Antitumor Effect of Brusatol in Acute Lymphoblastic Leukemia Models Is Triggered by Reactive Oxygen Species Accumulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines Culture and Characterization
2.2. Metabolic Activity by Resazurin Assay
2.3. Cell Death Evaluation
2.4. Cell Cycle Evaluation
2.5. Oxidative Stress Evaluation
2.6. Mitochondrial Membrane Potential Evaluation
2.7. NFE2L2 and KEAP1 Gene Expression Analysis
2.8. Statistical Analysis
3. Results
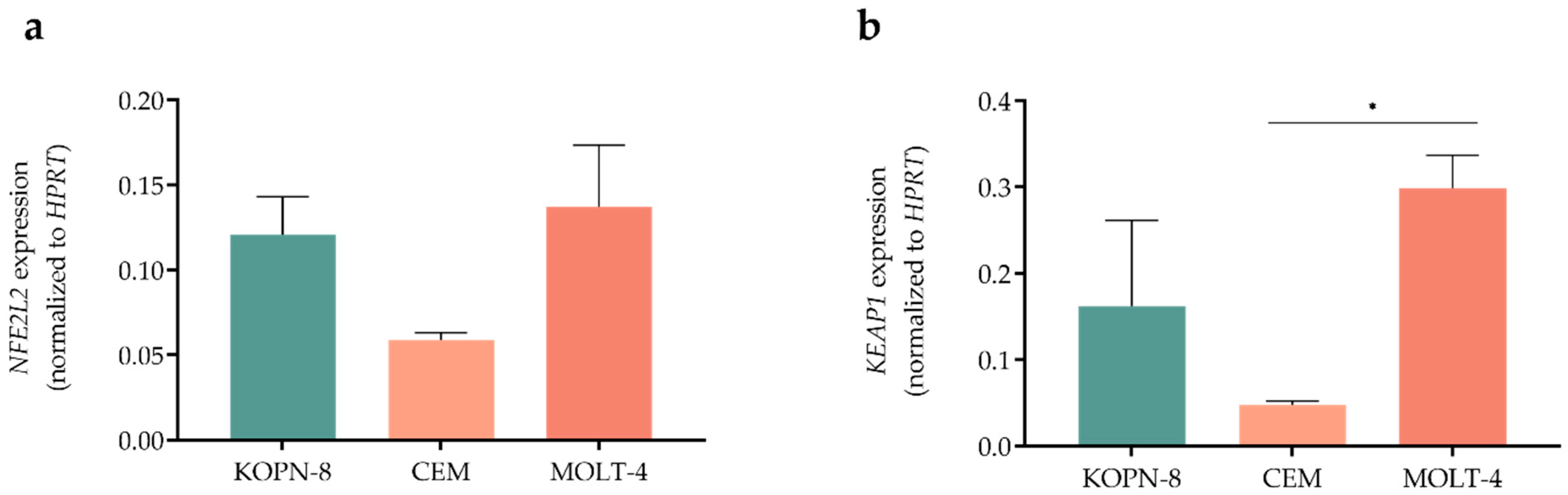
3.1. Acute Lymphoblastic Leukemia Cell Lines Showed Different Expression Levels of NFE2L2 and KEAP1 Genes
3.2. Brusatol Reduced Metabolic Activity in All Acute Lymphoblastic Leukemia Cell Lines
3.3. Proapoptotic Effect of Brusatol in Acute Lymphoblastic Leukemia Cell Lines
3.4. Cell Cycle Arrest Induced by Brusatol in Acute Lymphoblastic Leukemia Cell Lines
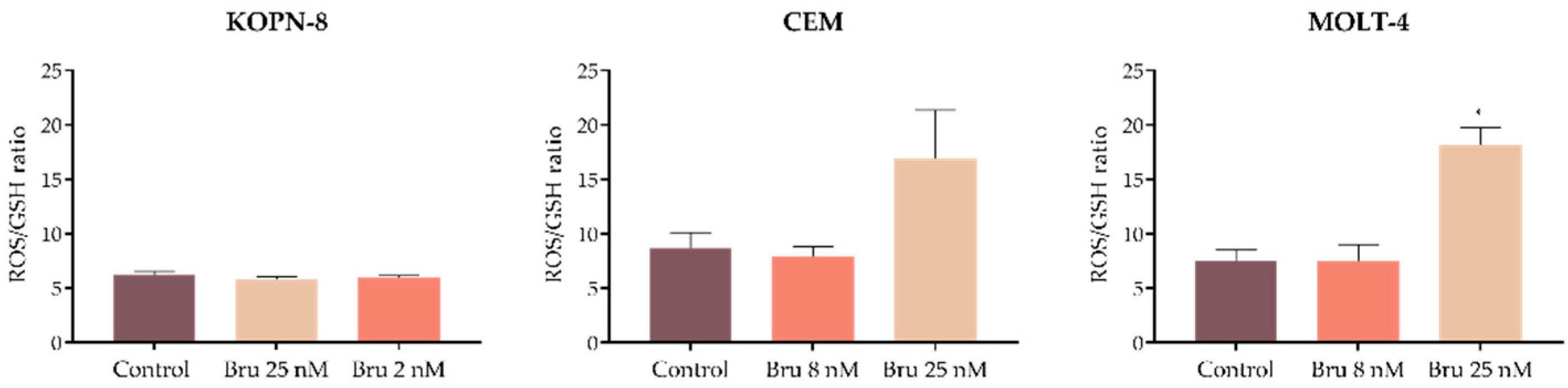
3.5. Brusatol Promotes Changes in the Intracellular Levels of ROS and GSH
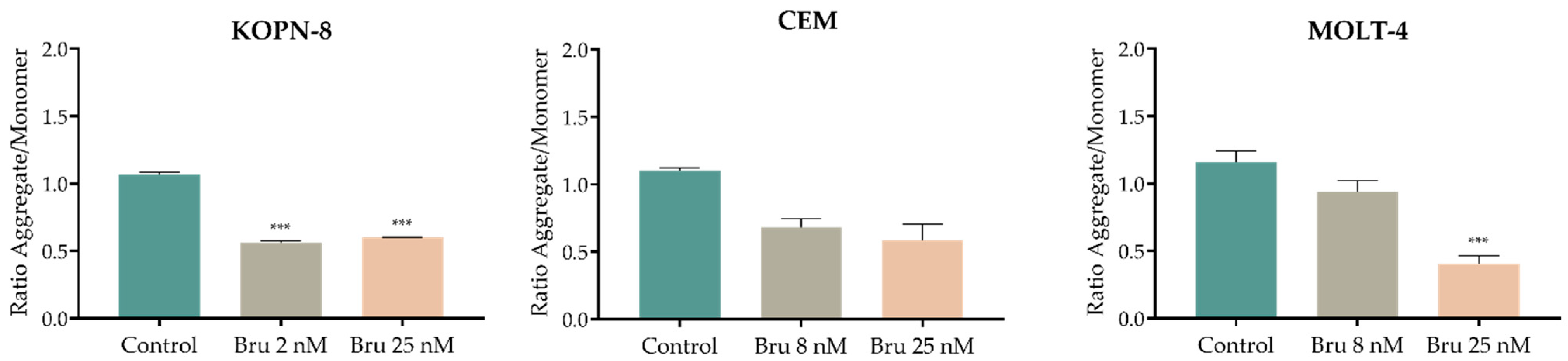
3.6. Effect of Brusatol on the Mitochondrial Membrane Potential (Δψmit)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malard, F.; Mohty, M. Acute lymphoblastic leukaemia. Lancet 2020, 395, 1146–1162. [Google Scholar] [CrossRef]
- Terwilliger, T.; Abdul-Hay, M. Acute lymphoblastic leukemia: A comprehensive review and 2017 update. Blood Cancer J. 2017, 7, e577. [Google Scholar] [CrossRef] [PubMed]
- Hunger, S.P.; Mullighan, C.G. Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mullighan, C.G.; Harvey, R.C.; Wu, G.; Chen, X.; Edmonson, M.; Buetow, K.H.; Carroll, W.L.; Chen, I.-M.; Devidas, M.; et al. Key pathways are frequently mutated in high-risk childhood acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood 2011, 118, 3080–3087. [Google Scholar] [CrossRef]
- Inaba, H.; Mullighan, C.G. Pediatric acute lymphoblastic leukemia. Haematologica 2020, 105, 2524–2539. [Google Scholar] [CrossRef] [PubMed]
- Sarmento-Ribeiro, A.B.; Proença, M.T.; Sousa, I.; Pereira, A.; Guedes, F.; Teixeira, A.; Oliveira, C.R. A possible role for oxidation stress in lymphoid leukaemias and therapeutic failure. Leuk. Res. 2012, 36, 1041–1048. [Google Scholar] [CrossRef]
