Therapeutic Neuromodulation toward a Critical State May Serve as a General Treatment Strategy
Abstract
:1. Introduction
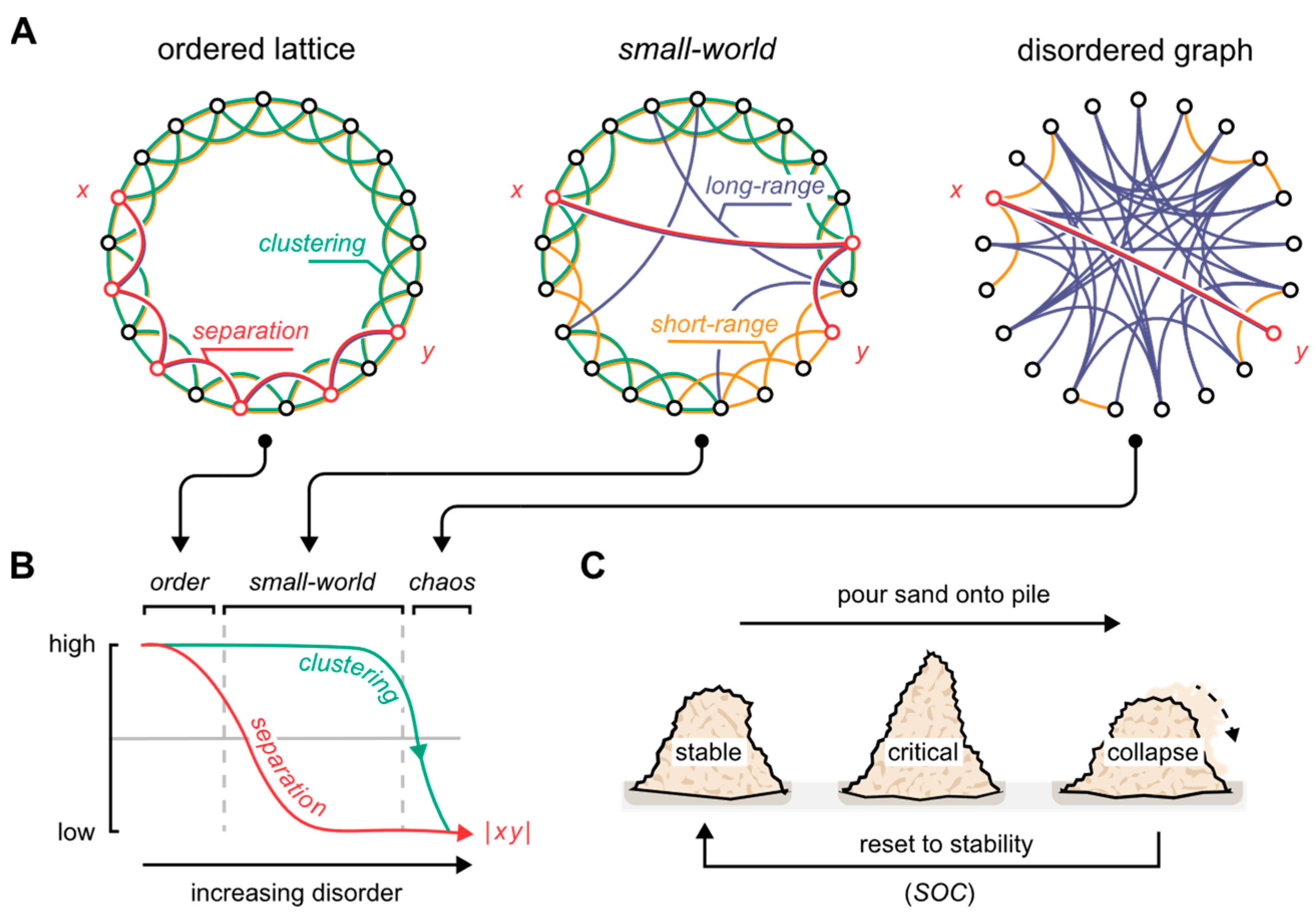
2. Neural Network Topology and the Critical State
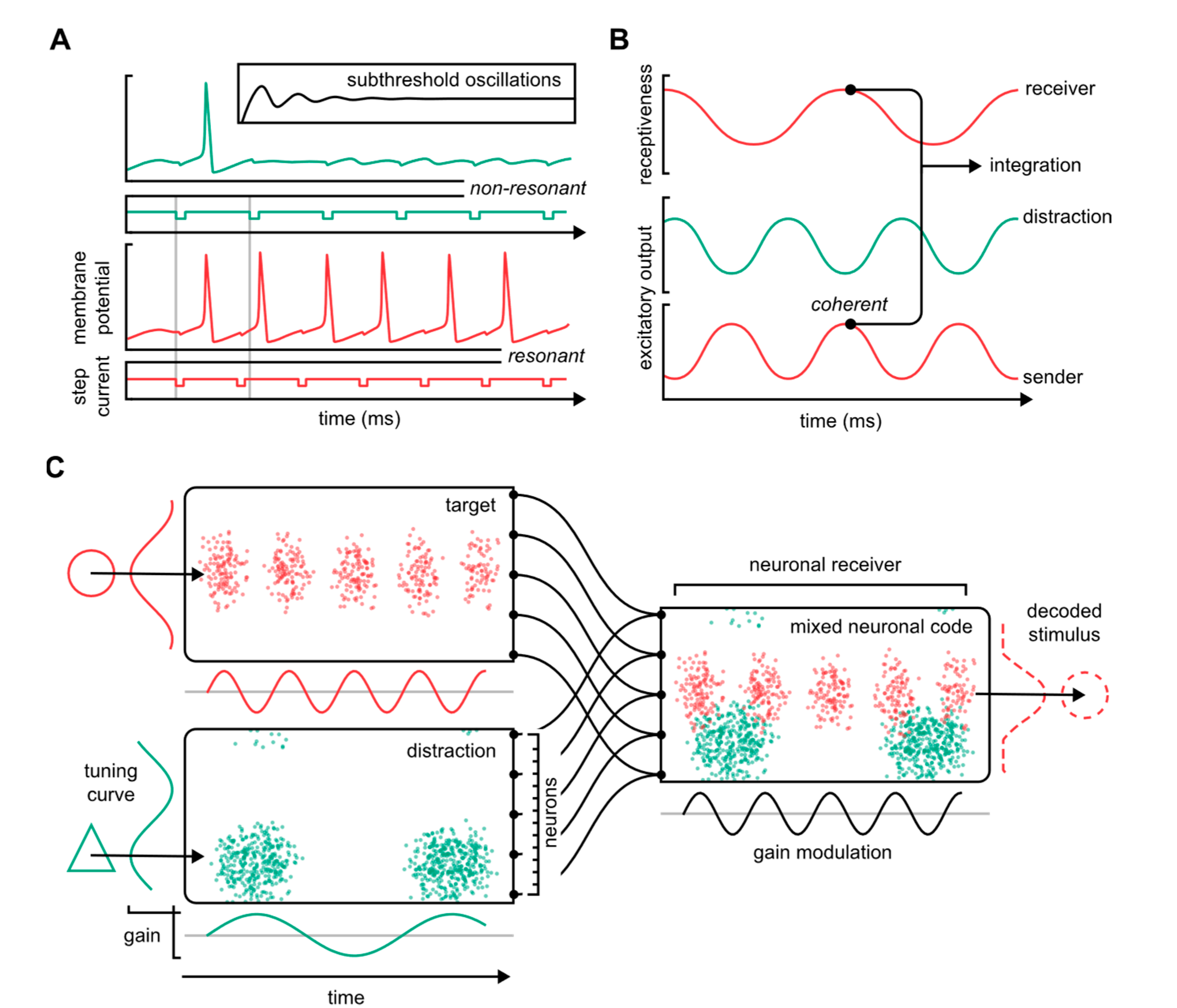
3. Brain Waves and Functional Topology: A Neuronal Communications Syntax
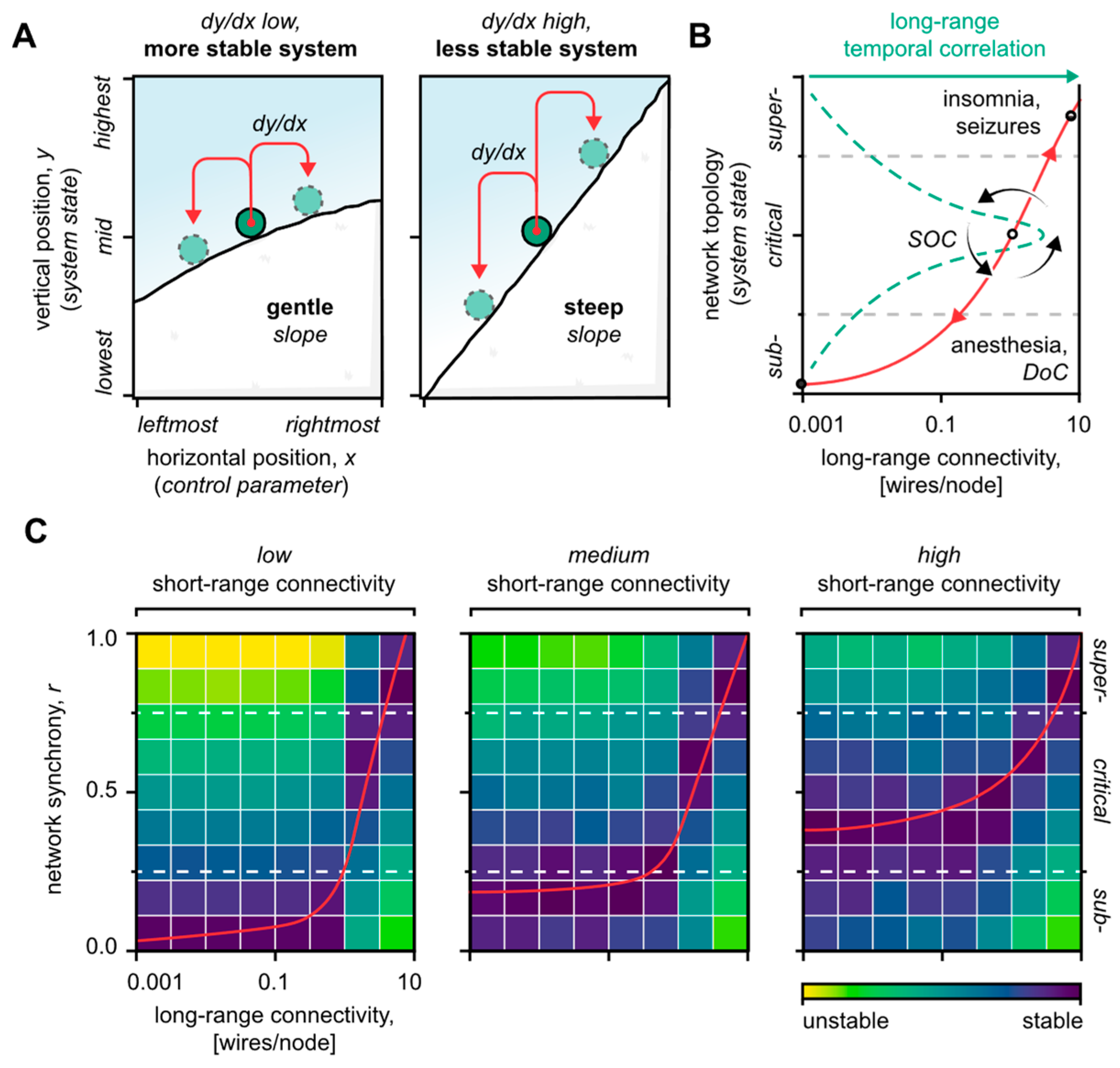
4. Reestablishing Critical System Dynamics: A General Strategy for Neuromodulation
4.1. Short-Range Neuronal Connectivity Affects the System’s Stability
4.2. Long-Range Neuronal Connectivity Defines the System’s State
4.3. Merits of Combinatorial Neuromodulation Strategies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Olesen, J.; Gustavsson, A.; Svensson, M.; Wittchen, H.-U.; Jönsson, B.; CDBE2010 Study Group; European Brain Council. The Economic Cost of Brain Disorders in Europe. Eur. J. Neurol. 2012, 19, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.; Schultz, S.R. Prospects for Optogenetic Augmentation of Brain Function. Front. Syst. Neurosci. 2015, 9, 157. [Google Scholar] [CrossRef]
- Mahmoudi, P.; Veladi, H.; Pakdel, F.G. Optogenetics, Tools and Applications in Neurobiology. J. Med. Signals Sens. 2017, 7, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-Y.; Yang, L.-H.; Stubbs, B.; Li, D.-J.; Tseng, P.-T.; Yeh, T.-C.; Chen, T.-Y.; Liang, C.-S.; Chu, C.-S. Efficacy and Tolerability of Deep Transcranial Magnetic Stimulation for Treatment-Resistant Depression: A Systematic Review and Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 99, 109850. [Google Scholar] [CrossRef] [PubMed]
- Larson, P.S. Deep Brain Stimulation for Movement Disorders. Neurotherapeutics 2014, 11, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Frießem, C.H.; Wiegand, T.; Eitner, L.; Maier, C.; Mainka, T.; Vollert, J.; Enax-Krumova, E.K. Effects of Spinal Cord and Peripheral Nerve Stimulation Reflected in Sensory Profiles and Endogenous Pain Modulation. Clin. J. Pain 2019, 35, 111–120. [Google Scholar] [CrossRef]
- Adil, S.M.; Charalambous, L.T.; Spears, C.A.; Kiyani, M.; Hodges, S.E.; Yang, Z.; Lee, H.-J.; Rahimpour, S.; Parente, B.; Greene, K.A.; et al. Impact of Spinal Cord Stimulation on Opioid Dose Reduction: A Nationwide Analysis. Neurosurgery 2020, 88, 193–201. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, M.; Breur, J.M.P.J.; Raphaël, M.F.; Vos, M.; Breugem, C.C.; Pasmans, S.G.M.A. Adverse Effects of Propranolol When Used in the Treatment of Hemangiomas: A Case Series of 28 Infants. J. Am. Acad. Dermatol. 2011, 65, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Corazzol, M.; Lio, G.; Lefevre, A.; Deiana, G.; Tell, L.; André-Obadia, N.; Bourdillon, P.; Guenot, M.; Desmurget, M.; Luauté, J.; et al. Restoring Consciousness with Vagus Nerve Stimulation. Curr. Biol. 2017, 27, R994–R996. [Google Scholar] [CrossRef]
- Collins, L.; Boddington, L.; Steffan, P.J.; McCormick, D. Vagus Nerve Stimulation Induces Widespread Cortical and Behavioral Activation. Curr. Biol. 2021, 31, 2088–2098. [Google Scholar] [CrossRef]
- Ghaffarpasand, F.; Razmkon, A.; Khalili, H. Deep Brain Stimulation in Patients with Traumatic Brain Injury; Facts and Figures. Bull. Emerg. Trauma 2014, 2, 101–102. [Google Scholar] [PubMed]
- Kundu, B.; Brock, A.A.; Englot, D.J.; Butson, C.R.; Rolston, J.D. Deep Brain Stimulation for the Treatment of Disorders of Consciousness and Cognition in Traumatic Brain Injury Patients: A Review. Neurosurg. Focus 2018, 45, E14. [Google Scholar] [CrossRef] [PubMed]
- Meaney, D.F.; Smith, D.H. Biomechanics of Concussion. Clin. Sports Med. 2011, 30, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Farzan, F.; Barr, M.S.; Sun, Y.; Fitzgerald, P.B.; Daskalakis, Z.J. Transcranial Magnetic Stimulation on the Modulation of Gamma Oscillations in Schizophrenia. Ann. N. Y. Acad. Sci. 2012, 1265, 25–35. [Google Scholar] [CrossRef]
