Diagnosis of Hymenoptera Venom Allergy: State of the Art, Challenges, and Perspectives
Abstract
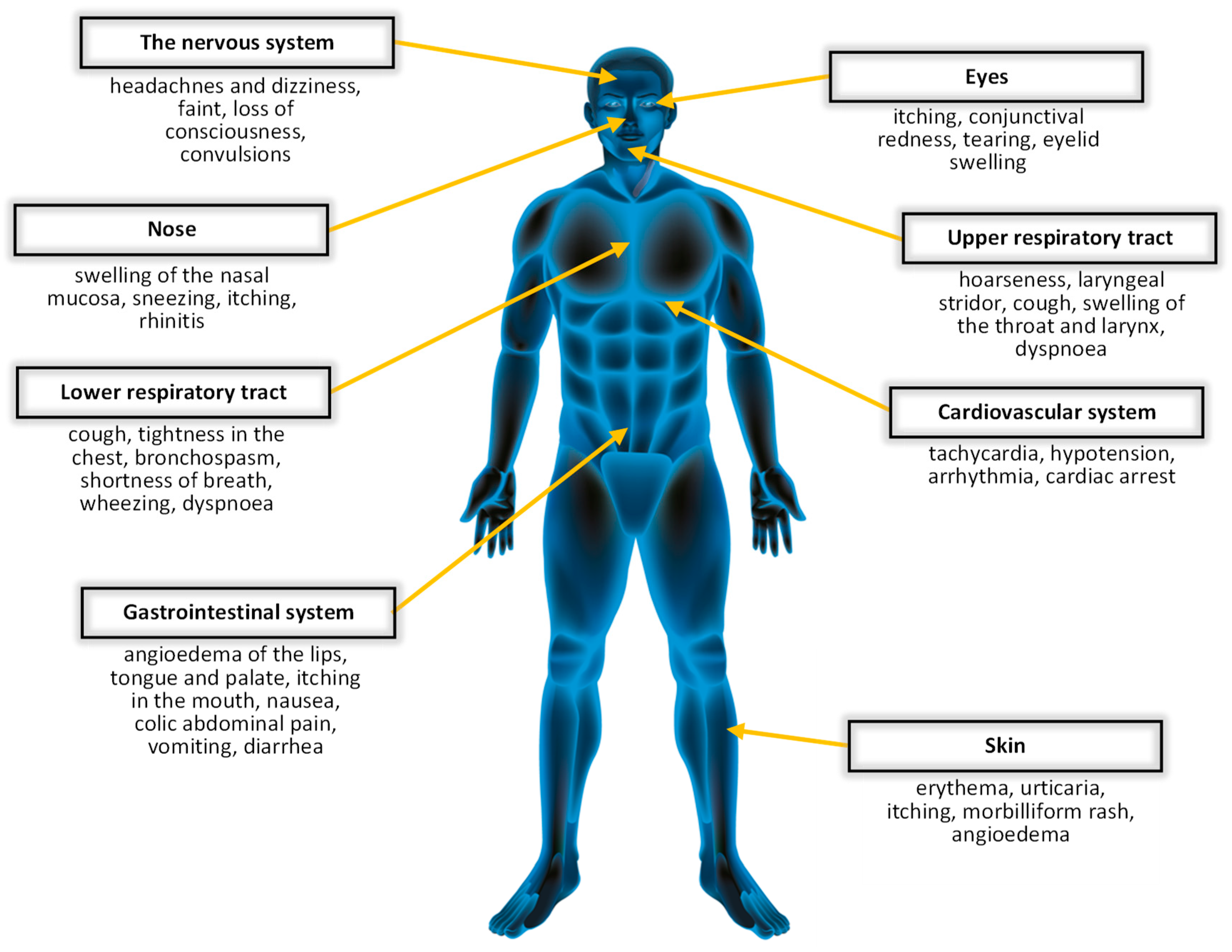
:1. Introduction
1.1. Hymenoptera
1.2. Hymenoptera Venoms
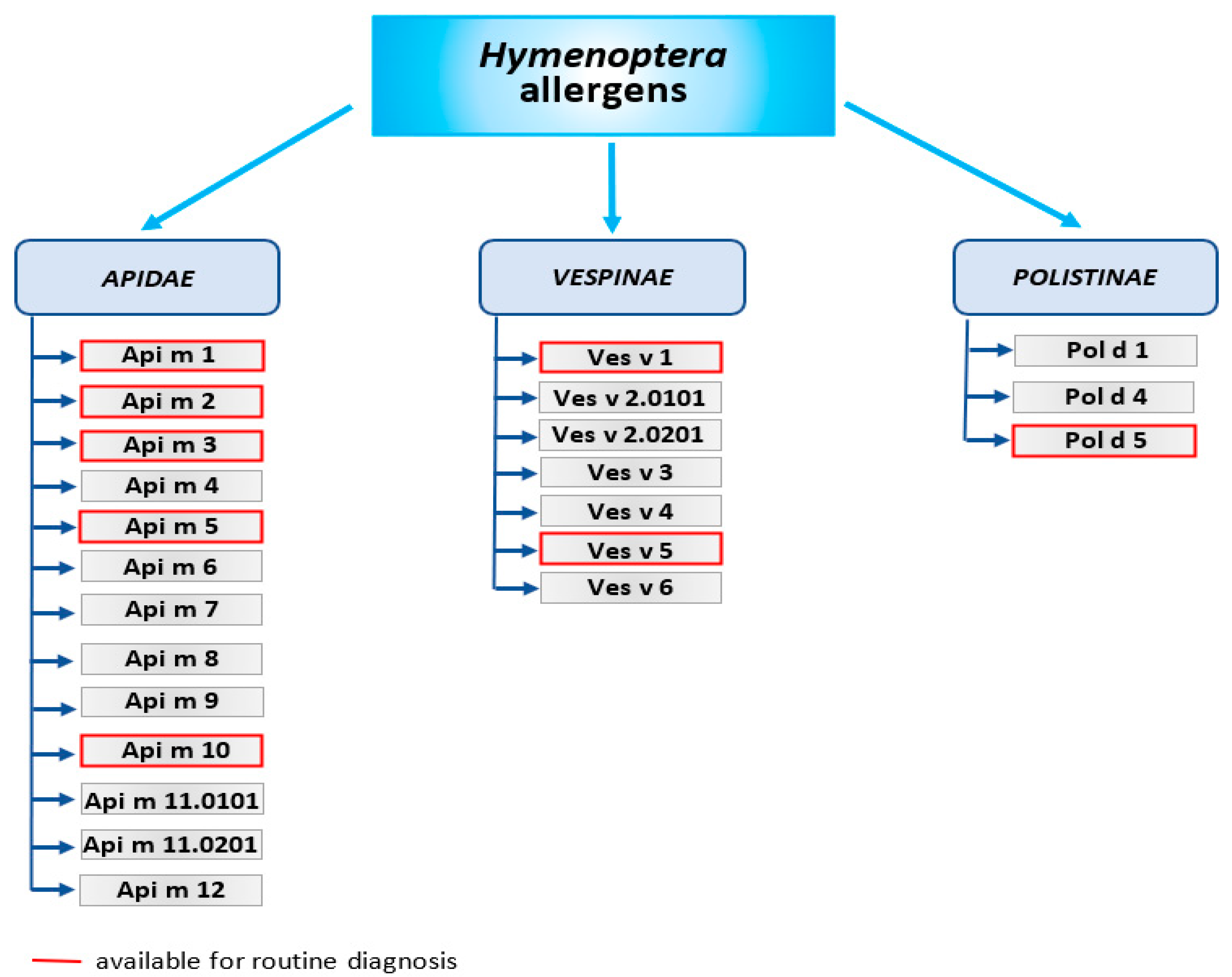
- Api m 1 is the phospholipase A2—the major allergen of honeybee venom. Phospholipase A2 is also contained in Bombus spp. venoms (Bom p 1 and Bom t 1), but the protein sequence identities between Apis mellifera and Bombus spp. is approximately 52–53%. Venoms of wasps contain phospholipase A1—Ves v 1 and Pol d 1, which differs in sequence and substrate specificity.
