Abstract
Blood pressure is determined by cardiac output and systemic vascular resistance, and mediators that induce vasoconstriction will increase systemic vascular resistance and thus elevate blood pressure. While peripheral vascular resistance reflects a complex interaction of multiple factors, vascular ion channels and transporters play important roles in the regulation of vascular tone by modulating the membrane potential of vascular cells. In vascular smooth muscle cells, chloride ions (Cl−) are a type of anions accumulated by anion exchangers and the anion–proton cotransporter system, and efflux of Cl− through Cl− channels depolarizes the membrane and thereby triggers vasoconstriction. Among these Cl− regulatory pathways, emerging evidence suggests that upregulation of the Ca2+-activated Cl− channel TMEM16A in the vasculature contributes to the increased vascular contractility and elevated blood pressure in hypertension. A robust accumulation of intracellular Cl− in vascular smooth muscle cells through the increased activity of Na+–K+–2Cl− cotransporter 1 (NKCC1) during hypertension has also been reported. Thus, the enhanced activity of both TMEM16A and NKCC1 could act additively and sequentially to increase vascular contractility and hence blood pressure in hypertension. In this review, we discuss recent findings regarding the role of Cl− in the regulation of vascular tone and arterial blood pressure and its association with hypertension, with a particular focus on TMEM16A and NKCC1.
1. Introduction
Hypertension is the most prevalent and important risk factor for cardiovascular disease around the world [1], and cardiovascular complications associated with hypertension accounted for 8.5 million deaths worldwide in 2015 [2]. Nevertheless, global control (<140/90 mmHg) rates among subjects with hypertension in 2019 were only 23% for women and 18% for men [3], and thus more effective treatment strategies for hypertension control are urgently needed.
Lifestyle modifications are recommended for the treatment and prevention of hypertension and hypertension-associated cardiovascular diseases for all subjects, including subjects with high normal blood pressure and patients who are taking antihypertensive agents [4]. In particular, the restriction of dietary sodium chloride (NaCl) has been one of the major focus points among lifestyle modifications for the treatment and prevention of hypertension [5,6]. Indeed, numerous animal and human studies have established a causal relationship between dietary NaCl intake and hypertension as well as hypertension-associated cardiovascular diseases [7,8,9].
While it is generally assumed that sodium ions (Na+) but not chloride ions (Cl−) play a critical role in NaCl-induced hypertension [10,11], the copresence of Na+ and Cl− has been reported to be requisite for the development or progression of hypertension in some animal models of hypertension, including desoxycorticosterone-induced hypertensive rats [12], Dahl salt-sensitive hypertensive rats [13,14] and stroke-prone spontaneously hypertensive rats [15]. Likewise, several studies have suggested the importance of Cl− in NaCl-induced hypertension in humans [16,17,18]. These animal and human studies suggest that Na+ alone may not be sufficient, and that Cl− may be indispensable or may act cooperatively with Na+ to give rise to NaCl-induced hypertension. A detailed description of the role of Cl− in NaCl-induced hypertension in animals and humans can be found in an excellent review by McCallum et al. [19].
The precise mechanisms by which Cl− contributes to the blood pressure rise in the above studies are yet to be determined, but the ability of Cl− to modify vascular contractility may play a role. In vascular smooth muscle cells, the intracellular concentration of Cl− is accumulated by anion exchangers and the anion–proton cotransporter system [20,21]. As the resting membrane potential of smooth muscle in vivo (e.g., −38 mV in the rat caudal artery [22]) is more negative than the reversal potential for Cl− (e.g., −18 mV in the guinea pig vas deferens [23]), the opening of Cl− channels leads to an efflux of Cl− and depolarizes the membrane potential, which would then increase the open probability of L-type Ca2+ channels to trigger smooth muscle constriction [20,24].
Thus, in situations with increased intracellular Cl− concentration or increased Cl− channel activity in vascular smooth muscle cells, the driving force for the efflux of Cl− is expected to increase, which in turn could facilitate membrane depolarization and vasoconstriction, and emerging evidence suggests that this scenario is indeed the case in some animal models of hypertension. In this review, we will discuss the possible involvement of Cl− in the pathogenesis of hypertension. Particular emphasis is given to the roles of Ca2+-activated Cl− channel transmembrane membrane 16A (TMEM16A; also known as Ano1) and Na+–K+–2Cl− cotransporter 1 (NKCC1) in the increased vascular contractility during hypertension.
2. Role of Chloride Ions in Regulation of Vascular Tone and Blood Pressure
The vascular tone in vivo is regulated by perivascular nerves, including sympathetic, parasympathetic and non-adrenergic non-cholinergic nerves, and the corelease of norepinephrine and ATP from the sympathetic nerve terminals causes vascular smooth muscle membrane depolarization and subsequent constriction [25,26,27,28,29]. Although multiple ionic mechanisms would underpin the nerve-mediated vascular smooth muscle depolarization, several previous studies have suggested that nerve-mediated and exogenously applied norepinephrine-evoked smooth muscle depolarization could be at least partly due to the generation of Ca2+-activated Cl− currents triggered by the Ca2+ release from the intracellular Ca2+ stores [24,30,31,32].
In addition to perivascular nerve-mediated regulation, myogenic response-mediated vascular smooth muscle depolarization and constriction in response to intravascular pressure change also contribute to the regulation of vascular tone [33]: in rat cerebral arteries, intravascular pressure-induced depolarization and constriction have been shown to be inhibited by two distinct Cl− channel blockers, indanyloxyacetic acid (IAA-94) and 4,4’-diisothiocyanatostilbene-2,2’-disulphonic acid (DIDS), suggesting that the efflux of Cl− ions through Cl− channels could contribute to the myogenic response-mediated vasoconstriction [34]. Indeed, in support of this observation, efflux of Cl− ions was associated with the myogenic constriction in the rat cerebral vascular bed [35]. Nevertheless, because subsequent studies performed in the rat cerebral arteries revealed that IAA-94 depresses L-type calcium current [36], and both IAA-94 and DIDS depress non-selective cationic current [37], the validity of the contribution of Cl− currents to the myogenic response was called into question.
As such, despite a significant amount of physiological and pharmacological evidence showing that vascular Cl− channels play a crucial role in regulating vascular tone, the absence of specific inhibitors and the lack of the molecular identities of the channels make it difficult to reach indisputable conclusions. Among other things, there has been a debate regarding the molecular identity of CaCCs ever since the initial report by Byrne and Large in 1987 [38]. Indeed, several proteins have been proposed as the molecular counterpart of CaCCs, and these include CLCA, CLC-3, TWEENTY and bestrophins [39]. However, three independent groups revealed in 2008 that the TMEM16A protein is a molecular counterpart for CaCCs [40,41,42].
Since these 2008 reports, many studies have confirmed that TMEM16A generates functional CaCC currents in a number of vascular smooth muscle cells and thereby regulates agonist-induced vasoconstriction [21,43,44,45]. Moreover, it has been revealed that TMEM16A also contributes to intravascular pressure-induced myogenic depolarization and vasoconstriction in the cerebral arteries and renal arterioles of rats [46,47]. Thus, it appears likely that the TMEM16A in vascular smooth muscle cells plays a critical role in regulating vascular tone and blood pressure. Support for this notion comes from the fact that conditional knockout mice of TMEM16A in vascular smooth muscle cells shows a complete deficiency of CaCC currents, decreased responsiveness to vasoconstrictor stimuli and reduced systemic blood pressure [48].
3. Alterations in Vascular Chloride Channels and Transporters in Hypertension
3.1. Ca2+-Activated Chloride Channels (CaCCs) in Vascular Smooth Muscle Cells
It is generally accepted that essential hypertension is characterized by an increased peripheral resistance [49,50]. The increased peripheral resistance in hypertension is determined by an integral and complex interplay between various pathogenic factors, including increased sympathetic nervous activity, enhanced calcium ion mobilization in vascular smooth muscle cells, increased calcium sensitivity of vascular smooth muscle cells and reduced production of endothelium-derived relaxing factors, to name a few [50,51]. Among these factors, alterations in the function of vascular ion channels during hypertension contribute to the increased peripheral resistance by shifting the membrane potential to depolarized levels [22,50,52].
While many studies have demonstrated downregulation of the expression and/or function of vascular potassium (K+) channels in hypertension [50,51,53,54], emerging evidence reveals an upregulation of expression and/or function of CaCCs in vascular smooth muscle cells of spontaneous hypertensive rats (SHRs), a genetic model of human essential hypertension. Although a previous study suggested an increased activity of CaCCs in vascular smooth muscle cells of SHRs [55], the molecular identity of the CaCCs observed in that study was unclear at the time. A subsequent study by Wang et al. for the first time revealed that TMEM16A is the molecular counterpart for the increased activity of CaCCs in vascular smooth muscle cells of SHRs, and that TMEM16A protein expression is significantly upregulated in the aorta, the carotid arteries, the hindlimb arteries and the mesenteric arteries of SHRs compared to those of normotensive Wistar Kyoto (WKY) rats [56] (Table 1). Consistent with the seminal findings of Wang and colleagues [56], the increased TMEM16A expression levels and the resultant potentiation of vasoconstrictions have also been reported in smooth muscle cells of the coronary arteries [57] and the renal arterioles [47] of SHRs (Table 1).

Table 1.
Alterations in vascular smooth muscle Ca2+-activated Cl− channels during hypertension.
Importantly, the increased expression and function of TMEM16A appear to be associated with blood pressure elevation in SHRs: the in vivo knockdown of TMEM16A by small interfering RNA (siRNA) transfection prevented blood pressure rise, and the in vivo inhibition of TMEM16A activity by T16Ainh-A01, a TMEM16A inhibitor, reduced blood pressure in SHRs [56] (Table 1). Similarly, a recent study in SHRs showed that in vitro treatment of mesenteric resistance arteries with TMinh-23, a small molecule inhibitor of vascular smooth muscle TMEM16A, blocked vascular smooth muscle constriction in response to vasoconstrictor stimuli, and in vivo treatment with TMinh-23 reduced blood pressure in SHRs with minimal blood pressure change in normotensive rats and mice [58] (Table 1). Although the greater blood pressure lowering effect of TMinh-23 in SHRs appears to be due to an increased sensitivity of TMEM16A to TMinh-23 [58], the mechanisms underlying the increased sensitivity of TMEM16A are unclear and warrant further investigations. Together, these findings implicate vascular smooth muscle CaCC TMEM16A as a possible contributor in the pathogenesis of hypertension in SHRs.
