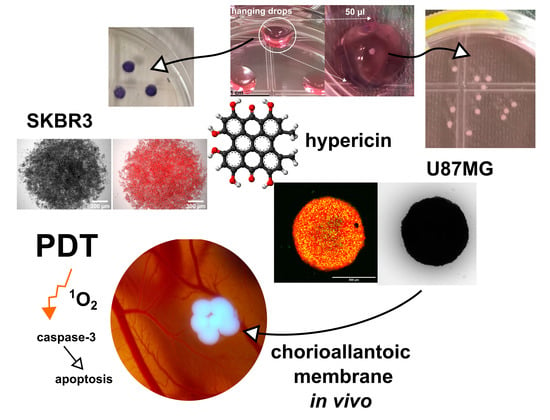
Spheroidal Model of SKBR3 and U87MG Cancer Cells for Live Imaging of Caspase-3 during Apoptosis Induced by Singlet Oxygen in Photodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Spheroids Preparation
2.2. Photodynamic Treatment of the Spheroids
2.3. Imaging of Spheroids
2.4. Assessment of Phototoxicity in 3D Spheroids
2.5. Flow Cytometric Analysis of Apoptosis and Caspase-3 Level
2.6. Singlet Oxygen Phosphorescence and Production Measurements
2.7. Ex Ovo CAM Model with Spheroids Preparation
2.8. PCR Analysis of CAM with Spheroids
3. Results
3.1. Assesment of 1O2, Caspase-3, and Apoptosis in 3D Model of SKBR3 Cells as Compact Aggregates
3.2. Assesment of 1O2 Production, Elevation of Caspase-3 and Apoptosis in Spontaneously Formed 3D Model of U87MG Cells
3.3. Assesment of Caspase-3 and LDH Level in U87MG Spheroids-Grown by Hanging Drop Method
3.4. Applicability of Large U87MG Spheroids for In Vivo Photodiagnostic Applications on Quail CAM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part Two—Cellular Signaling, Cell Metabolism and Modes of Cell Death. Photodiagn. Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Skovsen, E.; Snyder, J.W.; Lambert, J.D.C.; Ogilby, P.R. Lifetime and Diffusion of Singlet Oxygen in a Cell. J. Phys. Chem. B 2005, 109, 8570–8573. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Ouedraogo, G.D.; Kochevar, I.E. Downregulation of Epidermal Growth Factor Receptor Signaling by Singlet Oxygen through Activation of Caspase-3 and Protein Phosphatases. Oncogene 2003, 22, 4413–4424. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Demirs, J.T.; Kochevar, I.E. P38 Mitogen-Activated Protein Kinase Mediates Bid Cleavage, Mitochondrial Dysfunction, and Caspase-3 Activation during Apoptosis Induced by Singlet Oxygen but Not by Hydrogen Peroxide. J. Biol. Chem. 2000, 275, 25939–25948. [Google Scholar] [CrossRef] [PubMed]
- Huntošová, V.; Datta, S.; Lenkavská, L.; MáčAjová, M.; Bilčík, B.; Kundeková, B.; Čavarga, I.; Kronek, J.; Jutková, A.; Miškovský, P.; et al. Alkyl Chain Length in Poly(2-Oxazoline)-Based Amphiphilic Gradient Copolymers Regulates the Delivery of Hydrophobic Molecules: A Case of the Biodistribution and the Photodynamic Activity of the Photosensitizer Hypericin. Biomacromolecules 2021, 22, 4199–4216. [Google Scholar] [CrossRef] [PubMed]
- Lenkavska, L.; Blascakova, L.; Jurasekova, Z.; Macajova, M.; Bilcik, B.; Cavarga, I.; Miskovsky, P.; Huntosova, V. Benefits of Hypericin Transport and Delivery by Low- and High-Density Lipoproteins to Cancer Cells: From In Vitro to Ex Ovo. Photodiagn. Photodyn. Ther. 2019, 25, 214–224. [Google Scholar] [CrossRef]
- Bánó, G.; Staničová, J.; Jancura, D.; Marek, J.; Bánó, M.; Uličný, J.; Strejčková, A.; Miškovský, P. On the Diffusion of Hypericin in Dimethylsulfoxide/Water Mixtures-the Effect of Aggregation. J. Phys. Chem. B 2011, 115, 2417–2423. [Google Scholar] [CrossRef]
