The Pathological Mechanisms of Hearing Loss Caused by KCNQ1 and KCNQ4 Variants
Abstract
1. Introduction
2. The Pathologies of JLNS and DFNA2A Hearing Loss
3. The Pathological Roles of Kv7.1 and Kv7.4 Variants and Ongoing Pharmacological Strategies
4. Observations against a Haploinsufficiency-Based Pathological Mechanism
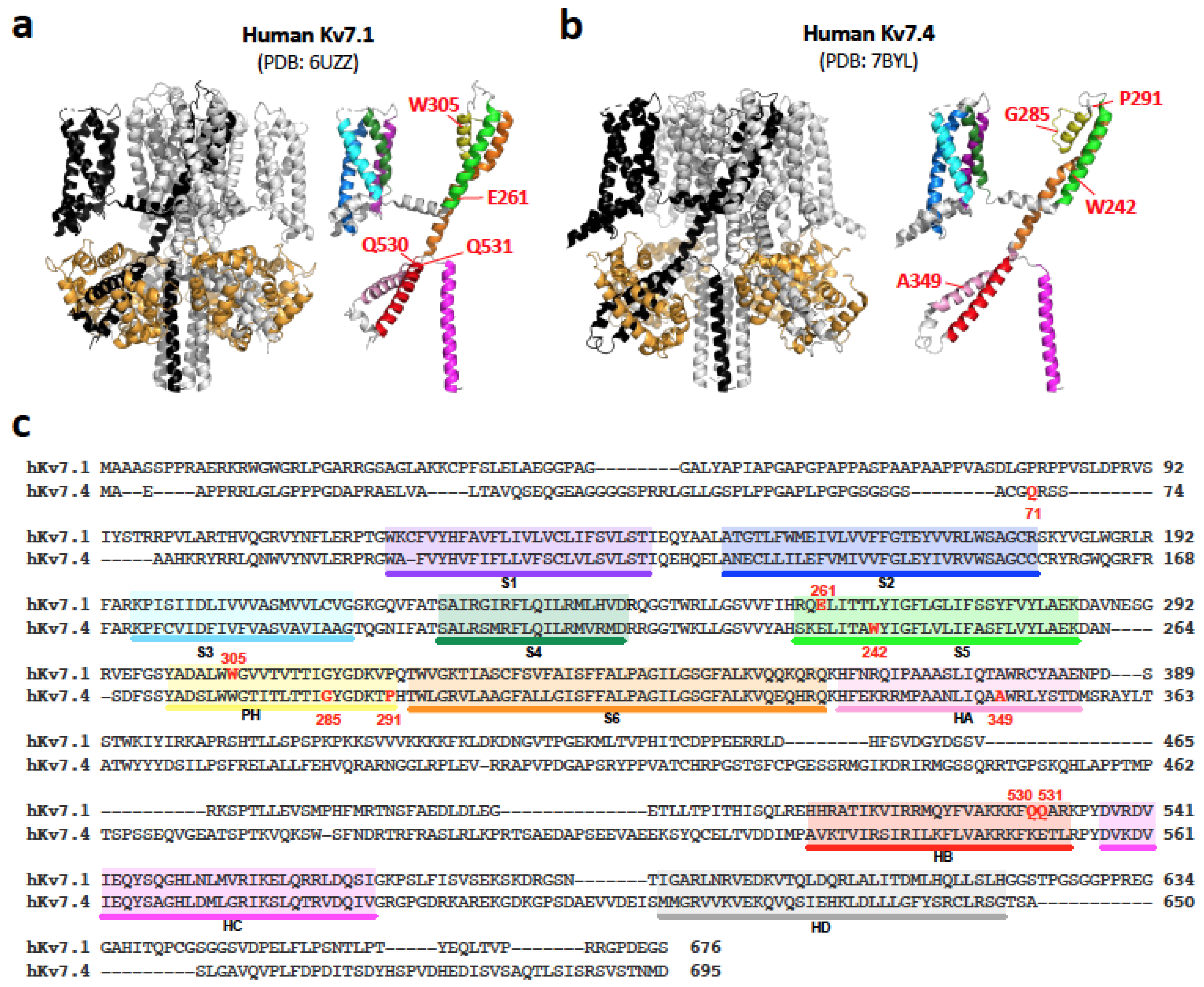
5. Identification of Cell Death-Inducing Cytotoxicity in Truncated Kv7.1 and Kv7.4 Variants
6. The Mechanism Underlying Cell-Death-Inducing Cytotoxicity
7. Updates on the Pathological Mechanisms of JLNS and DFNA2A
8. Remaining Questions and Future Directions
9. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maljevic, S.; Wuttke, T.V.; Seebohm, G.; Lerche, H. KV7 channelopathies. Pflug. Arch. 2010, 460, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Li, Y. KCNQ potassium channels in sensory system and neural circuits. Acta Pharmacol. Sin. 2016, 37, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Curran, M.E.; Splawski, I.; Burn, T.C.; Millholland, J.M.; VanRaay, T.J.; Shen, J.; Timothy, K.W.; Vincent, G.M.; de Jager, T.; et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat. Genet. 1996, 12, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Neyroud, N.; Tesson, F.; Denjoy, I.; Leibovici, M.; Donger, C.; Barhanin, J.; Faure, S.; Gary, F.; Coumel, P.; Petit, C.; et al. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat. Genet. 1997, 15, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Westenskow, P.; Splawski, I.; Timothy, K.W.; Keating, M.T.; Sanguinetti, M.C. Compound mutations: A common cause of severe long-QT syndrome. Circulation 2004, 109, 1834–1841. [Google Scholar] [CrossRef]
- Priori, S.G.; Schwartz, P.J.; Napolitano, C.; Bianchi, L.; Dennis, A.; De Fusco, M.; Brown, A.M.; Casari, G. A recessive variant of the Romano-Ward long-QT syndrome? Circulation 1998, 97, 2420–2425. [Google Scholar] [CrossRef]
- Lang, F.; Vallon, V.; Knipper, M.; Wangemann, P. Functional significance of channels and transporters expressed in the inner ear and kidney. Am. J. Physiol. Cell Physiol. 2007, 293, C1187–C1208. [Google Scholar] [CrossRef]
- Beisel, K.W.; Nelson, N.C.; Delimont, D.C.; Fritzsch, B. Longitudinal gradients of KCNQ4 expression in spiral ganglion and cochlear hair cells correlate with progressive hearing loss in DFNA2. Mol. Brain Res. 2000, 82, 137–149. [Google Scholar] [CrossRef]
- Kharkovets, T.; Hardelin, J.P.; Safieddine, S.; Schweizer, M.; El-Amraoui, A.; Petit, C.; Jentsch, T.J. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc. Natl. Acad. Sci. USA 2000, 97, 4333–4338. [Google Scholar] [CrossRef]
- Brownell, W.E.; Bader, C.R.; Bertrand, D.; de Ribaupierre, Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science 1985, 227, 194–196. [Google Scholar] [CrossRef]
- Evans, B.N.; Dallos, P. Stereocilia displacement induced somatic motility of cochlear outer hair cells. Proc. Natl. Acad. Sci. USA 1993, 90, 8347–8351. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Beurg, M.; Marcotti, W.; Fettiplace, R. Prestin-driven cochlear amplification is not limited by the outer hair cell membrane time constant. Neuron 2011, 70, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.M.; Dodson, K.M. Genetics of hearing loss: Focus on DFNA2. Appl. Clin. Genet. 2012, 5, 97–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stenson, P.D.; Mort, M.; Ball, E.V.; Evans, K.; Hayden, M.; Heywood, S.; Hussain, M.; Phillips, A.D.; Cooper, D.N. The Human Gene Mutation Database: Towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum. Genet. 2017, 136, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Wangemann, P. Supporting sensory transduction: Cochlear fluid homeostasis and the endocochlear potential. J. Physiol. 2006, 576, 11–21. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Zhao, X.; He, D.Z. Endolymphatic Pote.ential Measured From Developing and Adult Mouse Inner Ear. Front. Cell Neurosci 2020, 14, 584928. [Google Scholar] [CrossRef]
- Lee, M.P.; Ravenel, J.D.; Hu, R.J.; Lustig, L.R.; Tomaselli, G.; Berger, R.D.; Brandenburg, S.A.; Litzi, T.J.; Bunton, T.E.; Limb, C.; et al. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J. Clin. Investig. 2000, 106, 1447–1455. [Google Scholar] [CrossRef]
- Casimiro, M.C.; Knollmann, B.C.; Ebert, S.N.; Vary, J.C., Jr.; Greene, A.E.; Franz, M.R.; Grinberg, A.; Huang, S.P.; Pfeifer, K. Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen Syndrome. Proc. Natl. Acad. Sci. USA 2001, 98, 2526–2531. [Google Scholar] [CrossRef]