- Khodakarami, A.; Adibfar, S.; Karpisheh, V.; Abolhasani, S.; Jalali, P.; Mohammadi, H.; Gholizadeh Navashenaq, J.; Hojjat-Farsangi, M.; Jadidi-Niaragh, F. The molecular biology and therapeutic potential of Nrf2 in leukemia. Cancer Cell Int. 2022, 22, 241. [Google Scholar] [CrossRef]
- Cai, S.J.; Liu, Y.; Han, S.; Yang, C. Brusatol, an NRF2 inhibitor for future cancer therapeutic. Cell Biosci. 2019, 9, 45. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Z.; Chen, H.; Yu, X.; Wang, L.; Liu, X. Brusatol inhibits the growth of renal cell carcinoma by regulating the PTEN/PI3K/AKT pathway. J. Ethnopharmacol. 2022, 288, 115020. [Google Scholar] [CrossRef]
- Xiang, Y.; Ye, W.; Huang, C.; Lou, B.; Zhang, J.; Yu, D.; Huang, X.; Chen, B.; Zhou, M. Brusatol inhibits growth and induces apoptosis in pancreatic cancer cells via JNK/p38 MAPK/NF-κb/Stat3/Bcl-2 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 487, 820–826. [Google Scholar] [CrossRef]
- Xiang, Y.; Ye, W.; Huang, C.; Yu, D.; Chen, H.; Deng, T.; Zhang, F.; Lou, B.; Zhang, J.; Shi, K.; et al. Brusatol Enhances the Chemotherapy Efficacy of Gemcitabine in Pancreatic Cancer via the Nrf2 Signalling Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 2360427. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lai, Z.; Zheng, X.; Liao, H.; Xian, Y.; Li, Q.; Wu, J.; Ip, S.; Xie, Y.; Chen, J.; et al. Apoptotic activities of brusatol in human non-small cell lung cancer cells: Involvement of ROS-mediated mitochondrial-dependent pathway and inhibition of Nrf2-mediated antioxidant response. Toxicology 2021, 451, 152680. [Google Scholar] [CrossRef]
- Xing, S.; Nong, F.; Wang, Y.; Huang, D.; Qin, J.; Chen, Y.-F.; He, D.-H.; Wu, P.-E.; Huang, H.; Zhan, R.; et al. Brusatol has therapeutic efficacy in non-small cell lung cancer by targeting Skp1 to inhibit cancer growth and metastasis. Pharmacol. Res. 2022, 176, 106059. [Google Scholar] [CrossRef]
