Ventilation Induces Changes in Pulse Wave Transit Time in the Pulmonary Artery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Anesthesia
2.2. Ventilation
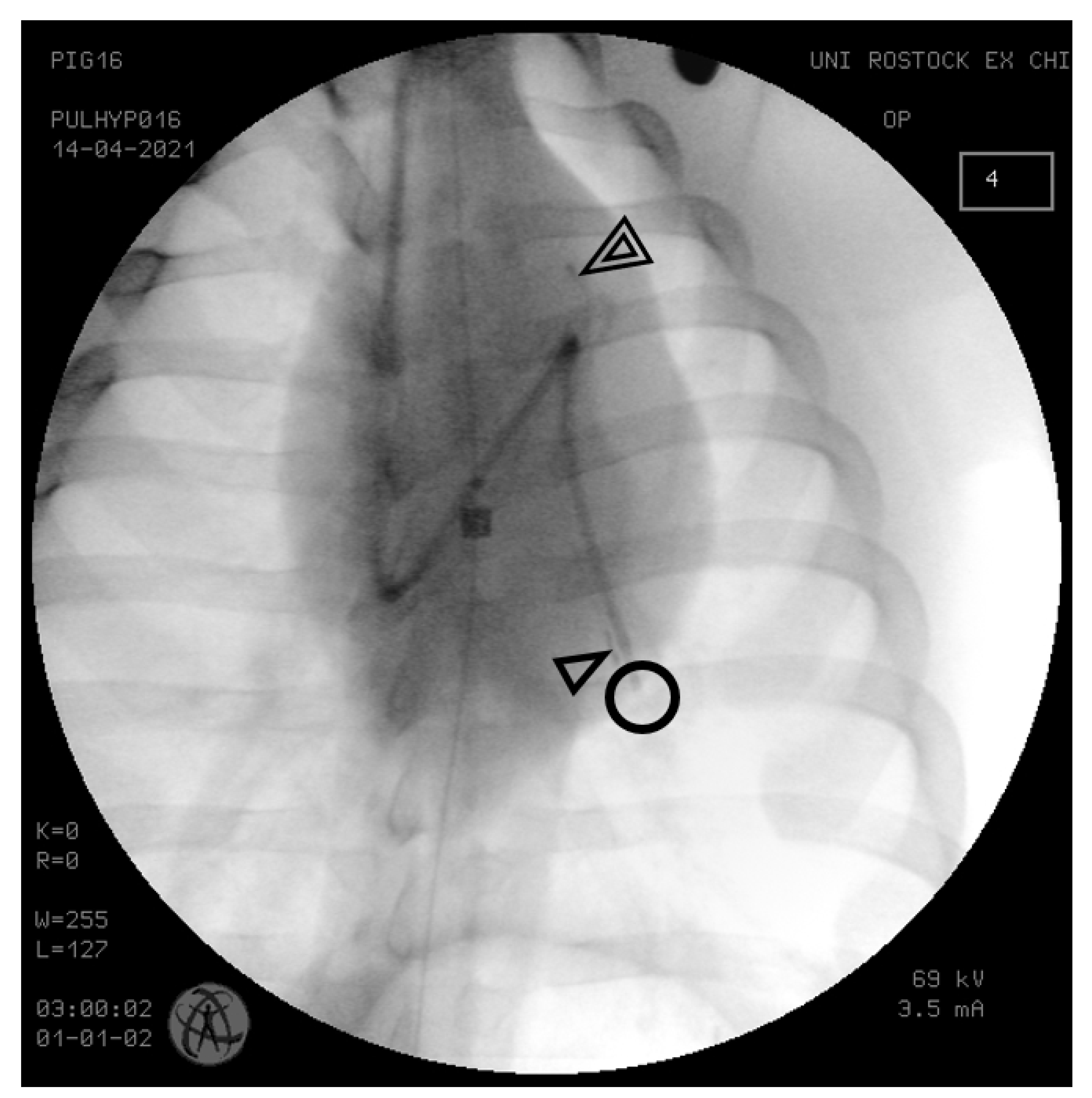
2.3. Instrumentation
2.4. Data Acquisition and Processing
2.5. Statistics
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.-S.H.; Bajwa, A.A.; White, R.D. Assessment of Pulmonary Arterial Hypertension by Magnetic Resonance Imaging. Tomography 2015, 1, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Wilson, N.; Ivy, D.D.; Ing, R.; Abman, S.; Browne, L.P.; Morgan, G.; Ross, M.; McLennan, D.; Barker, A.J.; et al. Noninvasive wave intensity analysis predicts functional worsening in children with pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H968–H977. [Google Scholar] [CrossRef]
- Proença, M.; Braun, F.; Solà, J.; Adler, A.; Lemay, M.; Thiran, J.-P.; Rimoldi, S.F. Non-invasive monitoring of pulmonary artery pressure from timing information by EIT: Experimental evaluation during induced hypoxia. Physiol. Meas. 2016, 37, 713–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller-Graf, F.; Merz, J.; Bandorf, T.; Albus, C.F.; Henkel, M.; Krukewitt, L.; Kühn, V.; Reuter, S.; Vollmar, B.; Pulletz, S.; et al. Correlation of Pulse Wave Transit Time with Pulmonary Artery Pressure in a Porcine Model of Pulmonary Hypertension. Biomedicines 2021, 9, 1212. [Google Scholar] [CrossRef]
- Pöhler, G.H.; Löffler, F.; Klimeš, F.; Behrendt, L.; Voskrebenzev, A.; González, C.C.; Westhoff-Bleck, M.; Wacker, F.; Vogel-Claussen, J. Validation of Phase-Resolved Functional Lung (PREFUL) Magnetic Resonance Imaging Pulse Wave Transit Time Compared to Echocardiography in Chronic Obstructive Pulmonary Disease. J. Magn. Reson. Imaging 2022, 56, 605–615. [Google Scholar] [CrossRef]
- Wibmer, T.; Rüdiger, S.; Scharnbeck, D.; Radermacher, M.; Markovic, S.; Stoiber, K.M.; Rottbauer, W.; Schumann, C. Pulmonary pulse transit time: A novel echocardiographic indicator of hemodynamic and vascular alterations in pulmonary hypertension and pulmonary fibrosis. Echocardiography 2015, 32, 904–911. [Google Scholar] [CrossRef]