- Api m 2—hyaluronidase, the second major allergen of bee venom. Due to the similarity structure of bee hyaluronidase and wasp hyaluronidase, this allergen is the main cause of cross-reactions of IgE antibodies against the venom of these insects. Wasp hyaluronidase exists in 2 isoforms—Ves v 2.0101 and Ves v 2.0201.
- Api m 3—acid phosphatase is an enzyme that catalyzes the hydrolysis of monoesters and anhydrides of phosphoric acid. Together with the Api m 1 and Api m 2, it is considered as one of the most potent venom allergens.
- Api m 4—melittin is a minor allergen but the main component of bee venom, accounting for about half (45–50%) of its dry weight. Melittin possesses strong hemolytic and antimicrobial properties. Api m 3 and Api m 4 have been identified only in bee venom so far.
- Api m 5—dipeptidyl peptidase-IV and its analogues (Ves v 3 and Pol d 3) are present in wasp venom and the European paper wasp venom. Ves v 3 and Pol d 3 are major allergens that show high cross-reactivity.
- Antigen 5—an allergen found in the venom of almost all Vespoidea insects; Ves v 5 is found in the venom of yellow jackets; and Pol d 5 is found in the venom of paper wasps. Its function is still unknown. However, it is known to be highly allergenic and responsible for cross-reactions. Antigen 5 is a key element in CRD (component-resolved diagnostics) to distinguish between bee and wasp venom allergy [25].
- Api m 6—putative protease inhibitor. It stands for only 1–2% of venom’s dry weight and is considered one of the weakest bee venom allergens.
- Api m 7—CUB (Clr/Cls, urchin EGF-like protein and bone morphogenic protein 1) serine protease, which shows high IgE-binding activity.
- Api m 8—carboxylesterase-6. Bee venom contains less than 1% of this allergen, and its function is not yet fully elucidated.
- Api m 9—venom serine carboxypeptidase that belongs to the peptidase S10 family. There are no reports on the immunogenic properties of Api m 9.
- Api m 10—icarpin. This carbohydrate-rich protein is identified as a key allergen due to its importance not only in the diagnosis of bee venom allergy, but also in immunotherapy. VIT may be ineffective in patients allergic to Api m 10 due to being underrepresented in some therapeutic extracts [26].
- Api m 11—occurs in two isoforms: major royal jelly protein (MRJP) 8 (Api m 11.0101a), and MRJP9 (Api m 11.0201a). MRJPs are a family of proteins identified only in the Apis spp. These proteins constitute about 90% of all royal jelly (RJ) proteins. Human IgE antibodies recognize MRJP1 present in the sera of patients allergic to RJ, as well as to bee venom.
- Api m 12—vitellogenin, 200 kDa peptide belonging to the vitellogenin family and cross-reactive to Ves v 6.
- Ves v 1 and Pol d 1—phospholipase A1, a peptide with hemolytic activity.
- Ves v 2 and Pol d 2—hyaluronidase (in Vespinae isoforms Ves v 2.0101, Ves v 2.0201 exist) hydrolyzes hyaluronic acid. This may cause a pathogenic reaction in allergic patients.
- Ves v 3 and Pol d 3—dipeptidyl peptidase-IV, peptides with high cross-reactivity, resulting from 76.1% sequence identity. Their function is activating or inactivating substrates by cleaving dipeptides.
- Ves v 5 and Pol d 5—antigen 5, considered the most important wasp allergen, but its function is still unknown.
- Ves v 6—vitellogenin, considered an IgE sensitizer.
