DNA Damage Response Differentially Affects BoHV-1 Gene Transcription in Cell Type-Dependent Manners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. Antibodies
2.3. Western Blot
2.4. Immunofluorescence Assay
2.5. Quantification of mRNA by qRT-PCR
3. Results
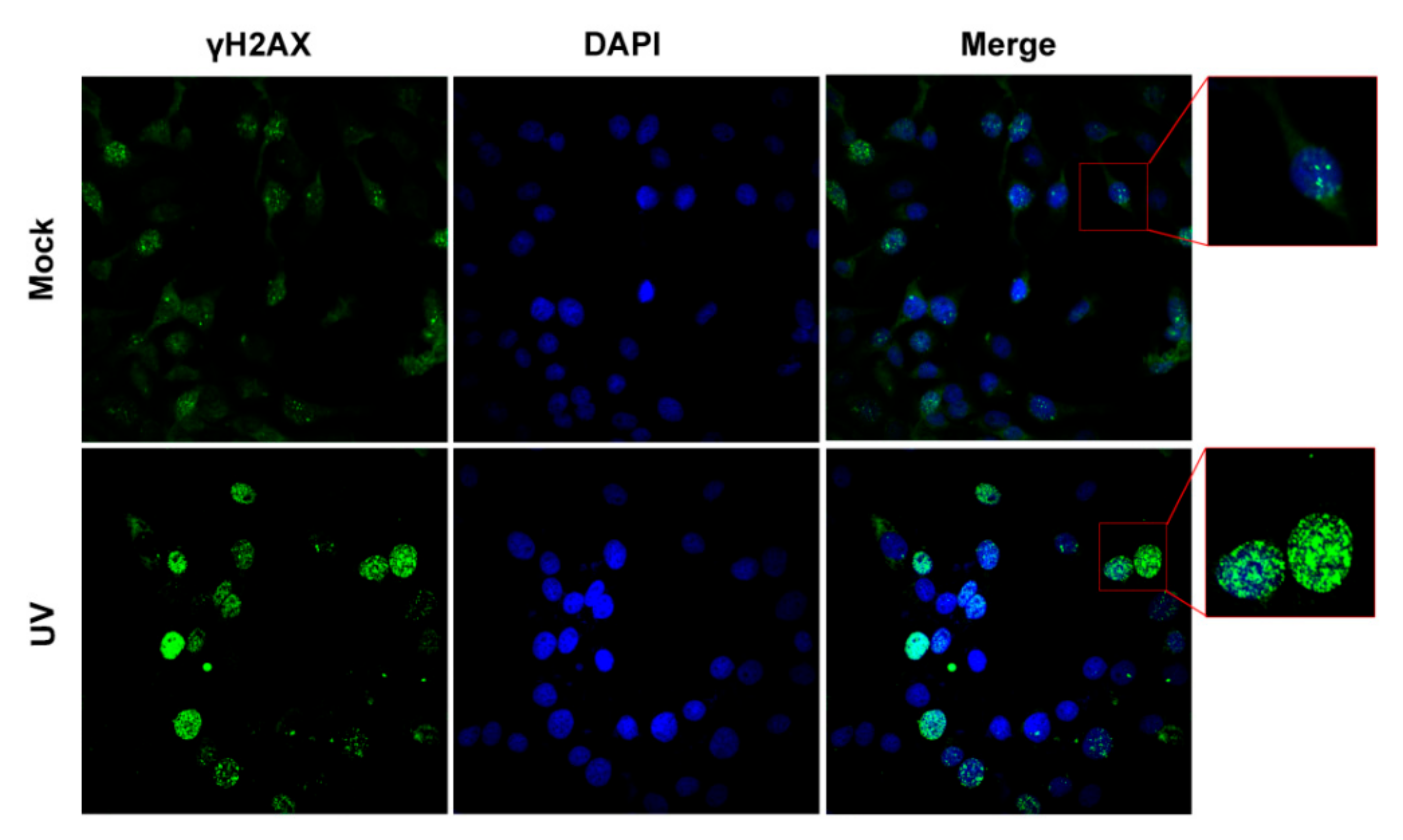
3.1. Define a Condition That Can Efficiently Induce DNA Damage Response (DDR) in Cell Cultures
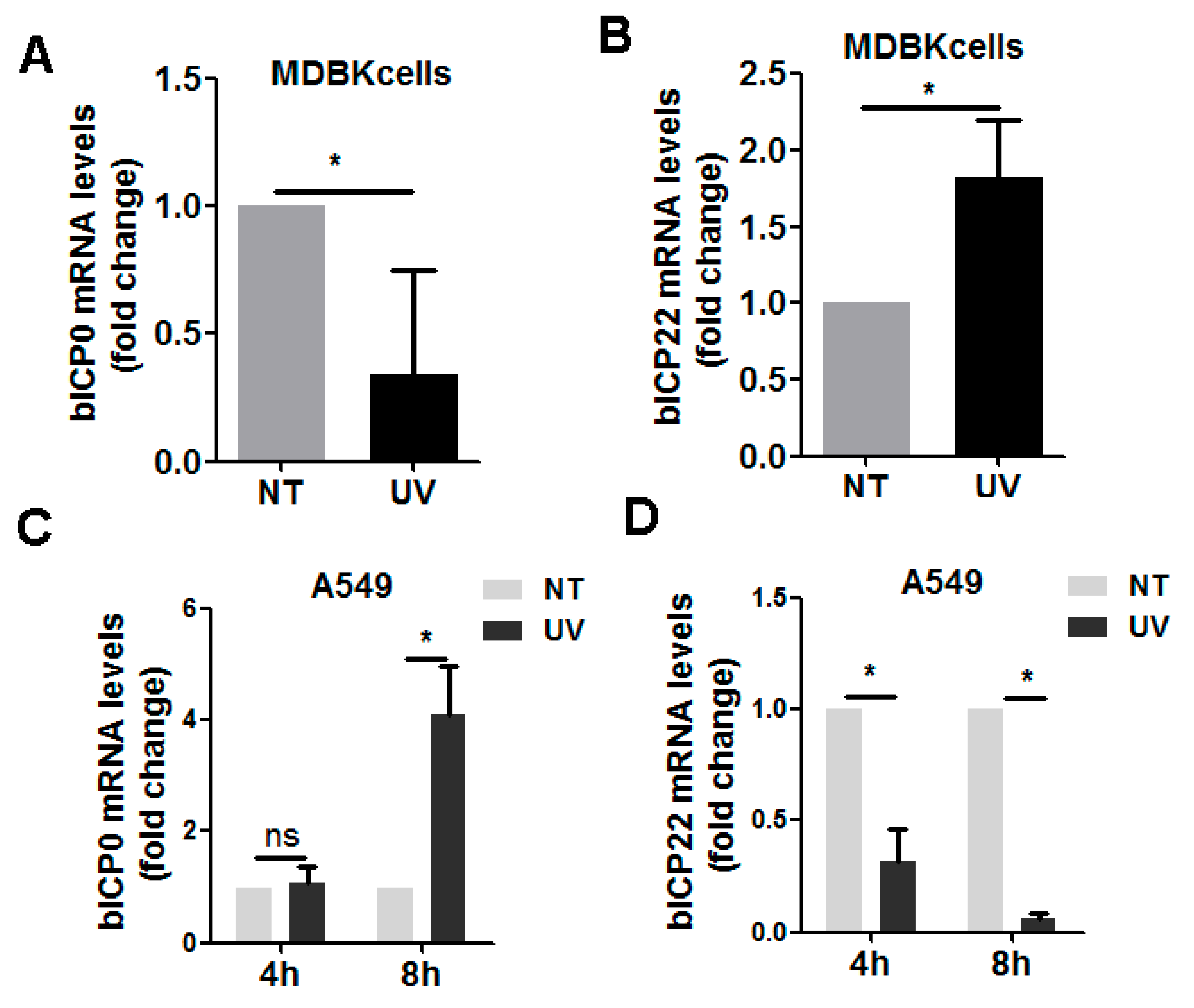
3.2. UV-Exposure-Induced DDR Has Differential Effects on Virus IE Transcription
3.3. UV-Induced DDR Prior to Virus Infection Has Effects on the Stabilization of Vial Glycoproteins gC and gD
3.4. BoHV-1 Productive Infection Has Effects on UV-Irradiation-Induced DDR Signaling γH2AX
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tikoo, S.K.; Campos, M.; Babiuk, L.A. Bovine herpesvirus 1 (bhv-1): Biology, pathogenesis, and control. Adv. Virus Res. 1995, 45, 191–223. [Google Scholar] [PubMed]
- Muylkens, B.; Thiry, J.; Kirten, P.; Schynts, F.; Thiry, E. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet. Res. 2007, 38, 181–209. [Google Scholar] [CrossRef]
- Jones, C.; Chowdhury, S. A review of the biology of bovine herpesvirus type 1 (bhv-1), its role as a cofactor in the bovine respiratory disease complex and development of improved vaccines. Anim. Health Res. Rev. 2007, 8, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, P.D.; Aich, P.; Manuja, A.; Hokamp, K.; Roche, F.M.; Brinkman, F.S.; Potter, A.; Babiuk, L.A.; Griebel, P.J. Effect of stress on viral-bacterial synergy in bovine respiratory disease: Novel mechanisms to regulate inflammation. Comp. Funct. Genom. 2005, 6, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Yates, W.D.; Babiuk, L.A.; Jericho, K.W. Viral-bacterial pneumonia in calves: Duration of the interaction between bovine herpesvirus 1 and pasteurella haemolytica. Can. J. Comp. Med. 1983, 47, 257–264. [Google Scholar] [PubMed]
- Newcomer, B.W.; Cofield, L.G.; Walz, P.H.; Givens, M.D. Prevention of abortion in cattle following vaccination against bovine herpesvirus 1: A meta-analysis. Prev. Vet. Med. 2017, 138, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Kumar, M.; Manohar, M.; Chauhan, R.S. Bovine herpes virus infections in cattle. Anim. Health Res. Rev. 2009, 10, 85–98. [Google Scholar] [CrossRef]