- Evans, J.P.; Winiarski, B.K.; Sutton, P.A.; Jones, R.P.; Ressel, L.; Duckworth, C.A.; Pritchard, M.D.; Lin, Z.-X.; Fretwell, V.L.; Tweedle, E.M.; et al. The Nrf2 inhibitor brusatol is a potent antitumour agent in an orthotopic mouse model of colorectal cancer. Oncotarget 2018, 9, 27104. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.A.; Cai, L.; Chen, Y.; Wang, S.; Zhang, Q.; Wang, C.; Tu, M.; Zhu, Z.; Li, Q.; Lu, X. Brusatol Inhibits Proliferation and Invasion of Glioblastoma by Down-Regulating the Expression of ECM1. Front. Pharmacol. 2021, 12, 775680. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hou, J.; Wang, J.; Wang, J.; Gao, J.; Bai, Y.E. Brusatol inhibits laryngeal cancer cell proliferation and metastasis via abrogating JAK2/STAT3 signaling mediated epithelial-mesenchymal transition. Life Sci. 2021, 284, 119907. [Google Scholar] [CrossRef]
- Lu, R.-J.; Zhao, G.-Z.; Jiang, R.; He, S.; Xu, H.; He, J.-M.; Sun, Y.; Wu, M.-N.; Ran, J.-H.; Chen, D.-L.; et al. Brusatol Inhibits Proliferation and Metastasis of Colorectal Cancer by Targeting and Reversing the RhoA/ROCK1 Pathway. BioMed Res. Int. 2022, 2022, 7132159. [Google Scholar] [CrossRef]
- Cheng, C.; Yuan, F.; Chen, X.-P.; Zhang, W.; Zhao, X.-L.; Jiang, Z.-P.; Zhou, H.-H.; Zhou, G.; Cao, S. Inhibition of Nrf2-mediated glucose metabolism by brusatol synergistically sensitizes acute myeloid leukemia to Ara-C. Biomed. Pharmacother. 2021, 142, 111652. [Google Scholar] [CrossRef]
- Vartanian, S.; Ma, T.P.; Lee, J.; Haverty, P.M.; Kirkpatrick, D.S.; Yu, K.; Stokoe, D. Application of Mass Spectrometry Profiling to Establish Brusatol as an Inhibitor of Global Protein Synthesis. Mol. Cell. Proteom. 2016, 15, 1220–1231. [Google Scholar] [CrossRef]
- Ren, D.; Villeneuve, N.F.; Jiang, T.; Wu, T.; Lau, A.; Toppin, H.A.; Zhang, D.D. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Natl. Acad. Sci. USA 2011, 108, 1433–1438. [Google Scholar] [CrossRef] [Green Version]