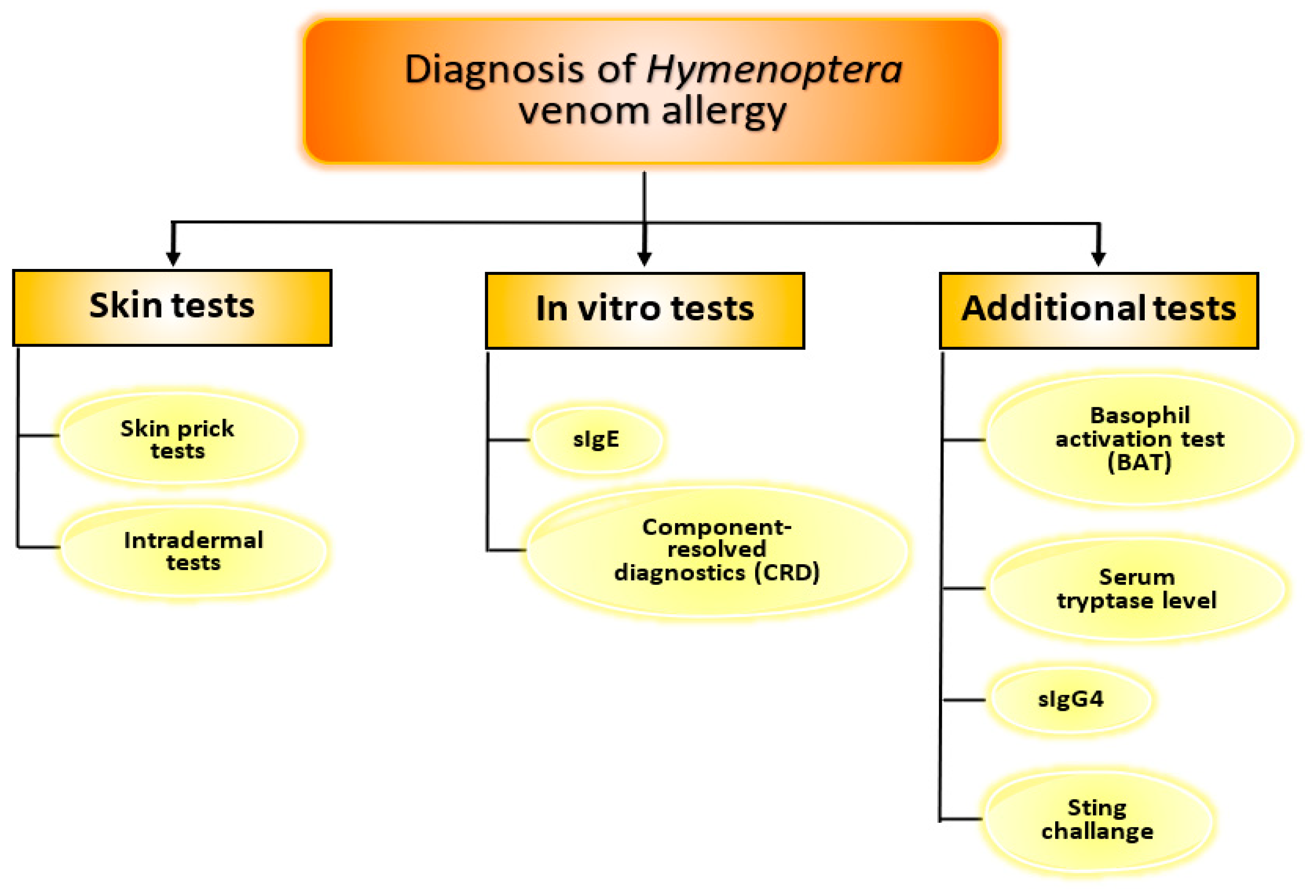
2. Diagnosis of Hymenoptera Venom Allergy
- rApi m 1, rApi m 2, rApi m 3, rApi m 5, rApi m 10;
- rVes v 1, rVes v 5;
- rPol d 5.
3. Additional Tests
4. Searching for New Diagnostic Capabilities of Hymenoptera Venom Allergy
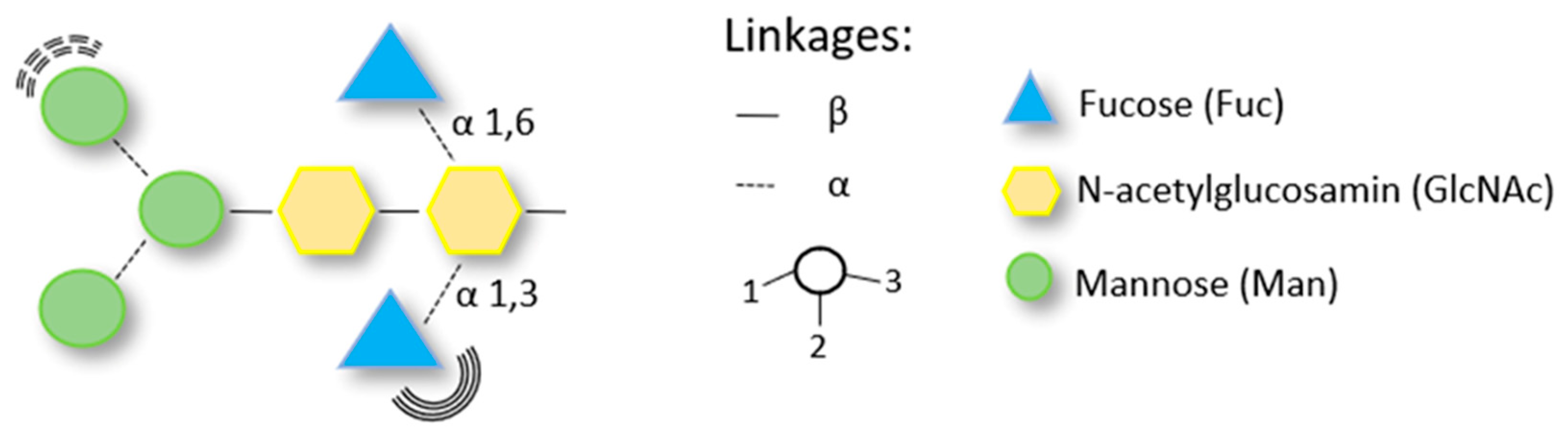
5. Diagnostic Challenge—Carbohydrate Determinants as the Cause of Cross-Reactivity in Patients Allergic to Hymenoptera Venom
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Worm, M.; Eckermann, O.; Dölle, S.; Aberer, W.; Beyer, K.; Hawranek, T.; Hompes, S.; Koehli, A.; Mahler, V.; Nemat, K.; et al. Triggers and Treatment of Anaphylaxis: An Analysis of 4,000 Cases from Germany, Austria and Switzerland. Dtsch. Arztebl. Int. 2014, 111, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Bilò, M.B. Anaphylaxis Caused by Hymenoptera Stings: From Epidemiology to Treatment. Allergy 2011, 66 (Suppl. S95), 35–37. [Google Scholar] [CrossRef]
- Blank, S.; Haemmerle, S.; Jaeger, T.; Russkamp, D.; Ring, J.; Schmidt-Weber, C.B.; Ollert, M. Prevalence of Hymenoptera Venom Allergy and Sensitization in the Population-Representative German KORA Cohort. Allergo J. Int. 2019, 28, 183–191. [Google Scholar] [CrossRef]
- Golden, D.B.K. Anaphylaxis to Insect Stings. Immunol. Allergy Clin. N. Am. 2015, 35, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Feás, X.; Vidal, C.; Remesar, S. What We Know about Sting-Related Deaths? Human Fatalities Caused by Hornet, Wasp and Bee Stings in Europe (1994–2016). Biology 2022, 11, 282. [Google Scholar] [CrossRef]
- Blank, S.; Pehlivanli, S.; Methe, H.; Schmidt-Weber, C.B.; Biedermann, T.; Horny, H.P.; Kristensen, T.; Amar, Y.; Köberle, M.; Brockow, K.; et al. Fatal Anaphylaxis Following a Hornet Sting in a Yellow Jacket Venom-Sensitized Patient with Undetected Monoclonal Mast Cell Activation Syndrome and without Previous History of a Systemic Sting Reaction. J. Allergy Clin. Immunol. Pract. 2020, 8, 401–403.e2. [Google Scholar] [CrossRef]
- Golden, D.B.K.; Demain, J.; Freeman, T.; Graft, D.; Tankersley, M.; Tracy, J.; Blessing-Moore, J.; Bernstein, D.; Dinakar, C.; Greenhawt, M.; et al. Stinging Insect Hypersensitivity: A Practice Parameter Update 2016. Ann. Allergy Asthma Immunol. 2017, 118, 28–54. [Google Scholar] [CrossRef]
- Tankersley, M.S.; Ledford, D.K. Stinging Insect Allergy: State of the Art 2015. J. Allergy Clin. Immunol. Pract. 2015, 3, 315–322. [Google Scholar] [CrossRef]
- Ludman, S.W.; Boyle, R.J. Stinging Insect Allergy: Current Perspectives on Venom Immunotherapy. J. Asthma Allergy 2015, 8, 75–86. [Google Scholar] [CrossRef]
- Sturm, G.J.; Arzt-Gradwohl, L.; Varga, E.M. Medical Algorithms: Diagnosis and Treatment of Hymenoptera Venom Allergy. Allergy 2019, 74, 2016–2018. [Google Scholar] [CrossRef]
- Baker, T.W.; Forester, J.P.; Johnson, M.L.; Stolfi, A.; Stahl, M.C. The HIT Study: Hymenoptera Identification Test--How Accurate Are People at Identifying Stinging Insects? Ann. Allergy Asthma Immunol. 2014, 113, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.W.; Forester, J.P.; Johnson, M.L.; Sikora, J.M.; Stolfi, A.; Stahl, M.C. Stinging Insect Identification: Are the Allergy Specialists Any Better than Their Patients? Ann. Allergy Asthma Immunol. 2016, 116, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Worm, M.; Moneret-Vautrin, A.; Scherer, K.; Lang, R.; Fernandez-Rivas, M.; Cardona, V.; Kowalski, M.L.; Jutel, M.; Poziomkowska-Gesicka, I.; Papadopoulos, N.G.; et al. First European Data from the Network of Severe Allergic Reactions (NORA). Allergy 2014, 69, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.Y.; Yi, M.H.; Son, M.; Lyu, D.; Lee, J.H.; Yong, T.S.; Park, J.W. IgE Reactivity of Recombinant Pac c 3 from the Asian Needle Ant (Pachycondyla Chinensis). Int. Arch. Allergy Immunol. 2016, 169, 93–100. [Google Scholar] [CrossRef]
