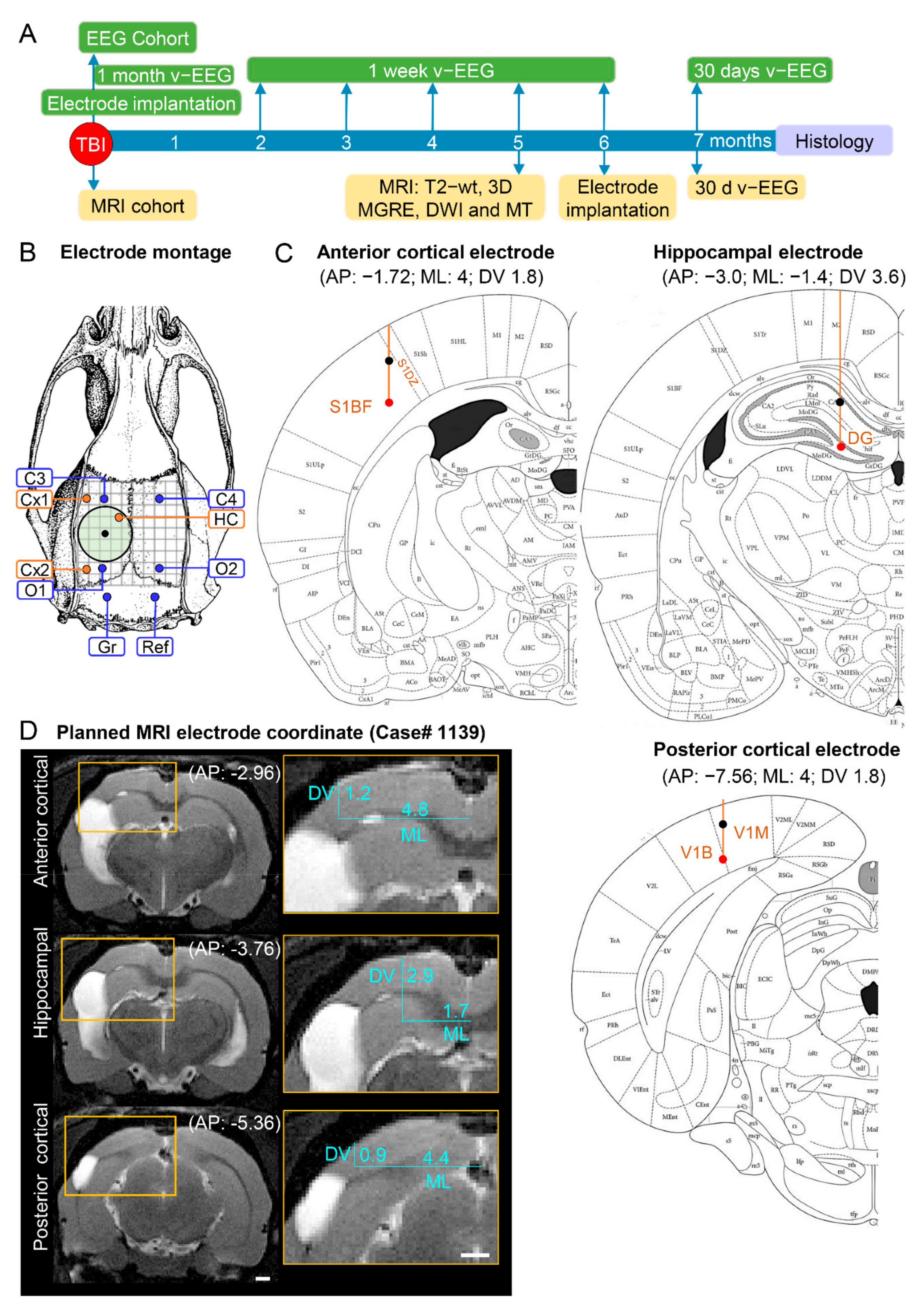
Figure 1.
Study design, electrode montage, and atlas or MRI-planned electrode coordinates. (
A) Study design. Following TBI, rats were divided into either the EEG or MRI cohort. The rats of the EEG cohort were implanted with electrodes after fully righting themselves following induction of TBI. The rats were followed up immediately afterward with 1 month video-EEG and then for 1 week monthly until the 6th post-TBI month. The rats of the MRI cohort were magnetic resonance-imaged at 5 months post-TBI and T2-wt images were used to calculate the coordinates of the intracerebral electrodes implanted at 6 months post-TBI. Both cohorts were continuously monitored with video-EEG for 30 days at 7 months post-TBI to diagnose epilepsy. At the end of the 7-month follow-up period, all rats were euthanized and the brains processed for histological identification of the location of the intracerebral electrodes. (
B) Electrode montage used in the study. Four epidural screw electrodes (C3, C4, O1 and O2), 3 intracerebral bipolar wire electrodes (anterior cortical Cx1, posterior cortical Cx2, and hippocampal HC), a ground (Gr) and reference (Ref) electrode. (
C) Atlas plates demonstrating the planned coordinates used in the EEG cohort to implant the anterior cortical, hippocampal, and posterior cortical electrodes. The black dot refers to the upper tip and the red dot to the lower tip of the bipolar electrode (1 mm apart). Reprinted/adapted with permission from [
25]. 2007, Elsevier Inc. (
D) MRI T2-wt images of rat 1139 demonstrating the planned-MRI coordinates of the intracerebral anterior cortical, hippocampal, and posterior cortical electrodes. The anteroposterior (AP) coordinate was determined by aligning the MR images with the atlas [
25]. The mediolateral (ML) and dorsoventral (DV) coordinates were determined using ImageJ software (version 1.47v, Wayne Rasband and contributors, National Institute of Health, USA).
Figure 1.
Study design, electrode montage, and atlas or MRI-planned electrode coordinates. (
A) Study design. Following TBI, rats were divided into either the EEG or MRI cohort. The rats of the EEG cohort were implanted with electrodes after fully righting themselves following induction of TBI. The rats were followed up immediately afterward with 1 month video-EEG and then for 1 week monthly until the 6th post-TBI month. The rats of the MRI cohort were magnetic resonance-imaged at 5 months post-TBI and T2-wt images were used to calculate the coordinates of the intracerebral electrodes implanted at 6 months post-TBI. Both cohorts were continuously monitored with video-EEG for 30 days at 7 months post-TBI to diagnose epilepsy. At the end of the 7-month follow-up period, all rats were euthanized and the brains processed for histological identification of the location of the intracerebral electrodes. (
B) Electrode montage used in the study. Four epidural screw electrodes (C3, C4, O1 and O2), 3 intracerebral bipolar wire electrodes (anterior cortical Cx1, posterior cortical Cx2, and hippocampal HC), a ground (Gr) and reference (Ref) electrode. (
C) Atlas plates demonstrating the planned coordinates used in the EEG cohort to implant the anterior cortical, hippocampal, and posterior cortical electrodes. The black dot refers to the upper tip and the red dot to the lower tip of the bipolar electrode (1 mm apart). Reprinted/adapted with permission from [
25]. 2007, Elsevier Inc. (
D) MRI T2-wt images of rat 1139 demonstrating the planned-MRI coordinates of the intracerebral anterior cortical, hippocampal, and posterior cortical electrodes. The anteroposterior (AP) coordinate was determined by aligning the MR images with the atlas [
25]. The mediolateral (ML) and dorsoventral (DV) coordinates were determined using ImageJ software (version 1.47v, Wayne Rasband and contributors, National Institute of Health, USA).
![Biomedicines 10 02295 g001]()
Figure 2.