In rat basilar arteries of 2-kidney, 2-clip (2K2C) renal hypertensive rats, exogenously applied angiotensin II (Ang II) induced vasoconstriction that was sensitive to T16Ainh-A01, and Ang II evoked TMEM16A-mediated CaCC currents in rat basilar smooth muscle cells [59]. These findings suggest that CaCC TMEM16A modulates the vasocontractility of basilar arteries of 2K2C renal hypertensive rats; however, in sharp contrast with SHRs, the activity of CaCCs was decreased gradually during the development of hypertension, and the CaCCs’ current density was negatively correlated with blood pressure levels, in basilar arteries of 2K2C renal hypertensive rats [60] (Table 1). Moreover, the TMEM16A protein expression in the smooth muscle layer of the basilar artery decreased during the development of hypertension in 2K2C renal hypertensive rats [59,60] (Table 1).
It is not clear why the activity and the expression of CaCC TMEM16A changed in the opposite direction between SHRs and 2K2C renal hypertensive rats, but the difference might be explained by the different levels of activity of the renin–angiotensin system (RAS) in the vasculature: while the plasma and tissue RASs are suppressed in SHRs [61], the RAS components—particularly the vascular Ang II concentration—are increased in 2K2C renal hypertensive rats [62]. As Ang II decreased TMEM16A expression in some vascular smooth muscle cells, including those from rat basilar arteries [59,60,63], an increase in vascular Ang II concentration in the basilar arteries of 2K2C renal hypertensive rats might downregulate TMEM16A expression and hence reduce the CaCCs’ current in this model.
It has been reported that the perivascular sympathetic nerves exert an abnormal trophic influence on the vascular smooth muscle membrane properties of SHRs [64], and a recent report showed that the expression and contractile function of the CaCC TMEM16A in rat arteries were reduced due to the trophic influence of sympathetic nerves during postnatal maturation [65]. Therefore, we speculate that the expression and function of CaCC TMEM16A might also be decreased along with the longer duration of hypertension in SHRs because of the persistent abnormal trophic influence of the sympathetic nerves. This hypothesis might be supported by the observation that the contribution of CaCCs to norepinephrine-induced vasoconstriction in the femoral arteries was decreased in 12-month-old SHRs compared to that of 6-month-old SHRs [66].
TMEM16A may modulate vascular contractility in cooperation with other ion channels in certain vascular beds. Thus, in rat mesenteric and tail arteries, TMEM16A modulates vascular contractility, at least in part, by positively regulating the expression and function of vascular L-type Ca2+ channels [67,68]. In another study in rat cerebral arterial smooth muscle cells, transient receptor potential canonical 6 channel (TRPC6) and TMEM16A were found to be spatially localized, and TRPC6 activation led to a local elevation of Ca2+, which in turn activated nearby TMEM16A, leading to vasoconstriction [69]. As the function and expression of both L-type Ca2+ channels [70,71] and TRPC6 [72] have been reported to be upregulated in hypertensive rats, it is intriguing to speculate that these mutual interactions of TMEM16A with other vascular ion channels function cooperatively to augment vasoconstriction and hence increase blood pressure in hypertension.
It has been reported that phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid of the plasma membrane, regulates ion channel activity in various cell types [73], and several studies reported that PIP2 acts as a positive modifier of TMEM16A [74,75,76]. By contrast, the TMEM16A-mediated CaCC current was not augmented, but rather inhibited by PIP2 in rat pulmonary artery smooth muscle cells [77]. The reason for the discrepancy is not clear. Nevertheless, a previous report suggests that a significant difference exists between WKY and SHR aortas regarding the PIP2 hydrolysis response following stimulation with norepinephrine [78], indicating the need for further research to understand the possible regulation of TMEM16A by PIP2 in blood vessels in hypertension.
Recent evidence suggests that inositol 1,4,5-trisphosphate receptors (IP3Rs) are spatially colocalized with TMEM16A proteins in nociceptive sensory neurons [79]. If the same holds true in vascular smooth muscle cells, IP3-induced Ca2+ release from intracellular Ca2+ stores would activate nearby TMEM16A, and alterations in this signaling pathway might contribute to the TMEM16A-mediated vasoconstriction in SHRs. Indeed, it has been reported that IP3R channels are upregulated in vascular smooth muscle in hypertension, resulting in enhanced IP3-induced Ca2+ release and increased vasoconstriction [80].
To sum up, while there is a growing body of evidence that CaCC TMEM16A contributes to the increased vascular contractility and elevated blood pressure in SHRs, it is currently unclear whether the upregulation of TMEM16A is specific to SHRs or is present in other hypertensive animal models, and further studies will be needed to clarify the molecular mechanisms that regulate TMEM16A activity during hypertension.
3.2. Ca2+-Activated Chloride Channels (CaCCs) in Vascular Endothelial Cells
In addition to their expression in vascular smooth muscle cells, CaCCs have been reported to be present in some vascular endothelial cells [81,82,83,84]. Although the physiological role of endothelial CaCCs is still not well understood, the endothelial CaCCs may contribute to the regulation of the resting membrane potential of the endothelial cells. Indeed, in mouse brain capillary endothelial cells, pharmacological blockade or knockdown of TMEM16A with siRNA induced membrane hyperpolarization, suggesting that the activation of endothelial CaCCs acts to depolarize the membrane potential of the endothelial cells [83]. Further support for this notion comes from the study by Yamamoto et al. [85]. They found that, in the isolated endothelium of guinea pig mesenteric arteries, ACh increased the intracellular concentration of Ca2+, which subsequently activated endothelial small conductance Ca2+-activated K+ channels (SKCas), intermediate conductance KCa (IKCa) and CaCC simultaneously, and the endothelium-dependent hyperpolarization (EDH) through the activation of both SKCa and IKCa was counteracted by the opposing membrane depolarization evoked by the activation of CaCCs [85].
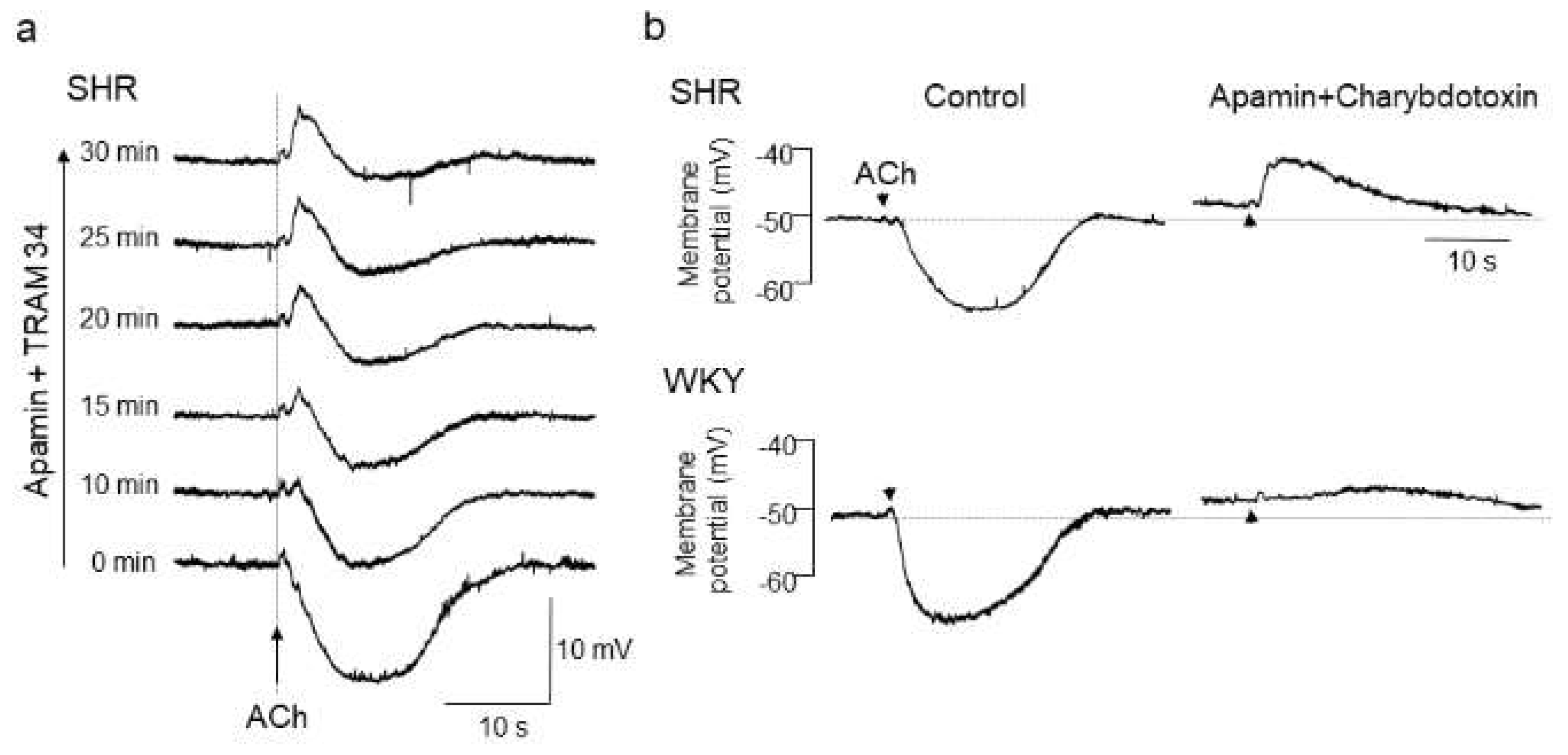
With respect to the alteration of endothelial CaCCs in hypertension, we have previously shown a functional upregulation of endothelial CaCCs in mesenteric resistance arteries of SHRs [86]. In that study, after blockade of EDH with KCa channel inhibitors, iontophoresed acetylcholine (ACh) evoked a rapid and substantial membrane depolarization in mesenteric resistance arteries of SHRs, but only negligible slow depolarization was detected in those of WKY rats [86,87] (Figure 1).