- Bilò, M.B.; Pravettoni, V.; Bignardi, D.; Bonadonna, P.; Mauro, M.; Novembre, E.; Quercia, O.; Cilia, M.; Cortellini, G.; Costantino, M.T.; et al. Hymenoptera Venom Allergy: Management of Children and Adults in Clinical Practice. J. Investig. Allergol. Clin. Immunol. 2019, 29, 180–205. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C. The Asian Wasp Vespa Velutina Nigrithorax: Entomological and Allergological Characteristics. Clin. Exp. Allergy 2022, 52, 489–498. [Google Scholar] [CrossRef]
- Feás, X. Human Fatalities Caused by Hornet, Wasp and Bee Stings in Spain: Epidemiology at State and Sub-State Level from 1999 to 2018. Biology 2021, 10, 73. [Google Scholar] [CrossRef]
- Vidal, C.; Armisén, M.; Monsalve, R.; González-Vidal, T.; Lojo, S.; López-Freire, S.; Méndez, P.; Rodríguez, V.; Romero, L.; Galán, A.; et al. Anaphylaxis to Vespa Velutina Nigrithorax: Pattern of Sensitization for an Emerging Problem in Western Countries. J. Investig. Allergol. Clin. Immunol. 2021, 31, 228–235. [Google Scholar] [CrossRef]
- Khalil, A.; Elesawy, B.H.; Ali, T.M.; Ahmed, O.M. Bee Venom: From Venom to Drug. Molecules 2021, 26, 4941. [Google Scholar] [CrossRef]
- Pucca, M.B.; Cerni, F.A.; Oliveira, I.S.; Jenkins, T.P.; Argemí, L.; Sørensen, C.V.; Ahmadi, S.; Barbosa, J.E.; Laustsen, A.H. Bee Updated: Current Knowledge on Bee Venom and Bee Envenoming Therapy. Front. Immunol. 2019, 10, 2090. [Google Scholar] [CrossRef]
- van Vaerenbergh, M.; Debyser, G.; Devreese, B.; de Graaf, D.C. Exploring the Hidden Honeybee (Apis Mellifera) Venom Proteome by Integrating a Combinatorial Peptide Ligand Library Approach with FTMS. J. Proteomics 2014, 99, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Danneels, E.L.; van Vaerenbergh, M.; Debyser, G.; Devreese, B.; de Graaf, D.C. Honeybee Venom Proteome Profile of Queens and Winter Bees as Determined by a Mass Spectrometric Approach. Toxins 2015, 7, 4468–4483. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Wahed, A.A.; Khalifa, S.A.M.; Sheikh, B.Y.; Farag, M.A.; Saeed, A.; Larik, F.A.; Koca-Caliskan, U.; AlAjmi, M.F.; Hassan, M.; Wahabi, H.A.; et al. Bee Venom Composition: From Chemistry to Biological Activity. Stud. Nat. Prod. Chem. 2019, 60, 459–484. [Google Scholar] [CrossRef]
- Burzyńska, M.; Piasecka-kwiatkowska, D. A Review of Honeybee Venom Allergens and Allergenicity. Int. J. Mol. Sci. 2021, 22, 8371. [Google Scholar] [CrossRef] [PubMed]
- Blank, S.; Bazon, M.L.; Grosch, J.; Schmidt-Weber, C.B.; Brochetto-Braga, M.R.; Bilò, M.B.; Jakob, T. Antigen 5 Allergens of Hymenoptera Venoms and Their Role in Diagnosis and Therapy of Venom Allergy. Curr. Allergy Asthma Rep. 2020, 20, 58. [Google Scholar] [CrossRef]
- Frick, M.; Fischer, J.; Helbling, A.; Ruëff, F.; Wieczorek, D.; Ollert, M.; Pfützner, W.; Müller, S.; Huss-Marp, J.; Dorn, B.; et al. Predominant Api m 10 Sensitization as Risk Factor for Treatment Failure in Honey Bee Venom Immunotherapy. J. Allergy Clin. Immunol. 2016, 138, 1663–1671.e9. [Google Scholar] [CrossRef]
- Sturm, G.J.; Kranzelbinder, B.; Schuster, C.; Sturm, E.M.; Bokanovic, D.; Vollmann, J.; Crailsheim, K.; Hemmer, W.; Aberer, W. Sensitization to Hymenoptera Venoms Is Common, but Systemic Sting Reactions Are Rare. J. Allergy Clin. Immunol. 2014, 133, 1635–1643.e1. [Google Scholar] [CrossRef]
- Mosbech, H.; Tang, L.; Linneberg, A. Insect Sting Reactions and Specific IgE to Venom and Major Allergens in a General Population. Int. Arch. Allergy Immunol. 2016, 170, 194–200. [Google Scholar] [CrossRef]
- Vachová, M.; Panzner, P.; Malkusová, I.; Hanzlíková, J.; Vlas, T. Utility of Laboratory Testing for the Diagnosis of Hymenoptera Venom Allergy. Allergy Asthma Proc. 2016, 37, 248–255. [Google Scholar] [CrossRef]
- Pfaar, O.; Bachert, C.; Bufe, A.; Buhl, R.; Ebner, C.; Eng, P.; Friedrichs, F.; Fuchs, T.; Hamelmann, E.; Hartwig-Bade, D.; et al. Guideline on Allergen-Specific Immunotherapy in IgE-Mediated Allergic Diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto-Rhino-Laryngology, Head and Neck. Allergo J. Int. 2014, 23, 28–67. [Google Scholar] [CrossRef]