- Rivas, A.; Francis, H.W. Inner ear abnormalities in a Kcnq1 (Kvlqt1) knockout mouse: A model of Jervell and Lange-Nielsen syndrome. Otol. Neurotol. 2005, 26, 415–424. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, J.; Li, Q.; Kim, Y.; Zhou, B.; Wang, Y.; Li, H.; Lin, X. Virally mediated Kcnq1 gene replacement therapy in the immature scala media restores hearing in a mouse model of human Jervell and Lange-Nielsen deafness syndrome. EMBO Mol. Med. 2015, 7, 1077–1086. [Google Scholar] [CrossRef]
- Kharkovets, T.; Dedek, K.; Maier, H.; Schweizer, M.; Khimich, D.; Nouvian, R.; Vardanyan, V.; Leuwer, R.; Moser, T.; Jentsch, T.J. Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. EMBO J. 2006, 25, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Carignano, C.; Barila, E.P.; Rias, E.I.; Dionisio, L.; Aztiria, E.; Spitzmaul, G. Inner Hair Cell and Neuron Degeneration Contribute to Hearing Loss in a DFNA2-Like Mouse Model. Neuroscience 2019, 410, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.J.; Clark, K.A.; Holton, J.M.; Minor, D.L., Jr. Structural insight into KCNQ (Kv7) channel assembly and channelopathy. Neuron 2007, 53, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Wiener, R.; Haitin, Y.; Shamgar, L.; Fernandez-Alonso, M.C.; Martos, A.; Chomsky-Hecht, O.; Rivas, G.; Attali, B.; Hirsch, J.A. The KCNQ1 (Kv7.1) COOH terminus, a multitiered scaffold for subunit assembly and protein interaction. J. Biol. Chem. 2008, 283, 5815–5830. [Google Scholar] [CrossRef]
- Xu, Q.; Minor, D.L., Jr. Crystal structure of a trimeric form of the K(V)7.1 (KCNQ1) A-domain tail coiled-coil reveals structural plasticity and context dependent changes in a putative coiled-coil trimerization motif. Protein Sci. 2009, 18, 2100–2114. [Google Scholar] [CrossRef]
- Xu, Q.; Chang, A.; Tolia, A.; Minor, D.L., Jr. Structure of a Ca2+/CaM:Kv7.4 (KCNQ4) B-helix complex provides insight into M current modulation. J. Mol. Biol. 2013, 425, 378–394. [Google Scholar] [CrossRef]
- Sachyani, D.; Dvir, M.; Strulovich, R.; Tria, G.; Tobelaim, W.; Peretz, A.; Pongs, O.; Svergun, D.; Attali, B.; Hirsch, J.A. Structural basis of a Kv7.1 potassium channel gating module: Studies of the intracellular c-terminal domain in complex with calmodulin. Structure 2014, 22, 1582–1594. [Google Scholar] [CrossRef]
- Sun, J.; MacKinnon, R. Cryo-EM Structure of a KCNQ1/CaM Complex Reveals Insights into Congenital Long QT Syndrome. Cell 2017, 169, 1042–1050 e1049. [Google Scholar] [CrossRef]
- Sun, J.; MacKinnon, R. Structural Basis of Human KCNQ1 Modulation and Gating. Cell 2020, 180, 340–347.e9. [Google Scholar] [CrossRef]
- Li, T.; Wu, K.; Yue, Z.; Wang, Y.; Zhang, F.; Shen, H. Structural Basis for the Modulation of Human KCNQ4 by Small-Molecule Drugs. Mol. Cell 2021, 81, 25–37.e4. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, H.; Chen, Y.; Dong, S.; Wang, F.; Wang, S.; Li, G.L.; Shu, Y.; Xu, F. Structural insights into the lipid and ligand regulation of a human neuronal KCNQ channel. Neuron 2022, 110, 237–247.e4. [Google Scholar] [CrossRef]
- Salata, J.J.; Jurkiewicz, N.K.; Wang, J.; Evans, B.E.; Orme, H.T.; Sanguinetti, M.C. A novel benzodiazepine that activates cardiac slow delayed rectifier K+ currents. Mol. Pharmacol. 1998, 54, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Salata, J.J.; Wang, J.; Wu, Y.; Yan, G.X.; Liu, T.; Marinchak, R.A.; Kowey, P.R. Increasing I(Ks) corrects abnormal repolarization in rabbit models of acquired LQT2 and ventricular hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H664–H670. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, T.V.; Lerche, H. Novel anticonvulsant drugs targeting voltage-dependent ion channels. Expert Opin. Investig. Drugs 2006, 15, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Sun, H.; Li, M. Zinc pyrithione-mediated activation of voltage-gated KCNQ potassium channels rescues epileptogenic mutants. Nat. Chem. Biol. 2007, 3, 287–296. [Google Scholar] [CrossRef]
- Leitner, M.G.; Feuer, A.; Ebers, O.; Schreiber, D.N.; Halaszovich, C.R.; Oliver, D. Restoration of ion channel function in deafness-causing KCNQ4 mutants by synthetic channel openers. Br. J. Pharmacol. 2012, 165, 2244–2259. [Google Scholar] [CrossRef]
- Tian, C.; Zhu, R.; Zhu, L.; Qiu, T.; Cao, Z.; Kang, T. Potassium channels: Structures, diseases, and modulators. Chem. Biol. Drug Des. 2014, 83, 1–26. [Google Scholar] [CrossRef]
- Imbrici, P.; Liantonio, A.; Camerino, G.M.; De Bellis, M.; Camerino, C.; Mele, A.; Giustino, A.; Pierno, S.; De Luca, A.; Tricarico, D.; et al. Therapeutic Approaches to Genetic Ion Channelopathies and Perspectives in Drug Discovery. Front. Pharmacol. 2016, 7, 121. [Google Scholar] [CrossRef]