Anterior intracortical electrode—schematic representations of the atlas, histological, and MRI-guided coordinates in each rat of the EEG and MRI cohorts. (A) Anteroposterior (AP) coordinate. In the EEG cohort (electrode operation right after injury), the fixed atlas-based target AP coordinate of −1.72 mm from the bregma was applied to implant the electrodes (orange dots). In the MRI cohort (electrode operation 5 months after injury), the target AP coordinate was individually determined using the 5-month in vivo T2-weighted MR images. The target coordinate fluctuated depending on the extent of the TBI (traumatic brain injury)-induced lesion. Note the anterior shift (y-axis) in the histologically verified “true” AP coordinate (blue dots) relative to the target coordinate (orange dots) in both cohorts. The deviations were comparable between the sham and TBI animals (p > 0.05). Animal numbers are shown on the x-axis. (B) Mediolateral (ML) coordinate. In the EEG cohort, the fixed atlas-based target ML coordinate at 4 mm lateral to midline was targeted. In the MRI cohort, the target ML coordinate was individually determined using the 5-month in vivo MRI. Note a small deviation of the histologically verified “true” ML coordinate from the target coordinate in both cohorts. (C) Dorsoventral (DV) coordinate. In both cohorts, the lower tip of the bipolar electrode was aimed to layer V in the selected AP and ML coordinates (see above). Electrode tips were located in the cortex in 83% (36/43) of the EEG cohort and 77% (24/31) of the MRI cohort. Importantly, even though the lower tip in the remaining cases went down into the external capsule or corpus callosum, the upper tip of the bipolar electrode, being 1 mm higher in the EEG and 0.5 mm in the MRI cohort, was still recording in the cortex. The percentages of electrode locations in the sham-operated and TBI animals are shown on the right side of the panel. (D) Dot plots of the AP and ML shift in the histological AP and ML coordinate, and % of electrode in the targeted layer V (number of cases in brackets). Note posterior and medial shift of some cases from the target (vertical dashed line). y-axis represents distance from target coordinate (Y = 0) or % of cases in targeted area. Abbreviations: cavity, cortical lesion cavity; cc, corpus callosum; cg, cingulum; S, subiculum; ec, external capsule; HC, hippocampus; and V, ventricle.
Figure 2.
Anterior intracortical electrode—schematic representations of the atlas, histological, and MRI-guided coordinates in each rat of the EEG and MRI cohorts. (A) Anteroposterior (AP) coordinate. In the EEG cohort (electrode operation right after injury), the fixed atlas-based target AP coordinate of −1.72 mm from the bregma was applied to implant the electrodes (orange dots). In the MRI cohort (electrode operation 5 months after injury), the target AP coordinate was individually determined using the 5-month in vivo T2-weighted MR images. The target coordinate fluctuated depending on the extent of the TBI (traumatic brain injury)-induced lesion. Note the anterior shift (y-axis) in the histologically verified “true” AP coordinate (blue dots) relative to the target coordinate (orange dots) in both cohorts. The deviations were comparable between the sham and TBI animals (p > 0.05). Animal numbers are shown on the x-axis. (B) Mediolateral (ML) coordinate. In the EEG cohort, the fixed atlas-based target ML coordinate at 4 mm lateral to midline was targeted. In the MRI cohort, the target ML coordinate was individually determined using the 5-month in vivo MRI. Note a small deviation of the histologically verified “true” ML coordinate from the target coordinate in both cohorts. (C) Dorsoventral (DV) coordinate. In both cohorts, the lower tip of the bipolar electrode was aimed to layer V in the selected AP and ML coordinates (see above). Electrode tips were located in the cortex in 83% (36/43) of the EEG cohort and 77% (24/31) of the MRI cohort. Importantly, even though the lower tip in the remaining cases went down into the external capsule or corpus callosum, the upper tip of the bipolar electrode, being 1 mm higher in the EEG and 0.5 mm in the MRI cohort, was still recording in the cortex. The percentages of electrode locations in the sham-operated and TBI animals are shown on the right side of the panel. (D) Dot plots of the AP and ML shift in the histological AP and ML coordinate, and % of electrode in the targeted layer V (number of cases in brackets). Note posterior and medial shift of some cases from the target (vertical dashed line). y-axis represents distance from target coordinate (Y = 0) or % of cases in targeted area. Abbreviations: cavity, cortical lesion cavity; cc, corpus callosum; cg, cingulum; S, subiculum; ec, external capsule; HC, hippocampus; and V, ventricle.
![Biomedicines 10 02295 g002]()
Figure 3.
(A–F) Electrode tracts. Histological images from the coronal thionine-stained sections of 6 rats, showing the tracts of the bipolar intracortical electrodes and location of the lower electrode tip (filled arrowhead). Roman numerals indicate the cortical layers. In panels (A,B) the electrode tip is located in layer V of the perilesional cortex. Note the electrode track-related lesion on the surface of the brain in panel (A) (open filled arrow). In panel (A), the electrode tip is within 500 µm from the edge of the TBI-induced lesion cavity (asterisk). In panel (C), the electrode tip is in the external capsule (ec). In panel (D) the electrode tip is within the cortical lesion, close to the angular bundle. Open arrow points to the electrode path associated neurodegeneration. In panel (E), the electrode tip is close to the edge of the lesion cavity (asterisk). In panel (F), the electrode tip is within the angular bundle (closed arrowhead). The open arrowhead points to the location of the upper electrode in layer IV (open arrow). The dark staining indicates iron deposits (arrowheads) adjacent to the electrode path. Scale bar = 500 µm.
Figure 3.
(A–F) Electrode tracts. Histological images from the coronal thionine-stained sections of 6 rats, showing the tracts of the bipolar intracortical electrodes and location of the lower electrode tip (filled arrowhead). Roman numerals indicate the cortical layers. In panels (A,B) the electrode tip is located in layer V of the perilesional cortex. Note the electrode track-related lesion on the surface of the brain in panel (A) (open filled arrow). In panel (A), the electrode tip is within 500 µm from the edge of the TBI-induced lesion cavity (asterisk). In panel (C), the electrode tip is in the external capsule (ec). In panel (D) the electrode tip is within the cortical lesion, close to the angular bundle. Open arrow points to the electrode path associated neurodegeneration. In panel (E), the electrode tip is close to the edge of the lesion cavity (asterisk). In panel (F), the electrode tip is within the angular bundle (closed arrowhead). The open arrowhead points to the location of the upper electrode in layer IV (open arrow). The dark staining indicates iron deposits (arrowheads) adjacent to the electrode path. Scale bar = 500 µm.
![Biomedicines 10 02295 g003]()
Figure 4.
Location of the lower tip of the anterior and posterior intracortical electrodes on atlas plates and unfolded cortical maps. (A) In the EEG cohort (upper panel), the dorsoventral (DV) location of at least 1 of the tips of all anterior bipolar electrodes (atlas plate: bregma −1.4 mm) was within the primary somatosensory cortex (S1) and that of the posterior electrode (lower panel; atlas plate: bregma −6.8 mm) was within the visual cortex. Each dot represents 1 bipolar electrode. (B) An unfolded map (UFM) showing the location of electrode tracks in the EEG cohort as seen from the surface of the brain. The intersection of the electrode path with cortical layer V was used as reference. The UFMs confirmed the location of the anterior electrode paths in the S1 and posterior electrode paths in the visual cortex. (C) Atlas plate showing the DV locations of the anterior (upper panel) and posterior (lower panel) intracortical electrodes in the MRI cohort. As in the EEG cohort, the anterior electrode was in S1 and the posterior electrode was in the visual cortex. (D) A UFM showing the location of electrode tracks in the MRI cohort as seen from the surface of the brain. All electrode tracks were within S1 or the visual cortex. Note that in the MRI cohort, we used the 5-month in vivo MRI to adjust the electrode coordinates to target the perilesional cortex and to avoid lesion cavities, underlying brain areas, or ventricles. As expected, this resulted in a more heterogeneous distribution of electrode paths than in the EEG cohort with atlas-based fixed coordinates. Atlas plates and UFMs were generated using the Paxinos rat brain atlas (6th edition).