- Bilò, B.M.; Bonifazi, F. Hymenoptera Venom Immunotherapy. Immunotherapy 2011, 3, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Vos, B.; Köhler, J.; Müller, S.; Stretz, E.; Ruëff, F.; Jakob, T. Spiking Venom with RVes v 5 Improves Sensitivity of IgE Detection in Patients with Allergy to Vespula Venom. J. Allergy Clin. Immunol. 2013, 131, 1225–1227.e1. [Google Scholar] [CrossRef] [PubMed]
- Möbs, C.; Ipsen, H.; Mayer, L.; Slotosch, C.; Petersen, A.; Würtzen, P.A.; Hertl, M.; Pfützner, W. Birch Pollen Immunotherapy Results in Long-Term Loss of Bet v 1-Specific TH2 Responses, Transient TR1 Activation, and Synthesis of IgE-Blocking Antibodies. J. Allergy Clin. Immunol. 2012, 130, 1108–1116.e6. [Google Scholar] [CrossRef]
- van de Veen, W.; Stanic, B.; Wirz, O.F.; Jansen, K.; Globinska, A.; Akdis, M. Role of Regulatory B Cells in Immune Tolerance to Allergens and Beyond. J. Allergy Clin. Immunol. 2016, 138, 654–665. [Google Scholar] [CrossRef]
- Blank, S.; Seismann, H.; Michel, Y.; McIntyre, M.; Cifuentes, L.; Braren, I.; Grunwald, T.; Darsow, U.; Ring, J.; Bredehorst, R.; et al. Api m 10, a Genuine A. Mellifera Venom Allergen, Is Clinically Relevant but Underrepresented in Therapeutic Extracts. Allergy 2011, 66, 1322–1329. [Google Scholar] [CrossRef]
- Alfaya Arias, T.; Soriano Gómis, V.; Soto Mera, T.; Vega Castro, A.; Vega Gutiérrez, J.M.; Alonso Llamazares, A.; Antolín Amérigo, D.; Carballada Gonzalez, F.J.; Dominguez Noche, C.; Gutierrez Fernandez, D.; et al. Key Issues in Hymenoptera Venom Allergy: An Update. J. Investig. Allergol. Clin. Immunol. 2017, 27, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.R.; Johansen, N.; Petersen, A.B.; Fromberg-Nielsen, J.; Haeberli, G. Hymenoptera Venom Allergy: Analysis of Double Positivity to Honey Bee and Vespula Venom by Estimation of IgE Antibodies to Species-Specific Major Allergens Api M1 and Ves V5. Allergy Eur. J. Allergy Clin. Immunol. 2009, 64, 543–548. [Google Scholar] [CrossRef]
- Blank, S.; Bilò, M.B.; Ollert, M. Component-Resolved Diagnostics to Direct in Venom Immunotherapy: Important Steps towards Precision Medicine. Clin. Exp. Allergy 2018, 48, 354–364. [Google Scholar] [CrossRef]
- Bilò, M.B.; Ollert, M.; Blank, S. The Role of Component-Resolved Diagnosis in Hymenoptera Venom Allergy. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 614–622. [Google Scholar] [CrossRef]
- Ollert, M.; Blank, S. Anaphylaxis to Insect Venom Allergens: Role of Molecular Diagnostics. Curr. Allergy Asthma Rep. 2015, 15, 26. [Google Scholar] [CrossRef]
- Jakob, T.; Müller, U.; Helbling, A.; Spillner, E. Component Resolved Diagnostics for Hymenoptera Venom Allergy. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Köhler, J.; Blank, S.; Müller, S.; Bantleon, F.; Frick, M.; Huss-Marp, J.; Lidholm, J.; Spillner, E.; Jakob, T. Component Resolution Reveals Additional Major Allergens in Patients with Honeybee Venom Allergy. J. Allergy Clin. Immunol. 2014, 133, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.C.; Pfender, N.; Weckesser, S.; Huss-Marp, J.; Jakob, T. Added Value of IgE Detection to RApi m 1 and RVes v 5 in Patients with Hymenoptera Venom Allergy. J. Allergy Clin. Immunol. 2011, 127, 265–267. [Google Scholar] [CrossRef]
- Müller, U.; Schmid-Grendelmeier, P.; Hausmann, O.; Helbling, A. IgE to Recombinant Allergens Api m 1, Ves v 1, and Ves v 5 Distinguish Double Sensitization from Crossreaction in Venom Allergy. Allergy 2012, 67, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Korošec, P.; Valenta, R.; Mittermann, I.; Čelesnik, N.; Šilar, M.; Zidarn, M.; Košnik, M. High Sensitivity of CAP-FEIA RVes v 5 and RVes v 1 for Diagnosis of Vespula Venom Allergy. J. Allergy Clin. Immunol. 2012, 129, 1406–1408. [Google Scholar] [CrossRef]
- Šelb, J.; Bidovec Stojković, U.; Bajrović, N.; Kopač, P.; Eržen, R.; Zidarn, M.; Košnik, M.; Korošec, P. Limited Ability of Recombinant Hymenoptera Venom Allergens to Resolve IgE Double Sensitization. J. Allergy Clin. Immunol. Pract. 2018, 6, 2118–2120. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, R.I.; Vega, A.; Marqués, L.; Miranda, A.; Fernández, J.; Soriano, V.; Cruz, S.; Domínguez-Noche, C.; Sánchez-Morillas, L.; Armisen-Gil, M.; et al. Component-Resolved Diagnosis of Vespid Venom-Allergic Individuals: Phospholipases and Antigen 5s Are Necessary to Identify Vespula or Polistes Sensitization. Allergy 2012, 67, 528–536. [Google Scholar] [CrossRef]