- Miceli, F.; Soldovieri, M.V.; Ambrosino, P.; Manocchio, L.; Mosca, I.; Taglialatela, M. Pharmacological Targeting of Neuronal Kv7.2/3 Channels: A Focus on Chemotypes and Receptor Sites. Curr. Med. Chem. 2018, 25, 2637–2660. [Google Scholar] [CrossRef]
- Barrese, V.; Stott, J.B.; Greenwood, I.A. KCNQ-Encoded Potassium Channels as Therapeutic Targets. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 625–648. [Google Scholar] [CrossRef]
- Shin, D.H.; Jung, J.; Koh, Y.I.; Rim, J.H.; Lee, J.S.; Choi, H.J.; Joo, S.Y.; Yu, S.; Cha, D.H.; Lee, S.Y.; et al. A recurrent mutation in KCNQ4 in Korean families with nonsyndromic hearing loss and rescue of the channel activity by KCNQ activators. Hum. Mutat. 2019, 40, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wu, M.; Townsend, S.D.; Zou, B.; Long, S.; Daniels, J.S.; McManus, O.B.; Li, M.; Lindsley, C.W.; Hopkins, C.R. Discovery, Synthesis, and Structure Activity Relationship of a Series of N-Aryl- bicyclo[2.2.1]heptane-2-carboxamides: Characterization of ML213 as a Novel KCNQ2 and KCNQ4 Potassium Channel Opener. ACS Chem. Neurosci. 2011, 2, 572–577. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Hu, F.; Yang, J.; Guo, X.; Hou, X.; Ju, C.; Wang, K. Activation of Neuronal Voltage-Gated Potassium Kv7/KCNQ/M-Current by a Novel Channel Opener SCR2682 for Alleviation of Chronic Pain. J. Pharmacol. Exp. Ther. 2021, 377, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Kamada, F.; Kure, S.; Kudo, T.; Suzuki, Y.; Oshima, T.; Ichinohe, A.; Kojima, K.; Niihori, T.; Kanno, J.; Narumi, Y.; et al. A novel KCNQ4 one-base deletion in a large pedigree with hearing loss: Implication for the genotype-phenotype correlation. J. Hum. Genet. 2006, 51, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M.S.; Tack, D.; McMordie, S.J.; DeLuca, A.; Hur, I.A.; Nishimura, C.; Huygen, P.; Casavant, T.L.; Smith, R.J. Audioprofile-directed screening identifies novel mutations in KCNQ4 causing hearing loss at the DFNA2 locus. Genet. Med. 2008, 10, 797–804. [Google Scholar] [CrossRef]
- Wasano, K.; Mutai, H.; Obuchi, C.; Masuda, S.; Matsunaga, T. A novel frameshift mutation in KCNQ4 in a family with autosomal recessive non-syndromic hearing loss. Biochem. Biophys. Res. Commun. 2015, 463, 582–586. [Google Scholar] [CrossRef]
- Kojima, T.; Wasano, K.; Takahashi, S.; Homma, K. Cell death-inducing cytotoxicity in truncated KCNQ4 variants associated with DFNA2 hearing loss. Dis. Model. Mech. 2021, 14, dmm049015. [Google Scholar] [CrossRef]
- Tranebjaerg, L.; Bathen, J.; Tyson, J.; Bitner-Glindzicz, M. Jervell and Lange-Nielsen syndrome: A Norwegian perspective. Am. J. Med. Genet. 1999, 89, 137–146. [Google Scholar] [CrossRef]
- Huang, L.; Bitner-Glindzicz, M.; Tranebjaerg, L.; Tinker, A. A spectrum of functional effects for disease causing mutations in the Jervell and Lange-Nielsen syndrome. Cardiovasc. Res. 2001, 51, 670–680. [Google Scholar] [CrossRef]
- Wilson, A.J.; Quinn, K.V.; Graves, F.M.; Bitner-Glindzicz, M.; Tinker, A. Abnormal KCNQ1 trafficking influences disease pathogenesis in hereditary long QT syndromes (LQT1). Cardiovasc. Res. 2005, 67, 476–486. [Google Scholar] [CrossRef]
- Davis, S.N.; Wu, P.; Camci, E.D.; Simon, J.A.; Rubel, E.W.; Raible, D.W. Chloroquine kills hair cells in zebrafish lateral line and murine cochlear cultures: Implications for ototoxicity. Hear. Res. 2020, 395, 108019. [Google Scholar] [CrossRef] [PubMed]
- Vincent, G.M.; Timothy, K.W.; Leppert, M.; Keating, M. The spectrum of symptoms and QT intervals in carriers of the gene for the long-QT syndrome. N. Engl. J. Med. 1992, 327, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Vincent, G.M. Long QT syndrome. Cardiol. Clin. 2000, 18, 309–325. [Google Scholar] [CrossRef]
- Priori, S.G.; Napolitano, C.; Schwartz, P.J. Low penetrance in the long-QT syndrome: Clinical impact. Circulation 1999, 99, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, L.; Bryant, R.M.; Vincent, G.M.; Flippin, M.; Lee, J.C.; Brown, E.; Zimmerman, F.; Rozich, R.; Szafranski, P.; et al. KCNQ1 mutations in patients with a family history of lethal cardiac arrhythmias and sudden death. Clin. Genet. 2003, 63, 273–282. [Google Scholar] [CrossRef]
- Attili, V.S.; Bapsy, P.P.; Anupama, G.; Lokanatha, D. Irreversible sensorineural hearing loss due to Imatinib. Leuk. Res. 2008, 32, 991–992. [Google Scholar] [CrossRef]
- Wasif, K.; Wasif, N.; Saif, M.W. Imatinib-induced Ototoxicity in a Patient with Gastrointestinal Stromal Tumor (GIST). Cureus 2016, 8, e848. [Google Scholar] [CrossRef]
- Kubisch, C.; Schroeder, B.C.; Friedrich, T.; Lutjohann, B.; El-Amraoui, A.; Marlin, S.; Petit, C.; Jentsch, T.J. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 1999, 96, 437–446. [Google Scholar] [CrossRef]