- Boudewyn, M.A.; Scangos, K.; Ranganath, C.; Carter, C.S. Using Prefrontal Transcranial Direct Current Stimulation (TDCS) to Enhance Proactive Cognitive Control in Schizophrenia. Neuropsychopharmacology 2020, 45, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Gurkoff, G.G.; Izadi, A.; Berman, R.F.; Ekstrom, A.D.; Muizelaar, J.P.; Lyeth, B.G.; Shahlaie, K. Medial Septal Nucleus Theta Frequency Deep Brain Stimulation Improves Spatial Working Memory after Traumatic Brain Injury. J. Neurotrauma 2013, 30, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Carrera, E.; Tononi, G. Diaschisis: Past, Present, Future. Brain 2014, 137 Pt 9, 2408–2422. [Google Scholar] [CrossRef]
- Tsuda, I. Toward an Interpretation of Dynamic Neural Activity in Terms of Chaotic Dynamical Systems. Behav. Brain Sci. 2001, 24, 793–810, discussion 810–848. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G. Rhythms of the Brain; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Akam, T.; Kullmann, D.M. Oscillations and Filtering Networks Support Flexible Routing of Information. Neuron 2010, 67, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Singer, W. Neuronal Synchrony: A Versatile Code for the Definition of Relations? Neuron 1999, 24, 49–65. [Google Scholar] [CrossRef] [Green Version]
- Singer, W. Cortical Dynamics Revisited. Trends Cogn. Sci. 2013, 17, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Akam, T.; Kullmann, D.M. Oscillatory Multiplexing of Population Codes for Selective Communication in the Mammalian Brain. Nat. Rev. Neurosci. 2014, 15, 111–122. [Google Scholar] [CrossRef]
- Colgin, L.L. Mechanisms and Functions of Theta Rhythms. Annu. Rev. Neurosci. 2013, 36, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Headley, D.B.; Paré, D. Common Oscillatory Mechanisms across Multiple Memory Systems. Npj Sci. Learn. 2017, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Wang, X.-J. Mechanisms of Gamma Oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Palmigiano, A.; Geisel, T.; Wolf, F.; Battaglia, D. Flexible Information Routing by Transient Synchrony. Nat. Neurosci. 2017, 20, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Shew, W.L.; Plenz, D. The Functional Benefits of Criticality in the Cortex. Neuroscientist 2012, 19, 88–100. [Google Scholar] [CrossRef]
- Cocchi, L.; Gollo, L.L.; Zalesky, A.; Breakspear, M. Criticality in the Brain: A Synthesis of Neurobiology, Models and Cognition. Prog. Neurobiol. 2017, 158, 132–152. [Google Scholar] [CrossRef]
- Zimmern, V. Why Brain Criticality Is Clinically Relevant: A Scoping Review. Front. Neural Circuits 2020, 14, 54. [Google Scholar] [CrossRef]
- Arvin, S.; Glud, A.; Yonehara, K. Short- and Long-Range Connections Differentially Modulate the Small-World Network’s Dynamics and State. Front. Comput. Neurosci. 2021, 15, 124. [Google Scholar]
- Wolfram, S. Universality and Complexity in Cellular Automata. Phys. D Nonlinear Phenom. 1984, 10, 1–35. [Google Scholar] [CrossRef]
- Wolfram, S. Cellular Automata as Models of Complexity. Nature 1984, 311, 419–424. [Google Scholar] [CrossRef]
- Watts, D.J.; Strogatz, S.H. Collective Dynamics of ‘Small-World’ Networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Bak, P. Self-Organized Criticality. Phys. A Stat. Mech. Its Appl. 1990, 163, 403–409. [Google Scholar] [CrossRef]
- Hesse, J.; Gross, T. Self-Organized Criticality as a Fundamental Property of Neural Systems. Front. Syst. Neurosci. 2014, 8, 166. [Google Scholar] [CrossRef]
- Barahona, M.; Pecora, L.M. Synchronization in Small-World Systems. Phys. Rev. Lett. 2002, 89, 054101. [Google Scholar] [CrossRef]
- Bassett, D.S.; Bullmore, E.T. Small-World Brain Networks Revisited. Neuroscientist 2017, 23, 499–516. [Google Scholar] [CrossRef]
- Colombo, M.A.; Wei, Y.; Ramautar, J.R.; Linkenkaer-Hansen, K.; Tagliazucchi, E.; Van Someren, E.J.W. More Severe Insomnia Complaints in People with Stronger Long-Range Temporal Correlations in Wake Resting-State EEG. Front. Physiol. 2016, 7, 576. [Google Scholar] [CrossRef]