- Huntosova, V.; Nadova, Z.; Dzurova, L.; Jakusova, V.; Sureau, F.; Miskovsky, P. Cell Death Response of U87 Glioma Cells on Hypericin Photoactivation Is Mediated by Dynamics of Hypericin Subcellular Distribution and Its Aggregation in Cellular Organelles. Photochem. Photobiol. Sci. 2012, 11, 1428–1436. [Google Scholar] [CrossRef]
- Varchola, J.; Želonková, K.; Chorvat, D.; Jancura, D.; Miskovsky, P.; Bánó, G. Singlet Oxygen Produced by Quasi-Continuous Photo-Excitation of Hypericin in Dimethyl-Sulfoxide. J. Lumin. 2016, 177, 17–21. [Google Scholar] [CrossRef]
- Buytaert, E.; Matroule, J.Y.; Durinck, S.; Close, P.; Kocanova, S.; Vandenheede, J.R.; De Witte, P.A.; Piette, J.; Agostinis, P. Molecular Effectors and Modulators of Hypericin-Mediated Cell Death in Bladder Cancer Cells. Oncogene 2008, 27, 1916–1929. [Google Scholar] [CrossRef] [Green Version]
- Mike, J.; Koval’, J.; Jendelovský, R.; Saková, V.; Uhrinová, I.; Kello, M.; Kuliková, L.; Fedoroĉko, P. The Role of P53 in the Efficiency of Photodynamic Therapy with Hypericin and Subsequent Long-Term Survival of Colon Cancer Cells. Photochem. Photobiol. Sci. 2009, 8, 1558–1567. [Google Scholar] [CrossRef]
- Pevna, V.; Wagnières, G.; Huntosova, V. Autophagy and Apoptosis Induced in U87 Mg Glioblastoma Cells by Hypericin-Mediated Photodynamic Therapy Can Be Photobiomodulated with 808 Nm Light. Biomedicines 2021, 9, 1703. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D.; Zhuang, Z.; Jin, K.; Zheng, L.; Yang, Q.; Guo, K. Hypericin-Mediated Photodynamic Therapy Induces Apoptosis in K562 Human Leukemia Cells through JNK Pathway Modulation. Mol. Med. Rep. 2015, 12, 6475–6482. [Google Scholar] [CrossRef]
- Helm, K.; Beyreis, M.; Mayr, C.; Ritter, M.; Jakab, M.; Kiesslich, T.; Plaetzer, K. In Vitro Cell Death Discrimination and Screening Method by Simple and Cost-Effective Viability Analysis. Cell. Physiol. Biochem. 2017, 41, 1011–1019. [Google Scholar] [CrossRef]
- Vasyutin, I.; Zerihun, L.; Ivan, C.; Atala, A. Bladder Organoids and Spheroids: Potential Tools for Normal and Diseased Tissue Modelling. Anticancer Res. 2019, 39, 1105–1118. [Google Scholar] [CrossRef]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in Vitro Model of Human Development and Disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef]
- Hempfling, L.; Adamus, A.; Wagner, B.R.; Engel, N.; Seitz, G. A New Valid Rhabdomyosarcoma Spheroid Culture Model for in Vitro Evaluation of Hypericin-Based Photodynamic Therapy. Pediatr. Blood Cancer 2021, 69, e29482. [Google Scholar] [CrossRef]
- Froehlich, K.; Haeger, J.D.; Heger, J.; Pastuschek, J.; Photini, S.M.; Yan, Y.; Lupp, A.; Pfarrer, C.; Mrowka, R.; Schleußner, E.; et al. Generation of Multicellular Breast Cancer Tumor Spheroids: Comparison of Different Protocols. J. Mammary Gland Biol. Neoplasia 2016, 21, 89–98. [Google Scholar] [CrossRef]
- Huygens, A.; Kamuhabwa, A.R.; Roskams, T.; Van Cleynenbreugel, B.; Van Poppel, H.; De Witte, P.A.M. Permeation of Hypericin in Spheroids Composed of Different Grade Transitional Cell Carcinoma Cell Lines and Normal Human Urothelial Cells. J. Urol. 2005, 174, 69–72. [Google Scholar] [CrossRef]