Figure 4.
Location of the lower tip of the anterior and posterior intracortical electrodes on atlas plates and unfolded cortical maps. (A) In the EEG cohort (upper panel), the dorsoventral (DV) location of at least 1 of the tips of all anterior bipolar electrodes (atlas plate: bregma −1.4 mm) was within the primary somatosensory cortex (S1) and that of the posterior electrode (lower panel; atlas plate: bregma −6.8 mm) was within the visual cortex. Each dot represents 1 bipolar electrode. (B) An unfolded map (UFM) showing the location of electrode tracks in the EEG cohort as seen from the surface of the brain. The intersection of the electrode path with cortical layer V was used as reference. The UFMs confirmed the location of the anterior electrode paths in the S1 and posterior electrode paths in the visual cortex. (C) Atlas plate showing the DV locations of the anterior (upper panel) and posterior (lower panel) intracortical electrodes in the MRI cohort. As in the EEG cohort, the anterior electrode was in S1 and the posterior electrode was in the visual cortex. (D) A UFM showing the location of electrode tracks in the MRI cohort as seen from the surface of the brain. All electrode tracks were within S1 or the visual cortex. Note that in the MRI cohort, we used the 5-month in vivo MRI to adjust the electrode coordinates to target the perilesional cortex and to avoid lesion cavities, underlying brain areas, or ventricles. As expected, this resulted in a more heterogeneous distribution of electrode paths than in the EEG cohort with atlas-based fixed coordinates. Atlas plates and UFMs were generated using the Paxinos rat brain atlas (6th edition).
![Biomedicines 10 02295 g004]()
Figure 5.
Posterior intracortical electrode—schematic representations of the atlas-based, histological, and MRI-guided coordinates in each rat of the EEG and MRI cohorts. (
A) Anteroposterior (AP) coordinate. In the EEG cohort (
n = 47, electrode operation right after injury), the fixed atlas-based target AP coordinate of −7.56 mm from the bregma was applied to implant the electrodes (orange dots). In the MRI cohort (
n = 40, electrode operation at 5 months postinjury), the target AP coordinate was individually determined using the in vivo 5-month T2-weighted MR images. The target coordinate fluctuated depending on the TBI (traumatic brain injury)-induced lesion extent. Note a mild anterior shift (
y-axis) in the histologically verified “true” AP coordinate (blue dots) relative to the target coordinate (orange dots) in both cohorts. In general, the anterior shift was less than that in a case of the anterior intra-cortical electrode (compare to
Figure 1). Animal numbers are shown on the
x-axis. (
B) Mediolateral (ML) coordinate. In the EEG cohort (
n = 47), the fixed atlas-based ML coordinate at 4 mm lateral to midline was targeted. In the MRI cohort (
n = 40), the target ML coordinate was individually determined using the 5-month MRI. Note almost a negligible deviation of the histologically verified “true” ML coordinate from the atlas-based (EEG cohort) or MRI-guided (MRI cohort) coordinates. (
C) Dorsoventral (DV) coordinate. In both cohorts, the lower tip of the bipolar electrode was targeted to layer V in the selected AP and ML coordinates (see above). In the EEG cohort, 46% (18/39), and in the MRI cohort, 66% (19/29) of the electrode tips in injured animals were in the cortex. Importantly, even though the lower tip in the remaining cases went down into the external capsule or corpus callosum, the upper tip of the bipolar electrode, being 1 mm higher in the EEG and 0.5 mm in the MRI cohort, was still recording in the cortex in 79% (31/39) of the rats in the EEG cohort and in 72% (21/29) in the MRI cohort. In 5 rats (3 sham, 2 TBI) in the EEG cohort and 10 rats (4 sham, 6 TBI) in the MRI cohort, the electrode was recording hippocampal rather than cortical activity, which affected the interpretation of the EEG data. The percentages of electrode locations in the sham-operated and TBI animals are shown on the right side of the panel. (
D) Dot plots of the AP and ML shift in the histological AP and ML coordinate, and % of electrode in the targeted layer V (number of cases in brackets). Note posterior shift of some TBI cases from the target (Y = 0). The
y-axis represents distance from target coordinate (Y = 0) or % of cases in targeted area. Note that in 4 animals in the EEG cohort and 2 in the MRI cohort, the DV location of the electrode tip could not be reliably determined in histological sections. Abbreviations: cavity, cortical lesion cavity; cc, corpus callosum; cg, cingulum; dcw, deep cerebral white matter; ec, external capsule; fmj, forceps major corpus callosum; HC, hippocampus; S, subiculum; V, ventricle.
Figure 5.
Posterior intracortical electrode—schematic representations of the atlas-based, histological, and MRI-guided coordinates in each rat of the EEG and MRI cohorts. (
A) Anteroposterior (AP) coordinate. In the EEG cohort (
n = 47, electrode operation right after injury), the fixed atlas-based target AP coordinate of −7.56 mm from the bregma was applied to implant the electrodes (orange dots). In the MRI cohort (
n = 40, electrode operation at 5 months postinjury), the target AP coordinate was individually determined using the in vivo 5-month T2-weighted MR images. The target coordinate fluctuated depending on the TBI (traumatic brain injury)-induced lesion extent. Note a mild anterior shift (
y-axis) in the histologically verified “true” AP coordinate (blue dots) relative to the target coordinate (orange dots) in both cohorts. In general, the anterior shift was less than that in a case of the anterior intra-cortical electrode (compare to
Figure 1). Animal numbers are shown on the
x-axis. (
B) Mediolateral (ML) coordinate. In the EEG cohort (
n = 47), the fixed atlas-based ML coordinate at 4 mm lateral to midline was targeted. In the MRI cohort (
n = 40), the target ML coordinate was individually determined using the 5-month MRI. Note almost a negligible deviation of the histologically verified “true” ML coordinate from the atlas-based (EEG cohort) or MRI-guided (MRI cohort) coordinates. (
C) Dorsoventral (DV) coordinate. In both cohorts, the lower tip of the bipolar electrode was targeted to layer V in the selected AP and ML coordinates (see above). In the EEG cohort, 46% (18/39), and in the MRI cohort, 66% (19/29) of the electrode tips in injured animals were in the cortex. Importantly, even though the lower tip in the remaining cases went down into the external capsule or corpus callosum, the upper tip of the bipolar electrode, being 1 mm higher in the EEG and 0.5 mm in the MRI cohort, was still recording in the cortex in 79% (31/39) of the rats in the EEG cohort and in 72% (21/29) in the MRI cohort. In 5 rats (3 sham, 2 TBI) in the EEG cohort and 10 rats (4 sham, 6 TBI) in the MRI cohort, the electrode was recording hippocampal rather than cortical activity, which affected the interpretation of the EEG data. The percentages of electrode locations in the sham-operated and TBI animals are shown on the right side of the panel. (
D) Dot plots of the AP and ML shift in the histological AP and ML coordinate, and % of electrode in the targeted layer V (number of cases in brackets). Note posterior shift of some TBI cases from the target (Y = 0). The
y-axis represents distance from target coordinate (Y = 0) or % of cases in targeted area. Note that in 4 animals in the EEG cohort and 2 in the MRI cohort, the DV location of the electrode tip could not be reliably determined in histological sections. Abbreviations: cavity, cortical lesion cavity; cc, corpus callosum; cg, cingulum; dcw, deep cerebral white matter; ec, external capsule; fmj, forceps major corpus callosum; HC, hippocampus; S, subiculum; V, ventricle.