- Quercia, O.; Cova, V.; Martini, M.; Cortellini, G.; Murzilli, F.; Bignardi, D.; Cilia, M.; Scarpa, A.; Bilò, M.B. CAP-Inhibition, Molecular Diagnostics, and Total IgE in the Evaluation of Polistes and Vespula Double Sensitization. Int. Arch. Allergy Immunol. 2018, 177, 365–369. [Google Scholar] [CrossRef]
- Gattinger, P.; Lupinek, C.; Kalogiros, L.; Silar, M.; Zidarn, M.; Korosec, P.; Koessler, C.; Novak, N.; Valenta, R.; Mittermann, I. The Culprit Insect but Not Severity of Allergic Reactions to Bee and Wasp Venom Can Be Determined by Molecular Diagnosis. PLoS ONE 2018, 13, e0199250. [Google Scholar] [CrossRef]
- Blank, S.; Etzold, S.; Darsow, U.; Schiener, M.; Eberlein, B.; Russkamp, D.; Wolf, S.; Graessel, A.; Biedermann, T.; Ollert, M.; et al. Component-Resolved Evaluation of the Content of Major Allergens in Therapeutic Extracts for Specific Immunotherapy of Honeybee Venom Allergy. Hum. Vaccines Immunother. 2017, 13, 2482–2489. [Google Scholar] [CrossRef]
- Korošec, P.; Šilar, M.; Eržen, R.; Čelesnik, N.; Bajrović, N.; Zidarn, M.; Košnik, M. Clinical Routine Utility of Basophil Activation Testing for Diagnosis of Hymenoptera-Allergic Patients with Emphasis on Individuals with Negative Venom-Specific IgE Antibodies. Int. Arch. Allergy Immunol. 2013, 161, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Balzer, L.; Pennino, D.; Blank, S.; Seismann, H.; Darsow, U.; Schnedler, M.; McIntyre, M.; Ollert, M.W.; Durham, S.R.; Spillner, E.; et al. Basophil Activation Test Using Recombinant Allergens: Highly Specific Diagnostic Method Complementing Routine Tests in Wasp Venom Allergy. PLoS ONE 2014, 9, e108619. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.; Brockow, K.; Darsow, U.; Ring, J.; Schmidt-Weber, C.B.; Grunwald, T.; Blank, S.; Ollert, M. Added Sensitivity of Component-Resolved Diagnosis in Hymenoptera Venom-Allergic Patients with Elevated Serum Tryptase and/or Mastocytosis. Allergy 2016, 71, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, P.; Bonifacio, M.; Lombardo, C.; Zanotti, R. Hymenoptera Allergy and Mast Cell Activation Syndromes. Curr. Allergy Asthma Rep. 2016, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- van de Veen, W.; Stanic, B.; Yaman, G.; Wawrzyniak, M.; Söllner, S.; Akdis, D.G.; Rückert, B.; Akdis, C.A.; Akdis, M. IgG4 Production Is Confined to Human IL-10-Producing Regulatory B Cells That Suppress Antigen-Specific Immune Responses. J. Allergy Clin. Immunol. 2013, 131, 1204–1212. [Google Scholar] [CrossRef]
- Bilo, B.M.; Rueff, F.; Mosbech, H.; Bonifazi, F.; Oude-Elberink, J.N.G. Diagnosis of Hymenoptera Venom Allergy. Allergy 2005, 60, 1339–1349. [Google Scholar] [CrossRef]
- Bloemen, K.; van den Heuvel, R.; Govarts, E.; Hooyberghs, J.; Nelen, V.; Witters, E.; Desager, K.; Schoeters, G. A New Approach to Study Exhaled Proteins as Potential Biomarkers for Asthma. Clin. Exp. Allergy 2011, 41, 346–356. [Google Scholar] [CrossRef]
- Holm, T.; Rutishauser, D.; Kai-Larsen, Y.; Lyutvinskiy, Y.; Stenius, F.; Zubarev, R.A.; Agerberth, B.; Alm, J.; Scheynius, A. Protein Biomarkers in Vernix with Potential to Predict the Development of Atopic Eczema in Early Childhood. Allergy 2014, 69, 104–112. [Google Scholar] [CrossRef]
- Won, K.K.; Hwang, H.R.; Do, H.K.; Phil, Y.L.; Yu, J.I.; Ryu, H.Y.; Sung, G.P.; Bae, K.H.; Sang, C.L. Glycoproteomic Analysis of Plasma from Patients with Atopic Dermatitis: CD5L and ApoE as Potential Biomarkers. Exp. Mol. 2008, 40, 677–685. [Google Scholar] [CrossRef]
- Molin, S.; Merl, J.; Dietrich, K.A.; Regauer, M.; Flaig, M.; Letulé, V.; Saucke, T.; Herzinger, T.; Ruzicka, T.; Hauck, S.M. The Hand Eczema Proteome: Imbalance of Epidermal Barrier Proteins. Br. J. Dermatol. 2015, 172, 994–1001. [Google Scholar] [CrossRef]