- Karathedath, S.; Rajamani, B.M.; Musheer Aalam, S.M.; Abraham, A.; Varatharajan, S.; Krishnamurthy, P.; Mathews, V.; Velayudhan, S.R.; Balasubramanian, P. Role of NF-E2 related factor 2 (Nrf2) on chemotherapy resistance in acute myeloid leukemia (AML) and the effect of pharmacological inhibition of Nrf2. PLoS ONE 2017, 12, e0177227. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shi, G.; Bian, C.; Nisar, M.F.; Guo, Y.; Wu, Y.; Li, W.; Huang, X.; Jiang, X.; Bartsch, J.W.; et al. UVA Irradiation Enhances Brusatol-Mediated Inhibition of Melanoma Growth by Downregulation of the Nrf2-Mediated Antioxidant Response. Oxid. Med. Cell. Longev. 2018, 2018, 9742154. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Drexler, H.G. Establishment and characterization of human B cell precursor-leukemia cell lines. Leuk. Res. 1998, 22, 567–579. [Google Scholar] [CrossRef]
- Quentmeier, H.; Pommerenke, C.; Dirks, W.G.; Eberth, S.; Koeppel, M.; MacLeod, R.A.F.; Nagel, S.; Steube, K.; Uphoff, C.C.; Drexler, H.G. The LL-100 panel: 100 cell lines for blood cancer studies. Sci. Rep. 2019, 9, 8218. [Google Scholar] [CrossRef] [PubMed]
- Salih, D.; Elena, B.; Qian, S.; Stephanie, H.; Eugen, T.; Cornelia, E.; Martin, E.; Rupert, H.; Te Geertruy, K.; Lisa, W.; et al. Therapeutic targeting of mutant p53 in pediatric acute lymphoblastic leukemia. Haematologica 2020, 105, 170–181. [Google Scholar] [CrossRef]
- Kawamura, M.; Ohnishi, H.; Guo, S.-X.; Sheng, X.M.; Minegishi, M.; Hanada, R.; Horibe, K.; Hongo, T.; Kaneko, Y.; Bessho, F.; et al. Alterations of the p53, p21, p16, p15 and RAS genes in childhood T-cell acute lymphoblastic leukemia. Leuk. Res. 1999, 23, 115–126. [Google Scholar] [CrossRef]
- Alves, R.; Gonçalves, A.C.; Jorge, J.; Almeida, A.M.; Sarmento-Ribeiro, A.B. Combination of Elacridar with Imatinib Modulates Resistance Associated with Drug Efflux Transporters in Chronic Myeloid Leukemia. Biomedicines 2022, 10, 1158. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Cortesão, E.; Oliveiros, B.; Alves, V.; Espadana, A.I.; Rito, L.; Magalhães, E.; Lobão, M.J.; Pereira, A.; Nascimento Costa, J.M.; et al. Oxidative stress and mitochondrial dysfunction play a role in myelodysplastic syndrome development, diagnosis, and prognosis: A pilot study. Free Radic. Res. 2015, 49, 1081–1094. [Google Scholar] [CrossRef]