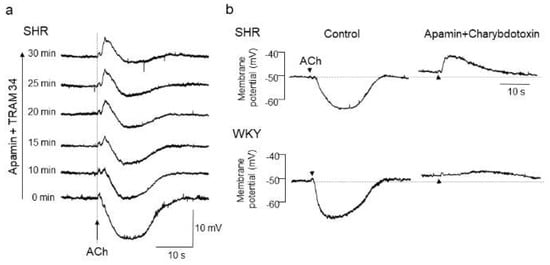
Figure 1.
Acetylcholine (ACh)-evoked depolarization in mesenteric arteries of spontaneously hypertensive rats (SHRs). (a) A hidden depolarization emerged after blockade of endothelium-dependent hyperpolarization (EDH) with apamin (0.5 μmol/L, a small-conductance Ca2+-sensitive K+ channel (KCa) inhibitor) plus TRAM34 (100 nmol/L, an intermediate-conductance KCa inhibitor) in mesenteric arteries of SHRs. All recordings were from the same cell. (b) ACh-evoked depolarization in the presence of apamin (0.5 μmol/L) plus charybdotoxin (60 nmol/L, a large and intermediate-conductance KCa inhibitor) was larger in amplitude and faster in time course in SHRs than in Wistar Kyoto (WKY) rats. Each paired recording was from the same preparation. Indomethacin (10 μmol/L) and Nω-nitro-L-arginine methyl ester (100 μmol/L) were present throughout. Arrows, application of ACh. Modified from Goto et al. [87].
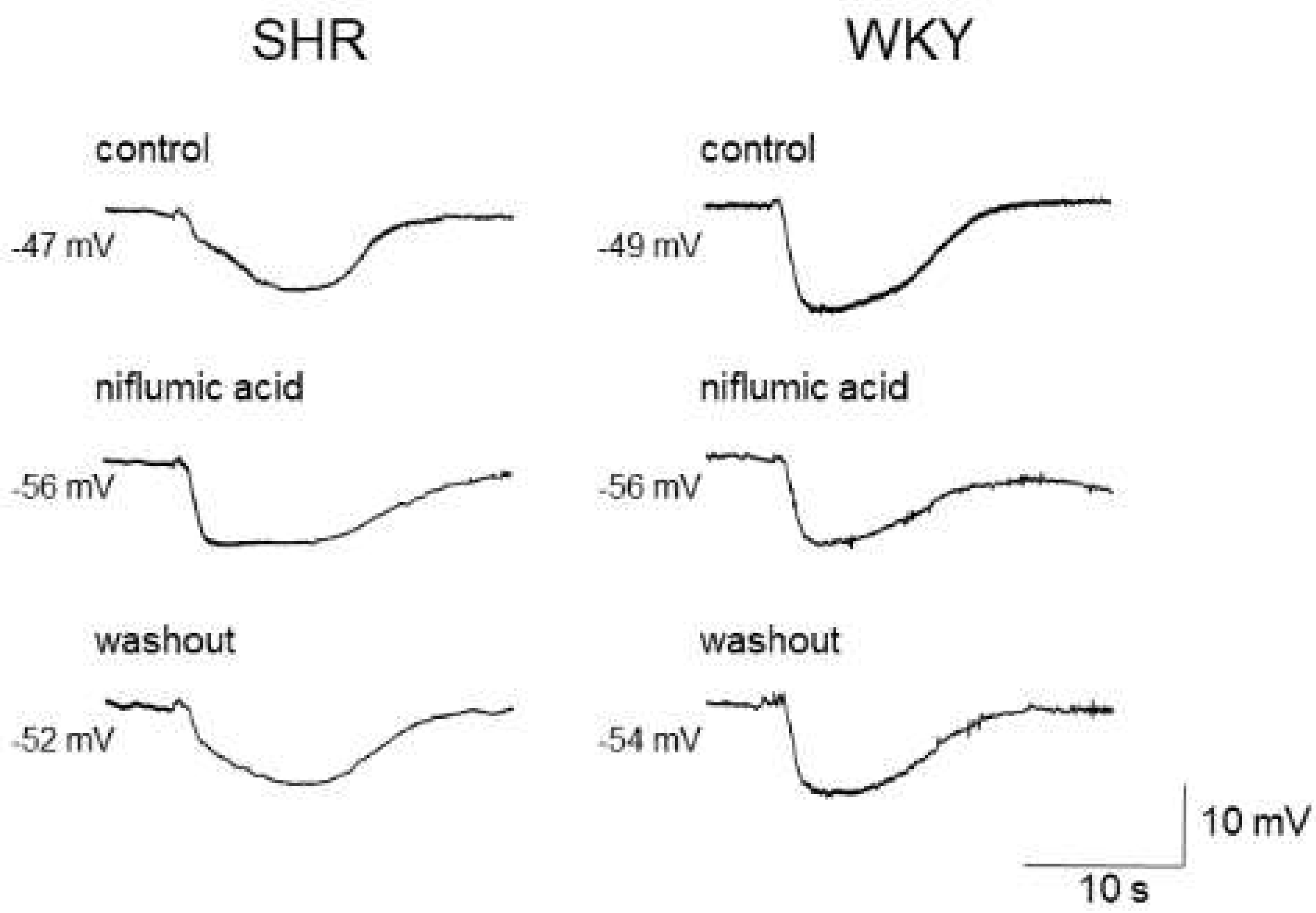
As the estimated reversal potential of the ACh-evoked depolarization in that study was −18 mV [86], which agrees closely with that reported for Cl− ions [23,88], and the ACh-evoked depolarization was abolished by endothelium denudation, or reduced either by replacement of external Cl− ions with impermeant anions or by treatment with the CaCC inhibitors niflumic acid or flufenamic acid, the ACh-evoked depolarization appears to be, at least in part, generated through the activation of endothelial CaCCs in the mesenteric resistance arteries of SHRs [86]. Moreover, the inhibition of the ACh-evoked depolarization by CaCC inhibitors improved the impaired ACh-induced EDH in mesenteric arteries of SHRs, suggesting that an increased activity of endothelial CaCCs may be responsible for the impairment of EDH (Figure 2) (Table 2).
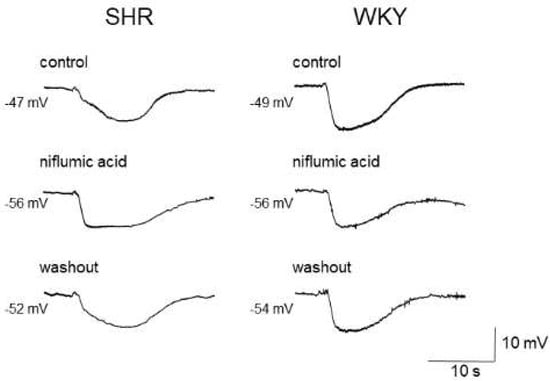
Figure 2.
Effects of niflumic acid on acetylcholine-induced, endothelium-dependent hyperpolarization (EDH) in mesenteric arteries of spontaneously hypertensive rats (SHRs) and Wistar Kyoto (WKY) rats. Niflumic acid (50 μmol/L) improved EDH in SHRs but not in WKY rats. Each paired recording was from the same preparation. Indomethacin (10 μmol/L) and Nω-nitro-L-arginine methyl ester (100 μmol/L) were present throughout.

Table 2.
Alterations in vascular endothelial Ca2+-activated Cl− channels during hypertension.
As endothelial cells and adjacent smooth muscle cells are electrically coupled via myoendothelial gap junctions in rat mesenteric arteries [89,90,91], the impaired EDH leads to attenuated EDH-mediated relaxation and hence to endothelial dysfunction in SHRs [86]. Although some studies have reported that the inhibition of volume-activated Cl− channels potentiates K+-induced, EDH-mediated relaxation in rat mesenteric arteries [92,93], the involvement of the volume-activated Cl− channels in the ACh-evoked depolarization in mesenteric arteries of SHRs is not likely because the volume-regulated Cl− channel inhibitor NPPB had no effect on the ACh-evoked depolarization in this vascular bed [86].
A negative causal link between the activity of endothelial CaCCs, specifically TMEM16A, and endothelial function has also been reported in other studies [82,84]. Thus, in Ang II-induced hypertensive mice, in which the expression of vascular endothelial TMEM16A is increased, the endothelial-specific TMEM16A knockout ameliorated endothelial function and lowered the systolic blood pressure, whereas the endothelial-specific TMEM16A overexpression deteriorated endothelial function and further elevated the systolic blood pressure [82], and these interactions appear to be related to the facilitating effects of TMEM16A on reactive oxygen species generation via Nox2-containing NADPH oxidase [82] (Table 2).
