Lipid-Based Drug Delivery Systems for Diseases Managements
Abstract
:1. Introduction
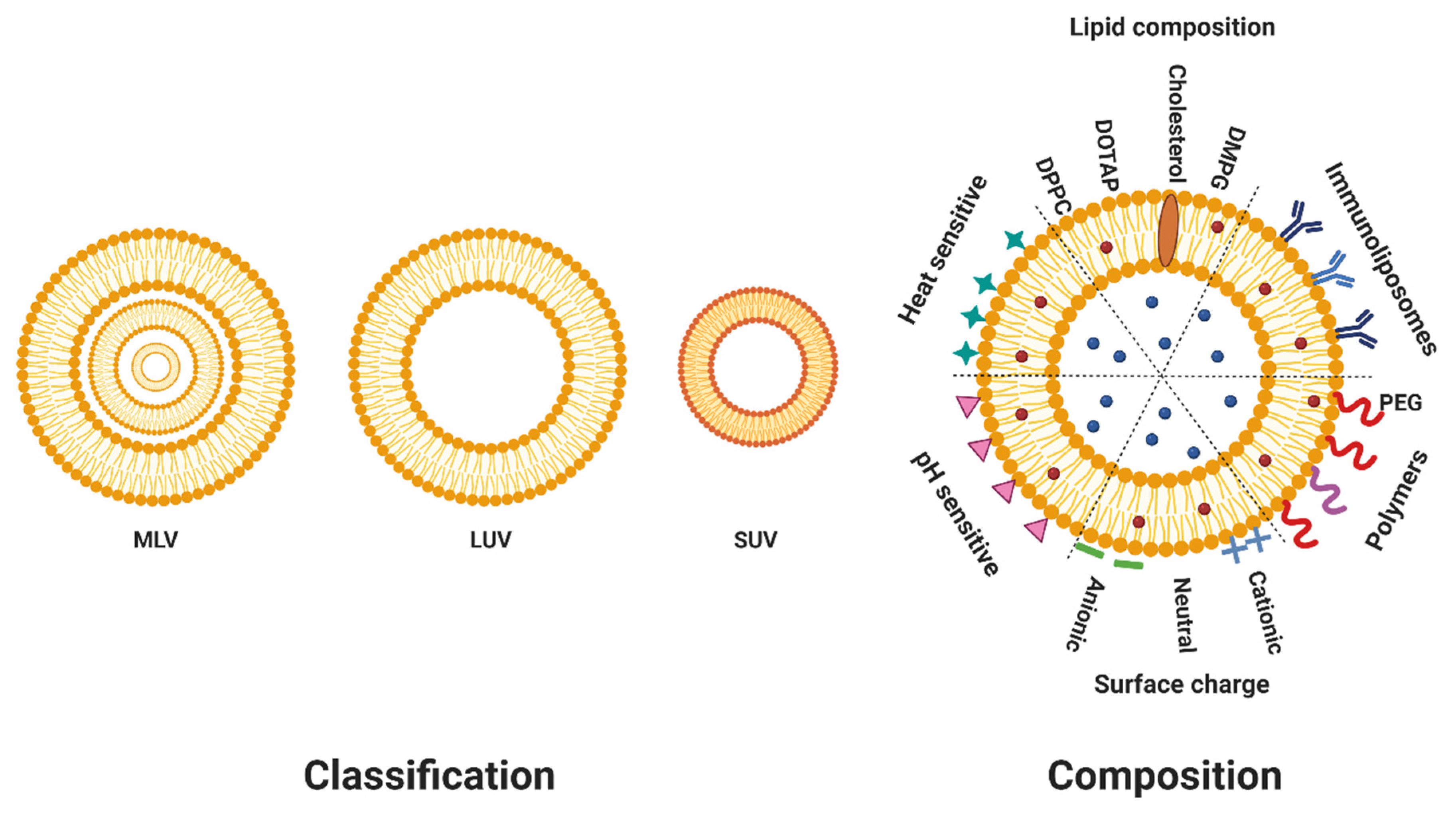
2. Composition of Liposomes
3. Classification of Liposomes
4. Methods of Preparation of Liposomal Therapeutics
4.1. Passive Loading Techniques
4.1.1. Thin Film Hydration and Dehydration–Rehydration Vesicles (DRV) Methods
4.1.2. Reverse Phase Evaporation Method (RPE)
4.1.3. Injection Method
4.1.4. Emulsion Method
4.1.5. Detergent Removal Method
4.1.6. Sonication
4.1.7. Membrane Extrusion
4.1.8. Microfluidization Method
4.1.9. Freeze-Drying Method
4.1.10. Heating Method
4.1.11. SuperLip Method
4.2. Active Encapsulation
5. Methods of Characterization
5.1. Particle Size
5.2. Lamellarity
5.3. Zeta Potential
5.4. Encapsulation Efficiency (EE)
6. Stabilization of Liposomes
7. Scale-Up of Liposomes
8. Mode of Action
9. Routes of Administration and Biodistribution
10. Advantages and Toxicity
11. Applications and Approved Treatments
11.1. Liposomes in Diagnostic
11.2. Liposomes for Brain Targeting
11.3. Liposomes as Vaccine Adjuvants
11.4. Liposomes in Eye Disease
11.5. Liposomes in Cancer Therapy
11.6. Liposomes as Delivery Systems for Antibiotics and Anti-Infectives
12. Conclusions
13. Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saraf, S.; Jain, A.; Tiwari, A.; Verma, A.; Panda, P.K.; Jain, S.K. Advances in Liposomal Drug Delivery to Cancer: An Overview. J. Drug Deliv. Sci. Technol. 2020, 56, 101549. [Google Scholar] [CrossRef]
- Jadhav, M.; Kalhapure, R.S.; Rambharose, S.; Mocktar, C.; Singh, S.; Kodama, T.; Govender, T. Novel Lipids with Three C18-Fatty Acid Chains and an Amino Acid Head Group for PH-Responsive and Sustained Antibiotic Delivery. Chem. Phys. Lipids 2018, 212, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.K.; Mutha, R.E.; Surana, S.J. Electrostatic Deposition Assisted Preparation, Characterization and Evaluation of Chrysin Liposomes for Breast Cancer Treatment. Drug Dev. Ind. Pharm. 2021, 47, 809–819. [Google Scholar] [CrossRef]
- Ong, S.G.M.; Ming, L.C.; Lee, K.S.; Yuen, K.H. Influence of the Encapsulation Efficiency and Size of Liposome on the Oral Bioavailability of Griseofulvin-Loaded Liposomes. Pharmaceutics 2016, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Obuobi, S.; Julin, K.; Fredheim, E.G.A.; Johannessen, M.; Škalko-Basnet, N. Liposomal Delivery of Antibiotic Loaded Nucleic Acid Nanogels with Enhanced Drug Loading and Synergistic Anti-Inflammatory Activity against S. Aureus Intracellular Infections. J. Control. Release 2020, 324, 620–632. [Google Scholar] [CrossRef]
- Alhariri, M.; Omri, A. Efficacy of Liposomal Bismuth-Ethanedithiol-Loaded Tobramycin after Intratracheal Administration in Rats with Pulmonary Pseudomonas aeruginosa Infection. Antimicrob. Agents Chemother. 2013, 57, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Iqbal, Z.; Panda, A.K.; Samim, M.; Talegaonkar, S.; Ahmad, F.J. Polymeric Nanoparticles as a Platform for Permeability Enhancement of Class III Drug Amikacin. Colloids Surf. B Biointerfaces 2018, 169, 206–213. [Google Scholar] [CrossRef]
