Neuroprotective Effects of the Lithium Salt of a Novel JNK Inhibitor in an Animal Model of Cerebral Ischemia–Reperfusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Compound Synthesis
2.3. Cerebral Ischemia Model
2.4. Ischemia/Reperfusion Protocol
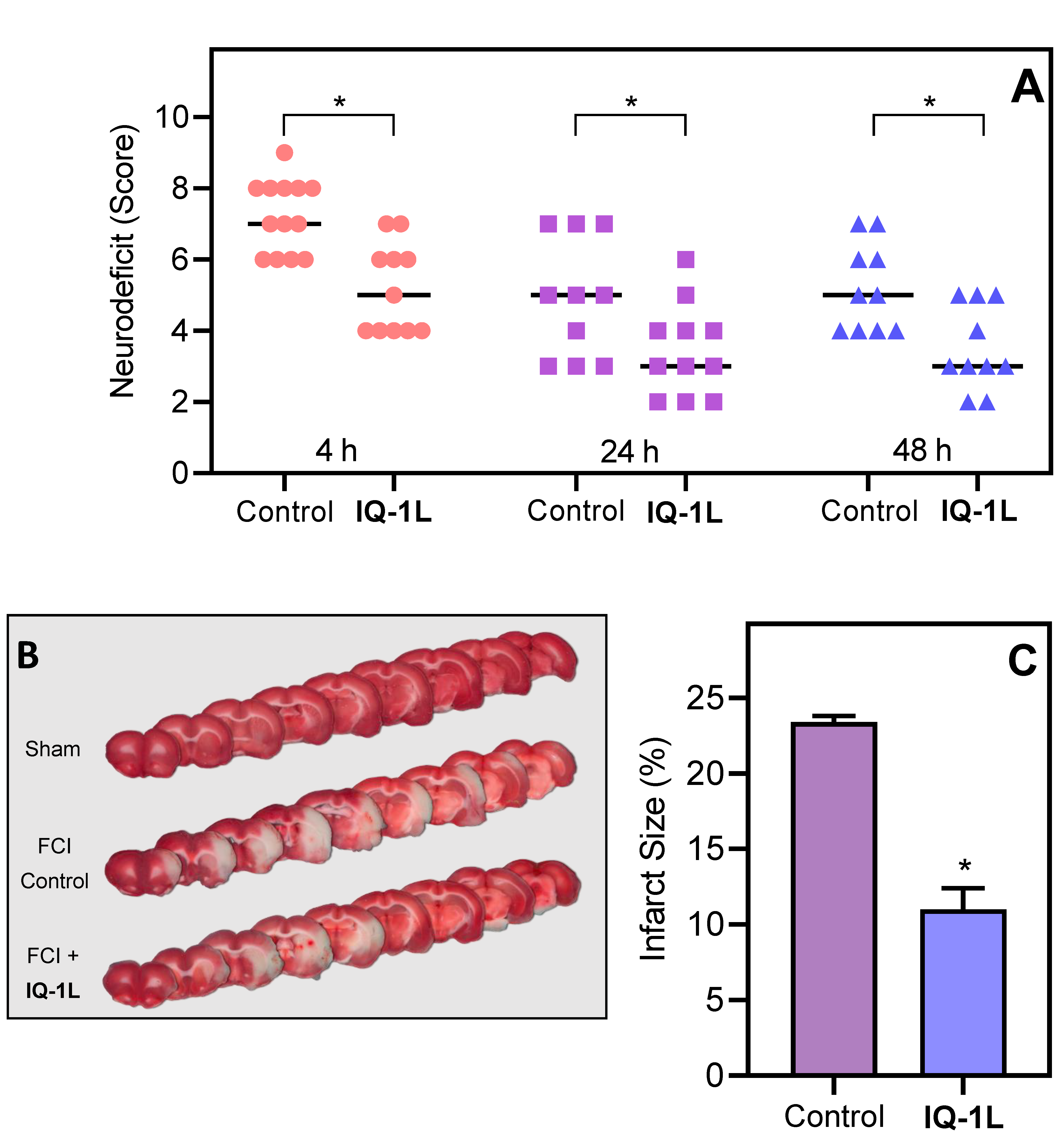
2.5. Neurological Deficit Evaluation
2.6. Assessment of Cerebral Infarct Size
2.7. Kinase Kd Determination
2.8. Cell Culture
2.9. Analysis of AP-1/NF-κB Activation
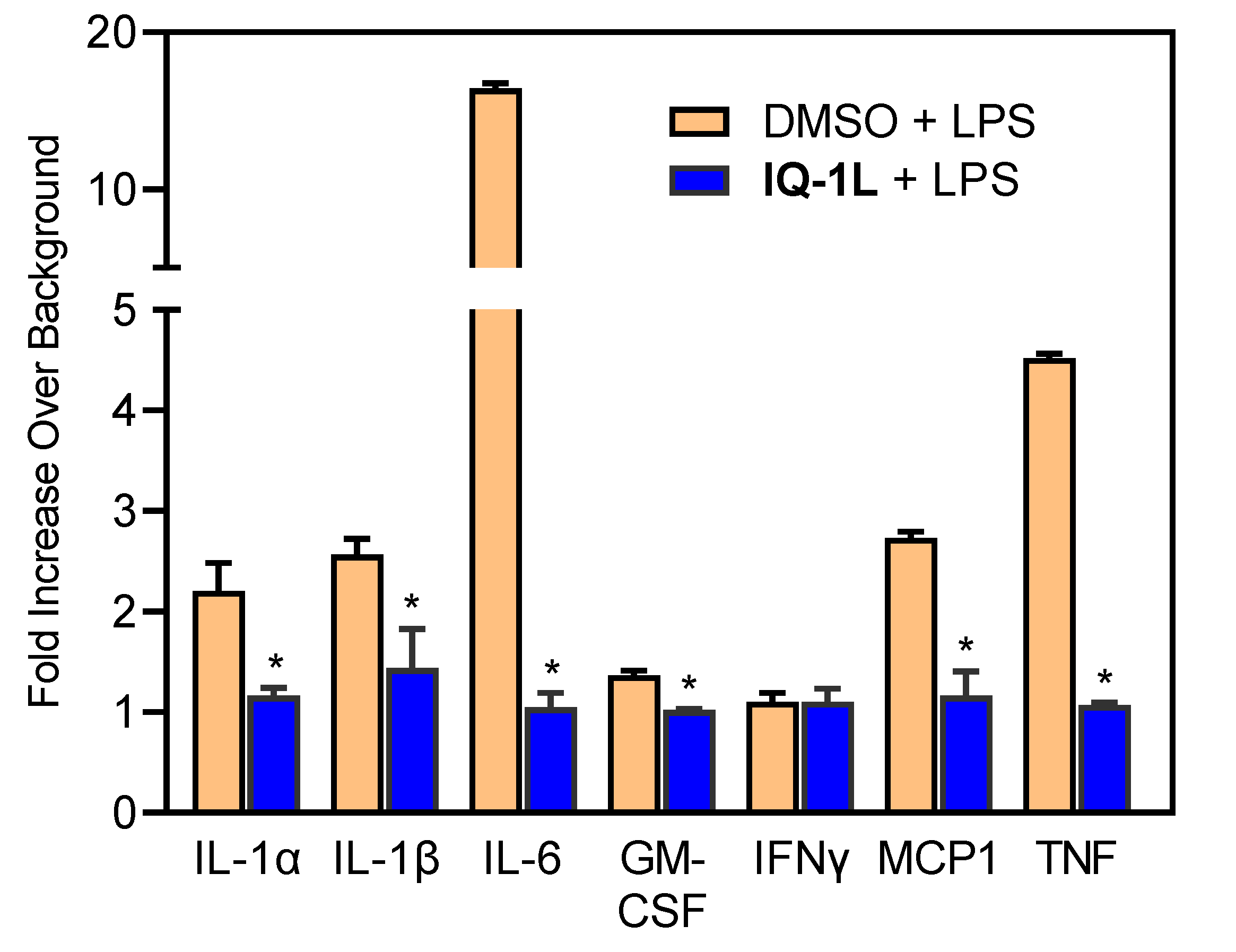
2.10. Cytokine Analysis
2.11. Cytotoxicity Assay
2.12. Western Blotting
2.13. Molecular Modeling
2.14. Statistical Analysis
3. Results and Discussion
3.1. Molecular Modeling
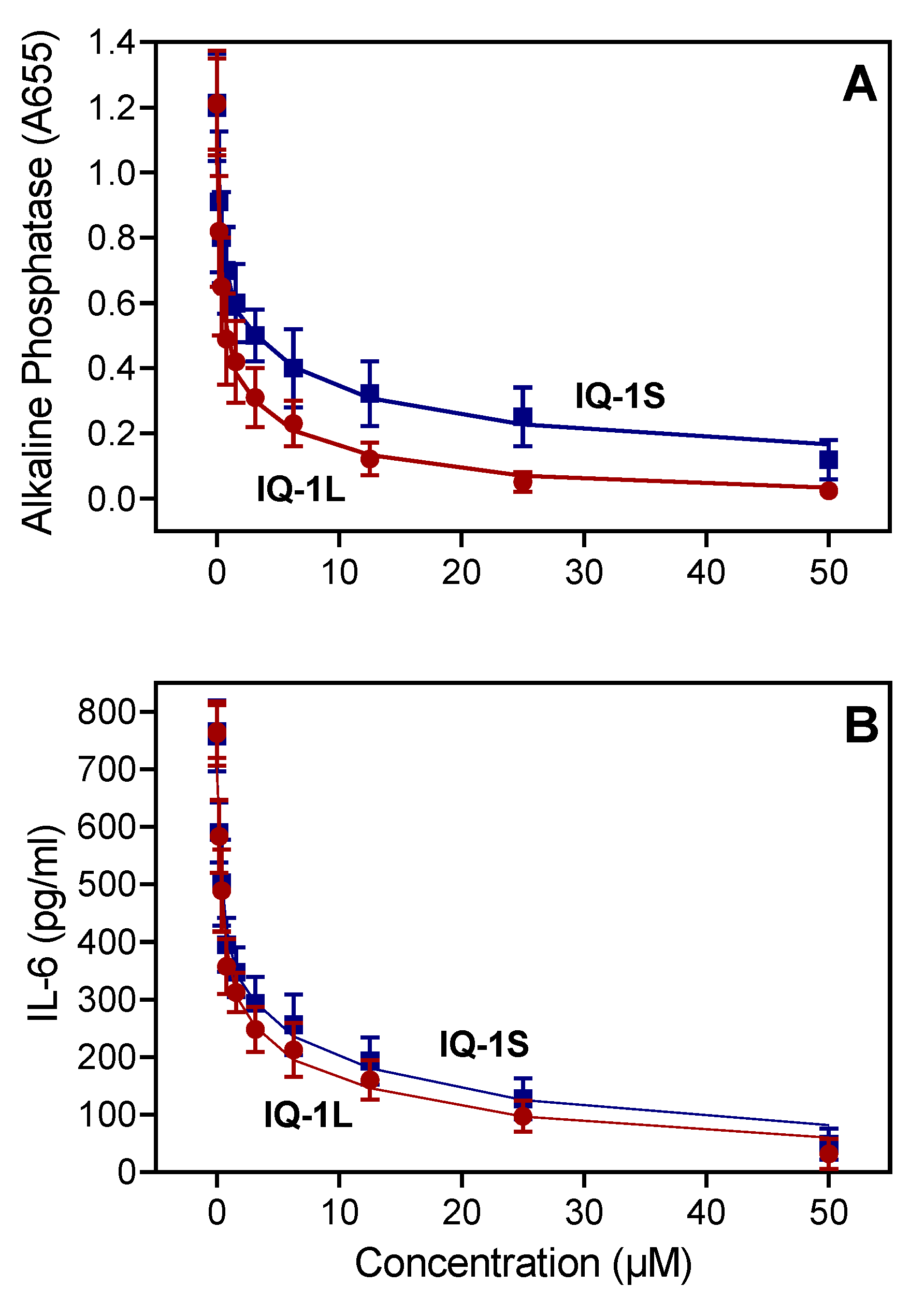
3.2. Affinity of IQ-1L for JNK
3.3. Therapeutic Effects of IQ-1L in FCI
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duris, K.; Splichal, Z.; Jurajda, M. The role of inflammatory response in stroke associated programmed cell death. Curr. Neuropharmacol. 2018, 16, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.L.; Manhas, N.; Raghubir, R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res. Rev. 2007, 54, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Veltkamp, R.; Gill, D. Clinical trials of immunomodulation in ischemic stroke. Neurotherapeutics 2016, 13, 791–800. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.S. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.H.; Liu, R.; Zhu, X.Q.; Smerin, D.; Zhong, Y.; Gu, L.J.; Fang, W.R.; Xiong, X.X. The Involvement and therapy target of immune cells after ischemic stroke. Front. Immunol. 2019, 10, 2167. [Google Scholar] [CrossRef]
- Ahnstedt, H.; McCullough, L.D. The impact of sex and age on T cell immunity and ischemic stroke outcomes. Cell Immunol. 2019, 345, 103960. [Google Scholar] [CrossRef]
- Wanrooy, B.J.; Wen, S.W.; Shim, R.; Wilson, J.L.; Prame Kumar, K.; Wong, C.H. Brain-associated innate leukocytes display diverse inflammatory states following experimental stroke. Immunol. Cell Biol. 2022, 100, 482–496. [Google Scholar] [CrossRef]
- Barone, F.C.; Schmidt, D.B.; Hillegass, L.M.; Price, W.J.; White, R.F.; Feuerstein, G.Z.; Clark, R.K.; Lee, E.V.; Griswold, D.E.; Sarau, H.M. Reperfusion increases neutrophils and leukotriene B4 receptor binding in rat focal ischemia. Stroke 1992, 23, 1337–1347. [Google Scholar] [CrossRef]