- Jorge, J.; Petronilho, S.; Alves, R.; Coucelo, M.; Gonçalves, A.C.; Nascimento Costa, J.M.; Sarmento-Ribeiro, A.B. Apoptosis induction and cell cycle arrest of pladienolide B in erythroleukemia cell lines. Investig. New Drugs 2020, 38, 369–377. [Google Scholar] [CrossRef]
- Hunger, S.P.; Lu, X.; Devidas, M.; Camitta, B.M.; Gaynon, P.S.; Winick, N.J.; Reaman, G.H.; Carroll, W.L. Improved Survival for Children and Adolescents with Acute Lymphoblastic Leukemia Between 1990 and 2005: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 1663–1669. [Google Scholar] [CrossRef]
- Kannan, S.; Irwin, M.E.; Herbrich, S.M.; Cheng, T.; Patterson, L.L.; Aitken, M.J.L.; Bhalla, K.; You, M.J.; Konopleva, M.; Zweidler-McKay, P.A.; et al. Targeting the NRF2/HO-1 Antioxidant Pathway in FLT3-ITD-Positive AML Enhances Therapy Efficacy. Antioxidants 2022, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Mata-Greenwood, E.; Cuendet, M.; Sher, D.; Gustin, D.; Stock, W.; Pezzuto, J.M. Brusatol-mediated induction of leukemic cell differentiation and G1 arrest is associated with down-regulation of c-myc. Leukemia 2002, 16, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Hwang, N.; Lang, F.; Zhou, L.; Wong, J.H.-Y.; Singh, R.K.; Jha, H.C.; El-Deiry, W.S.; Du, Y.; Robertson, E.S. Quassinoid analogs with enhanced efficacy for treatment of hematologic malignancies target the PI3Kγ isoform. Commun. Biol. 2020, 3, 267. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Kang, Q.; Pan, C.; Zhang, T.; Feng, C.; Chen, L.; Wei, S.; Wang, J. Nrf2 Overexpression Decreases Vincristine Chemotherapy Sensitivity Through the PI3K-AKT Pathway in Adult B-Cell Acute Lymphoblastic Leukemia. Front. Oncol. 2022, 12, 876556. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Q.; Wang, Y.; Du, L.; Xu, C.; Liu, Q. Brusatol Enhances the Radiosensitivity of A549 Cells by Promoting ROS Production and Enhancing DNA Damage. Int. J. Mol. Sci. 2016, 17, 997. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, Y.; Xu, J.; Lu, J.; Cai, L.; Li, Q.; Wang, C.; Su, Z. Brusatol Inhibits Tumor Growth and Increases the Efficacy of Cabergoline against Pituitary Adenomas. Oxid. Med. Cell. Longev. 2021, 2021, 6696015. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhou, M.; Shen, Y.; Long, Y.; Guo, Y.; Song, L.; Xiao, J. Poor treatment responses were related to poor outcomes in pediatric B cell acute lymphoblastic leukemia with KMT2A rearrangements. BMC Cancer 2022, 22, 859. [Google Scholar] [CrossRef]