- Yingyuad, P.; Sinthuvanich, C.; Leepasert, T.; Thongyoo, P.; Boonrungsiman, S. Preparation, Characterization and in Vitro Evaluation of Calothrixin B Liposomes. J. Drug Deliv. Sci. Technol. 2018, 44, 491–497. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From Bangham to Supercritical Fluids. Processes 2020, 8, 1022. [Google Scholar] [CrossRef]
- Abraham, T.; Mao, M.; Tan, C. Engineering Approaches of Smart, Bio-Inspired Vesicles for Biomedical Applications. Phys. Biol. 2018, 15, 061001. [Google Scholar] [CrossRef]
- Maja, L.; Željko, K.; Mateja, P. Sustainable Technologies for Liposome Preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and Innovation in the Manufacturing Process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef]
- Refaat, H.; Naguib, Y.W.; Elsayed, M.M.A.; Sarhan, H.A.A.; Alaaeldin, E. Modified Spraying Technique and Response Surface Methodology for the Preparation and Optimization of Propolis Liposomes of Enhanced Anti-Proliferative Activity against Human Melanoma Cell Line A375. Pharmaceutics 2019, 11, 558. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, S.; Li, J.; Wang, Y.; Su, Y.; Chi, D.; Wang, J.; Wang, X.; He, Z.; Lin, G.; et al. Simple Weak-Acid Derivatives of Paclitaxel for Remote Loading into Liposomes and Improved Therapeutic Effects. RSC Adv. 2020, 10, 27676–27687. [Google Scholar] [CrossRef]
- Mozafari, M.R. Liposomes: An Overview of Manufacturing Techniques. Cell. Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar]
- Meure, L.A.; Foster, N.R.; Dehghani, F. Conventional and Dense Gas Techniques for the Production of Liposomes: A Review. AAPS PharmSciTech 2008, 9, 798. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Nguyen, T.H.; Nguyen, D.H. Development and In Vitro Evaluation of Liposomes Using Soy Lecithin to Encapsulate Paclitaxel. Int. J. Biomater. 2017, 2017, 8234712. [Google Scholar] [CrossRef]
- Dave, V.; Gupta, A.; Singh, P.; Gupta, C.; Sadhu, V.; Reddy, K.R. Synthesis and Characterization of Celecoxib Loaded PEGylated Liposome Nanoparticles for Biomedical Applications. Nano-Struct. Nano-Objects 2019, 18, 100288. [Google Scholar] [CrossRef]
- Bnyan, R.; Cesarini, L.; Khan, I.; Roberts, M.; Ehtezazi, T. The Effect of Ethanol Evaporation on the Properties of Inkjet Produced Liposomes. DARU J. Pharm. Sci. 2020, 28, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kazemabadi, F.Z.; Heydarinasab, A.; Akbarzadeh, A.; Ardjmand, M. Preparation, Characterization and in Vitro Evaluation of PEGylated Nanoliposomal Containing Etoposide on Lung Cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.; Miladi, K.; Agusti, G.; Elaissari, A.; Fessi, H. Preparation of Liposomes: A Comparative Study between the Double Solvent Displacement and the Conventional Ethanol Injection—From Laboratory Scale to Large Scale. Colloids Surf. A Physicochem. Eng. Asp. 2017, 524, 71–78. [Google Scholar] [CrossRef]