- Baek, J.I.; Park, H.J.; Park, K.; Choi, S.J.; Lee, K.Y.; Yi, J.H.; Friedman, T.B.; Drayna, D.; Shin, K.S.; Kim, U.K. Pathogenic effects of a novel mutation (c.664_681del) in KCNQ4 channels associated with auditory pathology. Biochim. Biophys. Acta 2011, 1812, 536–543. [Google Scholar] [CrossRef]
- Kim, H.J.; Lv, P.; Sihn, C.R.; Yamoah, E.N. Cellular and molecular mechanisms of autosomal dominant form of progressive hearing loss, DFNA2. J. Biol. Chem. 2011, 286, 1517–1527. [Google Scholar] [CrossRef]
- Gao, Y.; Yechikov, S.; Vazquez, A.E.; Chen, D.; Nie, L. Impaired surface expression and conductance of the KCNQ4 channel lead to sensorineural hearing loss. J. Cell. Mol. Med. 2013, 17, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Lin, H.; Koh, Y.I.; Ryu, K.; Lee, J.S.; Rim, J.H.; Choi, H.J.; Lee, H.J.; Kim, H.Y.; Yu, S.; et al. Rare KCNQ4 variants found in public databases underlie impaired channel activity that may contribute to hearing impairment. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Zhang, J.; Pang, W.; Wang, S.; Lee, K.Y.; Cavaretta, J.P.; Walters, J.; Procko, E.; Tsai, N.P.; Chung, H.J. Reduced axonal surface expression and phosphoinositide sensitivity in Kv7 channels disrupts their function to inhibit neuronal excitability in Kcnq2 epileptic encephalopathy. Neurobiol. Dis. 2018, 118, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Mencia, A.; Gonzalez-Nieto, D.; Modamio-Hoybjor, S.; Etxeberria, A.; Aranguez, G.; Salvador, N.; Del Castillo, I.; Villarroel, A.; Moreno, F.; Barrio, L.; et al. A novel KCNQ4 pore-region mutation (p.G296S) causes deafness by impairing cell-surface channel expression. Hum. Genet. 2008, 123, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, K.M.; Yang, L.; Dong, Q.; Yu, J.T. Tauopathies: New perspectives and challenges. Mol. Neurodegener. 2022, 17, 28. [Google Scholar] [CrossRef]
- Wang, R.R.; Li, N.; Zhang, Y.H.; Wang, L.L.; Teng, S.Y.; Pu, J.L. Novel compound heterozygous mutations T2C and 1149insT in the KCNQ1 gene cause Jervell and Lange-Nielsen syndrome. Int. J. Mol. Med. 2011, 28, 41–46. [Google Scholar] [CrossRef]
- Nishimura, M.; Ueda, M.; Ebata, R.; Utsuno, E.; Ishii, T.; Matsushita, K.; Ohara, O.; Shimojo, N.; Kobayashi, Y.; Nomura, F. A novel KCNQ1 nonsense variant in the isoform-specific first exon causes both jervell and Lange-Nielsen syndrome 1 and long QT syndrome 1: A case report. BMC Med. Genet. 2017, 18, 66. [Google Scholar] [CrossRef]
- Wang, C.; Lu, Y.; Cheng, J.; Zhang, L.; Liu, W.; Peng, W.; Zhang, D.; Duan, H.; Han, D.; Yuan, H. Identification of KCNQ1 compound heterozygous mutations in three Chinese families with Jervell and Lange-Nielsen Syndrome. Acta Otolaryngol. 2017, 137, 522–528. [Google Scholar] [CrossRef]
- Vyas, B.; Puri, R.D.; Namboodiri, N.; Nair, M.; Sharma, D.; Movva, S.; Saxena, R.; Bohora, S.; Aggarwal, N.; Vora, A.; et al. KCNQ1 mutations associated with Jervell and Lange-Nielsen syndrome and autosomal recessive Romano-Ward syndrome in India-expanding the spectrum of long QT syndrome type 1. Am. J. Med. Genet. A 2016, 170, 1510–1519. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Moss, A.J.; Robinson, J.; Zareba, W.; Knilans, T.; Bowles, N.E.; Towbin, J.A. Compound heterozygous mutations in KvLQT1 cause Jervell and Lange-Nielsen syndrome. Mol. Genet. Metab. 2002, 75, 308–316. [Google Scholar] [CrossRef]
- Eldstrom, J.; Xu, H.; Werry, D.; Kang, C.; Loewen, M.E.; Degenhardt, A.; Sanatani, S.; Tibbits, G.F.; Sanders, C.; Fedida, D. Mechanistic basis for LQT1 caused by S3 mutations in the KCNQ1 subunit of IKs. J. Gen. Physiol. 2010, 135, 433–448. [Google Scholar] [CrossRef]
- Mohammad-Panah, R.; Demolombe, S.; Neyroud, N.; Guicheney, P.; Kyndt, F.; van den Hoff, M.; Baro, I.; Escande, D. Mutations in a dominant-negative isoform correlate with phenotype in inherited cardiac arrhythmias. Am. J. Hum. Genet. 1999, 64, 1015–1023. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chouabe, C.; Neyroud, N.; Richard, P.; Denjoy, I.; Hainque, B.; Romey, G.; Drici, M.D.; Guicheney, P.; Barhanin, J. Novel mutations in KvLQT1 that affect Iks activation through interactions with Isk. Cardiovasc. Res. 2000, 45, 971–980. [Google Scholar] [CrossRef]
- Park, K.H.; Piron, J.; Dahimene, S.; Merot, J.; Baro, I.; Escande, D.; Loussouarn, G. Impaired KCNQ1-KCNE1 and phosphatidylinositol-4,5-bisphosphate interaction underlies the long QT syndrome. Circ. Res. 2005, 96, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Sakaguchi, T.; Ding, W.G.; Watanabe, E.; Watanabe, I.; Nishio, Y.; Makiyama, T.; Ohno, S.; Akao, M.; Higashi, Y.; et al. Latent genetic backgrounds and molecular pathogenesis in drug-induced long-QT syndrome. Circ. Arrhythm. Electrophysiol. 2009, 2, 511–523. [Google Scholar] [CrossRef]