- Meisel, C.; Storch, A.; Hallmeyer-Elgner, S.; Bullmore, E.; Gross, T. Failure of Adaptive Self-Organized Criticality during Epileptic Seizure Attacks. PLoS Comput. Biol. 2012, 8, e1002312. [Google Scholar] [CrossRef]
- Meisel, C.; Bailey, K.; Achermann, P.; Plenz, D. Decline of Long-Range Temporal Correlations in the Human Brain during Sustained Wakefulness. Sci. Rep. 2017, 7, 11825. [Google Scholar] [CrossRef]
- Arviv, O.; Medvedovsky, M.; Sheintuch, L.; Goldstein, A.; Shriki, O. Deviations from Critical Dynamics in Interictal Epileptiform Activity. J. Neurosci. 2016, 36, 12276–12292. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Linden, D.E.J.; Singer, W.; Haenschel, C.; Lindner, M.; Maurer, K.; Rodriguez, E. Dysfunctional Long-Range Coordination of Neural Activity during Gestalt Perception in Schizophrenia. J. Neurosci. 2006, 26, 8168–8175. [Google Scholar] [CrossRef] [PubMed]
- Chennu, S.; Finoia, P.; Kamau, E.; Allanson, J.; Williams, G.B.; Monti, M.M.; Noreika, V.; Arnatkeviciute, A.; Canales-Johnson, A.; Olivares, F.; et al. Spectral Signatures of Reorganised Brain Networks in Disorders of Consciousness. PLoS Comput. Biol. 2014, 10, e1003887. [Google Scholar] [CrossRef] [PubMed]
- Singer, W. Development and Plasticity of Cortical Processing Architectures. Science 1995, 270, 758–764. [Google Scholar] [CrossRef]
- Madadi Asl, M.; Valizadeh, A.; Tass, P.A. Dendritic and Axonal Propagation Delays May Shape Neuronal Networks with Plastic Synapses. Front. Physiol. 2018, 9, 1849. [Google Scholar] [CrossRef]
- Dan, Y.; Poo, M.-M. Spike Timing-Dependent Plasticity of Neural Circuits. Neuron 2004, 44, 23–30. [Google Scholar] [CrossRef]
- Achard, S.; Salvador, R.; Whitcher, B.; Suckling, J.; Bullmore, E. A Resilient, Low-Frequency, Small-World Human Brain Functional Network with Highly Connected Association Cortical Hubs. J. Neurosci. 2006, 26, 63–72. [Google Scholar] [CrossRef]
- van den Heuvel, M.P.; Sporns, O. Rich-Club Organization of the Human Connectome. J. Neurosci. 2011, 31, 15775. [Google Scholar] [CrossRef]
- Bishop, G.H. Cyclic Changes in Excitability of the Optic Pathway of the Rabbit. Am. J. Physiol. Leg. Content 1932, 103, 213–224. [Google Scholar] [CrossRef]
- Llinás, R.R. Intrinsic Electrical Properties of Mammalian Neurons and CNS Function: A Historical Perspective. Front. Cell. Neurosci. 2014, 8, 320. [Google Scholar] [CrossRef]
- Izhikevich, E.M.; Desai, N.S.; Walcott, E.C.; Hoppensteadt, F.C. Bursts as a Unit of Neural Information: Selective Communication via Resonance. Trends Neurosci. 2003, 26, 161–167. [Google Scholar] [CrossRef]
- Buzsáki, G.; Watson, B.O. Brain Rhythms and Neural Syntax: Implications for Efficient Coding of Cognitive Content and Neuropsychiatric Disease. Dialogues Clin. Neurosci. 2012, 14, 345–367. [Google Scholar] [CrossRef]
- Wang, X.-J. Neurophysiological and Computational Principles of Cortical Rhythms in Cognition. Physiol. Rev. 2010, 90, 1195–1268. [Google Scholar] [CrossRef]
- Fries, P. Rhythms for Cognition: Communication through Coherence. Neuron 2015, 88, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Fries, P. A Mechanism for Cognitive Dynamics: Neuronal Communication through Neuronal Coherence. Trends Cogn. Sci. 2005, 9, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Fries, P.; Nikolić, D.; Singer, W. The Gamma Cycle. Trends Neurosci. 2007, 30, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G. Hippocampal Sharp Wave-Ripple: A Cognitive Biomarker for Episodic Memory and Planning. Hippocampus 2015, 25, 1073–1188. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G. The Brain from Inside Out; Oxford University Press: New York, NY, USA, 2019. [Google Scholar]