- Chiappori, A.; de Ferrari, L.; Folli, C.; Mauri, P.; Riccio, A.M.; Canonica, G.W. Biomarkers and Severe Asthma: A Critical Appraisal. Clin. Mol. Allergy 2015, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gottfries, J.; Barrenäs, F.; Benson, M. Identification of Novel Biomarkers in Seasonal Allergic Rhinitis by Combining Proteomic, Multivariate and Pathway Analysis. PLoS ONE 2011, 6, e23563. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.S.; Shin, S.Y.; Kim, J.H.; Lee, H.Y.; Palikhe, N.S.; Ye, Y.M.; Kim, S.H.; Park, H.S. Serum Lactoferrin Level as a Serologic Biomarker for Allergic Rhinitis. Clin. Exp. Allergy 2010, 40, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Palmigiano, A.; Mazzola, E.A.; Messina, A.; Milazzo, E.M.S.; Bortolotti, M.; Garozzo, D. Identification of Human Tear Fluid Biomarkers in Vernal Keratoconjunctivitis Using ITRAQ Quantitative Proteomics. Allergy 2014, 69, 254–260. [Google Scholar] [CrossRef] [PubMed]
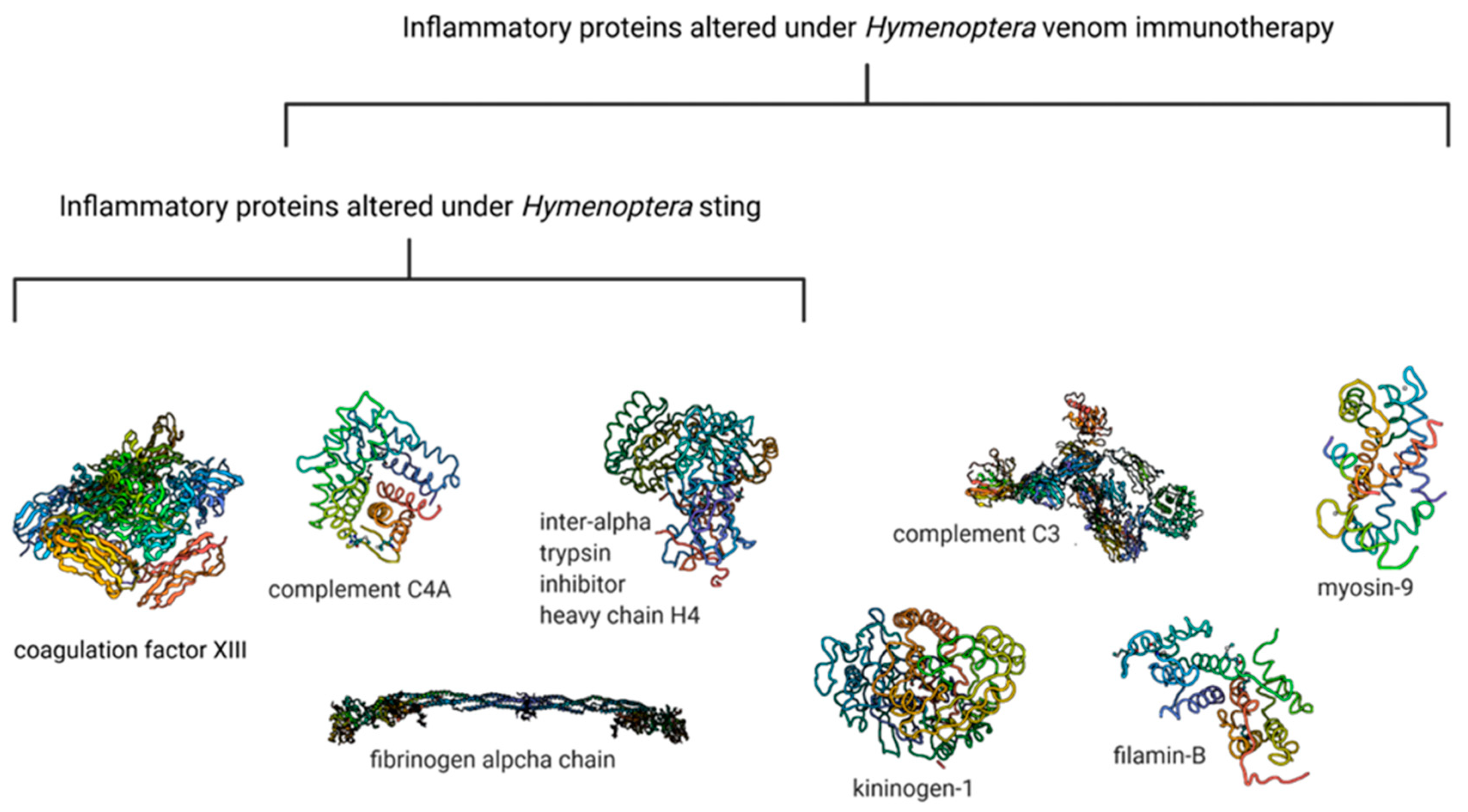
- Matuszewska, E.; Matysiak, J.; Brȩborowicz, A.; Olejniczak, K.; Kycler, Z.; Kokot, Z.J.; Matysiak, J. Proteomic Features Characterization of Hymenoptera Venom Allergy. Allergy Asthma Clin. Immunol. 2019, 15, 77. [Google Scholar] [CrossRef]
- Matysiak, J.; Matuszewska, E.; Kowalski, M.L.; Kosiński, S.W.; Smorawska-Sabanty, E.; Matysiak, J. Association between Venom Immunotherapy and Changes in Serum Protein—Peptide Patterns. Vaccines 2021, 9, 249. [Google Scholar] [CrossRef]
- Packi, K.; Matysiak, J.; Matuszewska, E.; Bręborowicz, A.; Kycler, Z.; Matysiak, J. New Biomarkers of Hymenoptera Venom Allergy in a Group of Inflammation Factors. Int. J. Environ. Res. Public Health 2021, 18, 4011. [Google Scholar] [CrossRef]
- Araki, S.; Yamamoto, Y.; Saito, R.; Kawakita, A.; Eguchi, M.; Goto, M.; Kubo, K.; Kawagoe, R.; Kawada, Y.; Kusuhara, K. Plasma but Not Serum Brain-Derived Neurotrophic Factor Concentration Is Decreased by Oral Glucose Tolerance Test-Induced Hyperglycemia in Children. J. Pediatric Endocrinol. Metab. 2017, 30, 525–530. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and Mast Cells in Allergic Disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef]
- Ahmad, S.; Azid, N.A.; Boer, J.C.; Lim, J.; Chen, X.; Plebanski, M.; Mohamud, R. The Key Role of TNF-TNFR2 Interactions in the Modulation of Allergic Inflammation: A Review. Front. Immunol. 2018, 9, 2572. [Google Scholar] [CrossRef] [PubMed]
- Polte, T.; Behrendt, A.K.; Hansen, G. Direct Evidence for a Critical Role of CD30 in the Development of Allergic Asthma. J. Allergy Clin. Immunol. 2006, 118, 942–948. [Google Scholar] [CrossRef]
- Croft, M. The TNF Family in T Cell Differentiation and Function—Unanswered Questions and Future Directions. Semin. Immunol. 2014, 26, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.M.; Na, H.H.; Park, J.S.; Ahn, B.S.; Kim, K.C. Identification of Cleaved Haptoglobin in the Serum of Bee Venom-Hypersensitive Patients. J. Acupunct. Meridian Stud. 2021, 14, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chai, L.; Liu, C.; Liu, M.; Han, L.; Li, C.; Guo, H.; Sun, Y.; Rao, X.; Xiao, M.; et al. Serum Metabolomics Analysis in Wasp Sting Patients. Biomed. Res. Int. 2018, 2018, 5631372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, J.; Chen, Y.; Li, Z.; Nie, H.; Peng, W.; Su, S. Altered Serum Metabolite Profiling and Relevant Pathway Analysis in Rats Stimulated by Honeybee Venom: New Insight into Allergy to Honeybee Venom. J. Agric. Food Chem. 2018, 66, 871–880. [Google Scholar] [CrossRef]
- Eržen, R.; Korošec, P.; Šilar, M.; Mušič, E.; Košnik, M. Carbohydrate Epitopes as a Cause of Cross-Reactivity in Patients Allergic to Hymenoptera Venom. Wien. Klin. Wochenschr. 2009, 121, 349–352. [Google Scholar] [CrossRef]
- Bousquet, J.; Müller, U.R.; Dreborg, S.; Jarisch, R.; Malling, H.-J.; Mosbech, H.; Urbanek, R.; Youlten, L. Immunotherapy with Hymenoptera Venoms. Position Paper of the Working Group on Immunotherapy of the European Academy of Allergy and Clinical Immunology. Allergy 1987, 42, 401–413. [Google Scholar] [CrossRef]