- Gehrmann, J.; Bonnekoh, P.; Miyazawa, T.; Hossmann, K.A.; Kreutzberg, G.W. Immunocytochemical study of an early microglial activation in ischemia. J. Cereb. Blood Flow Metab. 1992, 12, 257–269. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Tao, W.; Liu, M. Systematic review and meta-analysis of the efficacy of sphingosine-1-phosphate (S1P) receptor agonist FTY720 (fingolimod) in animal models of stroke. Int. J. Neurosci. 2013, 123, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, N.; Ren, L.; Yan, Y.; Sun, N.; Li, Y.J.; Han, W.; Xue, R.; Liu, Q.; Hao, J.; et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc. Natl. Acad. Sci. USA 2014, 111, 18315–18320. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, A.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef]
- Luo, G.; Huang, Y.; Mo, D.; Ma, N.; Gao, F.; Song, L.; Sun, X.; Xu, X.; Liu, L.; Huo, X.; et al. Tyrosol attenuates pro-inflammatory cytokines from cultured astrocytes and NF-κB activation in in vitro oxygen glucose deprivation. Neurochem. Int. 2018, 121, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Signore, A.P.; Yin, W.; Cao, G.; Yin, X.M.; Sun, F.; Luo, Y.; Graham, S.H.; Chen, J. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J. Cereb. Blood Flow Metab. 2005, 25, 694–712. [Google Scholar] [CrossRef]
- Murata, Y.; Fujiwara, N.; Seo, J.H.; Yan, F.; Liu, X.; Terasaki, Y.; Luo, Y.; Arai, K.; Ji, X.; Lo, E.H. Delayed inhibition of c-Jun N-terminal kinase worsens outcomes after focal cerebral ischemia. J. Neurosci. 2012, 32, 8112–8115. [Google Scholar] [CrossRef]
- Kuan, C.Y.; Burke, R.E. Targeting the JNK signaling pathway for stroke and Parkinson’s diseases therapy. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 63–67. [Google Scholar] [CrossRef]
- Gupta, S.; Barrett, T.; Whitmarsh, A.J.; Cavanagh, J.; Sluss, H.K.; Derijard, B.; Davis, R.J. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996, 15, 2760–2770. [Google Scholar] [CrossRef]
- Yamasaki, T.; Kawasaki, H.; Nishina, H. Diverse Roles of JNK and MKK Pathways in the Brain. J. Signal. Transduct. 2012, 2012, 459265. [Google Scholar] [CrossRef]
- Brecht, S.; Kirchhof, R.; Chromik, A.; Willesen, M.; Nicolaus, T.; Raivich, G.; Wessig, J.; Waetzig, V.; Goetz, M.; Claussen, M.; et al. Specific pathophysiological functions of JNK isoforms in the brain. Eur. J. Neurosci. 2005, 21, 363–377. [Google Scholar] [CrossRef]