- Jones, C. Bovine herpesvirus 1 counteracts immune responses and immune-surveillance to enhance pathogenesis and virus transmission. Front. Immunol. 2019, 10, 1008. [Google Scholar] [CrossRef]
- Langie, S.A.S.; Koppen, G.; Desaulniers, D.; Al-Mulla, F.; Al-Temaimi, R.; Amedei, A.; Azqueta, A.; Bisson, W.H.; Brown, D.; Brunborg, G.; et al. Causes of genome instability: The effect of low dose chemical exposures in modern society. Carcinogenesis 2015, 36, S61–S88. [Google Scholar] [CrossRef]
- Qiu, W.; Ding, X.; Li, S.; He, Y.; Zhu, L. Oncolytic bovine herpesvirus 1 inhibits human lung adenocarcinoma a549 cell proliferation and tumor growth by inducing DNA damage. Int. J. Mol. Sci. 2021, 22, 8582. [Google Scholar] [CrossRef]
- Basu, A.K. DNA damage, mutagenesis and cancer. Int. J. Mol. Sci. 2018, 19, 970. [Google Scholar] [CrossRef]
- Nagaria, P.; Robert, C.; Rassool, F.V. DNA double-strand break response in stem cells: Mechanisms to maintain genomic integrity. Biochim. Biophys. Acta 2013, 1830, 2345–2353. [Google Scholar] [CrossRef]
- Zhu, L.; Fu, X.; Yuan, C.; Jiang, X.; Zhang, G. Induction of oxidative DNA damage in bovine herpesvirus 1 infected bovine kidney cells (mdbk cells) and human tumor cells (a549 cells and u2os cells). Viruses 2018, 10, 393. [Google Scholar] [CrossRef]
- Ding, X.; Yuan, W.; Yang, H.; Liu, C.; Li, S.; Zhu, L. β-Catenin-Specific Inhibitor, iCRT14, Promotes BoHV-1 Infection-Induced DNA Damage in Human A549 Lung Adenocarcinoma Cells by Enhancing Viral Protein Expression. Int. J. Mol. Sci. 2022, 23, 2328. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K. Caspase-dependent and -independent cell death pathways after DNA damage (review). Oncol. Rep. 2005, 14, 595–599. [Google Scholar] [CrossRef]
- Ferreira, H.C.C.; de Araujo, E.N.; Rosado, N.C.L.; Fietto, J.L.R.; Santos, M.R.; Gomes, L.L.; Silva, L.M.N.; Bressan, G.C.; Martins, G.F.; Sreevatsan, S.; et al. Apoptosis in the late replication phase of bovine alphaherpesvirus 1 in experimentally infected calves. Braz. J. Microbiol. 2021, 52, 2529–2534. [Google Scholar] [CrossRef]