- Coyan, F.C.; Abderemane-Ali, F.; Amarouch, M.Y.; Piron, J.; Mordel, J.; Nicolas, C.S.; Steenman, M.; Merot, J.; Marionneau, C.; Thomas, A.; et al. A long QT mutation substitutes cholesterol for phosphatidylinositol-4,5-bisphosphate in KCNQ1 channel regulation. PLoS ONE 2014, 9, e93255. [Google Scholar] [CrossRef]
- Coto, E.; Garcia-Fernandez, F.J.; Calvo, D.; Salgado-Aranda, R.; Martin-Gonzalez, J.; Alonso, B.; Iglesias, S.; Gomez, J. An elderly Jervell and Lange-Nielsen patient heterozygous compound for two new KCNQ1 mutations. Am. J. Med. Genet. A 2017, 173, 749–752. [Google Scholar] [CrossRef]
- Tyson, J.; Tranebjaerg, L.; McEntagart, M.; Larsen, L.A.; Christiansen, M.; Whiteford, M.L.; Bathen, J.; Aslaksen, B.; Sorland, S.J.; Lund, O.; et al. Mutational spectrum in the cardioauditory syndrome of Jervell and Lange-Nielsen. Hum. Genet. 2000, 107, 499–503. [Google Scholar] [CrossRef]
- Neyroud, N.; Denjoy, I.; Donger, C.; Gary, F.; Villain, E.; Leenhardt, A.; Benali, K.; Schwartz, K.; Coumel, P.; Guicheney, P. Heterozygous mutation in the pore of potassium channel gene KvLQT1 causes an apparently normal phenotype in long QT syndrome. Eur. J. Hum. Genet. 1998, 6, 129–133. [Google Scholar] [CrossRef][Green Version]
- Chouabe, C.; Neyroud, N.; Guicheney, P.; Lazdunski, M.; Romey, G.; Barhanin, J. Properties of KvLQT1 K+ channel mutations in Romano-Ward and Jervell and Lange-Nielsen inherited cardiac arrhythmias. EMBO J. 1997, 16, 5472–5479. [Google Scholar] [CrossRef]
- Matsuda, S.; Ohnuki, Y.; Okami, M.; Ochiai, E.; Yamada, S.; Takahashi, K.; Osawa, M.; Okami, K.; Iida, M.; Mochizuki, H. Jervell and Lange-Nielsen syndrome with novel KCNQ1 and additional gene mutations. Hum. Genome Var. 2020, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Yang, Y.; Wang, J.; Gao, X.; Fan, M. The combined novel KCNQ1 frameshift I145Sfs*92 and nonsense W392X variants caused Jervell and Lange-Nielsen syndrome in a Chinese infant presenting with sustained foetal bradycardia. Europace 2020, 22, 1880–1884. [Google Scholar] [CrossRef] [PubMed]
- Harmer, S.C.; Mohal, J.S.; Royal, A.A.; McKenna, W.J.; Lambiase, P.D.; Tinker, A. Cellular mechanisms underlying the increased disease severity seen for patients with long QT syndrome caused by compound mutations in KCNQ1. Biochem. J. 2014, 462, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chen, S.; Wu, X.; Zhang, W.J.; Xie, W.; Jin, Y.; Xie, L.; Xu, K.; Bai, X.; Zhang, H.M.; et al. Jervell and Lange-Nielsen Syndrome due to a Novel Compound Heterozygous KCNQ1 Mutation in a Chinese Family. Neural. Plast. 2020, 2020, 3569359. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, D.; Gingell, R.L.; Moss, A.J.; Napolitano, C.; Priori, S.G.; Schwartz, P.J.; Kehoe, E.; Robinson, J.L.; Schulze-Bahr, E.; et al. Homozygous deletion in KVLQT1 associated with Jervell and Lange-Nielsen syndrome. Circulation 1999, 99, 1344–1347. [Google Scholar] [CrossRef]
- Amirian, A.; Zafari, Z.; Dalili, M.; Saber, S.; Karimipoor, M.; Dabbagh Bagheri, S.; Fazelifar, A.F.; Zeinali, S. Detection of a new KCNQ1 frameshift mutation associated with Jervell and Lange-Nielsen syndrome in 2 Iranian families. J. Arrhythm. 2018, 34, 286–290. [Google Scholar] [CrossRef]
- Al-Aama, J.Y.; Al-Ghamdi, S.; Bdier, A.Y.; Wilde, A.A.; Bhuiyan, Z.A. De novo mutation in the KCNQ1 gene causal to Jervell and Lange-Nielsen syndrome. Clin. Genet. 2014, 86, 492–495. [Google Scholar] [CrossRef]
- Chung, S.K.; MacCormick, J.M.; McCulley, C.H.; Crawford, J.; Eddy, C.A.; Mitchell, E.A.; Shelling, A.N.; French, J.K.; Skinner, J.R.; Rees, M.I. Long QT and Brugada syndrome gene mutations in New Zealand. Heart Rhythm. 2007, 4, 1306–1314. [Google Scholar] [CrossRef]
- Wei, J.; Fish, F.A.; Myerburg, R.J.; Roden, D.M.; George, A.L., Jr. Novel KCNQ1 mutations associated with recessive and dominant congenital long QT syndromes: Evidence for variable hearing phenotype associated with R518X. Hum. Mutat. 2000, 15, 387–388. [Google Scholar] [CrossRef]
- Gao, Y.; Li, C.; Liu, W.; Wu, R.; Qiu, X.; Liang, R.; Li, L.; Zhang, L.; Hu, D. Genotype-phenotype analysis of three Chinese families with Jervell and Lange-Nielsen syndrome. J. Cardiovasc. Dis. Res. 2012, 3, 67–75. [Google Scholar] [CrossRef]