- Du Percie Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Baydur, A.; Behrakis, P.K.; Zin, W.A.; Jaeger, M.; Milic-Emili, J. A simple method for assessing the validity of the esophageal balloon technique. Am. Rev. Respir. Dis. 1982, 126, 788–791. [Google Scholar] [CrossRef]
- Talmor, D.; Sarge, T.; O’Donnell, C.R.; Ritz, R.; Malhotra, A.; Lisbon, A.; Loring, S.H. Talmor Esophageal and transpulmonary pressures in acute respiratory failure. Crit. Care Med. 2006, 34, 1389. [Google Scholar]
- Kopeć, G.; Moertl, D.; Jankowski, P.; Tyrka, A.; Sobień, B.; Podolec, P. Pulmonary artery pulse wave velocity in idiopathic pulmonary arterial hypertension. Can. J. Cardiol. 2013, 29, 683–690. [Google Scholar] [CrossRef]
- Solà, J.; Vetter, R.; Renevey, P.; Chételat, O.; Sartori, C.; Rimoldi, S.F. Parametric estimation of pulse arrival time: A robust approach to pulse wave velocity. Physiol. Meas. 2009, 30, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Proença, M.; Braun, F.; Solà, J.; Thiran, J.-P.; Lemay, M. Noninvasive pulmonary artery pressure monitoring by EIT: A model-based feasibility study. Med. Biol. Eng. Comput. 2017, 55, 949–963. [Google Scholar] [CrossRef]
- Solà, J.; Adler, A.; Santos, A.; Tusman, G.; Sipmann, F.S.; Bohm, S.H. Non-invasive monitoring of central blood pressure by electrical impedance tomography: First experimental evidence. Med. Biol. Eng. Comput. 2011, 49, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Simmons, D.H.; Linde, L.M.; Miller, J.H.; O’reilly, R.J. Relation Between Lung Volume and Pulmonary Vascular Resistance. Circ. Res. 1961, 9, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Suresh, K.; Shimoda, L.A. Lung Circulation. Compr. Physiol. 2016, 6, 897–943. [Google Scholar] [CrossRef] [PubMed]
- Widrich, J.; Shetty, M. StatPearls: Physiology, Pulmonary Vascular Resistance; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hedenstierna, G.; Edmark, L. Effects of anesthesia on the respiratory system. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 273–284. [Google Scholar] [CrossRef]
- Hedenstierna, G.; Rothen, H.U. Atelectasis formation during anesthesia: Causes and measures to prevent it. J. Clin. Monit. Comput. 2000, 16, 329–335. [Google Scholar] [CrossRef]