![Biomedicines 10 02295 g005]()
Figure 6.
Histological confirmation of the success of MRI-guided electrode placement. Left panel (
A–
C): Anterior intracortical electrode MRI-planned coordinate, histological confirmation, and “virtual” electrode. Right panel: Posterior intracortical electrode MRI-panned coordinate histological confirmation, and “virtual” electrode. (
A) T2-weighted MRI and (
B) histological images showing the MRI-guided (insert) and histology-confirmed “true” location of the anterior intracortical electrode in rat 1139. The anteroposterior (AP) coordinate was estimated by aligning the magnetic resonance images with the rat brain atlas [
25]. The mediolateral (ML) and dorsoventral (DV) coordinates (inserts in (
A,
D)) were determined using ImageJ software (version 1.47v, Wayne Rasband and contributors, National Institute of Health, USA). Note that in this case, the confirmed AP location was about 1.8 mm more rostral than the planned location (−1.20 mm vs. −2.96). Lesion area is denoted in black-dashed-line circle. (
C) A “virtual” location of the electrode tip (black line) if the electrode had been implanted to the targeted atlas-based coordinate (−1.75 mm from bregma, 4 mm from midline, 1.8 mm from the surface of the brain). (
D) MRI-guided (insert) AP, ML and DV coordinates and (
E) histology-confirmed “true” location of the posterior intracortical electrode tip in rat 1139. The black-dashed-line circle denotes the lesion area. Note that the confirmed AP location was approximately 1.4 mm more rostral than the planned location (−3.96 vs. −5.36). Thus, even though both the anterior and posterior intracortical electrodes were more rostral than planned, their tips were recording EEG signals in the perilesional cortex. (
F) A “virtual” electrode (black line) at the atlas-based coordinates would have ended up in the lesion cavity. Scale bar in (
A,
D) = 1 mm, and in (
B,
C,
E,
F) = 2 mm.
Figure 6.
Histological confirmation of the success of MRI-guided electrode placement. Left panel (
A–
C): Anterior intracortical electrode MRI-planned coordinate, histological confirmation, and “virtual” electrode. Right panel: Posterior intracortical electrode MRI-panned coordinate histological confirmation, and “virtual” electrode. (
A) T2-weighted MRI and (
B) histological images showing the MRI-guided (insert) and histology-confirmed “true” location of the anterior intracortical electrode in rat 1139. The anteroposterior (AP) coordinate was estimated by aligning the magnetic resonance images with the rat brain atlas [
25]. The mediolateral (ML) and dorsoventral (DV) coordinates (inserts in (
A,
D)) were determined using ImageJ software (version 1.47v, Wayne Rasband and contributors, National Institute of Health, USA). Note that in this case, the confirmed AP location was about 1.8 mm more rostral than the planned location (−1.20 mm vs. −2.96). Lesion area is denoted in black-dashed-line circle. (
C) A “virtual” location of the electrode tip (black line) if the electrode had been implanted to the targeted atlas-based coordinate (−1.75 mm from bregma, 4 mm from midline, 1.8 mm from the surface of the brain). (
D) MRI-guided (insert) AP, ML and DV coordinates and (
E) histology-confirmed “true” location of the posterior intracortical electrode tip in rat 1139. The black-dashed-line circle denotes the lesion area. Note that the confirmed AP location was approximately 1.4 mm more rostral than the planned location (−3.96 vs. −5.36). Thus, even though both the anterior and posterior intracortical electrodes were more rostral than planned, their tips were recording EEG signals in the perilesional cortex. (
F) A “virtual” electrode (black line) at the atlas-based coordinates would have ended up in the lesion cavity. Scale bar in (
A,
D) = 1 mm, and in (
B,
C,
E,
F) = 2 mm.
![Biomedicines 10 02295 g006]()
Figure 7.
Hippocampal electrode—schematic representations of the atlas-based, histological, and MRI-guided coordinates in each rat of the EEG and MRI cohorts. (A) Anteroposterior (AP) coordinate. In the EEG cohort (electrode operation right after injury), the fixed atlas-based target AP coordinate of −3 mm from the bregma was applied to implant the electrodes (orange dots). In the MRI cohort (electrode operation 5 months after injury), the target AP coordinate was individually determined using the 5-month in vivo T2-weighted MR images. The target coordinate fluctuated, depending on the TBI-induced hippocampal structural abnormality. Note the anterior shift (y-axis) in the histologically verified “true” AP coordinate (blue dots) relative to the aimed target coordinate (orange dots) in both cohorts. Note the great variability in the anterior shift from animal to animal, particularly in the MRI cohort. Animal numbers are shown on the x-axis. (B) Mediolateral (ML) coordinate. In the EEG cohort, the fixed atlas-based target ML coordinate at 1.4 mm lateral to midline was targeted. In the MRI cohort, the target ML coordinate was individually determined using the 5-month MRI. Note only a very small deviation of the histologically defined “true” ML coordinate from the atlas-based (EEG cohort) or MRI-guided (MRI cohort) coordinates. (C) Dorsoventral (DV) coordinate. In both cohorts, the lower tip of the bipolar electrode was aimed at the hilus in the selected AP and ML coordinates (see above). In the EEG cohort, most of the tips were recording in the hippocampus proper or the dentate gyrus. In the EEG cohort, in only 19% (8/43) of TBI cases, the tip was either in fimbria, ventricle, or went through the septal hippocampus to the dorsal thalamus or to an unidentified location. In the MRI cohort, in only 14% (6/31) of TBI cases, the tip was outside the hippocampus or the dentate gyrus. The percentages of electrode locations in the sham-operated and TBI animals are shown on the right side of the panel. (D) Dot plots of the AP and ML shift in the histological AP and ML coordinate, and % of electrodes in the targeted dentate gyrus (number of cases in brackets). Note posterior shift of some cases from the target (vertical dashed line). The y-axis represents distance from target coordinate (Y = 0) or % of cases in targeted area. Abbreviations: alv, alveus; CA1, CA1 subfield of the hippocampus; CA3, CA3 (CA3b, CA3c) subfield of the hippocampus; gcl, granule cell layer (s-gcl, suprapyramidal blade, i-gcl, infrapyramidal blade); hf, hippocampal fissure; l-m, stratum lacunosum moleculare of CA1; mol, molecular layer of the dentate gyrus; V, ventricle.
Figure 7.
Hippocampal electrode—schematic representations of the atlas-based, histological, and MRI-guided coordinates in each rat of the EEG and MRI cohorts. (A) Anteroposterior (AP) coordinate. In the EEG cohort (electrode operation right after injury), the fixed atlas-based target AP coordinate of −3 mm from the bregma was applied to implant the electrodes (orange dots). In the MRI cohort (electrode operation 5 months after injury), the target AP coordinate was individually determined using the 5-month in vivo T2-weighted MR images. The target coordinate fluctuated, depending on the TBI-induced hippocampal structural abnormality. Note the anterior shift (y-axis) in the histologically verified “true” AP coordinate (blue dots) relative to the aimed target coordinate (orange dots) in both cohorts. Note the great variability in the anterior shift from animal to animal, particularly in the MRI cohort. Animal numbers are shown on the x-axis. (B) Mediolateral (ML) coordinate. In the EEG cohort, the fixed atlas-based target ML coordinate at 1.4 mm lateral to midline was targeted. In the MRI cohort, the target ML coordinate was individually determined using the 5-month MRI. Note only a very small deviation of the histologically defined “true” ML coordinate from the atlas-based (EEG cohort) or MRI-guided (MRI cohort) coordinates. (C) Dorsoventral (DV) coordinate. In both cohorts, the lower tip of the bipolar electrode was aimed at the hilus in the selected AP and ML coordinates (see above). In the EEG cohort, most of the tips were recording in the hippocampus proper or the dentate gyrus. In the EEG cohort, in only 19% (8/43) of TBI cases, the tip was either in fimbria, ventricle, or went through the septal hippocampus to the dorsal thalamus or to an unidentified location. In the MRI cohort, in only 14% (6/31) of TBI cases, the tip was outside the hippocampus or the dentate gyrus. The percentages of electrode locations in the sham-operated and TBI animals are shown on the right side of the panel. (D) Dot plots of the AP and ML shift in the histological AP and ML coordinate, and % of electrodes in the targeted dentate gyrus (number of cases in brackets). Note posterior shift of some cases from the target (vertical dashed line). The y-axis represents distance from target coordinate (Y = 0) or % of cases in targeted area. Abbreviations: alv, alveus; CA1, CA1 subfield of the hippocampus; CA3, CA3 (CA3b, CA3c) subfield of the hippocampus; gcl, granule cell layer (s-gcl, suprapyramidal blade, i-gcl, infrapyramidal blade); hf, hippocampal fissure; l-m, stratum lacunosum moleculare of CA1; mol, molecular layer of the dentate gyrus; V, ventricle.