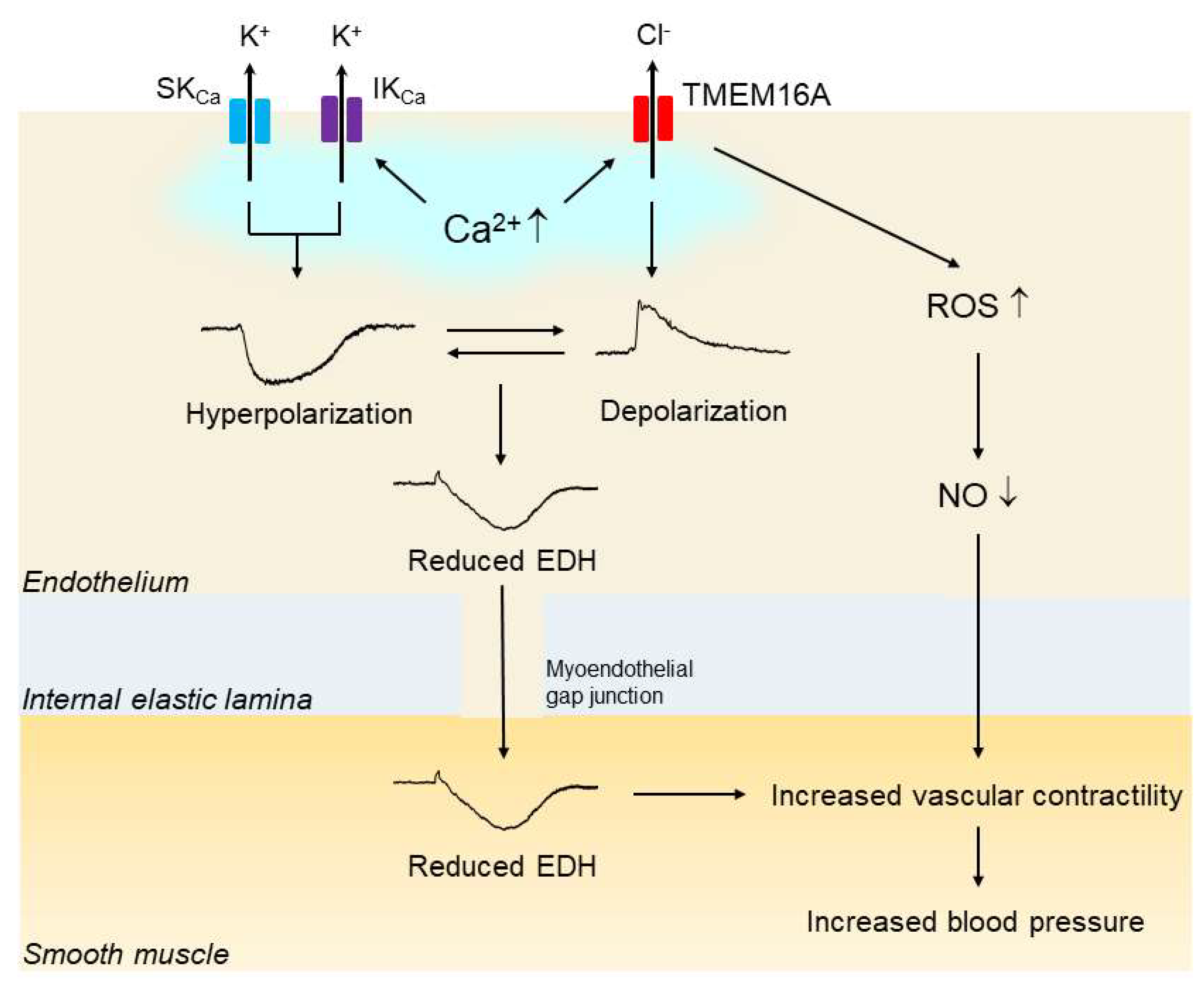
Another study showed that overexpression of TMEM16A in human pulmonary endothelial cells led to a decrease in ACh-induced NO production [84]. Taken together, these findings suggest that upregulation of endothelial CaCC TMEM16A may contribute to the impaired endothelial function, and if so, that it likely does so via a reduction in the activity of EDH and/or NO; finally, the results suggest that such a reduction in EDH and/or NO activity may be at least partly responsible for the elevated blood pressure in hypertension (Figure 3).
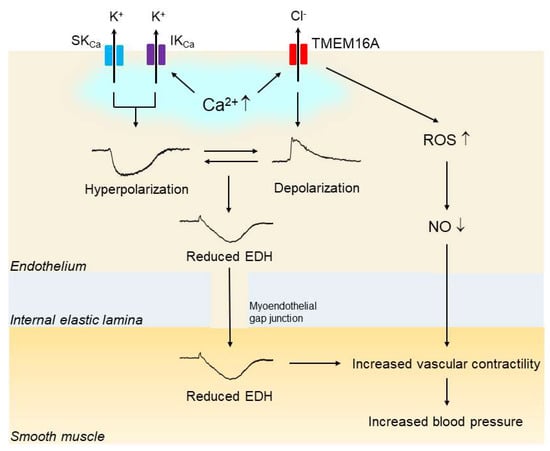
Figure 3.
Upregulation of endothelial TMEM16A impairs endothelial function in hypertension. In hypertension, the expression and function of vascular endothelial Ca2+-activated Cl— channel TMEM16A are increased. Endothelial stimulation with agonists and shear stress increases the intracellular Ca2+concentration, which subsequently activates endothelial small-conductance Ca2+-activated K+ channels (SKCas), intermediate-conductance KCa (IKCa) and TMEM16A simultaneously. The endothelium-dependent hyperpolarization (EDH) through the activation of both SKCa and IKCa is reduced by the opposing membrane depolarization evoked by the activation of TMEM16A. In addition, activation of TMEM16A may facilitate the generation of reactive oxygen species (ROS) through Nox2-containing NADPH oxidase, leading to reduced bioavailability of nitic oxide (NO). Impaired EDH and/or NO could be at least partly responsible for the blood pressure rise in hypertension.
3.3. Na+–K+–2Cl− Cotransporter1 (NKCC1)
NKCC1 located on vascular smooth muscle cells functions to accumulate intracellular Cl− [20,21]. The most compelling evidence of the functional role of NKCC1 in the regulation of vascular tone and arterial blood pressure comes from studies on NKCC1 knockout mice: the systolic blood pressure was significantly reduced in NKCC1 knockout mice compared to wild-type mice [94], and treatment with bumetanide, an inhibitor of NKCC1 [95], inhibited the vascular contractile activity and lowered mean arterial blood pressure in wild-type mice, with the effects being lost in NKCC1 knockout mice [94,96]. Thus, theoretically, an increase in the activity of the vascular smooth muscle NKCC1 could augment vascular contractility and subsequently lead to enhanced blood pressure, and this is indeed the case in several types of hypertensive rats.
In some experimental models of hypertensive rats, including SHRs [97,98,99], Milan hypertensive rats [100] and deoxycorticosterone acetate (DOCA) salt hypertensive rats [101], increase in the activity of NKCC in vascular smooth muscle cells has been reported (Table 3). Interestingly, Lee et al. reported that the mRNA and protein expression levels of NKCC1 were epigenetically upregulated in the aorta of SHRs due to Nkcc1 gene promoter hypomethylation [102], and the Nkcc1 gene promoter hypomethylation resulted from the decreased activity of DNA methyltransferase 3B [103] (Table 3). Likewise, an epigenetic upregulation of NKCC1 via histone modifications was reported in the aorta of Ang II-induced hypertensive rats [104] (Table 3).

Table 3.
Alterations in vascular smooth muscle NKCC1 during hypertension.
In addition to the epigenetic upregulation of NKCC1, another factor may also contribute to the increase in the activity of NKCC1 during hypertension. In fact, some studies have suggested the possible positive regulation of vascular NKCC1 by with-no-lysine kinase (WNK) and sterile-20-related praline–alanine-rich kinase (SPAK): heterozygous WNK1 knockout mouse aorta exhibited reduced phosphorylation of downstream SPAK and NKCC1, leading to decreased responses to vasoconstrictive stimuli [105]. Similarly, the aorta of SPAK knockout mice exhibited reduced phosphorylation of NKCC1 and decreased NKCC1-mediated vascular constriction, and the SPAK knockout mice had low blood pressure [106]. Moreover, activation of the WNK3/SPAK/NKCC1 pathway has been shown to be involved in both the Ang II-induced aortic constriction and Ang II-induced blood pressure rise in mice [107].
These observations suggest that the WNK/SPAK signaling pathway positively regulates the vascular NKCC1 toward vasoconstriction and hypertension. Interestingly, mutations of WNK have been found in patients with familial hyperkalemic hypertension, a form of monogenic hypertension [108]. Nevertheless, there is no evidence to date that demonstrates changes in the WNK/SPAK pathway in animal models of polygenic hypertension such as SHRs or in human essential hypertension.
The studies mentioned above have demonstrated that the expression and/or the function of NKCC1 are upregulated in vascular smooth muscle cells of hypertensive rats. Then, the question arises how the upregulation of NKCC in hypertension contributes to the augmented vascular contractility and increased blood pressure. It has been reported that the intracellular concentration of Cl− is increased in vascular smooth muscle cells of DOCA salt hypertensive rats because of the increase in the activity of NKCC [101].