- Gouda, A.; Sakr, O.S.; Nasr, M.; Sammour, O. Ethanol Injection Technique for Liposomes Formulation: An Insight into Development, Influencing Factors, Challenges and Applications. J. Drug Deliv. Sci. Technol. 2020, 61, 102174. [Google Scholar] [CrossRef]
- Salimi, A. Liposomes as a Novel Drug Delivery System: Fundamental and Pharmaceutical Application. Asian J. Pharm. (AJP) 2018, 12, S31–S41. [Google Scholar]
- Barba, A.A.; Bochicchio, S.; Dalmoro, A.; Lamberti, G. Lipid Delivery Systems for Nucleic-Acid-Based-Drugs: From Production to Clinical Applications. Pharmaceutics 2019, 11, 360. [Google Scholar] [CrossRef]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent Advances on Liposomal Nanoparticles: Synthesis, Characterization and Biomedical Applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome Production by Microfluidics: Potential and Limiting Factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef]
- Guimarães Sá Correia, M.; Briuglia, M.L.; Niosi, F.; Lamprou, D.A. Microfluidic Manufacturing of Phospholipid Nanoparticles: Stability, Encapsulation Efficacy, and Drug Release. Int. J. Pharm. 2017, 516, 91–99. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Wang, P.; DeMello, A.; Feng, L.; Zhu, X.; Wen, W.; Kodzius, R.; Gong, X. Synthesis of Biomaterials Utilizing Microfluidic Technology. Genes 2018, 9, 283. [Google Scholar] [CrossRef]
- Marín, D.; Alemán, A.; Sánchez-Faure, A.; Montero, P.; Gómez-Guillén, M.C. Freeze-Dried Phosphatidylcholine Liposomes Encapsulating Various Antioxidant Extracts from Natural Waste as Functional Ingredients in Surimi Gels. Food Chem. 2018, 245, 525–535. [Google Scholar] [CrossRef]
- Hussain, M.T.; Forbes, N.; Perrie, Y.; Malik, K.P.; Duru, C.; Matejtschuk, P. Freeze-Drying Cycle Optimization for the Rapid Preservation of Protein-Loaded Liposomal Formulations. Int. J. Pharm. 2020, 573, 118722. [Google Scholar] [CrossRef]
- Mortazavi, S.M.; Mohammadabadi, M.R.; Khosravi-Darani, K.; Mozafari, M.R. Preparation of Liposomal Gene Therapy Vectors by a Scalable Method without Using Volatile Solvents or Detergents. J. Biotechnol. 2007, 129, 604–613. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. A Versatile Supercritical Assisted Process for the One-Shot Production of Liposomes. J. Supercrit. Fluids 2019, 146, 136–143. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Scognamiglio, M.; Reverchon, E. Control of Liposomes Diameter at Micrometric and Nanometric Level Using a Supercritical Assisted Technique. J. CO2 Util. 2019, 32, 119–127. [Google Scholar] [CrossRef]
- Situ, W.; Song, X.; Luo, S.; Liang, Y. A Nano-Delivery System for Bioactive Ingredients Using Supercritical Carbon Dioxide and Its Release Behaviors. Food Chem. 2017, 228, 219–225. [Google Scholar] [CrossRef]
- Zucker, D.; Marcus, D.; Barenholz, Y.; Goldblum, A. Liposome Drugs’ Loading Efficiency: A Working Model Based on Loading Conditions and Drug’s Physicochemical Properties. J. Control. Release 2009, 139, 73–80. [Google Scholar] [CrossRef]