- Takagi, K. Information-Based Principle Induces Small-World Topology and Self-Organized Criticality in a Large Scale Brain Network. Front. Comput. Neurosci. 2018, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Huang, D.; Singer, W.; Nikolic, D. A Small World of Neuronal Synchrony. Cereb. Cortex 2008, 18, 2891–2901. [Google Scholar] [CrossRef]
- Massobrio, P.; Pasquale, V.; Martinoia, S. Self-Organized Criticality in Cortical Assemblies Occurs in Concurrent Scale-Free and Small-World Networks. Sci. Rep. 2015, 5, 10578. [Google Scholar] [CrossRef]
- Zhou, Y. Small World Properties Changes in Mild Traumatic Brain Injury. J. Magn. Reson. Imaging 2017, 46, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priesemann, V.; Valderrama, M.; Wibral, M.; Le Van Quyen, M. Neuronal Avalanches Differ from Wakefulness to Deep Sleep-Evidence from Intracranial Depth Recordings in Humans. PLoS Comput. Biol. 2013, 9, e1002985. [Google Scholar] [CrossRef]
- Demertzi, A.; Tagliazucchi, E.; Dehaene, S.; Deco, G.; Barttfeld, P.; Raimondo, F.; Martial, C.; Fernández-Espejo, D.; Rohaut, B.; Voss, H.U.; et al. Human Consciousness Is Supported by Dynamic Complex Patterns of Brain Signal Coordination. Sci. Adv. 2019, 5, eaat7603. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.N.; Lydic, R.; Schiff, N.D. General Anesthesia, Sleep, and Coma. N. Engl. J. Med. 2010, 363, 2638–2650. [Google Scholar] [CrossRef] [PubMed]
- Netoff, T.I.; Clewley, R.; Arno, S.; Keck, T.; White, J.A. Epilepsy in Small-World Networks. J. Neurosci. 2004, 24, 8075–8083. [Google Scholar] [CrossRef]
- Bak, P. How Nature Works: The Science of Self-Organized Criticality; Copernicus: New York, NY, USA, 1996. [Google Scholar]
- Hyde, T.M.; Weinberger, D.R. Seizures and Schizophrenia. Schizophr. Bull. 1997, 23, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Besag, F.M.C.; Vasey, M.J. Seizures and Epilepsy in Autism Spectrum Disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2020, 29, 483–500. [Google Scholar] [CrossRef]
- Fauser, S.; Essang, C.; Altenmüller, D.-M.; Staack, A.M.; Steinhoff, B.J.; Strobl, K.; Bast, T.; Schubert-Bast, S.; Stephani, U.; Wiegand, G.; et al. Long-Term Seizure Outcome in 211 Patients with Focal Cortical Dysplasia. Epilepsia 2015, 56, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, A.; Hadjichristodoulou, C.; Youroukos, S. Epilepsy in Patients with Cerebral Palsy. Dev. Med. Child Neurol. 1997, 39, 659–663. [Google Scholar] [CrossRef]
- Luppi, A.I.; Craig, M.M.; Pappas, I.; Finoia, P.; Williams, G.B.; Allanson, J.; Pickard, J.D.; Owen, A.M.; Naci, L.; Menon, D.K.; et al. Consciousness-Specific Dynamic Interactions of Brain Integration and Functional Diversity. Nat. Commun. 2019, 10, 4616. [Google Scholar] [CrossRef]
- Uhlhaas, P.J.; Singer, W. Neuronal Dynamics and Neuropsychiatric Disorders: Toward a Translational Paradigm for Dysfunctional Large-Scale Networks. Neuron 2012, 75, 963–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerster, M.; Berner, R.; Sawicki, J.; Zakharova, A.; Škoch, A.; Hlinka, J.; Lehnertz, K.; Schöll, E. FitzHugh-Nagumo Oscillators on Complex Networks Mimic Epileptic-Seizure-Related Synchronization Phenomena. Chaos 2020, 30, 123130. [Google Scholar] [CrossRef]
- Kopell, N.; Ermentrout, G.B.; Whittington, M.A.; Traub, R.D. Gamma Rhythms and Beta Rhythms Have Different Synchronization Properties. Proc. Natl. Acad. Sci. USA 2000, 97, 1867. [Google Scholar] [CrossRef]
- Hoy, K.E.; Bailey, N.W.; Arnold, S.L.; Fitzgerald, P.B. The Effect of Transcranial Direct Current Stimulation on Gamma Activity and Working Memory in Schizophrenia. Psychiatry Res. 2015, 228, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, H.; Wang, J.; Li, J.; Tian, Y.; Zheng, J.; He, M.; Xu, T.-L.; Wu, Z.-Y.; Li, X.-M.; et al. Cell Type-Differential Modulation of Prefrontal Cortical GABAergic Interneurons on Low Gamma Rhythm and Social Interaction. Sci. Adv. 2020, 6, eaay4073. [Google Scholar] [CrossRef]