- Laffon, E.; Laurent, F.; Bernard, V.; de Boucaud, L.; Ducassou, D.; Marthan, R. Noninvasive assessment of pulmonary arterial hypertension by MR phase-mapping method. J. Appl. Physiol. 2001, 90, 2197–2202. [Google Scholar] [CrossRef] [Green Version]
- Roeleveld, R.J.; Marcus, J.T.; Boonstra, A.; Postmus, P.E.; Marques, K.M.; Bronzwaer, J.G.F.; Vonk-Noordegraaf, A. A comparison of noninvasive MRI-based methods of estimating pulmonary artery pressure in pulmonary hypertension. J. Magn. Reson. Imaging 2005, 22, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Proença, M.; Braun, F.; Lemay, M.; Solà, J.; Adler, A.; Riedel, T.; Messerli, F.H.; Thiran, J.-P.; Rimoldi, S.F.; Rexhaj, E. Non-invasive pulmonary artery pressure estimation by electrical impedance tomography in a controlled hypoxemia study in healthy subjects. Sci. Rep. 2020, 10, 21462. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.R. Heart lung interactions during mechanical ventilation. Curr. Opin. Crit. Care 2012, 18, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Pinsky, M.R. Heart-lung interactions during mechanical ventilation: The basics. Ann. Transl. Med. 2018, 6, 349. [Google Scholar] [CrossRef]



| PWTT [ms] | sPAP [mmHg] | mPAP [mmHg] | dPAP [mmHg] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Expiration | Inspiration | p-Value | Expiration | Inspiration | p-Value | Expiration | Inspiration | p-Value | Expiration | Inspiration | p-Value | |
| 1 | 88.9 ± 3.4 | 81.8 ± 1.6 * | 0.001 | 25.7 ± 0.4 | 30.4 ± 0.9 * | 0.001 | 21.4 ± 0.3 | 25.5 ± 0.8 * | 0.001 | 15.4 ± 0.4 | 20.2 ± 1.0 * | 0.004 |
| 2 | 81.1 ± 54 | 57.8 ± 8.4 * | 0.001 | 21.1 ± 0.3 | 25.3 ± 0.9 * | 0.001 | 16.1 ± 0.1 | 19.4 ± 0.6 * | 0.001 | 10.7 ± 0.2 | 13.8 ± 1.1 * | 0.001 |
| 3 | 87.5 ± 1.2 | 83.5 ± 0.9 * | 0.001 | 21.8 ± 0.1 | 24.8 ± 0.4 * | 0.001 | 17.0 ± 0.2 | 19.9 ± 0.6 * | 0.001 | 10.3 ± 0.1 | 12.3 ± 1.3 * | 0.001 |
| 4 | 37.7 ± 1.3 | 36.7 ± 2.1 * | 0.001 | 19.3 ± 0.3 | 22.3 ± 0.5 * | 0.001 | 13.4 ± 0.3 | 16.8 ± 0.4 * | 0.001 | 7.7 ± 0.4 | 11.2 ± 1.9 * | 0.001 |
| 5 | 45.2 ± 1.3 | 44.3 ± 2.3 * | 0.001 | 28.2 ± 0.5 | 32.7 ± 1.2 * | 0.001 | 21.0 ± 0.4 | 26.4 ± 0.5 * | 0.001 | 13.9 ± 0.2 | 18.5 ± 1.6 * | 0.001 |