- Cern, A.; Marcus, D.; Tropsha, A.; Barenholz, Y.; Goldblum, A. New Drug Candidates for Liposomal Delivery Identified by Computer Modeling of Liposomes’ Remote Loading and Leakage. J. Control. Release 2017, 252, 18–27. [Google Scholar] [CrossRef]
- Zhou, S.; Li, J.; Yu, J.; Yang, L.; Kuang, X.; Wang, Z.; Wang, Y.; Liu, H.; Lin, G.; He, Z.; et al. A Facile and Universal Method to Achieve Liposomal Remote Loading of Non-Ionizable Drugs with Outstanding Safety Profiles and Therapeutic Effect. Acta Pharm. Sin. B 2020, 11, 258–270. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel Methods for Liposome Preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Lukowski, J.K.; Weaver, E.M.; Hummon, A.B. Analyzing Liposomal Drug Delivery Systems in Three-Dimensional Cell Culture Models Using MALDI Imaging Mass Spectrometry. Anal. Chem. 2017, 89, 8453–8458. [Google Scholar] [CrossRef] [PubMed]
- Peretz Damari, S.; Shamrakov, D.; Varenik, M.; Koren, E.; Nativ-Roth, E.; Barenholz, Y.; Regev, O. Practical Aspects in Size and Morphology Characterization of Drug-Loaded Nano-Liposomes. Int. J. Pharm. 2018, 547, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.M.; Felício, M.R.; Santos, N.C.; Gonçalves, S.; Domingues, M.M. Application of Light Scattering Techniques to Nanoparticle Characterization and Development. Front. Chem. 2018, 6, 237. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Bodycomb, J.; Travers, B.; Tatarkiewicz, K.; Travers, S.; Matyas, G.R.; Beck, Z. Particle Size Analyses of Polydisperse Liposome Formulations with a Novel Multispectral Advanced Nanoparticle Tracking Technology. Int. J. Pharm. 2019, 566, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Fehér, B.; Kitka, D.; Wacha, A.; Bóta, A.; Berényi, S.; Pipich, V.; Fraikin, J.-L. Size Measurement of Extracellular Vesicles and Synthetic Liposomes: The Impact of the Hydration Shell and the Protein Corona. Colloids Surf. B Biointerfaces 2020, 192, 111053. [Google Scholar] [CrossRef] [PubMed]
- Chibowski, E.; Szcześ, A. Zeta Potential and Surface Charge of DPPC and DOPC Liposomes in the Presence of PLC Enzyme. Adsorption 2016, 22, 755–765. [Google Scholar] [CrossRef]
- Guimarães, D.; Noro, J.; Loureiro, A.; Cavaco-Paulo, A.; Nogueira, E. Quantification of Drugs Encapsulated in Liposomes by 1H NMR. Colloids Surf. B Biointerfaces 2019, 179, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Susa, F.; Bucca, G.; Limongi, T.; Cauda, V.; Pisano, R. Enhancing the Preservation of Liposomes: The Role of Cryoprotectants, Lipid Formulations and Freezing Approaches. Cryobiology 2021, 98, 46–56. [Google Scholar] [CrossRef]
- Geng, S.; Yang, B.; Wang, G.; Qin, G.; Wada, S.; Wang, J.-Y. Two Cholesterol Derivative-Based PEGylated Liposomes as Drug Delivery System, Study on Pharmacokinetics and Drug Delivery to Retina. Nanotechnology 2014, 25, 275103. [Google Scholar] [CrossRef]