- Huygens, A.; Crnolatac, I.; Develter, J.; Van Cleynenbreugel, B.; Van der Kwast, T.; de Witte, P.A.M. Differential Accumulation of Hypericin in Spheroids Composed of T-24 Transitional Cell Carcinoma Cells Expressing Different Levels of E-Cadherin. J. Urol. 2008, 179, 2014–2019. [Google Scholar] [CrossRef]
- Aguilar Cosme, J.R.; Gagui, D.C.; Bryant, H.E.; Claeyssens, F. Morphological Response in Cancer Spheroids for Screening Photodynamic Therapy Parameters. Front. Mol. Biosci. 2021, 8, 784962. [Google Scholar] [CrossRef]
- Dubessy, C.; Merlin, J.L.; Marchal, C.; Guillemin, F. Spheroids in Radiobiology and Photodynamic Therapy. Crit. Rev. Oncol. Hematol. 2000, 36, 179–192. [Google Scholar] [CrossRef]
- Cogno, I.S.; Gilardi, P.; Comini, L.; Núñez-Montoya, S.C.; Cabrera, J.L.; Rivarola, V.A. Natural Photosensitizers in Photodynamic Therapy: In Vitro Activity against Monolayers and Spheroids of Human Colorectal Adenocarcinoma SW480 Cells. Photodiagn. Photodyn. Ther. 2020, 31, 101852. [Google Scholar] [CrossRef]
- Pevna, V.; Horvath, D.; Wagnieres, G.; Huntosova, V. Photobiomodulation and Photodynamic Therapy-Induced Switching of Autophagy and Apoptosis in Human Dermal Fibroblasts. J. Photochem. Photobiol. B 2022, 234, 112539. [Google Scholar] [CrossRef]
- Petrenčáková, M.; Filandr, F.; Hovan, A.; Yassaghi, G.; Man, P.; Kožár, T.; Schwer, M.-S.; Jancura, D.; Plückthun, A.; Novák, P.; et al. Photoinduced Damage of AsLOV2 Domain Is Accompanied by Increased Singlet Oxygen Production Due to Flavin Dissociation. Sci. Rep. 2020, 10, 4119. [Google Scholar] [CrossRef]
- Cheng, G.; Tse, J.; Jain, R.K.; Munn, L.L. Micro-Environmental Mechanical Stress Controls Tumor Spheroid Size and Morphology by Suppressing Proliferation and Inducing Apoptosis in Cancer Cells. PLoS ONE 2009, 4, e4632. [Google Scholar] [CrossRef]
- Sirenko, O.; Mitlo, T.; Hesley, J.; Luke, S.; Owens, W.; Cromwell, E.F. High-Content Assays for Characterizing the Viability and Morphology of 3D Cancer Spheroid Cultures. Assay Drug Dev. Technol. 2015, 13, 402–414. [Google Scholar] [CrossRef]
- Mirab, F.; Kang, Y.J.; Majd, S. Preparation and Characterization of Size-Controlled Glioma Spheroids Using Agarose Hydrogel Microwells. PLoS ONE 2019, 14, e0211078. [Google Scholar] [CrossRef]
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a Type of Three-Dimensional Cell Cultures—Examples of Methods of Preparation and the Most Important Application. Int. J. Mol. Sci. 2020, 21, 6225. [Google Scholar] [CrossRef]
- Bassler, M.C.; Rammler, T.; Wackenhut, F.; Oven-Krockhaus, S.Z.; Secic, I.; Ritz, R.; Meixner, A.J.; Marc, B. Accumulation and Penetration Behavior of Hypericin in Glioma Tumor Spheroids Studied by Fluorescence Microscopy and Confocal Fluorescence Lifetime Imaging Microscopy. Anal. Bioanal. Chem. 2022, 414, 4849–4860. [Google Scholar] [CrossRef]