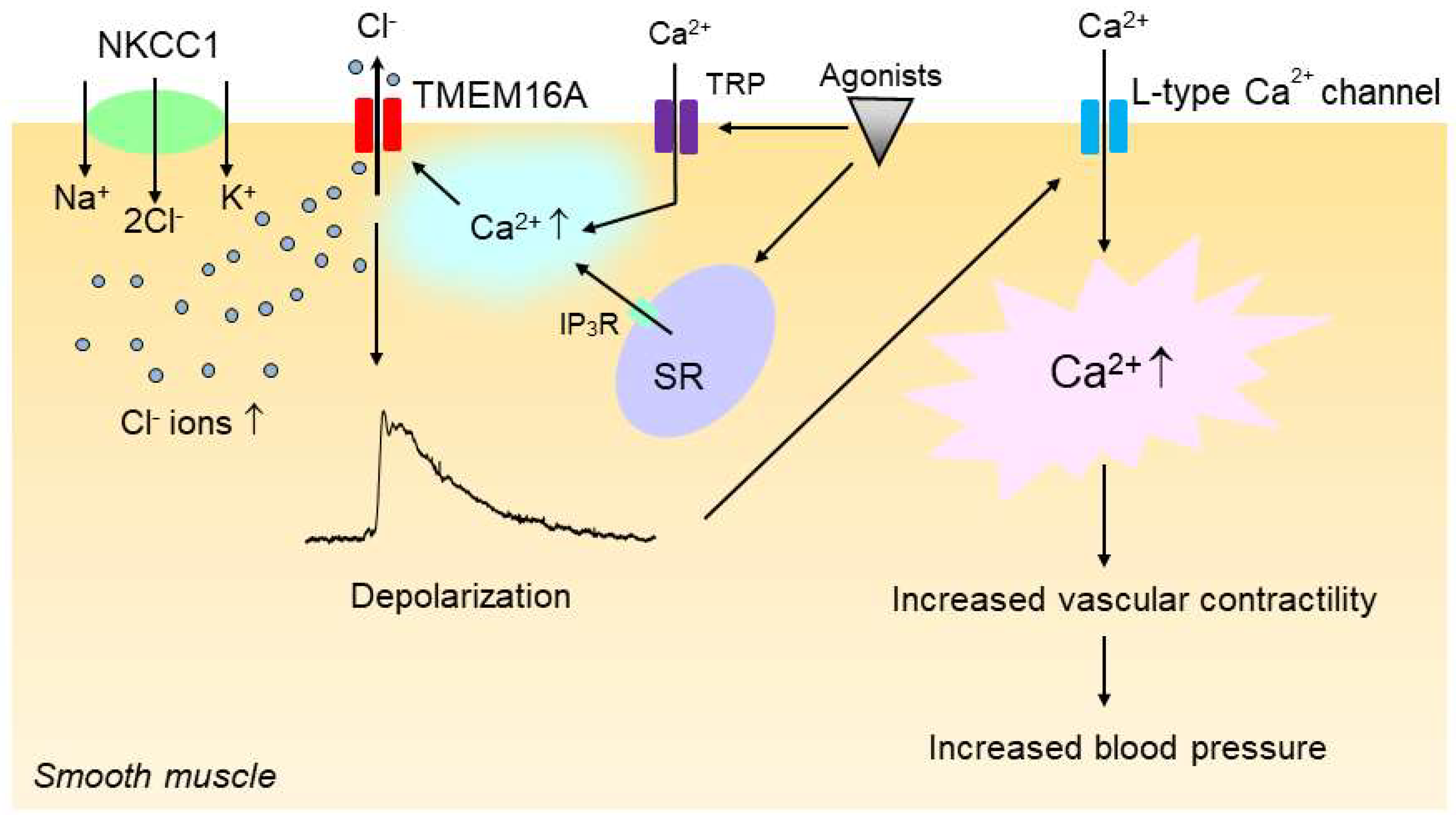
The increase in the intracellular Cl− concentration would increase the driving force for Cl− efflux via Cl− channels such as CaCCs upon vasoconstrictor stimulation, and the increase in Cl− efflux would make the membrane potential more depolarized [20], which in turn would enhance the open probability of voltage-gated L-type Ca2+ channels, leading to an increase in vascular tone. In support of this notion, we have shown that the inhibition of the NKCC with bumetanide, an inhibitor of NKCC1 [95], significantly reduced the CaCC-mediated membrane depolarization and constriction in vascular smooth muscle of SHRs [86]. Since, as discussed in the previous section, CaCCs are also functionally upregulated in the vasculature of hypertensive rats, we propose that the enhanced activities of NKCC1 and CaCCs act additively and sequentially to increase vascular contractility and hence blood pressure in hypertension (Figure 4).
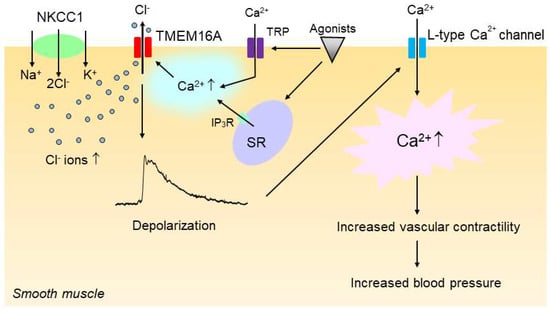
Figure 4.
Upregulation of smooth muscle Na+–K+–2Cl− cotransporter 1 (NKCC1) and TMEM16A additively and sequentially increases vascular contractility in hypertension. In hypertension, the intracellular concentration of Cl− is increased in vascular smooth muscle cells because of the increased activity of NKCC1. The increase in the intracellular Cl− concentration then increases the driving force for Cl− efflux via the Ca2+-activated Cl− channel TMEM16A when TMEM16A is activated by intracellular Ca2+ rise upon stimulation with vasoconstricting agonists, which in turn induces membrane depolarization. TMEM16A might be regulated by a local Ca2+ increase that could be generated by IP3R channels on the sarcoplasmic reticulum (SR) and/or transient receptor potential (TRP) channels. The membrane depolarization would then enhance the open probability of voltage-gated L-type Ca2+ channels, leading to an increase in vascular contractility and blood pressure.
4. Clinical Perspectives
While many animal studies suggest that the upregulation of TMEM16A and NKCC1 could contribute to the increased vascular contractility and elevated blood pressure in hypertension as mentioned in the preceding sections, there is very little information concerning their possible involvement in the pathogenesis of hypertension in humans. Interestingly, however, two independent population-based studies reported that some genetic variants of TMEM16A were significantly associated with hypertension in humans [57,109]. Further exploration of the functional impact of the SNP in the TMEM16A coding region could provide a clue to understand the pathophysiological role of TMEM16A in human hypertension.
In addition, it has been reported that there was a positive association between hyperchloremia and in-hospital mortality in hospitalized patients [110]. Moreover, in a recent study in patients with chronic kidney disease, hyperchloremia was an independent predictor of hypertension and proteinuria [111]. Taking these observational studies together, it might be possible to speculate that hyperchloremia might lead to blood pressure elevation and hence to poor prognosis. By contrast, in outpatients with hypertension [112] or chronic heart failure [113], hypochloremia was a predictor of mortality [112,113] but the level of Cl− was not associated with the level of blood pressure [112]. Thus, while these findings indicate that serum Cl− alterations are associated with poor prognosis in patients with elevated cardiovascular risk, the ability of changes in serum Cl− concentration to affect blood pressure is not clear. Further studies are needed to clarify the role of serum Cl− concentrations on blood pressure regulation and its association with long-term prognosis in patients with elevated cardiovascular risk.
5. Conclusions
Accumulating experimental evidence suggests that Cl− plays an important role in the regulation of vascular tone through its ability to depolarize vascular smooth muscle cells, and the increased contribution of Cl− to arterial constriction appears to be associated with the development and progression of hypertension. Of note, there is a growing body of evidence that the upregulation of CaCC TMEM16A in the vasculature contributes to the increased vascular contractility and elevated blood pressure in genetically hypertensive rats. In addition, the increased activity of NKCC1 may also promote hypertension as the result of a robust accumulation of intracellular Cl− in vascular cells.
Nevertheless, much remains to be determined about the precise molecular mechanisms underlying the increased activity of TMEM16A and NKCC1 as well as their interactions with other signaling pathways during hypertension, and most importantly the pathophysiological roles of these molecules in human hypertension. Further exploration of the arterial tone regulation by Cl− may facilitate a better understanding of the pathogenesis of hypertension, which may help to develop a novel therapeutic strategy to tackle hypertension and hypertension-associated cardiovascular diseases.
Author Contributions
K.G. was responsible for the conceptualization, literature research, and writing of the manuscript. T.K. assisted in the writing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI grant number JP21K11023. The APC was funded by JSPS KAKENHI grant number JP21K11023.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number JP21K11023.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lawes, C.M.; Hoorn, S.V.; Rodgers, A. Global Burden of Blood-Pressure-Related Disease, 2001. Lancet 2008, 371, 1513–1518. [Google Scholar] [CrossRef]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global Epidemiology, Health Burden and Effective Interventions for Elevated Blood Pressure and Hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide Trends in Hypertension Prevalence and Progress in Treatment and Control from 1990 to 2019: A Pooled Analysis of 1201 Population-Representative Studies with 104 Million Participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Umemura, S.; Arima, H.; Arima, S.; Asayama, K.; Dohi, Y.; Hirooka, Y.; Horio, T.; Hoshide, S.; Ikeda, S.; Ishimitsu, T.; et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens. Res. 2019, 42, 1235–1481. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihashi, T. Dietary Salt Intake in Japan-Past, Present, and Future. Hypertens. Res. 2022, 45, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Veronesi, M.; Fogacci, F. Dietary Intervention to Improve Blood Pressure Control: Beyond Salt Restriction. High Blood Press. Cardiovasc. Prev. 2021, 28, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Intersalt. Intersalt: An International Study of Electrolyte Excretion and Blood. BMJ 1988, 297, 319–328. [Google Scholar]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J. Global Sodium Consumption and Death from Cardiovascular Causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef]
- Meneton, P.; Jeunemaitre, X.; De Wardener, H.E.; Macgregor, G.A. Links between Dietary Salt Intake, Renal Salt Handling, Blood Pressure, and Cardiovascular Diseases. Physiol. Rev. 2005, 85, 679–715. [Google Scholar] [CrossRef]
- Dahl, L.K.; Love, R.A. Evidence for Relationship between Sodium (Chloride) Intake and Human Essential Hypertension. AMA Arch. Intern. Med. 1954, 94, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Kempner, W. Treatment of Hypertensive Vascular Disease with Rice Diet. Am. J. Med. 1948, 4, 545–577. [Google Scholar] [CrossRef]
- Kurtz, T.W.; Morris, R.C. Dietary Chloride as a Determinant of “Sodium-Dependent” Hypertension. Science 1983, 222, 1139–1141. [Google Scholar] [CrossRef] [PubMed]
