results | Ref. |
|---|---|---|---|---|
| Venous blood | 114 CRC patients (58 patients with and 56 patients without metastasis) | MiR-29a was significantly increased in CRC patients with metastasis than in those without. | AUC: 80.3% Sensitivity: 75% Specificity: 75% | [73] |
| Serum | 55 CRC patients and 55 normal controls | The serum level of miR-29b was lower in CRC as compared to the normal controls and inversely correlated with the advanced tumor stages. | AUC: 87% Sensitivity: 77% Specificity: 75% | [68] |
| Tissue Plasma | 200 CRC patients and 400 normal controls | The level of miR-29b in plasma and tissue was highly correlated and significantly lower in CRC versus the normal controls. | Tissue: AUC: 88.3% Sensitivity: 81.6% Specificity: 84.9% Plasma: AUC: 74.3% Sensitivity: 61.4% Specificity: 72.5% | [69] |
| Feces | 80 CRC patients and 51 normal controls | The level of miR-29a in feces was significantly lower in CRC versus the normal controls. | AUC: 77.7% Sensitivity: 85% Specificity: 61% | [71] |
| Serum | 160 colorectal neoplasms patients and 77 normal controls | The level of miR-29a in serum was significantly lower in colorectal neoplasms | AUC: 74.1% | [70] |
| Tissues | 245 CRC patients (34 stages I, 63 stages II, 104 stage III, and 44 stages IV) | MiR-29b expression was significantly decreased in tumor versus normal tissues | Higher miR-29b is associated with higher 5-year DFS and OS. | [74] |
| Tissues | 110 CRC patients (51 stages I and 59 stages II) | The level of miR-29a was a positive predictive factor for non-recurrence in stage II CRC. | Higher miR-29a is associated with longer DFS. Sensitivity: 67% Specificity: 88% | [75] |
| Sample | Sample Size & Methods | Outcomes | Results | Ref. |
|---|---|---|---|---|
| Serum |
| MiR-29c was overexpressed in serum samples | MiR-29c was correlated to the advanced stage and OS time in BC patients. | [36] |
| Urine |
| MiR-29a was upregulated in BC patients. | MiR-29a-3p in combination with six other miRNAs was used for the diagnosis of BC. AUC: 92.3% Sensitivity: 82% Specificity: 96% | [35] |
| Tissue |
| MiR-29c was downregulated in BC. MiR-29c inhibited cell proliferation, migration, and cell cycle progression, and induce apoptosis through AKT signaling. | MiR-29c was inversely associated with bladder tumor stages. | [78] |
| Tissue |
| MiR-29b and miR-29c were downregulated in BC tumors | Higher miR-29c levels were correlated with longer DFS. | [79] |
| Specimen |
| MiR-29c was significantly under-expressed in progressed tumors. | High expression of miR-29c was associated with a better prognosis. | [80] |
| Sample | Sample Size & Methods | Outcome | Results | Refs |
|---|---|---|---|---|
| Serum |
| MiR-29a: lower in NAFLD patient MiR-29c: unchanged MiR-29b: undetectable | For miR-29a: AUC: 0.679 Sensitivity: 60.87% Specificity: 82.35% | [91] |
| Tissue |
| MiR-29a-5p was associated with early HCC recurrence, resulting in lower OS | AUC: 0.708 Sensitivity: 74.2% Specificity: 68.2% | [92] |
| Venous blood |
| MiR-29a-3p was higher in both early and late stages of HCC | AUC: 0.71 (95%CI = 0.62–0.78) | [90] |
| Tissue |
| MiR-29a was downregulated in HCC samples MiR-29a targeted SIRT1 and suppressed the HCC cell cycle and proliferation. | Lower miR-29a is associated with higher tumor size, vascular invasion, poor DFS | [93] |
| Specimen |
| MiR-29a was dramatically decreased in HCC tissues | miR-29a targeted to SPARC, downstream of AKT/mTOR to suppress cell growth. | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.P.; Suman, K.H.; Nguyen, T.B.; Nguyen, H.T.; Do, D.N. The Role of miR-29s in Human Cancers—An Update. Biomedicines 2022, 10, 2121. https://doi.org/10.3390/biomedicines10092121
Nguyen TTP, Suman KH, Nguyen TB, Nguyen HT, Do DN. The Role of miR-29s in Human Cancers—An Update. Biomedicines. 2022; 10(9):2121. https://doi.org/10.3390/biomedicines10092121
Chicago/Turabian StyleNguyen, Thuy T. P., Kamrul Hassan Suman, Thong Ba Nguyen, Ha Thi Nguyen, and Duy Ngoc Do. 2022. "The Role of miR-29s in Human Cancers—An Update" Biomedicines 10, no. 9: 2121. https://doi.org/10.3390/biomedicines10092121
APA StyleNguyen, T. T. P., Suman, K. H., Nguyen, T. B., Nguyen, H. T., & Do, D. N. (2022). The Role of miR-29s in Human Cancers—An Update. Biomedicines, 10(9), 2121. https://doi.org/10.3390/biomedicines10092121






