Sex Differences in Microglia Activation in a Rodent Model of Preterm Hypoxic Ischemic Injury with Caffeine Treatment
Abstract
1. Introduction
2. Materials and Methods
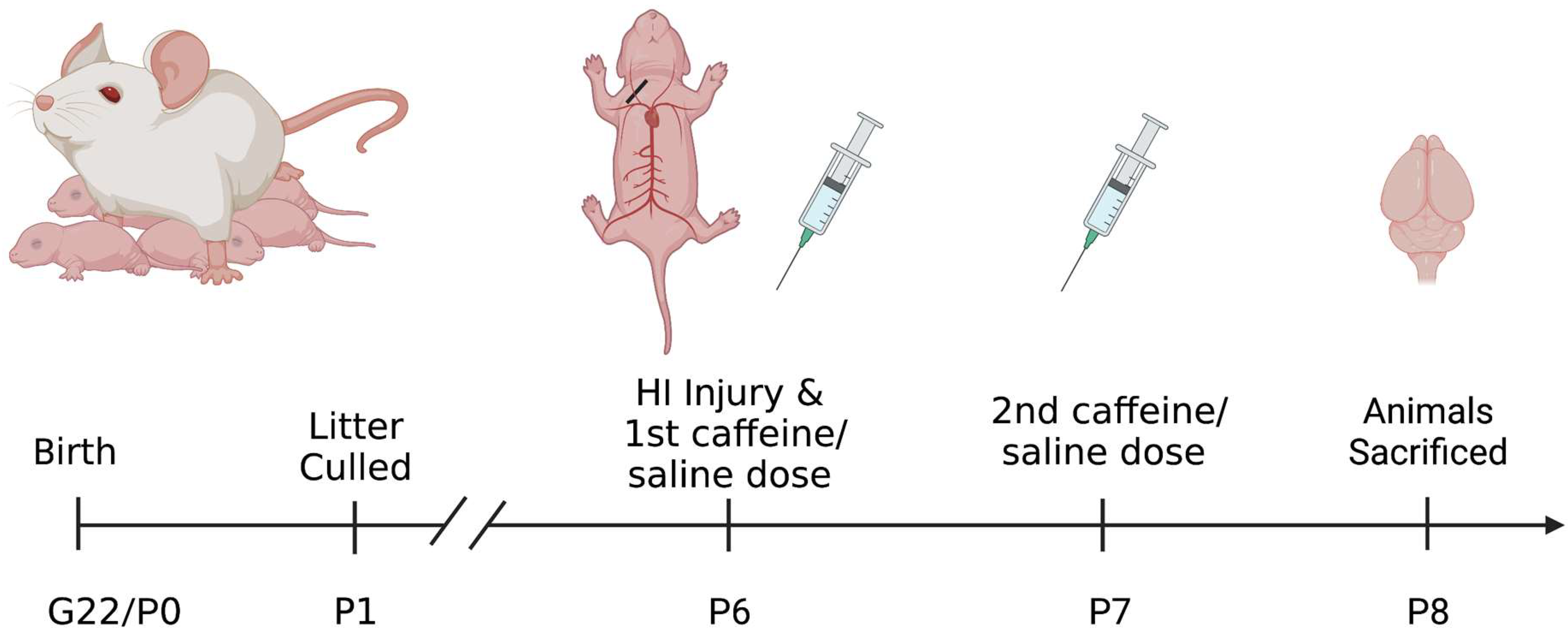
2.1. Subjects
2.2. HI Induction
2.3. Caffeine Treatment
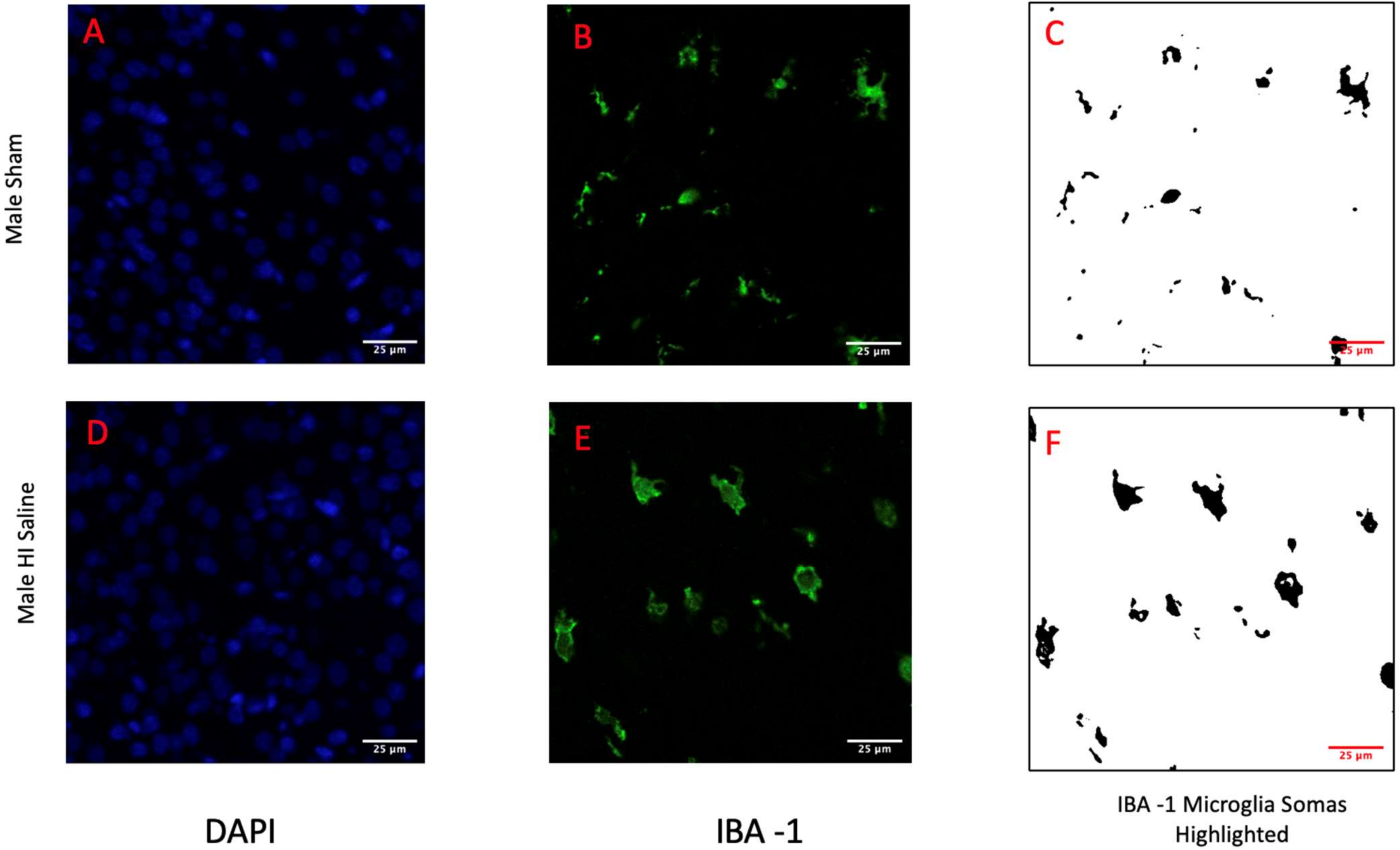
2.4. IBA-1 and DAPI Staining
2.5. Analysis
3. Results
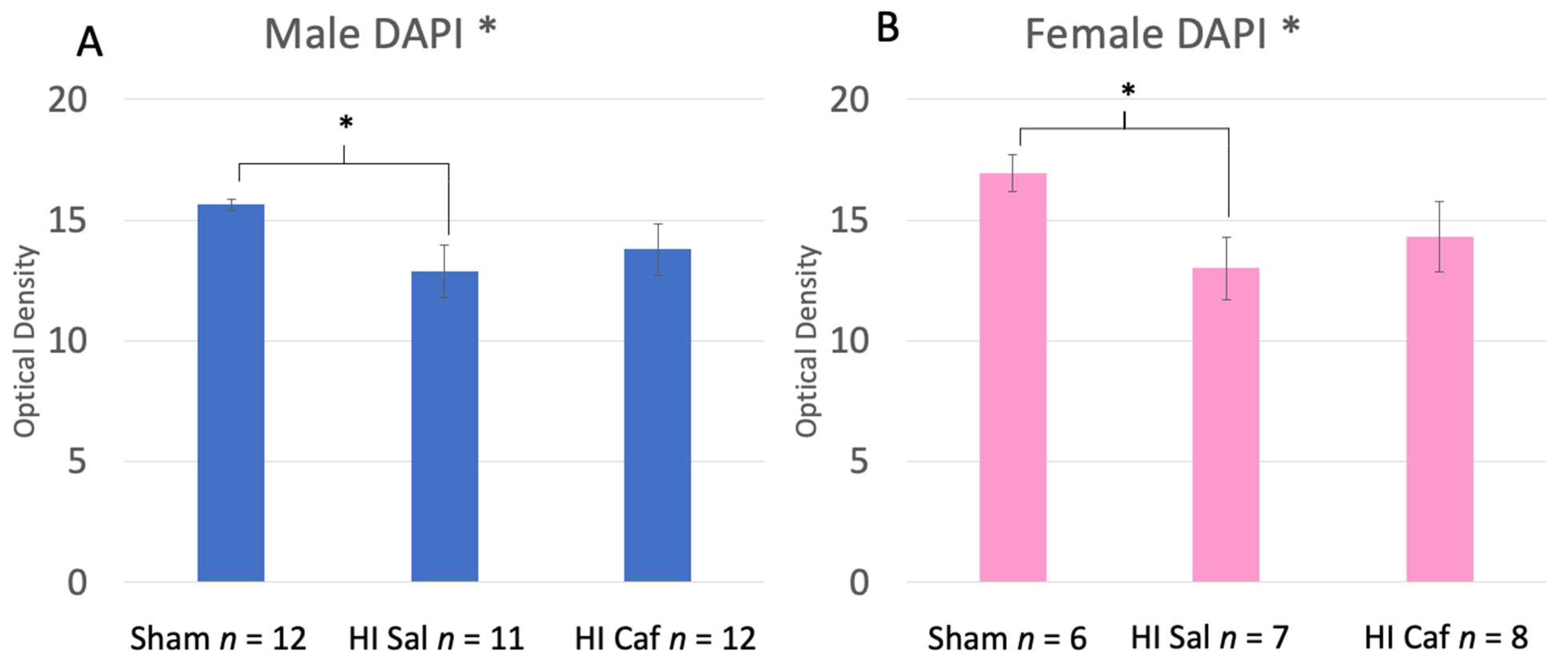
3.1. DAPI