- Kotchen, T.A.; Luke, R.G.; Ott, C.E.; Galla, J.H.; Whitescarver, S. Effect of Chloride on Renin and Blood Pressure Responses to Sodium Chloride. Ann. Intern. Med. 1983, 98 Pt 2, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Whitescarver, S.A.; Holtzclaw, B.J.; Downs, J.H.; Ott, C.E.; Sowers, J.R.; Kotchen, T.A. Effect of Dietary Chloride on Salt-Sensitive and Renin-Dependent Hypertension. Hypertension 1986, 8, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Schmidlin, O.; Yi, S.L.; Bollen, A.W.; Morris, R.C. Genetically Determined Chloride-Sensitive Hypertension and Stroke. Proc. Natl. Acad. Sci. USA 1997, 94, 14748–14752. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, T.W.; Al-Bander, H.A.; Morris, R.C. “Salt-Sensitive” Essential Hypertension in Men. Is the Sodium Ion Alone Important? N. Engl. J. Med. 1987, 317, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Luft, F.C.; Zemel, M.B.; Sowers, J.A.; Fine Berg, N.S.; Weinberger, M.H. Sodium Bicarbonate and Sodium Chloride: Effects on Blood Pressure and Electrolyte Homeostasis in Normal and Hypertensive Man. J. Hypertens. 1990, 8, 663–670. [Google Scholar] [CrossRef]
- Shore, A.C.; Markandu, N.D.; MacGregor, G.A. A Randomized Crossover Study to Compare the Blood Pressure Response to Sodium Loading with and without Chloride in Patients with Essential Hypertension. J. Hypertens. 1988, 6, 613–617. [Google Scholar] [CrossRef] [PubMed]
- McCallum, L.; Lip, S.; Padmanabhan, S. The Hidden Hand of Chloride in Hypertension. Pflug. Arch. 2015, 467, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Chipperfield, A.R.; Harper, A.A. Chloride in Smooth Muscle. Prog. Biophys. Mol. Biol. 2000, 74, 175–221. [Google Scholar] [CrossRef]
- Bulley, S.; Jaggar, J.H. Cl− Channels in Smooth Muscle Cells. Pflug. Arch. 2014, 466, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Bryant, H.J.; Harder, D.R.; Pamnani, M.B.; Haddy, F.J. In Vivo Membrane Potentials of Smooth Muscle Cells in the Caudal Artery of the Rat. Am. J. Physiol. 1985, 249 Pt 1, C78–C83. [Google Scholar] [CrossRef]
- Aickin, C.C.; Brading, A.F. Measurement of Intracellular Chloride in Guinea-pig Vas Deferens by Ion Analysis, 36chloride and Micro-electrodes. J. Physiol. 1982, 326, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Large, W.A.; Wang, Q. Characteristics and Physiological Role of the Ca(2+)-Activated Cl− Conductance in Smooth Muscle. Am. J. Physiol. 1996, 271 Pt 1, C435–C454. [Google Scholar] [CrossRef]
- Goto, K.; Fujii, K.; Onaka, U.; Abe, I.; Fujishima, M. Effects of Adrenomedullin and PAMP on Membrane Potential and Neurotransmission. Peptides 2000, 21, 257–263. [Google Scholar] [CrossRef]
- Brock, M.A.; Cunnane, T.C. Effects of Ca2+ Concentration and Ca2+ Channel Blockers on Noradrenaline Release and Purinergic Neuroeffector Transmission in Rat Tail Artery. Br. J. Pharmacol. 1999, 126, 11–18. [Google Scholar] [CrossRef][Green Version]
- Burnstock, G.; Ralevic, V. New Insights into the Local Regulation of Blood Flow by Perivascular Nerves and Endothelium. Br. J. Plast. Surg. 1994, 47, 527–543. [Google Scholar] [CrossRef]
- Hill, C.E.; Phillips, J.K.; Sandow, S.L. Heterogeneous Control of Blood Flow amongst Different Vascular Beds. Med. Res. Rev. 2001, 21, 1–60. [Google Scholar] [CrossRef]
- Plane, F.; Garland, C.J. Electrophysiology of Cerebral Blood Vessels. Pharmacol. Ther. 1992, 56, 341–358. [Google Scholar] [CrossRef]
- Gould, D.J.; Hill, C.E. Alpha-Adrenoceptor Activation of a Chloride Conductance in Rat Iris Arterioles. Am. J. Physiol. 1996, 271 Pt 2, H2469–H2476. [Google Scholar] [CrossRef]
- Kitamura, K.; Yamazaki, J. Chloride Channels and Their Functional Roles in Smooth Muscle Tone in the Vasculature. Jpn. J. Pharmacol. 2001, 85, 351–357. [Google Scholar] [CrossRef]
- Leblanc, N.; Ledoux, J.; Saleh, S.; Sanguinetti, A.; Angermann, J.; O’Driscoll, K.; Britton, F.; Perrino, B.A.; Greenwood, I.A. Regulation of Calcium-Activated Chloride Channels in Smooth Muscle Cells: A Complex Picture Is Emerging. Can. J. Physiol. Pharmacol. 2005, 83, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Hill, M.A. Signaling Mechanisms Underlying the Vascular Myogenic Response. Physiol. Rev. 1999, 79, 387–423. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.T.; Conway, M.A.; Knot, H.J.; Brayden, J.E. Chloride Channel Blockers Inhibit Myogenic Tone in Rat Cerebral Arteries. J. Physiol. 1997, 502 Pt 2, 259–264. [Google Scholar] [CrossRef]
- Doughty, J.M.; Langton, P.D. Measurement of Chloride Flux Associated with the Myogenic Response in Rat Cerebral Arteries. J. Physiol. 2001, 534 Pt 3, 753–761. [Google Scholar] [CrossRef]
- Doughty, J.M.; Miller, A.L.; Langton, P.D. Non-Specificity of Chloride Channel Blockers in Rat Cerebral Arteries: Block of the L-Type Calcium Channel. J. Physiol. 1998, 507 Pt 2, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.G.; Nelson, M.T.; Eckman, D.M.; Brayden, J.E. Swelling-Activated Cation Channels Mediate Depolarization of Rat Cerebrovascular Smooth Muscle by Hyposmolarity and Intravascular Pressure. J. Physiol. 2000, 527 Pt 1, 139–148. [Google Scholar] [CrossRef]
- Byrne, N.G.; Large, W.A. Membrane Mechanism Associated with Muscarinic Receptor Activation in Single Cells Freshly Dispersed from the Rat Anococcygeus Muscle. Br. J. Pharmacol. 1987, 92, 371–379. [Google Scholar] [CrossRef]
- Matchkov, V.V. Mechanisms of Cellular Synchronization in the Vascular Wall. Mechanisms of Vasomotion. Dan. Med. Bull. 2010, 57, B4191. [Google Scholar]
- Yang, Y.D.; Cho, H.; Koo, J.Y.; Tak, M.H.; Cho, Y.; Shim, W.S.; Park, S.P.; Lee, J.; Lee, B.; Kim, B.M.; et al. TMEM16A Confers Receptor-Activated Calcium-Dependent Chloride Conductance. Nature 2008, 455, 1210–1215. [Google Scholar] [CrossRef]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J.V. TMEM16A, a Membrane Protein Associated with Calcium-Dependent Chloride Channel Activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.C.; Cheng, T.; Jan, Y.N.; Jan, L.Y. Expression Cloning of TMEM16A as a Calcium-Activated Chloride Channel Subunit. Cell 2008, 134, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Dam, V.S.; Boedtkjer, D.M.B.; Aalkjaer, C.; Matchkov, V. The Bestrophin- and TMEM16A-Associated Ca(2+)- Activated Cl(–) Channels in Vascular Smooth Muscles. Channels 2014, 8, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Hawn, M.B.; Akin, E.; Hartzell, H.C.; Greenwood, I.A.; Leblanc, N. Molecular Mechanisms of Activation and Regulation of ANO1-Encoded Ca2+-Activated Cl− Channels. Channels 2021, 15, 569–603. [Google Scholar] [CrossRef]
- Wray, S.; Prendergast, C.; Arrowsmith, S. Calcium-Activated Chloride Channels in Myometrial and Vascular Smooth Muscle. Front. Physiol. 2021, 12, 1805. [Google Scholar] [CrossRef]
- Bulley, S.; Neeb, Z.P.; Burris, S.K.; Bannister, J.P.; Thomas-Gatewood, C.M.; Jangsangthong, W.; Jaggar, J.H. TMEM16A/ANO1 Channels Contribute to the Myogenic Response in Cerebral Arteries. Circ. Res. 2012, 111, 1027–1036. [Google Scholar] [CrossRef]
- Yip, K.P.; Balasubramanian, L.; Kan, C.; Wang, L.; Liu, R.; Ribeiro-Silva, L.; Sham, J.S.K. Intraluminal Pressure Triggers Myogenic Response via Activation of Calcium Spark and Calcium-Activated Chloride Channel in Rat Renal Afferent Arteriole. Am. J. Physiol. Renal Physiol. 2018, 315, F1592–F1600. [Google Scholar] [CrossRef]
- Heinze, C.; Seniuk, A.; Sokolov, M.V.; Huebner, A.K.; Klementowicz, A.E.; Szijártó, I.A.; Schleifenbaum, J.; Vitzthum, H.; Gollasch, M.; Ehmke, H.; et al. Disruption of Vascular Ca2+-Activated Chloride Currents Lowers Blood Pressure. J. Clin. Investig. 2014, 124, 675–686. [Google Scholar] [CrossRef]
- Mulvany, M.J.; Halpern, W. Contractile Properties of Small Arterial Resistance Vessels in Spontaneously Hypertensive and Normotensive Rats. Circ. Res. 1977, 41, 19–26. [Google Scholar] [CrossRef]
- Pintérová, M.; Kuneš, J.; Zicha, J. Altered Neural and Vascular Mechanisms in Hypertension. Physiol. Res. 2011, 60, 381–402. [Google Scholar] [CrossRef]
- Goto, K.; Ohtsubo, T.; Kitazono, T. Endothelium-Dependent Hyperpolarization (EDH) in Hypertension: The Role of Endothelial Ion Channels. Int. J. Mol. Sci. 2018, 19, 315. [Google Scholar] [CrossRef] [PubMed]