- Straumann, F.; Bucher, C.; Wüthrich, B. Double Sensitization to Honeybee and Wasp Venom: Immunotherapy with One or with Both Venoms? Value of FEIA Inhibition for the Identification of the Cross-Reacting Ige Antibodies in Double-Sensitized Patients to Honeybee and Wasp Venom. Int. Arch. Allergy Immunol. 2000, 123, 268–274. [Google Scholar] [CrossRef]
- Aalberse, R.C.; Koshte, V.; Clemens, J.G.J. Immunoglobulin E Antibodies That Crossreact with Vegetable Foods, Pollen, and Hymenoptera Venom. J. Allergy Clin. Immunol. 1981, 68, 356–364. [Google Scholar] [CrossRef]
- Brehler, R.; Grundmann, S.; Stöcker, B. Cross-Reacting Carbohydrate Determinants and Hymenoptera Venom Allergy. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 360–364. [Google Scholar] [CrossRef]
- Altmann, F. The Role of Protein Glycosylation in Allergy. Int. Arch. Allergy Immunol. 2007, 142, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Kochuyt, A.M.; van Hoeyveld, E.M.; Stevens, E.A.M. Prevalence and Clinical Relevance of Specific Immunoglobulin E to Pollen Caused by Sting-Induced Specific Immunoglobulin E to Cross-Reacting Carbohydrate Determinants in Hymenoptera Venoms. Clin. Exp. Allergy 2005, 35, 441–447. [Google Scholar] [CrossRef] [PubMed]
- van Ree, R. Carbohydrate Epitopes and Their Relevance for the Diagnosis and Treatment of Allergic Diseases. Int. Arch. Allergy Immunol. 2002, 129, 189–197. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, M.J.; van Ree, R.; Aalberse, R.C.; Akkerdaas, J.; Koppelman, S.J.; Jansen, H.M.; van der Zee, J.S. Poor Biologic Activity of Cross-Reactive IgE Directed to Carbohydrate Determinants of Glycoproteins. J. Allergy Clin. Immunol. 1997, 100, 327–334. [Google Scholar] [CrossRef]
- Mari, A.; Ooievaar-De Heer, P.; Scala, E.; Giani, M.; Pirrotta, L.; Zuidmeer, L.; Bethell, D.; van Ree, R. Evaluation by Double-Blind Placebo-Controlled Oral Challenge of the Clinical Relevance of IgE Antibodies against Plant Glycans. Allergy 2008, 63, 891–896. [Google Scholar] [CrossRef]
- Mertens, M.; Amler, S.; Moerschbacher, B.M.; Brehler, R. Cross-Reactive Carbohydrate Determinants Strongly Affect the Results of the Basophil Activation Test in Hymenoptera-Venom Allergy. Clin. Exp. Allergy 2010, 40, 1333–1345. [Google Scholar] [CrossRef]
- Fötisch, K.; Altmann, F.; Haustein, D.; Vieths, S. Involvement of Carbohydrate Epitopes in the IgE Response of Celery-Allergic Patients. Int. Arch. Allergy Immunol. 1999, 120, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Vieths, S.; Lüttkopf, D.; Reindl, J.; Anliker, M.D.; Wüthrich, B.; Ballmer-Weber, B.K. Allergens in Celery and Zucchini. Allergy 2002, 57 (Suppl. S72), 100–105. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr Allergy Immunol 2016, 27 (Suppl. S23), 1–250. [Google Scholar] [CrossRef]
- Mari, A. IgE to Cross-Reactive Carbohydrate Determinants: Analysis of the Distribution and Appraisal of the in Vivo and in Vitro Reactivity. Int. Arch. Allergy Immunol. 2002, 129, 286–295. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matysiak, J.; Matuszewska, E.; Packi, K.; Klupczyńska-Gabryszak, A. Diagnosis of Hymenoptera Venom Allergy: State of the Art, Challenges, and Perspectives. Biomedicines 2022, 10, 2170. https://doi.org/10.3390/biomedicines10092170
Matysiak J, Matuszewska E, Packi K, Klupczyńska-Gabryszak A. Diagnosis of Hymenoptera Venom Allergy: State of the Art, Challenges, and Perspectives. Biomedicines. 2022; 10(9):2170. https://doi.org/10.3390/biomedicines10092170
Chicago/Turabian StyleMatysiak, Joanna, Eliza Matuszewska, Kacper Packi, and Agnieszka Klupczyńska-Gabryszak. 2022. "Diagnosis of Hymenoptera Venom Allergy: State of the Art, Challenges, and Perspectives" Biomedicines 10, no. 9: 2170. https://doi.org/10.3390/biomedicines10092170
APA StyleMatysiak, J., Matuszewska, E., Packi, K., & Klupczyńska-Gabryszak, A. (2022). Diagnosis of Hymenoptera Venom Allergy: State of the Art, Challenges, and Perspectives. Biomedicines, 10(9), 2170. https://doi.org/10.3390/biomedicines10092170