- Davies, C.; Tournier, C. Exploring the function of the JNK (c-Jun N-terminal kinase) signalling pathway in physiological and pathological processes to design novel therapeutic strategies. Biochem. Soc. Trans. 2012, 40, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, M.; Anfinogenova, Y.; Atochina-Vasserman, E.N.; Schepetkin, I.A.; Atochin, D.N. c-Jun N-Terminal Kinases (JNKs) in Myocardial and Cerebral Ischemia/Reperfusion Injury. Front. Pharmacol. 2018, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef]
- Hayashi, T.; Sakai, K.; Sasaki, C.; Zhang, W.R.; Warita, H.; Abe, K. c-Jun N-terminal kinase (JNK) and JNK interacting protein response in rat brain after transient middle cerebral artery occlusion. Neurosci. Lett. 2000, 284, 195–199. [Google Scholar] [CrossRef]
- Irving, E.A.; Bamford, M. Role of mitogen- and stress-activated kinases in ischemic injury. J. Cereb. Blood Flow Metab. 2002, 22, 631–647. [Google Scholar] [CrossRef]
- Borsello, T.; Clarke, P.G.; Hirt, L.; Vercelli, A.; Repici, M.; Schorderet, D.F.; Bogousslavsky, J.; Bonny, C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 2003, 9, 1180–1186. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.I.; Hanks, T.S.; Kochetkova, I.; Pascual, D.W.; Jutila, M.A.; Quinn, M.T. Identification and characterization of a novel class of c-Jun N-terminal kinase inhibitors. Mol. Pharmacol. 2012, 81, 832–845. [Google Scholar] [CrossRef]
- Krenitsky, V.P.; Delgado, M.; Nadolny, L.; Sahasrabudhe, K.; Ayala, L.; Clareen, S.S.; Hilgraf, R.; Albers, R.; Kois, A.; Hughes, K.; et al. Aminopurine based JNK inhibitors for the prevention of ischemia reperfusion injury. Bioorg. Med. Chem. Lett. 2012, 22, 1427–1432. [Google Scholar] [CrossRef]
- Gehringer, M.; Muth, F.; Koch, P.; Laufer, S.A. c-Jun N-terminal kinase inhibitors: A patent review (2010–2014). Exp. Opin. Therapeut Patents 2015, 25, 849–872. [Google Scholar] [CrossRef]
- Guan, Q.H.; Pei, D.S.; Liu, X.M.; Wang, X.T.; Xu, T.L.; Zhang, G.Y. Neuroprotection against ischemic brain injury by SP600125 via suppressing the extrinsic and intrinsic pathways of apoptosis. Brain. Res. 2006, 1092, 36–46. [Google Scholar] [CrossRef]
- Koch, P.; Gehringer, M.; Laufer, S.A. Inhibitors of c-Jun N-terminal kinases: An update. J. Med. Chem. 2015, 58, 72–95. [Google Scholar] [CrossRef] [PubMed]