- Fiorito, F.; Nocera, F.P.; Cantiello, A.; Iovane, V.; Lambiase, S.; Piccolo, M.; Ferraro, M.G.; Santamaria, R.; De Martino, L. Bovine herpesvirus-1 infection in mouse neuroblastoma (neuro-2a) cells. Vet. Microbiol. 2020, 247, 108762. [Google Scholar] [CrossRef]
- Rensetti, D.E.; Marin, M.S.; Moran, P.E.; Odeon, A.C.; Verna, A.E.; Perez, S.E. Bovine herpesvirus type 5 replication and induction of apoptosis in vitro and in the trigeminal ganglion of experimentally-infected cattle. Comp. Immunol. Microbiol. Infect. Dis. 2018, 57, 8–14. [Google Scholar] [CrossRef]
- Fiorito, F.; Iovane, V.; Marullo, A.; Costagliola, A.; Granato, G.E.; De Martino, L. 2,3,7,8-tetrachlorodibenzo-p-dioxin influences bovine herpesvirus 1 replication through upregulation of sirt3 and cytoskeletal reorganization. Vet. Res. Commun. 2017, 41, 299–306. [Google Scholar] [CrossRef]
- Cardoso, T.C.; Rosa, A.C.; Ferreira, H.L.; Okamura, L.H.; Oliveira, B.R.; Vieira, F.V.; Silva-Frade, C.; Gameiro, R.; Flores, E.F. Bovine herpesviruses induce different cell death forms in neuronal and glial-derived tumor cell cultures. J. Neurovirol. 2016, 22, 725–735. [Google Scholar] [CrossRef] [Green Version]
- Hanon, E.; Lambot, M.; Hoornaert, S.; Lyaku, J.; Pastoret, P.P. Bovine herpesvirus 1-induced apoptosis: Phenotypic characterization of susceptible peripheral blood mononuclear cells. Arch. Virol. 1998, 143, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Georgoulis, A.; Vorgias, C.E.; Chrousos, G.P.; Rogakou, E.P. Genome instability and gammah2ax. Int. J. Mol. Sci. 2017, 18, 1979. [Google Scholar] [CrossRef] [PubMed]
- Carusillo, A.; Mussolino, C. DNA damage: From threat to treatment. Cells 2020, 9, 1665. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yu, Y.; Jiang, X.; Yuan, W.; Zhu, G. First report of bovine herpesvirus 1 isolation from bull semen samples in China. Acta Virol. 2017, 6, 483–486. [Google Scholar] [CrossRef]