- Vojdani, S.; Amirsalari, S.; Milanizadeh, S.; Molaei, F.; Ajalloueyane, M.; Khosravi, A.; Hamzehzadeh, L.; Ghasemi, M.M.; Talee, M.R.; Abbaszadegan, M.R. Mutation Screening of KCNQ1 and KCNE1 Genes in Iranian Patients With Jervell and Lange-Nielsen Syndrome. Fetal Pediatr. Pathol. 2019, 38, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Splawski, I.; Timothy, K.W.; Vincent, G.M.; Atkinson, D.L.; Keating, M.T. Molecular basis of the long-QT syndrome associated with deafness. N. Engl. J. Med. 1997, 336, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Kubota, T.; Yoshida, H.; Tsuji, K.; Makiyama, T.; Yamada, S.; Kuga, K.; Yamaguchi, I.; Kita, T.; Horie, M. A novel mutation associated with Jervell and Lange-Nielsen syndrome in a Japanese family. Circ. J. 2008, 72, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Safka Brozkova, D.; Uhrova Meszarosova, A.; Lassuthova, P.; Varga, L.; Stanek, D.; Borecka, S.; Lastuvkova, J.; Cejnova, V.; Raskova, D.; Lhota, F.; et al. The Cause of Hereditary Hearing Loss in GJB2 Heterozygotes-A Comprehensive Study of the GJB2/DFNB1 Region. Genes 2021, 12, 684. [Google Scholar] [CrossRef]
- Iwasa, Y.I.; Nishio, S.Y.; Usami, S.I. Comprehensive Genetic Analysis of Japanese Autosomal Dominant Sensorineural Hearing Loss Patients. PLoS ONE 2016, 11, e0166781. [Google Scholar] [CrossRef]
- Su, C.C.; Yang, J.J.; Shieh, J.C.; Su, M.C.; Li, S.Y. Identification of novel mutations in the KCNQ4 gene of patients with nonsyndromic deafness from Taiwan. Audiol. Neurootol. 2007, 12, 20–26. [Google Scholar] [CrossRef]
- Walls, W.D.; Moteki, H.; Thomas, T.R.; Nishio, S.Y.; Yoshimura, H.; Iwasa, Y.; Frees, K.L.; Nishimura, C.J.; Azaiez, H.; Booth, K.T.; et al. A comparative analysis of genetic hearing loss phenotypes in European/American and Japanese populations. Hum. Genet. 2020, 139, 1315–1323. [Google Scholar] [CrossRef]
- Sloan-Heggen, C.M.; Bierer, A.O.; Shearer, A.E.; Kolbe, D.L.; Nishimura, C.J.; Frees, K.L.; Ephraim, S.S.; Shibata, S.B.; Booth, K.T.; Campbell, C.A.; et al. Comprehensive genetic testing in the clinical evaluation of 1119 patients with hearing loss. Hum. Genet. 2016, 135, 441–450. [Google Scholar] [CrossRef]
- Naito, T.; Nishio, S.Y.; Iwasa, Y.; Yano, T.; Kumakawa, K.; Abe, S.; Ishikawa, K.; Kojima, H.; Namba, A.; Oshikawa, C.; et al. Comprehensive genetic screening of KCNQ4 in a large autosomal dominant nonsyndromic hearing loss cohort: Genotype-phenotype correlations and a founder mutation. PLoS ONE 2013, 8, e63231. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, Q.; Jia, Y.; Shu, Y.; Yang, J.; Yang, H.; Yan, Z. Molecular basis and restoration of function deficiencies of Kv7.4 variants associated with inherited hearing loss. Hear. Res. 2020, 388, 107884. [Google Scholar] [CrossRef]
- Uehara, D.T.; Freitas, E.L.; Alves, L.U.; Mazzeu, J.F.; Auricchio, M.T.; Tabith, A., Jr.; Monteiro, M.L.; Rosenberg, C.; Mingroni-Netto, R.C. A novel KCNQ4 mutation and a private IMMP2L-DOCK4 duplication segregating with nonsyndromic hearing loss in a Brazilian family. Hum. Genome Var. 2015, 2, 15038. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Choi, H.B.; Koh, Y.I.; Rim, J.H.; Choi, H.J.; Kim, S.H.; Lee, J.H.; An, J.; Kim, A.; Lee, J.S.; et al. Whole-exome sequencing identifies two novel mutations in KCNQ4 in individuals with nonsyndromic hearing loss. Sci. Rep. 2018, 8, 16659. [Google Scholar] [CrossRef] [PubMed]
- Namba, K.; Mutai, H.; Kaneko, H.; Hashimoto, S.; Matsunaga, T. In silico modeling of the pore region of a KCNQ4 missense mutant from a patient with hearing loss. BMC Res. Notes. 2012, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Van Hauwe, P.; Coucke, P.J.; Ensink, R.J.; Huygen, P.; Cremers, C.W.; Van Camp, G. Mutations in the KCNQ4 K+ channel gene, responsible for autosomal dominant hearing loss, cluster in the channel pore region. Am. J. Med. Genet. 2000, 93, 184–187. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Yi, Y.; Gao, Y.; Liu, Q.; Wang, D.; Li, Q.; Lan, L.; Li, N.; Guan, J.; et al. Targeted high-throughput sequencing identifies pathogenic mutations in KCNQ4 in two large Chinese families with autosomal dominant hearing loss. PLoS ONE 2014, 9, e103133. [Google Scholar] [CrossRef]
- Plevova, P.; Paprskarova, M.; Tvrda, P.; Turska, P.; Slavkovsky, R.; Mrazkova, E. STRC Deletion is a Frequent Cause of Slight to Moderate Congenital Hearing Impairment in the Czech Republic. Otol. Neurotol. 2017, 38, e393–e400. [Google Scholar] [CrossRef]
- Sommen, M.; Schrauwen, I.; Vandeweyer, G.; Boeckx, N.; Corneveaux, J.J.; van den Ende, J.; Boudewyns, A.; De Leenheer, E.; Janssens, S.; Claes, K.; et al. DNA Diagnostics of Hereditary Hearing Loss: A Targeted Resequencing Approach Combined with a Mutation Classification System. Hum. Mutat. 2016, 37, 812–819. [Google Scholar] [CrossRef]