- Ding, Y.; Tang, W.; Pei, F.; Fu, L.; Ma, P.; Bai, J.; Lin, M.; Liu, Y.; Hou, Q. Brusatol Derivative–34 Attenuates Allergic Airway Inflammation Via Inhibition of the Spleen Tyrosine Kinase Pathway. Front. Pharmacol. 2021, 12, 587417. [Google Scholar] [CrossRef]
- Joseph, P.L.; Anella, Y.; Patrick, A.B.; Lisa, M.N.; Asen, B.; Min, W.; Allyson, S.; Stacey, T.; Sarah, K.T. Combinatorial efficacy of entospletinib and chemotherapy in patient-derived xenograft models of infant acute lymphoblastic leukemia. Haematologica 2020, 106, 1067–1078. [Google Scholar] [CrossRef]
- Mishra, B.P.; Ansari, K.I.; Mandal, S.S. Dynamic association of MLL1, H3K4 trimethylation with chromatin and Hox gene expression during the cell cycle. FEBS J. 2009, 276, 1629–1640. [Google Scholar] [CrossRef]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue of Somatic Mutations In Cancer. Nucleic Acids Res. 2018, 47, D941–D947. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Dai, N.; He, Q.; Guo, P.; Xiang, Y.; Zhang, Q.; Hong, Z.; Zhang, Q. Comprehensive anti-tumor effect of Brusatol through inhibition of cell viability and promotion of apoptosis caused by autophagy via the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomed. Pharmacother. 2018, 105, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Jasek-Gajda, E.; Jurkowska, H.; Jasińska, M.; Lis, G.J. Targeting the MAPK/ERK and PI3K/AKT Signaling Pathways Affects NRF2, Trx and GSH Antioxidant Systems in Leukemia Cells. Antioxidants 2020, 9, 633. [Google Scholar] [CrossRef] [PubMed]
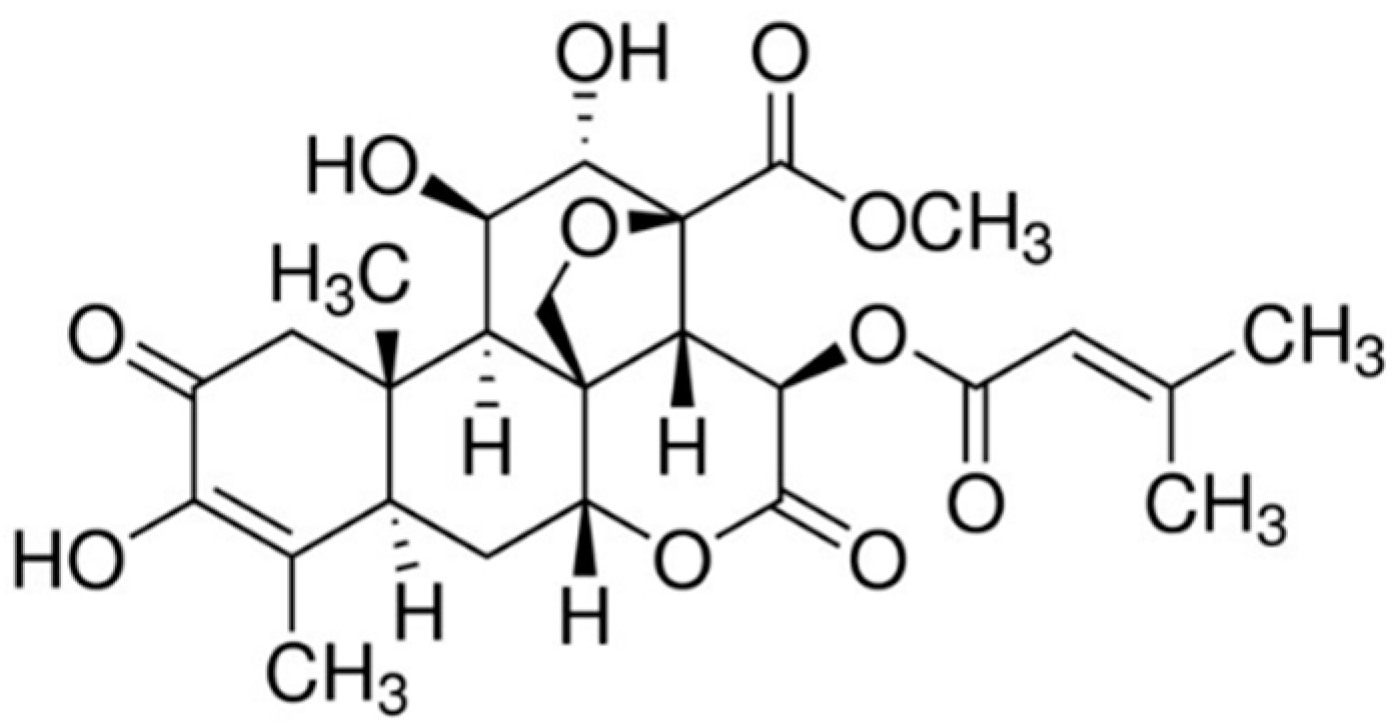



| Sub-G1 (%) | G0/G1 (%) | S (%) | G2/M (%) | ||
|---|---|---|---|---|---|
| KOPN-8 | Control | 0.2 ± 0.2 | 68.4 ± 1.5 | 27.0 ± 1.5 | 4.6 ± 0.4 |
| Bru 2 nM | 0.6 ± 0.6 | 71.6 ± 2.1 | 22.8 ± 1.7 | 5.8 ± 1.0 | |
| Bru 25 nM | 0.0 ± 0.0 | 91.4 ± 1.3 *** | 6.6 ± 1.0 *** | 2.0 ± 0.3 | |