![Biomedicines 10 02295 g007]()
Figure 8.
Anteroposterior and mediolateral shrinkage of the brain. In vivo magnetic resonance 3D multigradient echo (MGRE) images acquired at 5 months after TBI were used to estimate cortical shrinkage in the MRI cohort. (A1–A4) coronal, sagittal (ipsilateral and contralateral) and horizontal MGRE images of a sham rat (A1) and TBI rats (A2–A4). Anteroposterior (AP) cortical shrinkage was estimated by measuring the distance between the rostral and caudal cortical surface (double-headed arrows) in the sagittal slice at 4 mm from the midline both ipsilaterally (orange) and contralaterally (white). Note the change in the shape of the ipsilateral cortex (sagittal images) in TBI rats, indicating the TBI-induced cortical atrophy (see also turquoise arrows in (A3,A4)). Mediolateral (ML) shrinkage was assessed by measuring the distance between the midline and the lateral edge of the cortex (turquoise double headed arrow) in a horizontal slice at 1.7 mm below the pial surface at AP level −1.56 (corresponding to the targeted location of the anterior intracortical electrode tip). (B) A dot plot showing the ipsilateral (orange) and contralateral (blue) cortical AP lengths (y-axis) in the sham and TBI groups (x-axis). Note that both the ipsilateral and contralateral cortical AP lengths were reduced in TBI rats compared with sham-operated animals. Also, in the TBI group, the cortical AP length was shorter ipsilaterally than contralaterally. (C) A paired dot plot showing that the ipsilateral vs. contralateral shrinkage in each rat. The greater the ipsilateral shrinkage, the greater the contralateral shrinkage in the TBI compared with sham group. Arrows point to the 3 cases illustrated in panels (A1–A4). (D) A dot plot showing the ipsilateral and contralateral cortical ML lengths in sham-operated and TBI rats. Note that both the ipsilateral and contralateral cortical ML lengths were reduced in TBI rats compared with sham-operated animals. Also, in the TBI group, the cortical ML length was shorter ipsilaterally than contralaterally. Statistical significance: *** p < 0.001 compared with the contralateral hemisphere (Wilcoxon signed-rank test); ### p < 0.001, # p < 0.05 compared with the sham group (Mann–Whitney U test).
Figure 8.
Anteroposterior and mediolateral shrinkage of the brain. In vivo magnetic resonance 3D multigradient echo (MGRE) images acquired at 5 months after TBI were used to estimate cortical shrinkage in the MRI cohort. (A1–A4) coronal, sagittal (ipsilateral and contralateral) and horizontal MGRE images of a sham rat (A1) and TBI rats (A2–A4). Anteroposterior (AP) cortical shrinkage was estimated by measuring the distance between the rostral and caudal cortical surface (double-headed arrows) in the sagittal slice at 4 mm from the midline both ipsilaterally (orange) and contralaterally (white). Note the change in the shape of the ipsilateral cortex (sagittal images) in TBI rats, indicating the TBI-induced cortical atrophy (see also turquoise arrows in (A3,A4)). Mediolateral (ML) shrinkage was assessed by measuring the distance between the midline and the lateral edge of the cortex (turquoise double headed arrow) in a horizontal slice at 1.7 mm below the pial surface at AP level −1.56 (corresponding to the targeted location of the anterior intracortical electrode tip). (B) A dot plot showing the ipsilateral (orange) and contralateral (blue) cortical AP lengths (y-axis) in the sham and TBI groups (x-axis). Note that both the ipsilateral and contralateral cortical AP lengths were reduced in TBI rats compared with sham-operated animals. Also, in the TBI group, the cortical AP length was shorter ipsilaterally than contralaterally. (C) A paired dot plot showing that the ipsilateral vs. contralateral shrinkage in each rat. The greater the ipsilateral shrinkage, the greater the contralateral shrinkage in the TBI compared with sham group. Arrows point to the 3 cases illustrated in panels (A1–A4). (D) A dot plot showing the ipsilateral and contralateral cortical ML lengths in sham-operated and TBI rats. Note that both the ipsilateral and contralateral cortical ML lengths were reduced in TBI rats compared with sham-operated animals. Also, in the TBI group, the cortical ML length was shorter ipsilaterally than contralaterally. Statistical significance: *** p < 0.001 compared with the contralateral hemisphere (Wilcoxon signed-rank test); ### p < 0.001, # p < 0.05 compared with the sham group (Mann–Whitney U test).
![Biomedicines 10 02295 g008]()
Figure 9.
(A–C) Electrode tracts. Histological images from the coronal thionine-stained sections of 3 rats, showing the tracts of the bipolar intracortical electrodes and locations of the lower electrode tip (filled arrowhead). The target of the lower tip was the hilus. In panel (A), the electrode tip is in the suprapyramidal blade of the granule cell layer. In panel (B), the tip went through the dentate gyrus down to the dorsal thalamus. In panel (C), the tip is in the infrapyramidal blade of the granule cell layer. Note the electrode-path associated lesion in CA1 (open arrow). (D1–D3) Coronal, sagittal and horizontal in vivo magnetic resonance 3D multigradient echo (MGRE) images of the ipsilateral and contralateral hippocampus were used to assess hippocampal shrinkage after traumatic brain injury (TBI). (D1–D3) Hippocampal distortion and shrinkage. Panel (D1): A sham-operated experimental control (1107). Panels (D2,D3): Two rats with TBI (1028, 1144). The anteroposterior (AP) shift of the hippocampus was assessed by measuring the distance from the rostral edge of the frontal cortex to the rostral edge of the hippocampus at 1.4 mm from the midline in the horizontal slice 2.8 mm below the surface of the brain (left hemisphere: orange double-headed arrow; right hemisphere: white double-headed arrow). Mediolateral (ML) shrinkage was assessed by measuring the distance from the brain midline to the lateral edge of the hippocampus in the same horizontal plane (2.8 mm below the surface of the brain, turquoise double-headed arrow) in a slice sampled at AP level −2.8 mm, corresponding to the AP level of the atlas-based target coordinate. In both TBI rats (D2,D3), the distance from the frontal pole to the rostral edge of the hippocampus was longer than that in the sham-operated animal, indicating retraction of the septal hippocampus caudally. The ML length in TBI rats (D2,D3) was shorter than that in the sham-operated animal (D1), indicating a shift toward midline. (E) A dot plot showing the ipsilateral (orange) and contralateral (blue) anteroposterior lengths (y-axis) in the sham and TBI groups (x-axis). Note that both the ipsilateral and contralateral cortical AP lengths were increased in TBI rats compared with sham-operated animals. Also, in the TBI group, the cortical AP length was greater ipsilaterally than contralaterally. (F) A paired dot plot showing the ipsilateral vs. contralateral backward “movement” in each rat. The greater the ipsilateral “movement”, the greater the contralateral “movement”. Arrows point to the 3 cases illustrated in panels (D1–D3). (G) A dot plot showing the ipsilateral and contralateral hippocampal ML lengths in sham-operated and TBI rats. Note that both the ipsilateral and contralateral cortical ML lengths were reduced in TBI rats compared with sham-operated animals. Also, in the TBI group, the ML length was shorter ipsilaterally than contralaterally. Statistical significance: ##, p < 0.05; ###, p < 0.001 as compared to the sham group (Mann–Whitney U test); *** p < 0.001 compared with the contralateral hemisphere (Wilcoxon signed-rank test). Scale bar in (A–C) = 500 µm.