- Righes Marafiga, J.; Vendramin Pasquetti, M.; Calcagnotto, M.E. GABAergic Interneurons in Epilepsy: More than a Simple Change in Inhibition. Epilepsy Behav. 2021, 121. [Google Scholar] [CrossRef]
- Williams, S.; Boksa, P. Gamma Oscillations and Schizophrenia. J. Psychiatry Neurosci. 2010, 35, 75–77. [Google Scholar] [CrossRef]
- McLachlan, R.S. Vagus Nerve Stimulation for Intractable Epilepsy: A Review. J. Clin. Neurophysiol. 1997, 14, 358–368. [Google Scholar] [CrossRef]
- Cao, J.; Lu, K.-H.; Powley, T.L.; Liu, Z. Vagal Nerve Stimulation Triggers Widespread Responses and Alters Large-Scale Functional Connectivity in the Rat Brain. PLoS ONE 2017, 12, e0189518. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.-H.; Nahas, Z.; Lomarev, M.; Denslow, S.; Lorberbaum, J.P.; Bohning, D.E.; George, M.S. A Review of Functional Neuroimaging Studies of Vagus Nerve Stimulation (VNS). J. Psychiatr. Res. 2003, 37, 443–455. [Google Scholar] [CrossRef]
- Frolov, N.; Hramov, A. Extreme Synchronization Events in a Kuramoto Model: The Interplay between Resource Constraints and Explosive Transitions. Chaos 2021, 31, 063103. [Google Scholar] [CrossRef]
- Sharon, O.; Fahoum, F.; Nir, Y. Transcutaneous Vagus Nerve Stimulation in Humans Induces Pupil Dilation and Attenuates Alpha Oscillations. J. Neurosci. 2021, 41, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.-J.; Sun, L.-Z.; Xu, C.-B.; Xie, Y.; Pan, M.-Y.; Ran, J.; Hu, Y.; Nong, B.-X.; Shen, Q.; Huang, H.; et al. The Clinical Effect of Vagus Nerve Stimulation in the Treatment of Patients with a Minimally Conscious State. J. Neurorestoratol. 2020, 8, 160–171. [Google Scholar] [CrossRef]
- Fornai, F.; Ruffoli, R.; Giorgi, F.S.; Paparelli, A. The Role of Locus Coeruleus in the Antiepileptic Activity Induced by Vagus Nerve Stimulation. Eur. J. Neurosci. 2011, 33, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Dahlström, A.B.; Jonsson, G.; Marcellino, D.; Guescini, M.; Dam, M.; Manger, P.; Agnati, L. The Discovery of Central Monoamine Neurons Gave Volume Transmission to the Wired Brain. Prog. Neurobiol. 2010, 90, 82–100. [Google Scholar] [CrossRef]
- Krahl, S.E.; Clark, K.B.; Smith, D.C.; Browning, R.A. Locus Coeruleus Lesions Suppress the Seizure-Attenuating Effects of Vagus Nerve Stimulation. Epilepsia 1998, 39, 709–714. [Google Scholar] [CrossRef]
- Giorgi, F.S.; Ferrucci, M.; Lazzeri, G.; Pizzanelli, C.; Lenzi, P.; Alessandrl, M.G.; Murri, L.; Fornai, F. A Damage to Locus Coeruleus Neurons Converts Sporadic Seizures into Self-Sustaining Limbic Status Epilepticus. Eur. J. Neurosci. 2003, 17, 2593–2601. [Google Scholar] [CrossRef]
- Aaronson, S.T.; Carpenter, L.L.; Conway, C.R.; Reimherr, F.W.; Lisanby, S.H.; Schwartz, T.L.; Moreno, F.A.; Dunner, D.L.; Lesem, M.D.; Thompson, P.M.; et al. Vagus Nerve Stimulation Therapy Randomized to Different Amounts of Electrical Charge for Treatment-Resistant Depression: Acute and Chronic Effects. Brain Stimul. 2013, 6, 631–640. [Google Scholar] [CrossRef]
- Conway, C.R.; Kumar, A.; Xiong, W.; Bunker, M.; Aaronson, S.T.; Rush, A.J. Chronic Vagus Nerve Stimulation Significantly Improves Quality of Life in Treatment-Resistant Major Depression. J. Clin. Psychiatry 2018, 79, 18m12178. [Google Scholar] [CrossRef]
- Afrasiabi, M.; Redinbaugh, M.J.; Phillips, J.M.; Kambi, N.A.; Mohanta, S.; Raz, A.; Haun, A.M.; Saalmann, Y.B. Consciousness Depends on Integration between Parietal Cortex, Striatum, and Thalamus. Cell Syst. 2021, 12, 363–373.e11. [Google Scholar] [CrossRef]
- Smith, D.H.; Nonaka, M.; Miller, R.; Leoni, M.; Chen, X.H.; Alsop, D.; Meaney, D.F. Immediate Coma Following Inertial Brain Injury Dependent on Axonal Damage in the Brainstem. J. Neurosurg. 2000, 93, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browne, K.D.; Chen, X.-H.; Meaney, D.F.; Smith, D.H. Mild Traumatic Brain Injury and Diffuse Axonal Injury in Swine. J. Neurotrauma 2011, 28, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Gennarelli, T.A.; Thibault, L.E.; Adams, J.H.; Graham, D.I.; Thompson, C.J.; Marcincin, R.P. Diffuse Axonal Injury and Traumatic Coma in the Primate. Ann. Neurol. 1982, 12, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Min, B.-K. A Thalamic Reticular Networking Model of Consciousness. Theor. Biol. Med. Model. 2010, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M. The Thalamic Dynamic Core Theory of Conscious Experience. Conscious. Cogn. 2011, 20, 464–486. [Google Scholar] [CrossRef]