| CEM | Control | 1.2 ± 0.4 | 43.8 ± 1.6 | 43.6 ± 2.3 | 12.6 ± 0.8 |
| Bru 8 nM | 2.6 ± 0.5 | 58.2 ± 2.2 * | 32.4 ± 2.4 | 9.4 ± 1.1 | |
| Bru 25 nM | 9.2 ± 2.6 *** | 62.0 ± 4.8 ** | 31.8 ± 4.5 | 6.2 ± 1.0 * | |
| MOLT-4 | Control | 0.8 ± 0.3 | 44.4 ± 1.9 | 44.8 ± 2.1 | 10.8 ± 0.7 |
| Bru 8 nM | 2.0 ± 0.5 | 59.6 ± 2.0 * | 30.4 ± 0.9 * | 10.0 ± 1.4 | |
| Bru 25 nM | 11.2 ± 3.4 * | 60.8 ± 3.2 * | 33.2 ± 3.6 | 6.0 ± 1.9 |
| Peroxides (DCFH2-DA Fluorescence MFI) | Superoxide Anion (DHE Fluorescence MFI) | Reduced Glutathione (MO Fluorescence MFI) | ||
|---|---|---|---|---|
| KOPN-8 | Control | 474.8 ± 8.2 | 221.6 ± 6.7 | 113.8 ± 4.5 |
| Bru 2 nM | 390.2 ± 24.1 | 238.8 ± 11.5 | 106.2 ± 4.3 | |
| Bru 25 nM | 294.2 ± 10.2 | 106.2 ± 4.3 *** | 69.6 ± 2.0 *** | |
| CEM | Control | 598.2 ± 69.0 | 259.2 ± 81.5 | 104.8 ± 14.3 |
| Bru 8 nM | 562.0 ± 80.9 | 324.4 ± 65.8 | 115.6 ± 9.3 | |
| Bru 25 nM | 503.0 ± 69.4 | 466.2 ± 69.6 | 71.4 ± 16.3 | |
| MOLT-4 | Control | 585.0 ± 104.7 | 164.6 ± 40.2 | 101.2 ± 11.0 |
| Bru 8 nM | 484.8 ± 67.3 | 259.2 ± 65.0 | 110.8 ± 13.5 | |
| Bru 25 nM | 588.4 ± 79.4 | 520.8 ± 73.6 *** | 63.4 ± 7.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorge, J.; Magalhães, N.; Alves, R.; Lapa, B.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B. Antitumor Effect of Brusatol in Acute Lymphoblastic Leukemia Models Is Triggered by Reactive Oxygen Species Accumulation. Biomedicines 2022, 10, 2207. https://doi.org/10.3390/biomedicines10092207
Jorge J, Magalhães N, Alves R, Lapa B, Gonçalves AC, Sarmento-Ribeiro AB. Antitumor Effect of Brusatol in Acute Lymphoblastic Leukemia Models Is Triggered by Reactive Oxygen Species Accumulation. Biomedicines. 2022; 10(9):2207. https://doi.org/10.3390/biomedicines10092207
Chicago/Turabian StyleJorge, Joana, Nisa Magalhães, Raquel Alves, Beatriz Lapa, Ana Cristina Gonçalves, and Ana Bela Sarmento-Ribeiro. 2022. "Antitumor Effect of Brusatol in Acute Lymphoblastic Leukemia Models Is Triggered by Reactive Oxygen Species Accumulation" Biomedicines 10, no. 9: 2207. https://doi.org/10.3390/biomedicines10092207