- Carboni, S.; Boschert, U.; Gaillard, P.; Gotteland, J.P.; Gillon, J.Y.; Vitte, P.A. AS601245, a c-Jun NH2-terminal kinase (JNK) inhibitor, reduces axon/dendrite damage and cognitive deficits after global cerebral ischaemia in gerbils. Br. J. Pharmacol. 2008, 153, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kirpotina, L.N.; Hammaker, D.; Kochetkova, I.; Khlebnikov, A.I.; Lyakhov, S.A.; Firestein, G.S.; Quinn, M.T. Anti-inflammatory effects and joint protection in collagen-induced arthritis after treatment with IQ-1S, a selective c-Jun N-terminal Kinase Inhibitor. J. Pharmacol. Exp. Ther. 2015, 353, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Xia, X.; Zhao, Y.; Zhang, S.; Zhang, Y.; Wang, J. JNK selective inhibitor, IQ-1S, protects the mice against lipopolysaccharides-induced sepsis. Bioorg. Med. Chem. 2021, 30, 115945. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Stokes, J.M.; Eastman, R.T.; Itkin, Z.; Zakharov, A.V.; Collins, J.J.; Jaakkola, T.S.; Barzilay, R. Deep learning identifies synergistic drug combinations for treating COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2105070118. [Google Scholar] [CrossRef]
- Atochin, D.N.; Schepetkin, I.A.; Khlebnikov, A.I.; Seledtsov, V.I.; Swanson, H.; Quinn, M.T.; Huang, P.L. A novel dual NO-donating oxime and c-Jun N-terminal kinase inhibitor protects against cerebral ischemia-reperfusion injury in mice. Neurosci. Lett. 2016, 618, 45–49. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Chernysheva, G.A.; Aliev, O.I.; Smol’iakova, V.I.; Fomina, T.I.; Osipenko, A.N.; Rydchenko, V.S.; Anfinogenova, Y.J.; Khlebnikov, A.I.; Schepetkin, I.A.; et al. Protective Effects of a new C-Jun N-terminal kinase inhibitor in the model of global cerebral ischemia in rats. Molecules 2019, 24, 1722. [Google Scholar] [CrossRef]
- Matveevskaya, V.V.; Pavlov, D.I.; Sukhikh, T.S.; Gushchin, A.L.; Ivanov, A.Y.; Tennikova, T.B.; Sharoyko, V.V.; Baykov, S.V.; Benassi, E.; Potapov, A.S. Arene-ruthenium(II) Complexes Containing 11H-Indeno[1,2-b]quinoxalin-11-one Derivatives and Tryptanthrin-6-oxime: Synthesis, characterization, cytotoxicity, and catalytic transfer hydrogenation of aryl ketones. ACS Omega 2020, 5, 11167–11179. [Google Scholar] [CrossRef]
- Ozbolat, G.; Yegani, A.A. Synthesis, characterization, biological activity and electrochemistry studies of iron(III) complex with curcumin-oxime ligand. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1834–1842. [Google Scholar] [CrossRef]