- Coucke, P.J.; Van Hauwe, P.; Kelley, P.M.; Kunst, H.; Schatteman, I.; Van Velzen, D.; Meyers, J.; Ensink, R.J.; Verstreken, M.; Declau, F.; et al. Mutations in the KCNQ4 gene are responsible for autosomal dominant deafness in four DFNA2 families. Hum. Mol. Genet. 1999, 8, 1321–1328. [Google Scholar] [CrossRef]
- Talebizadeh, Z.; Kelley, P.M.; Askew, J.W.; Beisel, K.W.; Smith, S.D. Novel mutation in the KCNQ4 gene in a large kindred with dominant progressive hearing loss. Hum. Mutat. 1999, 14, 493–501. [Google Scholar] [CrossRef]
- Bal, M.; Zhang, J.; Zaika, O.; Hernandez, C.C.; Shapiro, M.S. Homomeric and heteromeric assembly of KCNQ (Kv7) K+ channels assayed by total internal reflection fluorescence/fluorescence resonance energy transfer and patch clamp analysis. J. Biol. Chem. 2008, 283, 30668–30676. [Google Scholar] [CrossRef]
- Smedley, D.; Smith, K.R.; Martin, A.; Thomas, E.A.; McDonagh, E.M.; Cipriani, V.; Ellingford, J.M.; Arno, G.; Tucci, A.; Vandrovcova, J.; et al. 100,000 Genomes Pilot on Rare-Disease Diagnosis in Health Care—Preliminary Report. N. Engl. J. Med. 2021, 385, 1868–1880. [Google Scholar] [CrossRef]
- Arnett, J.; Emery, S.B.; Kim, T.B.; Boerst, A.K.; Lee, K.; Leal, S.M.; Lesperance, M.M. Autosomal dominant progressive sensorineural hearing loss due to a novel mutation in the KCNQ4 gene. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, Y.; Gao, X.; Xu, J.; Dai, P.; Zhu, Q.; Yuan, Y. A novel pore-region mutation, c.887G > A (p.G296D) in KCNQ4, causing hearing loss in a Chinese family with autosomal dominant non-syndromic deafness 2. BMC Med. Genet. 2017, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.; Lenarduzzi, S.; Cappellani, S.; Pecile, V.; Morgutti, M.; Orzan, E.; Ghiselli, S.; Ambrosetti, U.; Brumat, M.; Gajendrarao, P.; et al. Genomic Studies in a Large Cohort of Hearing Impaired Italian Patients Revealed Several New Alleles, a Rare Case of Uniparental Disomy (UPD) and the Importance to Search for Copy Number Variations. Front. Genet. 2018, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Choi, H.B.; Park, M.; Choi, I.S.; An, J.; Kim, A.; Kim, E.; Kim, N.; Han, J.H.; Kim, M.Y.; et al. Novel KCNQ4 variants in different functional domains confer genotype- and mechanism-based therapeutics in patients with nonsyndromic hearing loss. Exp. Mol. Med. 2021, 53, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Boucher, S.; Tai, F.W.J.; Delmaghani, S.; Lelli, A.; Singh-Estivalet, A.; Dupont, T.; Niasme-Grare, M.; Michel, V.; Wolff, N.; Bahloul, A.; et al. Ultrarare heterozygous pathogenic variants of genes causing dominant forms of early-onset deafness underlie severe presbycusis. Proc. Natl. Acad. Sci. USA 2020, 117, 31278–31289. [Google Scholar] [CrossRef]
- Khan, A.; Han, S.; Wang, R.; Ansar, M.; Ahmad, W.; Zhang, X. Sequence variants in genes causing nonsyndromic hearing loss in a Pakistani cohort. Mol. Genet. Genom. Med. 2019, 7, e917. [Google Scholar] [CrossRef]
- Van Laer, L.; Carlsson, P.I.; Ottschytsch, N.; Bondeson, M.L.; Konings, A.; Vandevelde, A.; Dieltjens, N.; Fransen, E.; Snyders, D.; Borg, E.; et al. The contribution of genes involved in potassium-recycling in the inner ear to noise-induced hearing loss. Hum. Mutat. 2006, 27, 786–795. [Google Scholar] [CrossRef]
- Mehregan, H.; Mohseni, M.; Akbari, M.; Jalalvand, K.; Arzhangi, S.; Nikzat, N.; Kahrizi, K.; Najmabadi, H. Novel Mutations in KCNQ4, LHFPL5 and COCH Genes in Iranian Families with Hearing Impairment. Arch. Iran. Med. 2019, 22, 189–197. [Google Scholar]
- Miyagawa, M.; Naito, T.; Nishio, S.Y.; Kamatani, N.; Usami, S. Targeted exon sequencing successfully discovers rare causative genes and clarifies the molecular epidemiology of Japanese deafness patients. PLoS ONE 2013, 8, e71381. [Google Scholar] [CrossRef]
- Wu, C.C.; Lin, Y.H.; Lu, Y.C.; Chen, P.J.; Yang, W.S.; Hsu, C.J.; Chen, P.L. Application of massively parallel sequencing to genetic diagnosis in multiplex families with idiopathic sensorineural hearing impairment. PLoS ONE 2013, 8, e57369. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Matsunaga, T.; Namba, K.; Mutai, H.; Inoue, Y.; Ogawa, K. Moderate hearing loss associated with a novel KCNQ4 non-truncating mutation located near the N-terminus of the pore helix. Biochem. Biophys. Res. Commun. 2013, 432, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.J.; Lewis, K.L.; Ng, D.; Singh, L.N.; Wynter, J.; Brewer, C.; Brooks, B.P.; Brownell, I.; Candotti, F.; Gonsalves, S.G.; et al. Individualized iterative phenotyping for genome-wide analysis of loss-of-function mutations. Am. J. Hum. Genet. 2015, 96, 913–925. [Google Scholar] [CrossRef]