| PWTT [ms] | sPAP [mmHg] | mPAP [mmHg] | dPAP [mmHg] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ventilation | Apnoe | p-Value | Ventilation | Apnoe | p-Value | Ventilation | Apnoe | p-Value | Ventilation | Apnoe | p-Value | |
| 1 | 84.6 ± 5.8 | 71.3 ± 1.4 * | 0.001 | 26.9 ± 2.1 | 29.7 ± 0.6 * | 0.001 | 22.2 ± 2.0 | 23.8 ± 0.2 * | 0.001 | 15.9 ± 2.3 | 16.2 ± 0.4 * | 0.001 |
| 2 | 73.3 ± 14.7 | 80.9 ± 0.8 * | 0.001 | 22.4 ± 1.8 | 21.3 ± 0.5 * | 0.001 | 16.9 ± 1.5 | 16.9 ± 0.2 * | 0.001 | 11.7 ± 1.9 | 11.5 ± 0.4 * | 0.001 |
| 3 | 82.2 ± 7.1 | 62.5 ± 1.2 * | 0.001 | 22.5 ± 1.4 | 24.4 ± 0.2 * | 0.001 | 17.7 ± 1.3 | 18.9 ± 0.3 * | 0.001 | 11.0 ± 1.7 | 12.5 ± 0.4 * | 0.001 |
| 4 | 37.8 ± 2.9 | 51.8 ± 0.7 * | 0.001 | 19.7 ± 1.4 | 19.0 ± 0.4 * | 0.001 | 13.9 ± 1.7 | 13.1 ± 0.3 * | 0.001 | 8.2 ± 2.9 | 7.9 ± 0.3 * | 0.001 |
| 5 | 44.6 ± 2.0 | 46.4 ± 1.1 * | 0.001 | 29.1 ± 2.3 | 27.6 ± 0.5 * | 0.001 | 22.0 ± 2.3 | 20.7 ± 0.4 * | 0.001 | 15.4 ± 2.7 | 13.5 ± 0.4 * | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller-Graf, F.; Frenkel, P.; Albus, C.F.; Henkel, M.; Reuter, S.; Vollmar, B.; Tusman, G.; Adler, A.; Pulletz, S.; Böhm, S.H.; et al. Ventilation Induces Changes in Pulse Wave Transit Time in the Pulmonary Artery. Biomedicines 2023, 11, 182. https://doi.org/10.3390/biomedicines11010182
Mueller-Graf F, Frenkel P, Albus CF, Henkel M, Reuter S, Vollmar B, Tusman G, Adler A, Pulletz S, Böhm SH, et al. Ventilation Induces Changes in Pulse Wave Transit Time in the Pulmonary Artery. Biomedicines. 2023; 11(1):182. https://doi.org/10.3390/biomedicines11010182
Chicago/Turabian StyleMueller-Graf, Fabian, Paul Frenkel, Chiara Felicitas Albus, Maike Henkel, Susanne Reuter, Brigitte Vollmar, Gerardo Tusman, Andy Adler, Sven Pulletz, Stephan H. Böhm, and et al. 2023. "Ventilation Induces Changes in Pulse Wave Transit Time in the Pulmonary Artery" Biomedicines 11, no. 1: 182. https://doi.org/10.3390/biomedicines11010182
APA StyleMueller-Graf, F., Frenkel, P., Albus, C. F., Henkel, M., Reuter, S., Vollmar, B., Tusman, G., Adler, A., Pulletz, S., Böhm, S. H., Zitzmann, A., & Reuter, D. A. (2023). Ventilation Induces Changes in Pulse Wave Transit Time in the Pulmonary Artery. Biomedicines, 11(1), 182. https://doi.org/10.3390/biomedicines11010182