Figure 9.
(A–C) Electrode tracts. Histological images from the coronal thionine-stained sections of 3 rats, showing the tracts of the bipolar intracortical electrodes and locations of the lower electrode tip (filled arrowhead). The target of the lower tip was the hilus. In panel (A), the electrode tip is in the suprapyramidal blade of the granule cell layer. In panel (B), the tip went through the dentate gyrus down to the dorsal thalamus. In panel (C), the tip is in the infrapyramidal blade of the granule cell layer. Note the electrode-path associated lesion in CA1 (open arrow). (D1–D3) Coronal, sagittal and horizontal in vivo magnetic resonance 3D multigradient echo (MGRE) images of the ipsilateral and contralateral hippocampus were used to assess hippocampal shrinkage after traumatic brain injury (TBI). (D1–D3) Hippocampal distortion and shrinkage. Panel (D1): A sham-operated experimental control (1107). Panels (D2,D3): Two rats with TBI (1028, 1144). The anteroposterior (AP) shift of the hippocampus was assessed by measuring the distance from the rostral edge of the frontal cortex to the rostral edge of the hippocampus at 1.4 mm from the midline in the horizontal slice 2.8 mm below the surface of the brain (left hemisphere: orange double-headed arrow; right hemisphere: white double-headed arrow). Mediolateral (ML) shrinkage was assessed by measuring the distance from the brain midline to the lateral edge of the hippocampus in the same horizontal plane (2.8 mm below the surface of the brain, turquoise double-headed arrow) in a slice sampled at AP level −2.8 mm, corresponding to the AP level of the atlas-based target coordinate. In both TBI rats (D2,D3), the distance from the frontal pole to the rostral edge of the hippocampus was longer than that in the sham-operated animal, indicating retraction of the septal hippocampus caudally. The ML length in TBI rats (D2,D3) was shorter than that in the sham-operated animal (D1), indicating a shift toward midline. (E) A dot plot showing the ipsilateral (orange) and contralateral (blue) anteroposterior lengths (y-axis) in the sham and TBI groups (x-axis). Note that both the ipsilateral and contralateral cortical AP lengths were increased in TBI rats compared with sham-operated animals. Also, in the TBI group, the cortical AP length was greater ipsilaterally than contralaterally. (F) A paired dot plot showing the ipsilateral vs. contralateral backward “movement” in each rat. The greater the ipsilateral “movement”, the greater the contralateral “movement”. Arrows point to the 3 cases illustrated in panels (D1–D3). (G) A dot plot showing the ipsilateral and contralateral hippocampal ML lengths in sham-operated and TBI rats. Note that both the ipsilateral and contralateral cortical ML lengths were reduced in TBI rats compared with sham-operated animals. Also, in the TBI group, the ML length was shorter ipsilaterally than contralaterally. Statistical significance: ##, p < 0.05; ###, p < 0.001 as compared to the sham group (Mann–Whitney U test); *** p < 0.001 compared with the contralateral hemisphere (Wilcoxon signed-rank test). Scale bar in (A–C) = 500 µm.
![Biomedicines 10 02295 g009]()
Figure 10.
Location of electrode tip at 5 months postinjury without prior MRI analysis. Photomicrographs of thionine-stained coronal brain sections of 4 animals; (
A) #1019, (
B) #1139, (
C) #1158 and (
D) #1036 in the MRI cohort with electrode implantations at 5 months after TBI. Left panels: MRI-guided placement of the posterior cortical electrode. Note that all electrodes are within the perilesional cortex. Right panels: The location of the electrode tip (arrow), if the electrode was implanted according to the targeted atlas-based coordinates (−7.56 mm from bregma, 4 mm from midline, 1.8 mm from the surface of the brain). Note that in all rats except 1019, the electrode tip ended in the lesion cavity.
Table 2 summarizes the locations for all cases. Scale bar = 2 mm.
Figure 10.
Location of electrode tip at 5 months postinjury without prior MRI analysis. Photomicrographs of thionine-stained coronal brain sections of 4 animals; (
A) #1019, (
B) #1139, (
C) #1158 and (
D) #1036 in the MRI cohort with electrode implantations at 5 months after TBI. Left panels: MRI-guided placement of the posterior cortical electrode. Note that all electrodes are within the perilesional cortex. Right panels: The location of the electrode tip (arrow), if the electrode was implanted according to the targeted atlas-based coordinates (−7.56 mm from bregma, 4 mm from midline, 1.8 mm from the surface of the brain). Note that in all rats except 1019, the electrode tip ended in the lesion cavity.
Table 2 summarizes the locations for all cases. Scale bar = 2 mm.
Figure 11.
Distance of the intracortical electrodes from the edge of the cortical lesion cavity. (A) An unfolded cortical map of a rat 1064, showing the cytoarchitectonic distribution of the cortical lesion (blue outline) and the location of the anterior (brown filled circle in the S1BF) and posterior (yellow filled circle in the V2L) intracortical electrodes. Note that the lesion had progressed laterally and caudally. Consequently, the posterior electrode was closer to the lesion cavity edge than the anterior electrode. (B) A scatter plot showing the cortical lesion area in the EEG and MRI cohorts (each dot represents 1 rat). The lesion area was comparable between cohorts (p > 0.05). (C) In the EEG cohort, the distance from the electrode tip to the lesion cavity edge (layer V intersection was used as reference) was similar between the anterior and posterior intracortical electrodes (p > 0.05). (D) In the MRI cohort, the distance from the anterior intracortical electrode tip to the cavity edge was slightly greater than that from the EEG cohort (p < 0.05). (E) In the EEG cohort, the larger the lesion, the closer the posterior electrode tip to the lesion cavity edge (p < 0.001). (F) In the MRI cohort, the larger the lesion, the closer the posterior electrode tip to the cavity edge (p < 0.001). Abbreviations: S1BF, primary somatosensory barrel field; V2L, secondary visual cortex lateral area. Statistical significance: #, p < 0.05 compared with the EEG cohort (Mann–Whitney U test).
Figure 11.
Distance of the intracortical electrodes from the edge of the cortical lesion cavity. (A) An unfolded cortical map of a rat 1064, showing the cytoarchitectonic distribution of the cortical lesion (blue outline) and the location of the anterior (brown filled circle in the S1BF) and posterior (yellow filled circle in the V2L) intracortical electrodes. Note that the lesion had progressed laterally and caudally. Consequently, the posterior electrode was closer to the lesion cavity edge than the anterior electrode. (B) A scatter plot showing the cortical lesion area in the EEG and MRI cohorts (each dot represents 1 rat). The lesion area was comparable between cohorts (p > 0.05). (C) In the EEG cohort, the distance from the electrode tip to the lesion cavity edge (layer V intersection was used as reference) was similar between the anterior and posterior intracortical electrodes (p > 0.05). (D) In the MRI cohort, the distance from the anterior intracortical electrode tip to the cavity edge was slightly greater than that from the EEG cohort (p < 0.05). (E) In the EEG cohort, the larger the lesion, the closer the posterior electrode tip to the lesion cavity edge (p < 0.001). (F) In the MRI cohort, the larger the lesion, the closer the posterior electrode tip to the cavity edge (p < 0.001). Abbreviations: S1BF, primary somatosensory barrel field; V2L, secondary visual cortex lateral area. Statistical significance: #, p < 0.05 compared with the EEG cohort (Mann–Whitney U test).