- Yu, J.Y.; Chuesiang, P.; Shin, G.H.; Park, H.J. Post-Processing Techniques for the Improvement of Liposome Stability. Pharmaceutics 2021, 13, 1023. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Yang, S.-C.; Liu, X.-L.; Gao, Y.; Dong, X.; Lai, X.; Zhu, M.-H.; Feng, H.-Y.; Zhu, X.-D.; Lu, Q.; et al. Nanobowl-Supported Liposomes Improve Drug Loading and Delivery. Nano Lett. 2020, 20, 4177–4187. [Google Scholar] [CrossRef]
- Roces, C.B.; Port, E.C.; Daskalakis, N.N.; Watts, J.A.; Aylott, J.W.; Halbert, G.W.; Perrie, Y. Rapid Scale-up and Production of Active-Loaded PEGylated Liposomes. Int. J. Pharm. 2020, 586, 119566. [Google Scholar] [CrossRef]
- Gkionis, L.; Campbell, R.A.; Aojula, H.; Harris, L.K.; Tirella, A. Manufacturing Drug Co-Loaded Liposomal Formulations Targeting Breast Cancer: Influence of Preparative Method on Liposomes Characteristics and in Vitro Toxicity. Int. J. Pharm. 2020, 590, 119926. [Google Scholar] [CrossRef]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. PH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [Google Scholar] [CrossRef]
- Kang, J.H.; Jang, W.Y.; Ko, Y.T. The Effect of Surface Charges on the Cellular Uptake of Liposomes Investigated by Live Cell Imaging. Pharm. Res. 2017, 34, 704–717. [Google Scholar] [CrossRef]
- Kolašinac, R.; Kleusch, C.; Braun, T.; Merkel, R.; Csiszár, A. Deciphering the Functional Composition of Fusogenic Liposomes. Int. J. Mol. Sci. 2018, 19, 346. [Google Scholar] [CrossRef]
- Islam Shishir, M.R.; Karim, N.; Gowd, V.; Zheng, X.; Chen, W. Liposomal Delivery of Natural Product: A Promising Approach in Health Research. Trends Food Sci. Technol. 2019, 85, 177–200. [Google Scholar] [CrossRef]
- Hussain, A.; Singh, S.; Sharma, D.; Webster, T.J.; Shafaat, K.; Faruk, A. Elastic Liposomes as Novel Carriers: Recent Advances in Drug Delivery. Int. J. Nanomed. 2017, 12, 5087–5108. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Zamboni, W.C. Concept and Clinical Evaluation of Carrier-Mediated Anticancer Agents. Oncologist 2008, 13, 248–260. [Google Scholar] [CrossRef]
- Fanciullino, R.; Ciccolini, J. Liposome-Encapsulated Anticancer Drugs: Still Waiting for the Magic Bullet? Curr. Med. Chem. 2009, 16, 4361–4373. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-J.; Ju, R.-J.; Zeng, F.; Qi, X.-R.; Lu, W.-L. Liposomes in Drug Delivery: Status and Advances. In Liposome-Based Drug Delivery Systems; Lu, W.-L., Qi, X.-R., Eds.; Biomaterial Engineering; Springer: Berlin/Heidelberg, Germany, 2021; pp. 3–24. ISBN 978-3-662-49320-5. [Google Scholar]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.-C. Immunological and Toxicological Considerations for the Design of Liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef] [Green Version]