- Ren, B.; Guo, C.; Liu, R.Z.; Bian, Z.Y.; Liu, R.C.; Huang, L.F.; Tang, J.J. Imidazolylacetophenone oxime-based multifunctional neuroprotective agents: Discovery and structure-activity relationships. Eur. J. Med. Chem. 2022, 228, 114031. [Google Scholar] [CrossRef]
- Nonaka, S.; Chuang, D.M. Neuroprotective effects of chronic lithium on focal cerebral ischemia in rats. Neuroreport 1998, 9, 2081–2084. [Google Scholar] [CrossRef] [PubMed]
- Chuang, D.M.; Chen, R.W.; Chalecka-Franaszek, E.; Ren, M.; Hashimoto, R.; Senatorov, V.; Kanai, H.; Hough, C.; Hiroi, T.; Leeds, P. Neuroprotective effects of lithium in cultured cells and animal models of diseases. Bipolar Disord. 2002, 4, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Mohammadianinejad, S.E.; Majdinasab, N.; Sajedi, S.A.; Abdollahi, F.; Moqaddam, M.M.; Sadr, F. The effect of lithium in post-stroke motor recovery: A double-blind, placebo-controlled, randomized clinical trial. Clin. Neuropharmacol. 2014, 37, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Culman, J.; Blume, A.; Brecht, S.; Gohlke, P. Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death. Stroke 2003, 34, 1287–1292. [Google Scholar] [CrossRef]
- Ren, M.; Senatorov, V.V.; Chen, R.W.; Chuang, D.M. Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. Proc. Natl. Acad. Sci. USA 2003, 100, 6210–6215. [Google Scholar] [CrossRef]
- Kim, Y.R.; van Meer, M.P.A.; Tejima, E.; Murata, Y.; Mandeville, J.B.; Dai, G.; Chuang, D.M.; Rosen, B.R.; Lo, E.H. Functional MRI of delayed chronic lithium treatment in rat focal cerebral ischemia. Stroke 2008, 39, 439–447. [Google Scholar] [CrossRef]
- Bian, Q.M.; Shi, T.; Chuang, D.M.; Qian, Y.I. Lithium reduces ischemia-induced hippocampal CA1 damage and behavioral deficits in gerbils. Brain Res. 2007, 1184, 270–276. [Google Scholar] [CrossRef]
- Yan, X.B.; Wang, S.S.; Hou, H.L.; Ji, R.; Zhou, J.N. Lithium improves the behavioral disorder in rats subjected to transient global cerebral ischemia. Behav. Brain Res. 2007, 177, 282–289. [Google Scholar] [CrossRef]
- Yan, X.B.; Hou, H.L.; Wu, L.M.; Liu, J.; Zhou, J.N. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology 2007, 53, 487–495. [Google Scholar] [CrossRef]
- Manji, H.K.; Lenox, R.H. Lithium: A molecular transducer of mood-stabilization in the treatment of bipolar disorder. Neuropharmacology 1998, 19, 161–166. [Google Scholar] [CrossRef]