![Biomedicines 10 02295 g011]()
Table 1.
Summary of the location of the dorsoventral tip of the intracerebral electrodes.
Table 1.
Summary of the location of the dorsoventral tip of the intracerebral electrodes.
| | EEG Cohort
Atlas-Based
% (n) | MRI Cohort
Atlas-Based without MRI Guidance
% (n) | MRI Cohort
with MRI Guidance
% (n) | EEG and MRI Cohorts
% (n) |
|---|
| | Sham | TBI | All | Sham | TBI | All | Sham | TBI | All | Sham | TBI | All |
|---|
| Anterior cortical electrode | | | | | | | | | | | | |
| Within 1 mm of target coordinate # | 29% (4/14) | 26% (11/43) | 26% (15/57) | 100% (11/11) | 100% (31/31) | 100% (42/42) | 55% (6/11) | 32% (10/31) | 38% (16/42) | 42% (10/25) | 28% (21/74) | 31% (31/99) |
| In cortex | 79% (11/14) | 84% (36/43) | 83% (47/57) | 100% (11/11) | 32% (10/31) | 50% (21/42) | 73% * (8/11) | 77% *** (24/31) | 76% (32/42) | 76% (19/25) | 81% (60/74) | 78% (79/99) |
| In brain | 100% (14/14) | 100% (43/43) | 100% (57/57) | 100% (11/11) | 52% (16/31) | 64% (27/42) | 100% (11/11) | 100% *** (31/31) | 100% (42/42) | 100% (25/25) | 100% (74/74) | 100% (99/99) |
| Not recording in brain | 0% (0/14) | 0% (0/43) | 0% (0/57) | 0% (0/111) | 48% (15/31) | 36% (15/42) | 0% (0/11) | 0% *** (0/31) | 0% (0/42) | 0% (0/25) | 0% (0/74) | 0% (0/99) |
| Hippocampal electrode | | | | | | | | | | | | |
| Within 1 mm of target coordinate # | 93% (13/14) | 77% (33/43) | 81% (46/57) | 100% (11/11) | 100% (31/31) | 100% (42/42) | 82% (9/11) | 52% (16/31) | 60% (25/42) | 88% (22/25) | 66% (49/74) | 72% (71/99) |
| In hippocampus proper/DG | 86% (12/14) | 81% (35/43) | 82% (47/57) | 100% (11/11) | 100% (31/31) | 100% (42/42) | 100% (11/11) | 81% * (25/31) | 86% (36/42) | 92% (23/25) | 81% (60/74) | 84% (83/99) |
| In brain | 93% (13/14) | 93% (41/43) | 95% (54/57) | 100% (11/11) | 100% (31/31) | 100% (42/42) | 100% (11/11) | 84% * (26/31) | 88% (37/42) | 96% (24/25) | 91% (67/74) | 92% (91/99) |
| Not recording in brain | 7% (1/14) | 5% (2/43) | 5% (3/57) | 0% (0/111) | 0% (0/31) | 0% (42/42) | 0% (0/11) | 16% * (5/39) | 12% (5/42) | 28% (7/25) | 10% (7/74) | 8% (8/99) |
| Posterior cortical electrode | | | | | | | | | | | | |
| Within 1 mm of target coordinate # | 43% (6/14) | 51% (20/39) | 49% (26/53) | 100% (11/11) | 100% (31/31) | 100% (42/42) | 36% (4/11) | 21% (6/29) | 25% (10/40) | 40% (10/25) | 38% (26/68) | 41% (38/93) |
| In cortex | 21% (3/14) | 46% (18/39) | 40% (21/53) | 91% (10/11) | 42% (13/31) | 55% (23/42) | 46% * (5/11) | 66% * (19/29) | 60% (24/40) | 32% (8/25) | 54% (37/68) | 48% (45/93) |
| In brain | 100% (14/14) | 97% (34/35) | 90% (48/53) | 100% (11/11) | 42% (13/31) | 57% (24/42) | 100% (11/11) | 93% *** (27/29) | 95% (38/40) | 100% (25/25) | 90% (61/68) | 92% (86/93) |
| Not recording in brain | 0% (0/14) | 13% (5/39) | 10% (5/53) | 0% (0/11) | 58% (18/31) | 43% (18/42) | 0% (0//11) | 7% *** (2/29) | 5% (2/40) | 0% (0/25) | 10% (7/68) | 8% (7/93) |
Table 2.
Location of the “virtual electrode”. Summary of the locations of the posterior intracortical electrodes in the MRI cohort, if implanted according to the atlas-based coordinates. Note that 58% (18/31) of the lower electrode tips were in the cortex while 42% (13/31) of the lower tips were in the lesion cavity. After MRI-guidance, 71% (22/31) of the lower tips were recording in the cortex. In 5 additional cases, the upper tip (0.5 dorsal to the lower tip) was expected to record in the cortex, resulting in a total of 87% (27/31) of the electrodes recording in the cortex. Only 1 electrode was not recording in the brain.
Table 2.
Location of the “virtual electrode”. Summary of the locations of the posterior intracortical electrodes in the MRI cohort, if implanted according to the atlas-based coordinates. Note that 58% (18/31) of the lower electrode tips were in the cortex while 42% (13/31) of the lower tips were in the lesion cavity. After MRI-guidance, 71% (22/31) of the lower tips were recording in the cortex. In 5 additional cases, the upper tip (0.5 dorsal to the lower tip) was expected to record in the cortex, resulting in a total of 87% (27/31) of the electrodes recording in the cortex. Only 1 electrode was not recording in the brain.
| | Atlas-Based | MRI-Guided |
|---|
| Animal | AP Level | DV Location
(Distance from Lesion Edge) | AP Level | DV Location |
|---|
| 1008 | 7.56 | Lesion cavity | −4.68 | Layer VI |
| 1012 | 7.56 | Lesion cavity | −5.20 | CA1 |
| 1019 | 7.56 | Perilesional cortex (medial, 0 mm) | −4.20 | Layer V |
| 1024 | 7.56 | Lesion cavity | −4.68 | angular bundle |
| 1028 | 7.56 | Lesion cavity | −4.36 | Layer VI |
| 1029 | 7.56 | Lesion cavity | −4.36 | corpus callosum |
| 1031 | 7.56 | Lesion cavity | | not found |
| 1036 | 7.56 | Lesion cavity | −5.28 | Layer VI |
| 1038 | 7.56 | Perilesional cortex (medial, 0.7 mm) | −4.68 | alveus of the hippocampus |
| 1043 | 7.56 | Lesion cavity | −3.96 | Layer IV |
| 1046 | 7.56 | Lesion cavity | −4.08 | Layer V |
| 1090 | 7.56 | Perilesional cortex (medial, 0.53 mm) | −4.36 | Layer VI |
| 1095 | 7.56 | Perilesional cortex (medial, 0.32 mm) | −3.96 | Layer VI |
| 1099 | 7.56 | Perilesional cortex (medial, 0.93 mm) | −4.20 | Layer VI |
| 1103 | 7.56 | Perilesional cortex (caudal, 0.2 mm) | −4.20 | Layer V |
| 1104 | 7.56 | Perilesional cortex (medial, 0.73 mm) | −5.28 | Layer V |
| 1105 | 7.56 | Lesion cavity | −4.68 | Layer VI |
| 1138 | 7.56 | Perilesional cortex (medial, 0.71 mm) | −3.96 | Layer V |
| 1139 | 7.56 | Lesion cavity | −5.40 | Layer V |
| 1140 | 7.56 | Perilesional cortex (medial, 1.1 mm) | −5.52 | Layer VI |
| 1142 | 7.56 | Lesion cavity | −4.20 | corpus callosum |
| 1144 | 7.56 | Lesion cavity | −4.80 | ventricle |
| 1145 | 7.56 | Perilesional cortex (medial, 0.63 mm) | −4.20 | CA1 |
| 1149 | 7.56 | Lesion cavity | −4.20 | Layer VI |
| 1150 | 7.56 | Lesion cavity | −4.20 | Layer VI |
| 1152 | 7.56 | Perilesional cortex (medial, 0.91 mm) | −4.68 | Layer V |
| 1153 | 7.56 | Lesion cavity | −4.20 | Layer VI |
| 1154 | 7.56 | Lesion cavity | −5.40 | Layer VI |
| 1156 | 7.56 | Perilesional cortex (medial, 0.28 mm) | −4.68 | Layer VI |
| 1158 | 7.56 | Lesion cavity | −4.20 | Layer VI |
| 1159 | 7.56 | Perilesional cortex (medial, 1.3 mm) | | not found |
Table 3.