- Gbian, D.L.; Omri, A. Current and Novel Therapeutic Strategies for the Management of Cystic Fibrosis. Expert Opin. Drug Deliv. 2021, 18, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Neun, B.W.; Barenholz, Y.; Szebeni, J.; Dobrovolskaia, M.A. Understanding the Role of Anti-PEG Antibodies in the Complement Activation by Doxil in Vitro. Molecules 2018, 23, 1700. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.A.; Pytlik, R.; Kozak, T.; Chhanabhai, M.; Gascoyne, R.; Lu, B.; Deitcher, S.R.; Winter, J.N. Vincristine Sulfate Liposomes Injection (Marqibo) in Heavily Pretreated Patients with Refractory Aggressive Non-Hodgkin Lymphoma. Cancer 2009, 115, 3475–3482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Leifer, F.; Rose, S.; Chun, D.Y.; Thaisz, J.; Herr, T.; Nashed, M.; Joseph, J.; Perkins, W.R.; DiPetrillo, K. Amikacin Liposome Inhalation Suspension (ALIS) Penetrates Non-Tuberculous Mycobacterial Biofilms and Enhances Amikacin Uptake Into Macrophages. Front. Microbiol. 2018, 9, 915. [Google Scholar] [CrossRef]
- Cipolla, D.; Blanchard, J.; Gonda, I. Development of Liposomal Ciprofloxacin to Treat Lung Infections. Pharmaceutics 2016, 8, 6. [Google Scholar] [CrossRef]
- Clancy, J.P.; Dupont, L.; Konstan, M.W.; Billings, J.; Fustik, S.; Goss, C.H.; Lymp, J.; Minic, P.; Quittner, A.L.; Rubenstein, R.C.; et al. Phase II Studies of Nebulised Arikace in CF Patients with Pseudomonas aeruginosa Infection. Thorax 2013, 68, 818–825. [Google Scholar] [CrossRef]
- Heirali, A.A.; Workentine, M.L.; Acosta, N.; Poonja, A.; Storey, D.G.; Somayaji, R.; Rabin, H.R.; Whelan, F.J.; Surette, M.G.; Parkins, M.D. The Effects of Inhaled Aztreonam on the Cystic Fibrosis Lung Microbiome. Microbiome 2017, 5, 51. [Google Scholar] [CrossRef]
- Aygun, F.; Aygun, F.D.; Varol, F.; Durak, C.; Cokugraş, H.; Camcioglu, Y.; Cam, H. Can Nebulised Colistin Therapy Improve Outcomes in Critically Ill Children with Multi-Drug Resistant Gram-Negative Bacterial Pneumonia? Antibiotics 2019, 8, 40. [Google Scholar] [CrossRef]
- Laurent, A.; Pantet, O.; Laurent, L.; Hirt-Burri, N.; de Buys Roessingh, A.; Raffoul, W.; Laurent, P.; Monod, M.; Applegate, L.A. Potency and Stability of Liposomal Amphotericin B Formulated for Topical Management of Aspergillus Spp. Infections in Burn Patients. Burn. Open 2020, 4, 110–116. [Google Scholar] [CrossRef]
- He, H.; Yuan, D.; Wu, Y.; Cao, Y. Pharmacokinetics and Pharmacodynamics Modeling and Simulation Systems to Support the Development and Regulation of Liposomal Drugs. Pharmaceutics 2019, 11, 110. [Google Scholar] [CrossRef] [Green Version]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An MRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Sonju, J.J.; Dahal, A.; Singh, S.S.; Jois, S.D. Peptide-Functionalized Liposomes as Therapeutic and Diagnostic Tools for Cancer Treatment. J. Control. Release 2021, 329, 624–644. [Google Scholar] [CrossRef]