- Foglietta, F.; Canaparo, R.; Cossari, S.; Panzanelli, P.; Dosio, F.; Serpe, L. Ultrasound Triggers Hypericin Activation Leading to Multifaceted Anticancer Activity. Pharmaceutics 2022, 14, 1102. [Google Scholar] [CrossRef] [PubMed]
- Vandepitte, J.; Roelants, M.; Van Cleynenbreugel, B.; Hettinger, K.; Lerut, E.; Van Poppel, H.; de Witte, P.A.M. Biodistribution and Photodynamic Effects of Polyvinylpyrrolidone-Hypericin Using Multicellular Spheroids Composed of Normal Human Urothelial and T24 Transitional Cell Carcinoma Cells. J. Biomed. Opt. 2011, 16, 018001. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, J.M. Reactive Oxygen Species in Photodynamic Therapy: Mechanisms of Their Generation and Potentiation. Adv. Inorg. Chem. 2017, 70, 343–394. [Google Scholar] [CrossRef]
- Hadjur, C.; Richard, M.J.; Parat, M.O.; Jardon, P.; Favier, A. Photodynamic Effects of Hypericin on Lipid Peroxidation and Antioxidant Status in Melanoma Cells. Photochem. Photobiol. 1996, 64, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.J.; Dong, J.F.; Zhang, X.B. Signal Interaction between Nitric Oxide and Hydrogen Peroxide in Heat Shock-Induced Hypericin Production of Hypericum Perforatum Suspension Cells. Sci. China Ser. C Life Sci. 2008, 51, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Diwu, Z.; William Lown, J. Photosensitization with Anticancer Agents EPR Studies of Photodynamic Action of Hypericin: Formation of Semiquinone Radical and Activated Oxygen Species on Illumination. Free Radic. Biol. Med. 1993, 14, 209–215. [Google Scholar] [CrossRef]
- Thomas, C.; MacGill, R.S.; Miller, G.C.; Pardini, R.S. Photoactivation of Hypericin Generates Singlet Oxygen in Mitochondria and Inhibits Succinoxidase. Photochem. Photobiol. 1992, 55, 47–53. [Google Scholar] [CrossRef]
- Gbur, P.; Dedic, R.; Chorvat, D.; Miskovsky, P.; Hala, J.; Jancura, D. Time-Resolved Luminescence and Singlet Oxygen Formation after Illumination of the Hypericin-Low-Density Lipoprotein Complex. Photochem. Photobiol. 2009, 85, 816–823. [Google Scholar] [CrossRef]
- Liu, H.; Carter, P.J.H.; Laan, A.C.; Eelkema, R.; Denkova, A.G. Singlet Oxygen Sensor Green Is Not a Suitable Probe for 1O2 in the Presence of Ionizing Radiation. Sci. Rep. 2019, 9, 8393. [Google Scholar] [CrossRef]
- Gollmer, A.; Arnbjerg, J.; Blaikie, F.H.; Pedersen, B.W.; Breitenbach, T.; Daasbjerg, K.; Glasius, M.; Ogilby, P.R. Singlet Oxygen Sensor Green®: Photochemical Behavior in Solution and in a Mammalian Cell. Photochem. Photobiol. 2011, 87, 671–679. [Google Scholar] [CrossRef]
- Majerník, M.; Jendželovský, R.; Babinčák, M.; Košuth, J.; Ševc, J.; Gombalová, Z.T.; Jendželovská, Z.; Buríková, M.; Fedoročko, P. Novel Insights into the Effect of Hyperforin and Photodynamic Therapy with Hypericin on Chosen Angiogenic Factors in Colorectal Micro-Tumors Created on Chorioallantoic Membrane. Int. J. Mol. Sci. 2019, 20, 3004. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Van Beijnum, J.R.; Van Berkel, M.; Van Den Bergh, H.; Griffioen, A.W. Vascular Regrowth Following Photodynamic Therapy in the Chicken Embryo Chorioallantoic Membrane. Angiogenesis 2010, 13, 281–292. [Google Scholar] [CrossRef]