- Ceyhan-Birsoy, O.; Murry, J.B.; Machini, K.; Lebo, M.S.; Yu, T.W.; Fayer, S.; Genetti, C.A.; Schwartz, T.S.; Agrawal, P.B.; Parad, R.B.; et al. Interpretation of Genomic Sequencing Results in Healthy and Ill Newborns: Results from the BabySeq Project. Am. J. Hum. Genet. 2019, 104, 76–93. [Google Scholar] [CrossRef] [PubMed]
| Variant * | Functional Study † | ||
|---|---|---|---|
| c.2T>C | translation starts from Met159? | [21] | [21] |
| c.115G>T | p.E39X | [22] | |
| c.546C>A | p.S182R | [23] | |
| c.557G>A | p.G186D | [24] | |
| c.604G>A | p.D202N | [25] | [26] |
| c.728G>A | p.R243H | [27] | [27,28,29,30,31,32,33] |
| c.775C>G | p.R259G | [34] | |
| c.783G>C | p.E261D | [35] | [29,31] |
| c.815G>A | p.G272D | [35] | |
| c.914G>C | p.W305S | [36] | [27,37] |
| c.1040T>G | p.L347R | [38] | |
| c.1051T>C | p.F351L | [24] | |
| c.1175G>A | p.W392X | [39] | |
| c.1588C>T | p.Q530X | [35] | [5,29,31,40,41] ‡ |
| c.1741A>T | p.K581X | [42] | |
| c.431delC | p.I145Sfs | [39] | |
| c.443delA | p.Y148Lfs | [24] | |
| c.451_452delCT | p.L151Gfs | [43] | |
| c.585delG | p.L196Sfs | [25] | |
| c.733_734delGG | p.G245Rfs | [44] | |
| c.820_830del11 | p.I274Vfs | [45] | |
| c.998_999delCT | p.S333Cfs | [46] | |
| c.1008delC | p.I337Sfs | [35] | [29,31] |
| c.1188delC | p.R397Gfs | [47] | |
| c.1319delT | p.V440Afs | [48] | |
| c.1356delG | p.L453Wfs | [49] | |
| c.567dupG | p.R190Afs | [50] | |
| c.1149dupT | p.A384Cfs | [21] | [21] |
| c.743_744delGGinsTC | p.W248F | [51] | [40,51] |
| c.1630_1635delCAGTACinsGTTGAGA | p.Q544Vfs | [4] | [27,37] |
| Variant * | Functional Study † | ||
|---|---|---|---|
| c.140T>C | p.L47P | [54] | [54] |
| c.343C>G | p.L115V | [55] | |
| c.463G>A | p.G155R | [56] | |
| c.546C>G | p.F182L | [57] | [58] |
| c.572C>T | p.A191V | [59] | |
| c.650T>A | p.M217K | [60] | |
| c.689T>A | p.V230E | [61] | [62] |
| c.701A>T | p.H234L | [63] | |
| c.709G>A | p.E237K | [59] | |
| c.725G>A | p.W242X | [64] | [41] ‡ |
| c.754G>C | p.A252P | [56] | |
| c.767T>G | p.V256G | [59] | |
| c.770A>G | p.Y257C | [59] | |
| c.773T>C | p.L258P | [59] | |
| c.778G>A | p.E260K | [64] | [62] |
| c.785A>T | p.D262V | [64] | [62] |
| c.796G>T | p.D266Y | [65] | [65] |
| c.808T>C | p.Y270H | [66] | [62] |
| c.821T>A | p.L274H | [67] | [58,68] |
| c.823T>C | p.W275R | [69] | [62] |
| c.824G>C | p.W275S | [70] | |
| c.827G>T | p.W276L | [71] | |
| c.827G>C | p.W276S | [72] | [58,68,73,74] |
| c.842T>C | p.L281S | [75] | [58,68] |
| c.842T>G | p.L281W | [59] | |
| c.853G>T | p.G285C | [72] | [58,68,73] |
| c.853G>A | p.G285S | [76] | [52,68,76,77] ¶ |
| c.857A>G | p.Y286C | [60] | |
| c.857A>C | p.Y286S | [78] | |
| c.859G>C | p.G287R | [79] | [62] |
| c.872C>T | p.P291L | [61] | [62] |
| c.871C>T | p.P291S | [61] | [62] |
| c.878C>T | p.T293I | [59] | |
| c.887G>A | p.G296D | [80] | |
| c.886G>A | p.G296S | [81] | [58,68,81] |
| c.889A>G | p.R297G | [59] | |
| c.891G>T | p.R297S | [61] | |
| c.947G>T | p.G316V | [82] | |
| c.956G>A | p.G319D | [83] | [83] |
| c.961G>A | p.G321S | [72] | [58,68] |
| c.992G>A | p.R331Q | [83] | [83] |
| c.992G>C | p.R331P | [59] | |
| c.1012C>G | p.R338G | [84] | |
| c.1012C>T | p.R338W | [59] | |
| c.1288G>A | p.E430K | [85] | |
| c.1316G>A | p.R439H | [84] | |
| c.1365G>T | p.H455Q | [86] | [74,81] |
| c.1498C>T | p.R500C | [59] | |
| c.1600A>G | p.I534V | [82] | |
| c.1647C>G | p.F549L | [87] | |
| c.1762G>C | p.G588R | [59] | |
| c.2014G>A | p.V672M | [88] | |
| c.2039C>T | p.S680F | [89] | [62] |
| c.211delC | p.Q71Sfs | [90] | [41] ‡ |
| c.212_224del13 | p.Q71Pfs | [72] | |
| c.261_269delCTACAACGT | p.Y88_V90del | [65] | [65] |
| c.664_681del18 | p.G222_L227del | [73] | [73] |
| c.806_808delCCT | p.S269del | [91] | [83] |
| c.811_816delGCCGAC | p.A271_D272del | [83] | [83] |
| c.1044_1051delTGCCTGGC | p.A349Pfs | [92] | [41] ‡ |
| c.1725delG | p.I576Sfs | [93] | |
| c.228_229dupGC | p.H77Rfs | [61] | |
| c.1671_1672dupACGAC | p.V558Tfs | [94] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homma, K. The Pathological Mechanisms of Hearing Loss Caused by KCNQ1 and KCNQ4 Variants. Biomedicines 2022, 10, 2254. https://doi.org/10.3390/biomedicines10092254
Homma K. The Pathological Mechanisms of Hearing Loss Caused by KCNQ1 and KCNQ4 Variants. Biomedicines. 2022; 10(9):2254. https://doi.org/10.3390/biomedicines10092254
Chicago/Turabian StyleHomma, Kazuaki. 2022. "The Pathological Mechanisms of Hearing Loss Caused by KCNQ1 and KCNQ4 Variants" Biomedicines 10, no. 9: 2254. https://doi.org/10.3390/biomedicines10092254
APA StyleHomma, K. (2022). The Pathological Mechanisms of Hearing Loss Caused by KCNQ1 and KCNQ4 Variants. Biomedicines, 10(9), 2254. https://doi.org/10.3390/biomedicines10092254