- Haupt, M.; Bahr, M.; Doeppner, T.R. Lithium beyond psychiatric indications: The reincarnation of a new old drug. Neural Regen. Res. 2021, 16, 2383–2387. [Google Scholar] [CrossRef] [PubMed]
- Rowe, M.K.; Chuang, D.M. Lithium neuroprotection: Molecular mechanisms and clinical implications. Expert Rev. Mol. Med. 2004, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible Middle Cerebral-Artery Occlusion without Craniectomy in Rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, Y.; Wang, W.; Gao, H.M. Rat model of focal cerebral ischemia in the dominant hemisphere. Int. J. Clin. Exp. Med. 2015, 8, 504–511. [Google Scholar] [PubMed]
- Karaman, M.W.; Herrgard, S.; Treiber, D.K.; Gallant, P.; Atteridge, C.E.; Campbell, B.T.; Chan, K.W.; Ciceri, P.; Davis, M.I.; Edeen, P.T.; et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008, 26, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Ehlert, S.; Mewes, J.M. r(2)SCAN-3c: A "Swiss army knife" composite electronic-structure method. J. Chem. Phys. 2021, 154, 064103. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Karabatsos, G.J.; Taller, R.A. Structural studies by nuclear magnetic resonance-XV. Conformations and configurations of oximes. Tetrahedron 1967, 23, 1079–1095. [Google Scholar] [CrossRef]
- Xie, X.L.; Gu, Y.; Fox, T.; Coll, J.T.; Fleming, M.A.; Markland, W.; Caron, P.R.; Wilson, K.P.; Su, M.S.S. Crystal structure of JNK3: A kinase implicated in neuronal apoptosis. Structure 1998, 6, 983–991. [Google Scholar] [CrossRef]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. The functional contrariety of JNK. Mol. Carcinog. 2007, 46, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Z.; Arumugam, T.V.; Cheng, Y.L.; Lee, J.H.; Chigurupati, S.; Mattson, M.P.; Basta, M. Combination therapy with low-dose IVIG and a C1-esterase inhibitor ameliorates brain damage and functional deficits in experimental ischemic stroke. Neuromol. Med. 2018, 20, 63–72. [Google Scholar] [CrossRef]
- Jiang, M.J.; Li, J.; Peng, Q.X.; Liu, Y.; Liu, W.; Luo, C.H.; Peng, J.; Li, J.K.; Yung, K.K.L.; Mo, Z.X. Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. J. Neuroinflamm. 2014, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.A.; Biggs, W.H.; Treiber, D.K.; Atteridge, C.E.; Azimioara, M.D.; Benedetti, M.G.; Carter, T.A.; Ciceri, P.; Edeen, P.T.; Floyd, M.; et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 2005, 23, 329–336. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Tumour necrosis factors receptor associated signalling molecules and their role in activtion of apoptosis, JNK and NF-kappaB. Ann. Rheum. Dis. 2000, 59 (Suppl. 1), i6–i16. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Toll-like receptors; their physiological role and signal transduction system. Int. Immunopharmacol. 2001, 1, 625–635. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Chernysheva, G.A.; Smolyakova, V.I.; Aliev, O.I.; Trofimova, E.S.; Sherstoboev, E.Y.; Osipenko, A.N.; Khlebnikov, A.I.; Anfinogenova, Y.J.; Schepetkin, I.A.; et al. Neuroprotective effects of a novel inhibitor of c-Jun N-terminal kinase in the rat model of transient focal cerebral ischemia. Cells 2020, 9, 1860. [Google Scholar] [CrossRef]
- Croci, D.M.; Sivanrupan, S.; Wanderer, S.; Agnoletto, G.J.; Chiappini, A.; Grüter, B.E.; Andereggen, L.; Mariani, L.; Taussky, P.; Marbacher, S. Preclinical and clinical role of interleukin-6 in the development of delayed cerebral vasospasm and neuronal cell death after subarachnoid hemorrhage: Towards a potential target therapy? Neurosurg. Rev. 2022, 45, 395–403. [Google Scholar] [CrossRef]
- Xu, X.H.; Zhang, H.L.; Han, R.; Gu, Z.L.; Qin, Z.H. Enhancement of neuroprotection and heat shock protein induction by combined prostaglandin A(1) and lithium in rodent models of focal ischemia. Brain Res. 2006, 1102, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Hua, Y.N.; Zhang, H.L.; Wu, J.C.; Miao, Y.Z.; Han, R.; Gu, Z.L.; Qin, Z.H. Greater stress protein expression enhanced by combined prostaglan-din A(1) and lithium in a rat model of focal ischemia. Acta Pharmacol. Sin. 2007, 28, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Gao, B.; Sheng, R.; Zhang, L.S.; Zhang, H.L.; Gu, Z.L.; Qin, Z.H. Synergistic effects of prostaglandin E1 and lithium in a rat model of cerebral ischemia. Acta Pharmacol. Sin. 2008, 29, 1141–1149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, J.; Zhang, G.Y. Lithium reduced N-methyl-D-aspartate receptor subunit 2A tyrosine phosphorylation and its interactions with Src and Fyn mediated by PSD-95 in rat hippocampus following cerebral ischemia. Neurosci. Lett. 2003, 348, 185–189. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, G.Y.; Liu, Y.; Yan, J.Z.; Hao, Z.B. Lithium suppressed Tyr-402 phosphorylation of proline-rich tyrosine kinase (Pyk2) and interactions of Pyk2 and PSD-95 with NR2A in rat hippocampus following cerebral ischemia. Neurosci. Res. 2004, 49, 357–362. [Google Scholar] [CrossRef]