- Afroz, S.; Garg, R.; Fodje, M.; van Drunen Littel-van den Hurk, S. The major tegument protein of bovine herpesvirus 1, vp8, interacts with DNA damage response proteins and induces apoptosis. J. Virol. 2018, 92, 15. [Google Scholar] [CrossRef]
- Zhu, L.; Jones, C. The high mobility group at-hook 1 protein stimulates bovine herpesvirus 1 productive infection. Virus Res. 2017, 238, 236–242. [Google Scholar] [CrossRef]
- Greinert, R.; Volkmer, B.; Henning, S.; Breitbart, E.W.; Greulich, K.O.; Cardoso, M.C.; Rapp, A. Uva-induced DNA double-strand breaks result from the repair of clustered oxidative DNA damages. Nucleic Acids Res. 2012, 40, 10263–10273. [Google Scholar] [CrossRef]
- Dhuppar, S.; Roy, S.; Mazumder, A. Gamma h2ax in the s phase after uv irradiation corresponds to DNA replication and does not report on the extent of DNA damage. Mol. Cell. Biol. 2020, 40, 20. [Google Scholar] [CrossRef]
- Mah, L.J.; El-Osta, A.; Karagiannis, T.C. Gammah2ax: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef]
- Jones, C. Alphaherpesvirus latency: Its role in disease and survival of the virus in nature. Adv. Virus Res. 1998, 51, 81–133. [Google Scholar]
- Sirbu, B.M.; Cortez, D. DNA damage response: Three levels of DNA repair regulation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012724. [Google Scholar] [CrossRef] [Green Version]
- Weitzman, M.D.; Fradet-Turcotte, A. Virus DNA replication and the host DNA damage response. Annu. Rev. Virol. 2018, 5, 141–164. [Google Scholar] [CrossRef]
- Smith, S.; Weller, S.K. Hsv-i and the cellular DNA damage response. Future Virol. 2015, 10, 383–397. [Google Scholar] [CrossRef]
- Lees-Miller, S.P.; Long, M.C.; Kilvert, M.A.; Lam, V.; Rice, S.A.; Spencer, C.A. Attenuation of DNA-dependent protein kinase activity and its catalytic subunit by the herpes simplex virus type 1 transactivator icp0. J. Virol. 1996, 70, 7471–7477. [Google Scholar] [CrossRef]
- Parkinson, J.; Lees-Miller, S.P.; Everett, R.D. Herpes simplex virus type 1 immediate-early protein vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J. Virol. 1999, 73, 650–657. [Google Scholar] [CrossRef]