- Yeo, S.S.; Chang, P.H.; Jang, S.H. The Ascending Reticular Activating System from Pontine Reticular Formation to the Thalamus in the Human Brain. Front. Hum. Neurosci. 2013, 7, 416. [Google Scholar] [CrossRef]
- Lutkenhoff, E.S.; Nigri, A.; Rossi Sebastiano, D.; Sattin, D.; Visani, E.; Rosazza, C.; D’Incerti, L.; Bruzzone, M.G.; Franceschetti, S.; Leonardi, M.; et al. EEG Power Spectra and Subcortical Pathology in Chronic Disorders of Consciousness. Psychol. Med. 2022, 52, 1491–1500. [Google Scholar] [CrossRef]
- Kinney, H.C.; Korein, J.; Panigrahy, A.; Dikkes, P.; Goode, R. Neuropathological Findings in the Brain of Karen Ann Quinlan. The Role of the Thalamus in the Persistent Vegetative State. N. Engl. J. Med. 1994, 330, 1469–1475. [Google Scholar] [CrossRef]
- Adams, J.H.; Graham, D.I.; Jennett, B. The Neuropathology of the Vegetative State after an Acute Brain Insult. Brain 2000, 123 Pt 7, 1327–1338. [Google Scholar] [CrossRef]
- Jennett, B.; Adams, J.H.; Murray, L.S.; Graham, D.I. Neuropathology in Vegetative and Severely Disabled Patients after Head Injury. Neurology 2001, 56, 486–490. [Google Scholar] [CrossRef]
- Fuller, P.M.; Sherman, D.; Pedersen, N.P.; Saper, C.B.; Lu, J. Reassessment of the Structural Basis of the Ascending Arousal System. J. Comp. Neurol. 2011, 519, 933–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luppi, A.I.; Cain, J.; Spindler, L.R.B.; Górska, U.J.; Toker, D.; Hudson, A.E.; Brown, E.N.; Diringer, M.N.; Stevens, R.D.; Massimini, M.; et al. Curing Coma Campaign and Its Contributing Collaborators. Mechanisms Underlying Disorders of Consciousness: Bridging Gaps to Move Toward an Integrated Translational Science. Neurocrit. Care 2021, 35 (Suppl. 1), 37–54. [Google Scholar] [CrossRef] [PubMed]
- Modolo, J.; Hassan, M.; Wendling, F.; Benquet, P. Decoding the Circuitry of Consciousness: From Local Microcircuits to Brain-Scale Networks. Netw Neurosci. 2020, 4, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Schiff, N.D. Central Thalamic Deep-Brain Stimulation in the Severely Injured Brain: Rationale and Proposed Mechanisms of Action. Ann. N. Y. Acad. Sci. 2009, 1157, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, J.-J.; Sontheimer, A.; Pereira, B.; Coste, J.; Rosenberg, S.; Sarret, C.; Coll, G.; Gabrillargues, J.; Jean, B.; Gillart, T.; et al. Deep Brain Stimulation in Five Patients with Severe Disorders of Consciousness. Ann. Clin. Transl. Neurol. 2018, 5, 1372–1384. [Google Scholar] [CrossRef]
- Ghalaenovi, H.; Fattahi, A.; Koohpayehzadeh, J.; Khodadost, M.; Fatahi, N.; Taheri, M.; Azimi, A.; Rohani, S.; Rahatlou, H. The Effects of Amantadine on Traumatic Brain Injury Outcome: A Double-Blind, Randomized, Controlled, Clinical Trial. Brain Inj. 2018, 32, 1050–1055. [Google Scholar] [CrossRef]
- Schiff, N.D. Recovery of Consciousness after Severe Brain Injury: The Role of Arousal Regulation Mechanisms and Some Speculation on the Heart-Brain Interface. Cleve. Clin. J. Med. 2010, 77 (Suppl. 3), S27–S33. [Google Scholar] [CrossRef]
- Schnakers, C.; Monti, M.M. Disorders of Consciousness after Severe Brain Injury: Therapeutic Options. Curr. Opin. Neurol. 2017, 30, 573–579. [Google Scholar] [CrossRef]
- Yamamoto, T.; Katayama, Y.; Kobayashi, K.; Oshima, H.; Fukaya, C.; Tsubokawa, T. Deep Brain Stimulation for the Treatment of Vegetative State. Eur. J. Neurosci. 2010, 32, 1145–1151. [Google Scholar] [CrossRef]