- Song, Y.; Li, W.; Meng, S.; Zhou, W.; Su, B.; Tang, L.; Zhao, Y.; Wu, X.; Yin, D.; Fan, M.; et al. Dual Integrin Avβ 3 and NRP-1-Targeting Paramagnetic Liposome for Tumor Early Detection in Magnetic Resonance Imaging. Nanoscale Res. Lett. 2018, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Huang, Z.; Youngquist, B.M.; Scott, J.W.; Niu, A.; Bojanowski, C.M.; Zwezdaryk, K.J.; Saba, N.S.; Fan, J.; Yin, X.-M.; et al. Liposome-Mediated Detection of SARS-CoV-2 RNA-Positive Extracellular Vesicles in Plasma. Nat. Nanotechnol. 2021, 16, 1039–1044. [Google Scholar] [CrossRef]
- Vieira, D.B.; Gamarra, L.F. Getting into the Brain: Liposome-Based Strategies for Effective Drug Delivery across the Blood–Brain Barrier. Int. J. Nanomed. 2016, 11, 5381–5414. [Google Scholar] [CrossRef]
- Kim, J.H.; Marks, F.; Clemens, J.D. Looking beyond COVID-19 Vaccine Phase 3 Trials. Nat. Med. 2021, 27, 205–211. [Google Scholar] [CrossRef]
- Malayala, S.V.; Mohan, G.; Vasireddy, D.; Atluri, P. Purpuric Rash and Thrombocytopenia After the MRNA-1273 (Moderna) COVID-19 Vaccine. Cureus 2021, 13, e14099. [Google Scholar] [CrossRef]
- López-Cano, J.J.; González-Cela-Casamayor, M.A.; Andrés-Guerrero, V.; Herrero-Vanrell, R.; Molina-Martínez, I.T. Liposomes as Vehicles for Topical Ophthalmic Drug Delivery and Ocular Surface Protection. Expert Opin. Drug Deliv. 2021, 18, 819–847. [Google Scholar] [CrossRef]
- Wang, C.-H.; Hsieh, Y.-H.; Powers, Z.M.; Kao, C.-Y. Defeating Antibiotic-Resistant Bacteria: Exploring Alternative Therapies for a Post-Antibiotic Era. Int. J. Mol. Sci. 2020, 21, 1061. [Google Scholar] [CrossRef] [Green Version]
- de Morais Ribeiro, L.N.; de Paula, E.; Rossi, D.A.; Monteiro, G.P.; Júnior, E.C.V.; Silva, R.R.; Franco, R.R.; Espíndola, F.S.; Goulart, L.R.; Fonseca, B.B. Hybrid Pectin-Liposome Formulation against Multi-Resistant Bacterial Strains. Pharmaceutics 2020, 12, 769. [Google Scholar] [CrossRef]
- Rukavina, Z.; Šegvić Klarić, M.; Filipović-Grčić, J.; Lovrić, J.; Vanić, Ž. Azithromycin-Loaded Liposomes for Enhanced Topical Treatment of Methicillin-Resistant Staphyloccocus aureus (MRSA) Infections. Int. J. Pharm. 2018, 553, 109–119. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Russo, A.; Peghin, M. Inhaled Liposomal Antimicrobial Delivery in Lung Infections. Drugs 2020, 80, 1309–1318. [Google Scholar] [CrossRef]


| Methods | Advantages | Disadvantages |
|---|---|---|
| DRV/thin film hydration | Simple, high EE for lipophilic drugs | Use of organic solvent, low EE for hydrophilic drugs, MLV formed, heterogeneity, difficulty scaling up, size reduction required |
| RPE | Simple, high EE for hydrophilic drugs | Challenge removing organic solvent, LUV and MLV formed, low EE for lipophilic drugs, not suitable for fragile molecules, size reduction required |
| Injection | Simple, SUV formed in one step, good reproducibility | Heterogeneity, low EE, possible degradation of active drug, difficulty removing ethanol, time consuming |
| Emulsion | High EE, control over liposomes size | Use of organic solvent, LUV formed, multiple steps |
| Detergent removal | No organic solvent used, proteins encapsulation, size uniformity, good reproducibility | LUV formed, poor EE of hydrophobic drugs, detergent residues, time consuming |