- Stekiel, W.J.; Contney, S.J.; Lombard, J.H. Small Vessel Membrane Potential, Sympathetic Input, and Electrogenic Pump Rate in SHR. Am. J. Physiol. 1986, 250 Pt 1, C547–C556. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Tominaga, M.; Ohmori, S.; Kobayashi, K.; Koga, T.; Takata, Y.; Fujishima, M. Decreased Endothelium-Dependent Hyperpolarization to Acetylcholine in Smooth Muscle of the Mesenteric Artery of Spontaneously Hypertensive Rats. Circ. Res. 1992, 70, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Fujii, K.; Abe, I. Impaired β-Adrenergic Hyperpolarization in Arteries from Prehypertensive Spontaneously Hypertensive Rats. Hypertension 2001, 37, 609–613. [Google Scholar] [CrossRef]
- Wang, Z.; Chai, Q.; Liu, Z.; Liu, D.; Chen, L. Chloride Channel Activity of Vascular Smooth Muscle in the Spontaneous Hypertensive Rats. Chin. J. Physiol. 2004, 47, 129–135. [Google Scholar] [PubMed]
- Wang, B.; Li, C.; Huai, R.; Qu, Z. Overexpression of ANO1/TMEM16A, an Arterial Ca2+-Activated Cl− Channel, Contributes to Spontaneous Hypertension. J. Mol. Cell. Cardiol. 2015, 82, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Askew Page, H.R.; Dalsgaard, T.; Baldwin, S.N.; Jepps, T.A.; Povstyan, O.; Olesen, S.P.; Greenwood, I.A. TMEM16A Is Implicated in the Regulation of Coronary Flow and Is Altered in Hypertension. Br. J. Pharmacol. 2019, 176, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Cil, O.; Chen, X.; Askew Page, H.R.; Baldwin, S.N.; Jordan, M.C.; Myat Thwe, P.; Anderson, M.O.; Haggie, P.M.; Greenwood, I.A.; Roos, K.P.; et al. A Small Molecule Inhibitor of the Chloride Channel TMEM16A Blocks Vascular Smooth Muscle Contraction and Lowers Blood Pressure in Spontaneously Hypertensive Rats. Kidney Int. 2021, 100, 311–320. [Google Scholar] [CrossRef]
- Li, R.S.; Wang, Y.; Chen, H.S.; Jiang, F.Y.; Tu, Q.; Li, W.J.; Yin, R.X. TMEM16A Contributes to Angiotensin II-Induced Cerebral Vasoconstriction via the RhoA/ROCK Signaling Pathway. Mol. Med. Rep. 2016, 13, 3691–3699. [Google Scholar] [CrossRef]
- Wang, M.; Yang, H.; Zheng, L.Y.; Zhang, Z.; Tang, Y.B.; Wang, G.L.; Du, Y.H.; Lv, X.F.; Liu, J.; Zhou, J.G.; et al. Downregulation of TMEM16A Calcium-Activated Chloride Channel Contributes to Cerebrovascular Remodeling during Hypertension by Promoting Basilar Smooth Muscle Cell Proliferation. Circulation 2012, 125, 697–707. [Google Scholar] [CrossRef]
- Shiono, K.; Sokabe, H. Renin-Angiotensin System in Spontaneously Hypertensive Rats. Am. J. Physiol. 1976, 231, 1295–1299. [Google Scholar] [CrossRef]
- Okamura, T.; Miyazaki, M.; Inagami, T.; Toda, N. Vascular Renin-Angiotensin System in Two-Kidney, One Clip Hypertensive Rats. Hypertension 1986, 8, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Zheng, B.; Yang, Z.; He, M.; Yue, L.Y.; Zhang, R.N.; Zhang, M.; Zhang, W.; Zhang, X.; Wen, J.K. TMEM16A and Myocardin Form a Positive Feedback Loop That Is Disrupted by KLF5 during Ang II-Induced Vascular Remodeling. Hypertension 2015, 66, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Abel, P.W.; Hermsmeyer, K. Sympathetic Cross-Innervation of SHR and Genetic Controls Suggests a Trophic Influence on Vascular Muscle Membranes. Circ. Res. 1981, 49, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Kostyunina, D.S.; Zhang, L.; Shvetsova, A.A.; Selivanova, E.K.; Tarasova, O.S.; Matchkov, V.V.; Gaynullina, D.K. Trophic Sympathetic Influence Weakens Pro-Contractile Role of Cl− Channels in Rat Arteries during Postnatal Maturation. Sci. Rep. 2020, 10, 20002. [Google Scholar] [CrossRef] [PubMed]
- Liskova, S.; Petrova, M.; Karen, P.; Behuliak, M.; Zicha, J. Contribution of Ca2+-Dependent Cl− Channels to Norepinephrine-Induced Contraction of Femoral Artery Is Replaced by Increasing EDCF Contribution during Ageing. Biomed Res. Int. 2014, 2014, 289361. [Google Scholar] [CrossRef]
- Jensen, A.B.; Joergensen, H.B.; Dam, V.S.; Kamaev, D.; Boedtkjer, D.; Füchtbauer, E.M.; Aalkjaer, C.; Matchkov, V.V. Variable Contribution of TMEM16A to Tone in Murine Arterial Vasculature. Basic Clin. Pharmacol. Toxicol. 2018, 123, 30–41. [Google Scholar] [CrossRef]
- Dam, V.S.; Boedtkjer, D.M.B.; Nyvad, J.; Aalkjaer, C.; Matchkov, V. TMEM16A Knockdown Abrogates Two Different Ca(2+)-Activated Cl(−) Currents and Contractility of Smooth Muscle in Rat Mesenteric Small Arteries. Pflug. Arch. 2014, 466, 1391–1409. [Google Scholar] [CrossRef]
- Wang, Q.; Dennis Leo, M.; Narayanan, D.; Kuruvilla, K.P.; Jaggar, J.H. Local Coupling of TRPC6 to ANO1/TMEM16A Channels in Smooth Muscle Cells Amplifies Vasoconstriction in Cerebral Arteries. Am. J. Physiol. Cell Physiol. 2016, 310, C1001–C1009. [Google Scholar] [CrossRef]
- Ohya, Y.; Abe, I.; Fujii, K.; Takata, Y.; Fujishima, M. Voltage-Dependent Ca2+ Channels in Resistance Arteries from Spontaneously Hypertensive Rats. Circ. Res. 1993, 73, 1090–1099. [Google Scholar] [CrossRef]
- Pesic, A.; Madden, J.A.; Pesic, M.; Rusch, N.J. High Blood Pressure Upregulates Arterial L-Type Ca2+ Channels: Is Membrane Depolarization the Signal? Circ. Res. 2004, 94, e97–e104. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.M.; Kim, A.; Lee, Y.J.; Lim, W.; Noh, Y.H.; Kim, E.J.; Kim, J.; Kim, T.K.; Park, S.W.; Kim, B.; et al. Enhancement of Receptor-Operated Cation Current and TRPC6 Expression in Arterial Smooth Muscle Cells of Deoxycorticosterone Acetate-Salt Hypertensive Rats. J. Hypertens. 2007, 25, 809–817. [Google Scholar] [CrossRef]
- Suh, B.C.; Hille, B. PIP2 Is a Necessary Cofactor for Ion Channel Function: How and Why? Annu. Rev. Biophys. 2008, 37, 175–195. [Google Scholar] [CrossRef]
- Ta, C.M.; Acheson, K.E.; Rorsman, N.J.G.; Jongkind, R.C.; Tammaro, P. Contrasting Effects of Phosphatidylinositol 4,5-Bisphosphate on Cloned TMEM16A and TMEM16B Channels. Br. J. Pharmacol. 2017, 174, 2984–2999. [Google Scholar] [CrossRef] [PubMed]
- Tembo, M.; Wozniak, K.L.; Bainbridge, R.E.; Carlson, A.E. Phosphatidylinositol 4,5-Bisphosphate (PIP 2) and Ca2+ Are Both Required to Open the Cl− Channel TMEM16A. J. Biol. Chem. 2019, 294, 12556–12564. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Chen, J. Specific PIP 2 Binding Promotes Calcium Activation of TMEM16A Chloride Channels. Commun. Biol. 2021, 4, 259. [Google Scholar] [CrossRef]
- Pritchard, H.A.T.; Leblanc, N.; Albert, A.P.; Greenwood, I.A. Inhibitory Role of Phosphatidylinositol 4,5-Bisphosphate on TMEM16A-Encoded Calcium-Activated Chloride Channels in Rat Pulmonary Artery. Br. J. Pharmacol. 2014, 171, 4311–4321. [Google Scholar] [CrossRef]
- Ek, T.P.; Campbell, M.D.; Deth, R.C. Reduction of Norepinephrine-Induced Tonic Contraction and Phosphoinositide Turnover in Arteries of Spontaneously Hypertensive Rats. A Possible Role for Protein Kinase C. Am. J. Hypertens. 1989, 2, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Shah, S.; Liu, Y.; Zhang, H.; Lees, M.; Fu, Z.; Lippiat, J.D.; Beech, D.J.; Sivaprasadarao, A.; Baldwin, S.A.; et al. Activation of the Cl− Channel ANO1 by Localized Calcium Signals in Nociceptive Sensory Neurons Requires Coupling with the IP3 Receptor. Sci. Signal. 2013, 6, ra73. [Google Scholar] [CrossRef]
- Abou-Saleh, H.; Pathan, A.R.; Daalis, A.; Hubrack, S.; Abou-Jassoum, H.; Al-Naeimi, H.; Rusch, N.J.; Machaca, K. Inositol 1,4,5-Trisphosphate (IP3) Receptor up-Regulation in Hypertension Is Associated with Sensitization of Ca2+ Release and Vascular Smooth Muscle Contractility. J. Biol. Chem. 2013, 288, 32941–32951. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Droogmans, G. Ion Channels and Their Functional Role in Vascular Endothelium. Physiol. Rev. 2001, 81, 1415–1459. [Google Scholar] [CrossRef]
- Ma, M.M.; Gao, M.; Guo, K.M.; Wang, M.; Li, X.Y.; Zeng, X.L.; Sun, L.; Lv, X.F.; Du, Y.H.; Wang, G.L.; et al. TMEM16A Contributes to Endothelial Dysfunction by Facilitating Nox2 NADPH Oxidase-Derived Reactive Oxygen Species Generation in Hypertension. Hypertension 2017, 69, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yasumoto, M.; Suzuki, Y.; Asai, K.; Imaizumi, Y.; Yamamura, H. TMEM16A Ca2+-Activated Cl− Channel Regulates the Proliferation and Migration of Brain Capillary Endothelial Cells. Mol. Pharmacol. 2020, 98, 61–71. [Google Scholar] [CrossRef]