- Schwartz, R.A.; Carson, C.T.; Schuberth, C.; Weitzman, M.D. Adeno-associated virus replication induces a DNA damage response coordinated by DNA-dependent protein kinase. J. Virol. 2009, 83, 6269–6278. [Google Scholar] [CrossRef]
- Lilley, C.E.; Carson, C.T.; Muotri, A.R.; Gage, F.H.; Weitzman, M.D. DNA repair proteins affect the lifecycle of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 2005, 102, 5844–5849. [Google Scholar] [CrossRef]
- Alekseev, O.; Donegan, W.E.; Donovan, K.R.; Limonnik, V.; Azizkhan-Clifford, J. Hsv-1 hijacks the host DNA damage response in corneal epithelial cells through icp4-mediated activation of atm. Investig. Ophth. Vis. Sci. 2020, 61, 39. [Google Scholar] [CrossRef]
- Mohni, K.N.; Dee, A.R.; Smith, S.; Schumacher, A.J.; Weller, S.K. Efficient herpes simplex virus 1 replication requires cellular atr pathway proteins. J. Virol. 2013, 87, 531–542. [Google Scholar] [CrossRef]
- Adeyemi, R.O.; Landry, S.; Davis, M.E.; Weitzman, M.D.; Pintel, D.J. Parvovirus minute virus of mice induces a DNA damage response that facilitates viral replication. PLoS Pathog. 2010, 6, e1001141. [Google Scholar] [CrossRef]
- Orba, Y.; Suzuki, T.; Makino, Y.; Kubota, K.; Tanaka, S.; Kimura, T.; Sawa, H. Large t antigen promotes jc virus replication in g2-arrested cells by inducing atm- and atr-mediated g2 checkpoint signaling. J. Biol. Chem. 2010, 285, 1544–1554. [Google Scholar] [CrossRef] [Green Version]
- Shah, G.A.; O’Shea, C.C. Viral and cellular genomes activate distinct DNA damage responses. Cell 2015, 162, 987–1002. [Google Scholar] [CrossRef]
- Filipponi, D.; Emelyanov, A.; Muller, J.; Molina, C.; Nichols, J.; Bulavin, D.V. DNA damage signaling-induced cancer cell reprogramming as a driver of tumor relapse. Mol. Cell 2019, 74, 651–663.e8. [Google Scholar] [CrossRef]
- Ui, A.; Chiba, N.; Yasui, A. Relationship among DNA double-strand break (dsb), dsb repair, and transcription prevents genome instability and cancer. Cancer Sci. 2020, 111, 1443–1451. [Google Scholar] [CrossRef]
- Zhu, L.; Thompson, J.; Ma, F.; Eudy, J.; Jones, C. Effects of the synthetic corticosteroid dexamethasone on bovine herpesvirus 1 productive infection. Virology 2017, 505, 71–79. [Google Scholar] [CrossRef]