| Sonication | Easy, formation of SUVs | Possible degradation of phospholipids, possible metallic pollution when using probe |
| Extrusion | Simple, size uniformity, SUV formation, good reproducibility | Use of high pressure, possible clogging of membrane, product loss, laborious, time consuming |
| Microfluidization | SUV formation in one step, continuous production, size uniformity, good EE [29] | Use of organic solvent, requires specific setup, high energy and pressure used, difficult large scale production, costly [30] |
| Freeze-drying | Storage stability, sterile liposomes, extended shelf-life | Applications might be limited when carbohydrates are used as cryo-protectants, low EE, potential damage to bilayer and size increase of liposomes due to membrane fusion [31,40] |
| Heating | No organic solvent used, sterile product [17], scalable | High temperature makes continuous manufacturing impractical, low EE, LUV formed, size reduction required |
| SuperLip | SUV formation in one step, continuous production, superior high EE, size uniformity, no organic solvent | Use of high pressure, more complex |
| Active encapsulation | High EE, stable retention of drug [39] | Only applies to amphipathic weak bases/acids, complex synthesis of some derivatives [16] |
| Product | Drug | Target Treatment | References |
|---|---|---|---|
| Doxil® | Doxorubicin | Breast and ovarian cancer | [7,65] |
| Marqibo® | Vincristine sulfate liposomes | Hodgkin’s lymphoma and leukemia | [66] |
| Arikayce® | Amikacin | MAC and PA infections | [67] |
| Lipoquin® | Ciprofloxacin | MAC infections | [68] |
| Pulmaquin® | Ciprofloxacin | PA lung infections | [68] |
| TOBI® | Tobramycin | PA infections | [69] |
| Cayston® | Aztreonam | Gram negative infections | [70] |
| Colobreathe® | Colistin | Gram negative infections | [71] |
| Ambisome® | Amphotericin B | Fungal infections | [72,73] |
| Exparel® | Bupivacaine | Pain management | [7] |
| Inflexal® | Inactivated hemagglutinin of influenza virus strains A and B | Influenza | [7] |
| Epaxal® | Formalin inactivated hepatitis A virus, strain RG-SB | Hepatitis A | [73] |
| Moderna COVID-19 | mRNA 1273 | Covid-19 | [74,75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gbian, D.L.; Omri, A. Lipid-Based Drug Delivery Systems for Diseases Managements. Biomedicines 2022, 10, 2137. https://doi.org/10.3390/biomedicines10092137
Gbian DL, Omri A. Lipid-Based Drug Delivery Systems for Diseases Managements. Biomedicines. 2022; 10(9):2137. https://doi.org/10.3390/biomedicines10092137
Chicago/Turabian StyleGbian, Douweh Leyla, and Abdelwahab Omri. 2022. "Lipid-Based Drug Delivery Systems for Diseases Managements" Biomedicines 10, no. 9: 2137. https://doi.org/10.3390/biomedicines10092137
APA StyleGbian, D. L., & Omri, A. (2022). Lipid-Based Drug Delivery Systems for Diseases Managements. Biomedicines, 10(9), 2137. https://doi.org/10.3390/biomedicines10092137