- Skofic Maurer, D.; Zabini, D.; Nagaraj, C.; Sharma, N.; Lengyel, M.; Nagy, B.M.; Frank, S.; Klepetko, W.; Gschwandtner, E.; Enyedi, P.; et al. Endothelial Dysfunction Following Enhanced TMEM16A Activity in Human Pulmonary Arteries. Cells 2020, 9, 1984. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Suzuki, H. Effects of Increased Intracellular Cl− Concentration on Membrane Responses to Acetylcholine in the Isolated Endothelium of Guinea Pig Mesenteric Arteries. J. Physiol. Sci. 2007, 57, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Edwards, F.R.; Hill, C.E. Depolarization Evoked by Acetylcholine in Mesenteric Arteries of Hypertensive Rats Attenuates Endothelium-Dependent Hyperpolarizing Factor. J. Hypertens. 2007, 25, 345–359. [Google Scholar] [CrossRef]
- Goto, K.; Rummery, N.M.; Grayson, T.H.; Hill, C.E. Attenuation of Conducted Vasodilation in Rat Mesenteric Arteries during Hypertension: Role of Inwardly Rectifying Potassium Channels. J. Physiol. 2004, 561, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Hirst, G.D.S.; Bramich, N.J.; Teramoto, N.; Suzuki, H.; Edwards, F.R. Regenerative Component of Slow Waves in the Guinea-Pig Gastric Antrum Involves a Delayed Increase in [Ca2+]i and Cl− Channels. J. Physiol. 2002, 540 Pt 3, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Sandow, S.L.; Hill, C.E. Incidence of Myoendothelial Gap Junctions in the Proximal and Distal Mesenteric Arteries of the Rat Is Suggestive of a Role in Endothelium-Derived Hyperpolarizing Factor–Mediated Responses. Circ. Res. 2000, 86, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Fujii, K.; Kansui, Y.; Abe, I.; Iida, M. Critical Role of Gap Junctions in Endothelium-Dependent Hyperpolarization in Rat Mesenteric Arteries. Clin. Exp. Pharmacol. Physiol. 2002, 29, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Mather, S.; Dora, K.A.; Sandow, S.L.; Winter, P.; Garland, C.J. Rapid Endothelial Cell-Selective Loading of Connexin 40 Antibody Blocks Endothelium-Derived Hyperpolarizing Factor Dilation in Rat Small Mesenteric Arteries. Circ. Res. 2005, 97, 399–407. [Google Scholar] [CrossRef]
- Doughty, J.M.; Boyle, J.P.; Langton, P.D. Blockade of Chloride Channels Reveals Relaxations of Rat Small Mesenteric Arteries to Raised Potassium. Br. J. Pharmacol. 2001, 132, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kwan, Y.W.; Chan, S.W.; Lee, S.M.Y.; Leung, G.P.H. Potentiation of EDHF-Mediated Relaxation by Chloride Channel Blockers. Acta Pharmacol. Sin. 2010, 31, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.W.; Flagella, M.; Sutliff, R.L.; Lorenz, J.N.; Nieman, M.L.; Weber, C.S.; Paul, R.J.; Shull, G.E. Decreased Blood Pressure and Vascular Smooth Muscle Tone in Mice Lacking Basolateral Na+-K+-2Cl− Cotransporter. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1846–H1855. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Roy, K.; Vidossich, P.; Cancedda, L.; De Vivo, M.; Forbush, B.; Cao, E. Structural Basis for Inhibition of the Cation-Chloride Cotransporter NKCC1 by the Diuretic Drug Bumetanide. Nat. Commun. 2022, 13, 2747. [Google Scholar] [CrossRef]
- Garg, P.; Martin, C.F.; Elms, S.C.; Gordon, F.J.; Wall, S.M.; Garland, C.J.; Sutliff, R.L.; O’Neill, W.C. Effect of the Na-K-2Cl Cotransporter NKCC1 on Systemic Blood Pressure and Smooth Muscle Tone. Am. J. Physiol. Heart Circ. Physiol. 2007, 292. [Google Scholar] [CrossRef]
- Orlov, S.N.; Resink, T.J.; Bernhardt, J.; Bühler, F.R. Na(+)-K+ Pump and Na(+)-K+ Co-Transport in Cultured Vascular Smooth Muscle Cells from Spontaneously Hypertensive and Normotensive Rats: Baseline Activity and Regulation. J. Hypertens. 1992, 10, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Denny, T.N.; Aviv, A. 22Na+ and 86Rb+ Transport in Vascular Smooth Muscle of SHR, Wistar Kyoto, and Wistar Rats. J. Cardiovasc. Pharmacol. 1988, 11, 722–729. [Google Scholar] [CrossRef]
- Tokushige, A.; Kino, M.; Tamura, H.; Hopp, L.; Searle, B.M.; Aviv, A. Bumetanide-Sensitive Sodium-22 Transport in Vascular Smooth Muscle Cell of the Spontaneously Hypertensive Rat. Hypertension 1986, 8, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Canessa, M.; Salazar, G.; Werner, E.; Vallega, G.; Gonzalez, A. Cell Growth and Na-K-Cl Cotransport Responses of Vascular Smooth Muscle Cells of Milan Rats. Hypertension 1994, 23 Pt 2, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.P.L.; Chipperfield, A.R.; Harper, A.A. Accumulation of Intracellular Chloride by (Na-K-Cl) Co-Transport in Rat Arterial Smooth Muscle Is Enhanced in Deoxycorticosterone Acetate (DOCA)/Salt Hypertension. J. Mol. Cell. Cardiol. 1993, 25, 233–237. [Google Scholar] [CrossRef]
- Lee, H.A.; Baek, I.; Seok, Y.M.; Yang, E.; Cho, H.M.; Lee, D.Y.; Hong, S.H.; Kim, I.K. Promoter Hypomethylation Upregulates Na+-K+-2Cl− Cotransporter 1 in Spontaneously Hypertensive Rats. Biochem. Biophys. Res. Commun. 2010, 396, 252–257. [Google Scholar] [CrossRef]
- Cho, H.M.; Lee, H.A.; Kim, H.Y.; Han, H.S.; Kim, I.K. Expression of Na+-K+-2Cl Cotransporter 1 Is Epigenetically Regulated during Postnatal Development of Hypertension. Am. J. Hypertens. 2011, 24, 1286–1293. [Google Scholar] [CrossRef]
- Cho, H.M.; Lee, D.Y.; Kim, H.Y.; Lee, H.A.; Seok, Y.M.; Kim, I.K. Upregulation of the Na(+)-K(+)-2Cl(−) Cotransporter 1 via Histone Modification in the Aortas of Angiotensin II-Induced Hypertensive Rats. Hypertens. Res. 2012, 35, 819–824. [Google Scholar] [CrossRef]
- Bergaya, S.; Faure, S.; Baudrie, V.; Rio, M.; Escoubet, B.; Bonnin, P.; Henrion, D.; Loirand, G.; Achard, J.M.; Jeunemaitre, X.; et al. WNK1 Regulates Vasoconstriction and Blood Pressure Response to α 1-Adrenergic Stimulation in Mice. Hypertension 2011, 58, 439–445. [Google Scholar] [CrossRef]
- Yang, S.S.; Lo, Y.F.; Wu, C.C.; Lin, S.W.; Yeh, C.J.; Chu, P.; Sytwu, H.K.; Uchida, S.; Sasaki, S.; Lin, S.H. SPAK-Knockout Mice Manifest Gitelman Syndrome and Impaired Vasoconstriction. J. Am. Soc. Nephrol. 2010, 21, 1868–1877. [Google Scholar] [CrossRef]
- Zeniya, M.; Sohara, E.; Kita, S.; Iwamoto, T.; Susa, K.; Mori, T.; Oi, K.; Chiga, M.; Takahashi, D.; Yang, S.; et al. Dietary Salt Intake Regulates WNK3-SPAK-NKCC1 Phosphorylation Cascade in Mouse Aorta through Angiotensin II. Hypertension 2013, 62, 872–878. [Google Scholar] [CrossRef]
- Murthy, M.; Kurz, T.; O’Shaughnessy, K.M. WNK Signalling Pathways in Blood Pressure Regulation. Cell. Mol. Life Sci. 2017, 74, 1261–1280. [Google Scholar] [CrossRef]
- Jin, H.-S.; Jung, D. Gender-Specific Association of the ANO1 Genetic Variations with Hypertension. Biomed. Sci. Lett. 2015, 21, 144–151. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Cheungpasitporn, W.; Hansrivijit, P.; Thirunavukkarasu, S.; Chewcharat, A.; Medaura, J.; Mao, M.A.; Kashani, K. Association of Serum Chloride Level Alterations with In-Hospital Mortality. Postgrad. Med. J. 2020, 96, 731–736. [Google Scholar] [CrossRef]
- Takahashi, A.; Maeda, K.; Sasaki, K.; Doi, S.; Nakashima, A.; Doi, T.; Masaki, T. Relationships of Hyperchloremia with Hypertension and Proteinuria in Patients with Chronic Kidney Disease. Clin. Exp. Nephrol. 2022, 26, 880–885. [Google Scholar] [CrossRef] [PubMed]
- McCallum, L.; Jeemon, P.; Hastie, C.E.; Patel, R.K.; Williamson, C.; Redzuan, A.M.; Dawson, J.; Sloan, W.; Muir, S.; Morrison, D.; et al. Serum Chloride Is an Independent Predictor of Mortality in Hypertensive Patients. Hypertension 2013, 62, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Bellino, M.C.; Massari, F.; Albanese, M.; Ursi, R.; Angelini, G.; Lisi, F.; Amato, L.; Scicchitano, P.; Guida, P.; Brunetti, N.D.; et al. Baseline and Incident Hypochloremia in Chronic Heart Failure Outpatients: Clinical Correlates and Prognostic Role. Eur. J. Intern. Med. 2021, 84, 32–37. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).