- Chalecka-Franaszek, E.; Chuang, D.M. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc. Natl. Acad. Sci. USA 1999, 96, 8745–8750. [Google Scholar] [CrossRef]
- Roh, M.S.; Eom, T.Y.; Zmijewska, A.A.; De Sarno, P.; Roth, K.A.; Jope, R.S. Hypoxia activates glycogen synthase kinase-3 in mouse brain in vivo: Protection by mood stabilizers and imipramine. Biol. Psychiat. 2005, 57, 278–286. [Google Scholar] [CrossRef]





| Cation (M+) | Z Isomer, Chelate vs. Non-Chelate | E Isomer, Chelate vs. Non-Chelate | Z,E Isomerization * |
|---|---|---|---|
| ΔG, kcal/mol | |||
| Li+ | −15.00 | −3.22 | 6.93 |
| Na+ | −12.12 | −5.36 | 1.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schepetkin, I.A.; Chernysheva, G.A.; Aliev, O.I.; Kirpotina, L.N.; Smol’yakova, V.I.; Osipenko, A.N.; Plotnikov, M.B.; Kovrizhina, A.R.; Khlebnikov, A.I.; Plotnikov, E.V.; et al. Neuroprotective Effects of the Lithium Salt of a Novel JNK Inhibitor in an Animal Model of Cerebral Ischemia–Reperfusion. Biomedicines 2022, 10, 2119. https://doi.org/10.3390/biomedicines10092119
Schepetkin IA, Chernysheva GA, Aliev OI, Kirpotina LN, Smol’yakova VI, Osipenko AN, Plotnikov MB, Kovrizhina AR, Khlebnikov AI, Plotnikov EV, et al. Neuroprotective Effects of the Lithium Salt of a Novel JNK Inhibitor in an Animal Model of Cerebral Ischemia–Reperfusion. Biomedicines. 2022; 10(9):2119. https://doi.org/10.3390/biomedicines10092119
Chicago/Turabian StyleSchepetkin, Igor A., Galina A. Chernysheva, Oleg I. Aliev, Liliya N. Kirpotina, Vera I. Smol’yakova, Anton N. Osipenko, Mark B. Plotnikov, Anastasia R. Kovrizhina, Andrei I. Khlebnikov, Evgenii V. Plotnikov, and et al. 2022. "Neuroprotective Effects of the Lithium Salt of a Novel JNK Inhibitor in an Animal Model of Cerebral Ischemia–Reperfusion" Biomedicines 10, no. 9: 2119. https://doi.org/10.3390/biomedicines10092119
APA StyleSchepetkin, I. A., Chernysheva, G. A., Aliev, O. I., Kirpotina, L. N., Smol’yakova, V. I., Osipenko, A. N., Plotnikov, M. B., Kovrizhina, A. R., Khlebnikov, A. I., Plotnikov, E. V., & Quinn, M. T. (2022). Neuroprotective Effects of the Lithium Salt of a Novel JNK Inhibitor in an Animal Model of Cerebral Ischemia–Reperfusion. Biomedicines, 10(9), 2119. https://doi.org/10.3390/biomedicines10092119