- Magrassi, L.; Maggioni, G.; Pistarini, C.; Di Perri, C.; Bastianello, S.; Zippo, A.G.; Iotti, G.A.; Biella, G.E.M.; Imberti, R. Results of a Prospective Study (CATS) on the Effects of Thalamic Stimulation in Minimally Conscious and Vegetative State Patients. J. Neurosurg. 2016, 125, 972–981. [Google Scholar] [CrossRef]
- Spritzer, S.D.; Kinney, C.L.; Condie, J.; Wellik, K.E.; Hoffman-Snyder, C.R.; Wingerchuk, D.M.; Demaerschalk, B.M. Amantadine for Patients with Severe Traumatic Brain Injury: A Critically Appraised Topic. Neurologist 2015, 19, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Cain, J.A.; Spivak, N.M.; Coetzee, J.P.; Crone, J.S.; Johnson, M.A.; Lutkenhoff, E.S.; Real, C.; Buitrago-Blanco, M.; Vespa, P.M.; Schnakers, C.; et al. Ultrasonic Thalamic Stimulation in Chronic Disorders of Consciousness. Brain Stimul. 2021, 14, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Hillary, F.G.; Slocomb, J.; Hills, E.C.; Fitzpatrick, N.M.; Medaglia, J.D.; Wang, J.; Good, D.C.; Wylie, G.R. Changes in Resting Connectivity during Recovery from Severe Traumatic Brain Injury. Int. J. Psychophysiol. 2011, 82, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Eaves, E.; Dams-O’Connor, K.; Ho, L.; Leung, E.; Wong, E.; Carpenter, D.; Ng, J.; Gordon, W.; Pasinetti, G. Diffuse Disconnectivity in TBi: A Resting State FMri anD Dti StuDy. Transl. Neurosci. 2012, 3, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hillary, F.G.; Roman, C.A.; Venkatesan, U.; Rajtmajer, S.M.; Bajo, R.; Castellanos, N.D. Hyperconnectivity Is a Fundamental Response to Neurological Disruption. Neuropsychology 2015, 29, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Iraji, A.; Chen, H.; Wiseman, N.; Welch, R.D.; O’Neil, B.J.; Haacke, E.M.; Liu, T.; Kou, Z. Compensation through Functional Hyperconnectivity: A Longitudinal Connectome Assessment of Mild Traumatic Brain Injury. Neural Plast. 2016, 2016, 4072402. [Google Scholar] [CrossRef]
- Xia, X.; Bai, Y.; Zhou, Y.; Yang, Y.; Xu, R.; Gao, X.; Li, X.; He, J. Effects of 10 Hz Repetitive Transcranial Magnetic Stimulation of the Left Dorsolateral Prefrontal Cortex in Disorders of Consciousness. Front. Neurol. 2017, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Maneeton, B.; Maneeton, N.; Woottiluk, P.; Likhitsathian, S. Repetitive Transcranial Magnetic Stimulation Combined with Antidepressants for the First Episode of Major Depressive Disorder. Curr. Neuropharmacol. 2020, 18, 852–860. [Google Scholar] [CrossRef]
- Best, S.R.D.; Pavel, D.G.; Haustrup, N. Combination Therapy with Transcranial Magnetic Stimulation and Ketamine for Treatment-Resistant Depression: A Long-Term Retrospective Review of Clinical Use. Heliyon 2019, 5, e02187. [Google Scholar] [CrossRef]
- Aaronson, S.T.; Sears, P.; Ruvuna, F.; Bunker, M.; Conway, C.R.; Dougherty, D.D.; Reimherr, F.W.; Schwartz, T.L.; Zajecka, J.M. A 5-Year Observational Study of Patients with Treatment-Resistant Depression Treated with Vagus Nerve Stimulation or Treatment as Usual: Comparison of Response, Remission, and Suicidality. Am. J. Psychiatry 2017, 174, 640–648. [Google Scholar] [CrossRef]
- Desbeaumes Jodoin, V.; Richer, F.; Miron, J.-P.; Fournier-Gosselin, M.-P.; Lespérance, P. Long-Term Sustained Cognitive Benefits of Vagus Nerve Stimulation in Refractory Depression. J. ECT 2018, 34, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Bender Pape, T.L.; Herrold, A.A.; Livengood, S.L.; Guernon, A.; Weaver, J.A.; Higgins, J.P.; Rosenow, J.M.; Walsh, E.; Bhaumik, R.; Pacheco, M.; et al. A Pilot Trial Examining the Merits of Combining Amantadine and Repetitive Transcranial Magnetic Stimulation as an Intervention for Persons with Disordered Consciousness After TBI. J. Head Trauma Rehabil. 2020, 35, 371–387. [Google Scholar] [CrossRef]