- Bhuvaneswari, R.; Gan, Y.Y.Y.; Yee, K.K.L.; Soo, K.C.; Olivo, M. Effect of Hypericin-Mediated Photodynamic Therapy on the Expression of Vascular Endothelial Growth Factor in Human Nasopharyngeal Carcinoma. Int. J. Mol. Med. 2007, 20, 421–428. [Google Scholar] [CrossRef]
- Koon, H.K.; Lo, K.W.; Leung, K.N.; Lung, M.L.; Chang, C.C.K.; Wong, R.N.S.; Leung, W.N.; Mak, N.K. Photodynamic Therapy-Mediated Modulation of Inflammatory Cytokine Production by Epstein-Barr Virus-Infected Nasopharyngeal Carcinoma Cells. Cell. Mol. Immunol. 2010, 7, 323–326. [Google Scholar] [CrossRef]
- Falk-Mahapatra, R.; Gollnick, S.O. Photodynamic Therapy and Immunity: An Update. Photochem. Photobiol. 2020, 96, 550–559. [Google Scholar] [CrossRef] [Green Version]











| Gene | Primers (5′-3′) | Tm (°C) |
|---|---|---|
| VEGF-A–for1 | CGG AAG CCC AAT GAA GTT ATC | 59.4 |
| VEGF-A–rev1 | GCA CAT CTC ATC AGA GGC ACA | 64.0 |
| Quek1-for1 | CAT CAA TGC GAA TCA TAC AGT TAA G | 60.9 |
| Quek1–rev1 | CAT TCA CAA GCA GGG TGA ATG | 59.4 |
| IL-8–for1 | CTG AGG TGC CAG TGC ATT AG | 63.5 |
| IL-8–rev1 | AGC ACA CCT CTC TTC CAT CC | 63.3 |
| TLR-7–for1 | AGA TGT TTT CTG GGC AGA CG | 60.0 |
| TLR-7–rev1 | AAT GAC TTC AAC CGG TTA CTG G | 60.0 |
| IFN-α–for1 | CCT TGC TCC TTC AAC GAC A | 64.1 |
| IFN-α–rev1 | CGC TGA GGA TAC TGA AGA GGT | 62.3 |
| GAPDH–for1 | GAA CGC CAT CAC TAT CTT CCA G | 62.1 |
| GAPDH–rev1 | GGG CTG AGA TGA TAA CAC GC | 60.5 |
| Protocol | Hanging Drop Method | Spontaneous Formation |
|---|---|---|
| untreated control | 860 ± 100 µm | 150 ± 30 µm |
| hypericin | 780 ± 50 µm | 150 ± 30 µm |
| +2 J/cm2 | 740 ± 40 µm | 120 ± 20 µm |
| +4 J/cm2 | 670 ± 70 µm | 120 ± 30 µm |
| +6 J/cm2 | 600 ± 80 µm | 110 ± 40 µm |
| +10 J/cm2 | 540 ± 50 µm | 100 ± 20 µm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pevná, V.; Máčajová, M.; Hovan, A.; Bánó, G.; Meta, M.; Bilčík, B.; Palková, J.; Huntošová, V. Spheroidal Model of SKBR3 and U87MG Cancer Cells for Live Imaging of Caspase-3 during Apoptosis Induced by Singlet Oxygen in Photodynamic Therapy. Biomedicines 2022, 10, 2141. https://doi.org/10.3390/biomedicines10092141
Pevná V, Máčajová M, Hovan A, Bánó G, Meta M, Bilčík B, Palková J, Huntošová V. Spheroidal Model of SKBR3 and U87MG Cancer Cells for Live Imaging of Caspase-3 during Apoptosis Induced by Singlet Oxygen in Photodynamic Therapy. Biomedicines. 2022; 10(9):2141. https://doi.org/10.3390/biomedicines10092141
Chicago/Turabian StylePevná, Viktória, Mariana Máčajová, Andrej Hovan, Gregor Bánó, Majlinda Meta, Boris Bilčík, Júlia Palková, and Veronika Huntošová. 2022. "Spheroidal Model of SKBR3 and U87MG Cancer Cells for Live Imaging of Caspase-3 during Apoptosis Induced by Singlet Oxygen in Photodynamic Therapy" Biomedicines 10, no. 9: 2141. https://doi.org/10.3390/biomedicines10092141