Cytoarchitectonic distribution of the TBI-induced cortical lesion in the EEG and MRI cohorts.
Table 3.
Cytoarchitectonic distribution of the TBI-induced cortical lesion in the EEG and MRI cohorts.
| | EEG Cohort | MRI Cohort |
|---|
| Cortical Area | Area (mm2)
(Mean ± SEM) | Min–Max | % of Rats | Area
(Mean ± SEM) | Min–Max | % of Rats |
|---|
| DLEnt | 0.27 ± 0.01 | 0.27–0.27 | 5 (2/43) | | | 0 (0/30) |
| PRh | 0.44 ± 0.11 | 0.01–1.35 | 44 (19/43) | 0.79 ± 0.16 | 0.10–1.61 | 37 (11/30) |
| Ect | 1.47 ± 0.20 | 0.01–3.82 | 77 (33/43) | 1.77 ± 0.33 | 0.08–5.11 | 70 (21/30) |
| DI | | | 0 (0/43) | 0.18 | 0.18–0.18 | 3 (1/30) |
| GI | | | 0 (0/43) | 0.12 ± 0.05 | 0.03–0.24 | 13 (4/30) # |
| TeA | 2.35 ± 0.17 | 0.01–3.92 | 91 (39/43) | 2.33 ± 0.26 | 0.01–3.92 | 87 (26/30) |
| AuV | 0.91 ± 0.09 | 0.01–2.45 | 93 (40/43) | 1.22 ± 0.15 | 0.02–2.62 | 90 (27/30) |
| Au1 | 3.77 ± 0.20 | 0.86–5.78 | 100 (43/43) | 4.32 ± 0.30 * | 0.66–5.86 | 100 (30/30) |
| AuD | 2.31 ± 0.06 | 0.58–2.61 | 100 (43/43) | 2.32 ± 0.09 | 1.04–2.61 | 100 (30/30) |
| S1ULp | 0.75 ± 0.09 | 0.03–1.98 | 93 (40/43) | 1.14 ± 0.12 * | 0.13–2.52 | 80 (24/30) |
| S1 | 1.16 ± 0.03 | 0.49–1.23 | 98 (42/43) | 1.09 ± 0.05 | 0.02–1.23 | 97 (29/30) |
| S1BF | 3.79 ± 0.36 | 0.56–10.44 | 98 (42/43) | 3.42 ± 0.41 | 0.16–8.68 | 93 (28/30) |
| S1DZ | 0.19 ± 0.06 | 0.02–0.3 | 9 (4/43) | 0.01 | 0.01–0.01 | 3 (1/30) |
| PtPR | 0.61 ± 0.03 | 0.06–0.75 | 95 (41/43) | 0.61 ± 0.04 | 0.01–0.75 | 97 (29/30) |
| PtPD | 0.47 ± 0.05 | 0.01–0.97 | 86 (37/43) | 0.47 ± 0.05 | 0.01–0.96 | 83 (25/30) |
| V2L | 4.62 ± 0.27 | 0.02–6.28 | 100 (43/43) | 4.60 ± 0.32 | 0.87–6.48 | 100 (30/30) |
| V1B | 2.12 ± 0.23 | 0.02–4.59 | 81 (35/43) | 2.19 ± 0.30 | 0.15–4.81 | 80 (24/30) |
| V1 | 0.73 ± 0.16 | 0–2.13 | 56 (24/43) | 0.37 ± 0.09 | 0.05–1.21 | 60 (18/30) |
| V1M | 1.42 ± 0.26 | 0.01–3.98 | 47 (20/43) | 1.32 ± 0.28 | 0.12–3.54 | 53 (16/30) |
| V2ML | 0.68 ± 0.29 | 0.03–2.7 | 21 (9/43) | 0.24 ± 0.14 | 0.01–0.76 | 17 (5/30) |
| V2MM | 0.76 ± 0.50 | 0.04–2.26 | 9 (4/43) | 0.28 ± 0.15 | 0.02–1.01 | 20 (6/30) |
| MPtA | 0.04 ± 0.02 | 0.02–0.06 | 5 (2/43) | | | 0 (0/30) |
| LPtA | 0.83 ± 0.22 | 0.06–1.62 | 16 (7/43) | 0.04 ± 0.03 | 0.01–0.09 | 10 (3/30) |
| S1Tr | 0.54 ± 0.27 | 0.2–1.09 | 7 (3/43) | | | 0 (0/30) |
| S1Sh | 0.01 | 0.01–0.01 | 2 (1/43) | | | 0 (0/30) |
| S1FL | 0.12 | 0.12–0.12 | 2 (1/43) | | | 0 (0/30) |
| S2 | 0.56 ± 0.07 | 0.04–2.10 | 95 (41/43) | 1.07 ± 0.17 * | 0.38–3.09 | 80 (24/30) # |
| RSD | 1.09 ± 0.91 | 0.17–2.0 | 5 (2/43) | 0.20 ± 0.08 | 0.02–0.42 | 13 (4/30) |
| RSGc | 0.37 | 0.37–0.37 | 2 (1/43) | | | 0 (0/30) |
| Sum of S1 | 4.89 ± 0.41 | 0.00–12.09 | (43/43) | 5.16 ± 0.55 | 0.00–11.55 | (30/30) |
| Total S | 5.43 ± 0.44 | 0.00–13.22 | (43/43) | 6.01 ± 0.65 | 0.00–12.59 | (30/30) |
| Sum of V1 | 2.80 ± 0.46 | 0.00–10.20 | (43/43) | 2.68 ± 0.49 | 0.00–9.12 | (30/30) |
| Sum of V2 | 4.84 ± 0.31 | 0.02–10.76 | (43/43) | 4.69 ± 0.34 | 0.87–7.71 | (30/30) |
| Total V | 7.63 ± 0.72 | 0.02–20.95 | (43/43) | 7.37 ± 0.78 | 0.87–15.82 | (30/30) |
| Total Au | 6.93 ± 0.34 | 1.44–10.84 | (43/43) | 7.73 ± 0.51 | 1.86–11.08 | (30/30) |
| Total Area | 25.33 ± 1.74 | 1.72–52.88 | (43/43) | 25.69 ± 2.32 | 5.66–47.61 | (30/30) |