- Dowdy, R.A.E.; Mansour, S.T.; Cottle, J.H.; Mabe, H.R.; Weprin, H.B.; Yarborough, L.E.; Ness, G.M.; Jacobs, T.M.; Cornelius, B.W. Cardiac Arrest Upon Induction of General Anesthesia. Anesth. Prog. 2021, 68, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Moitra, V.K.; Einav, S.; Thies, K.-C.; Nunnally, M.E.; Gabrielli, A.; Maccioli, G.A.; Weinberg, G.; Banerjee, A.; Ruetzler, K.; Dobson, G.; et al. Cardiac Arrest in the Operating Room: Resuscitation and Management for the Anesthesiologist: Part 1. Anesth. Analg. 2018, 126, 876–888. [Google Scholar] [CrossRef]
- Porche, K.; Maciel, C.B.; Lucke-Wold, B.; Robicsek, S.A.; Chalouhi, N.; Brennan, M.; Busl, K.M. Preoperative Prediction of Postoperative Urinary Retention in Lumbar Surgery: A Comparison of Regression to Multilayer Neural Network. J. Neurosurg. Spine 2022, 36, 32–41. [Google Scholar] [CrossRef]
- Clifton, G.L.; Valadka, A.; Zygun, D.; Coffey, C.S.; Drever, P.; Fourwinds, S.; Janis, L.S.; Wilde, E.; Taylor, P.; Harshman, K.; et al. Very Early Hypothermia Induction in Patients with Severe Brain Injury (the National Acute Brain Injury Study: Hypothermia II): A Randomised Trial. Lancet Neurol. 2011, 10, 131–139. [Google Scholar] [CrossRef]
- Thal, S.C.; Timaru-Kast, R.; Wilde, F.; Merk, P.; Johnson, F.; Frauenknecht, K.; Sebastiani, A.; Sommer, C.; Staib-Lasarzik, I.; Werner, C.; et al. Propofol Impairs Neurogenesis and Neurologic Recovery and Increases Mortality Rate in Adult Rats after Traumatic Brain Injury. Crit. Care Med. 2014, 42, 129–141. [Google Scholar] [CrossRef]
- Tepas, J.J., III.; Leaphart, C.L.; Pieper, P.; Beaulieu, C.L.; Spierre, L.R.; Tuten, J.D.; Celso, B.G. The Effect of Delay in Rehabilitation on Outcome of Severe Traumatic Brain Injury. J. Pediatr. Surg. 2009, 44, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Barra, M.E.; Izzy, S.; Sarro-Schwartz, A.; Hirschberg, R.E.; Mazwi, N.; Edlow, B.L. Stimulant Therapy in Acute Traumatic Brain Injury: Prescribing Patterns and Adverse Event Rates at 2 Level 1 Trauma Centers. J. Intensive Care Med. 2020, 35, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Hausburg, M.A.; Banton, K.L.; Roman, P.E.; Salgado, F.; Baek, P.; Waxman, M.J.; Tanner, A., II.; Yoder, J.; Bar-Or, D. Effects of Propofol on Ischemia-Reperfusion and Traumatic Brain Injury. J. Crit. Care 2020, 56, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Leunig, A.; Han, S.; Peterka, D.S.; Yuste, R. Prolonged Anesthesia Alters Brain Synaptic Architecture. Proc. Natl. Acad. Sci. USA 2021, 118, e2023676118. [Google Scholar] [CrossRef] [PubMed]
- Pandharipande, P.P.; Girard, T.D.; Jackson, J.C.; Morandi, A.; Thompson, J.L.; Pun, B.T.; Brummel, N.E.; Hughes, C.G.; Vasilevskis, E.E.; Shintani, A.K.; et al. BRAIN-ICU Study Investigators. Long-Term Cognitive Impairment after Critical Illness. N. Engl. J. Med. 2013, 369, 1306–1316. [Google Scholar] [CrossRef]
- Wutzl, B.; Golaszewski, S.M.; Leibnitz, K.; Langthaler, P.B.; Kunz, A.B.; Leis, S.; Schwenker, K.; Thomschewski, A.; Bergmann, J.; Trinka, E. Narrative Review: Quantitative EEG in Disorders of Consciousness. Brain Sci. 2021, 11, 697. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arvin, S.; Yonehara, K.; Glud, A.N. Therapeutic Neuromodulation toward a Critical State May Serve as a General Treatment Strategy. Biomedicines 2022, 10, 2317. https://doi.org/10.3390/biomedicines10092317
Arvin S, Yonehara K, Glud AN. Therapeutic Neuromodulation toward a Critical State May Serve as a General Treatment Strategy. Biomedicines. 2022; 10(9):2317. https://doi.org/10.3390/biomedicines10092317
Chicago/Turabian StyleArvin, Simon, Keisuke Yonehara, and Andreas Nørgaard Glud. 2022. "Therapeutic Neuromodulation toward a Critical State May Serve as a General Treatment Strategy" Biomedicines 10, no. 9: 2317. https://doi.org/10.3390/biomedicines10092317
APA StyleArvin, S., Yonehara, K., & Glud, A. N. (2022). Therapeutic Neuromodulation toward a Critical State May Serve as a General Treatment Strategy. Biomedicines, 10(9), 2317. https://doi.org/10.3390/biomedicines10092317