- Saira, K.; Zhou, Y.; Jones, C. The infected cell protein 0 encoded by bovine herpesvirus 1 (bicp0) induces degradation of interferon response factor 3 and, consequently, inhibits beta interferon promoter activity. J. Virol. 2007, 81, 3077–3086. [Google Scholar] [CrossRef]
- Mohni, K.N.; Smith, S.; Dee, A.R.; Schumacher, A.J.; Weller, S.K. Herpes simplex virus type 1 single strand DNA binding protein and helicase/primase complex disable cellular atr signaling. PLoS Pathog. 2013, 9, e1003652. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013, 332, 237–248. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 2006, 12, 440–450. [Google Scholar] [CrossRef]
- Rodrigues, R.; Cuddington, B.; Mossman, K. Bovine herpesvirus type 1 as a novel oncolytic virus. Cancer Gene Ther. 2010, 17, 344–355. [Google Scholar] [CrossRef]
- Cuddington, B.P.; Verschoor, M.; Ashkar, A.; Mossman, K.L. Enhanced efficacy with azacytidine and oncolytic bhv-1 in a tolerized cotton rat model of breast adenocarcinoma. Mol. Ther. Oncolytics 2015, 2, 15004. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Yuan, W.; Li, S.; Ding, X.; Zhu, L. DNA Damage Response Differentially Affects BoHV-1 Gene Transcription in Cell Type-Dependent Manners. Biomedicines 2022, 10, 2282. https://doi.org/10.3390/biomedicines10092282
Tang L, Yuan W, Li S, Ding X, Zhu L. DNA Damage Response Differentially Affects BoHV-1 Gene Transcription in Cell Type-Dependent Manners. Biomedicines. 2022; 10(9):2282. https://doi.org/10.3390/biomedicines10092282
Chicago/Turabian StyleTang, Linke, Weifeng Yuan, Shitao Li, Xiuyan Ding, and Liqian Zhu. 2022. "DNA Damage Response Differentially Affects BoHV-1 Gene Transcription in Cell Type-Dependent Manners" Biomedicines 10, no. 9: 2282. https://doi.org/10.3390/biomedicines10092282
APA StyleTang, L., Yuan, W., Li, S., Ding, X., & Zhu, L. (2022). DNA Damage Response Differentially Affects BoHV-1 Gene Transcription in Cell Type-Dependent Manners. Biomedicines, 10(9), 2282. https://doi.org/10.3390/biomedicines10092282