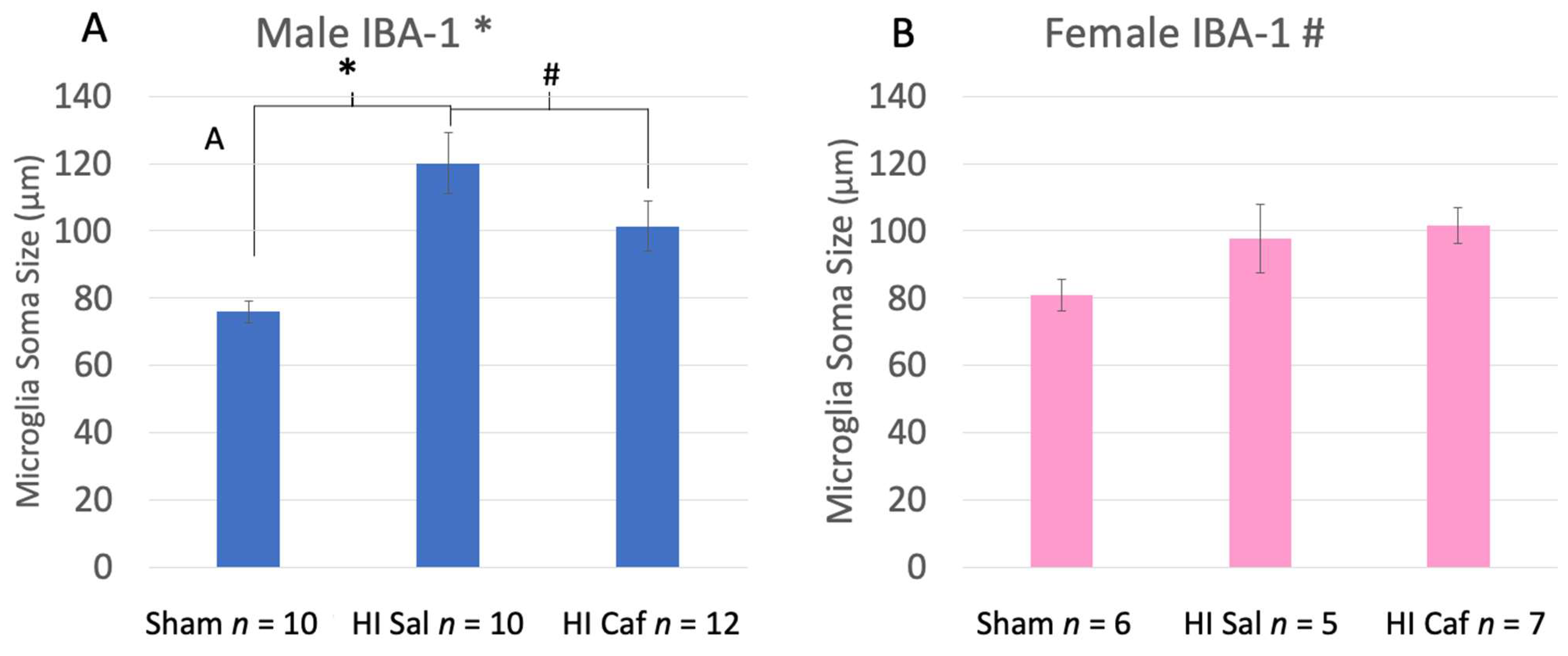
3.2. IBA-1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laptook, A.R. Birth Asphyxia and Hypoxic-Ischemic Brain Injury in the Preterm Infant. Clin. Perinatol. 2016, 43, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Novak, C.M.; Ozen, M.; Burd, I. Perinatal Brain Injury: Mechanisms, Prevention, and Outcomes. Clin Perinatol. 2018, 45, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Perinatal hypoxic-ischemic brain injury. Pediatr. Clin. N. Am. 1976, 23, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Aylward, G.P. Neurodevelopmental outcomes of infants born prematurely. J. Dev. Behav. Pediatr. JDBP 2014, 35, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, S. Therapeutic hypothermia for neonatal encephalopathy. Curr. Opin. Pediatr. 2015, 27, 152–157. [Google Scholar] [CrossRef]
- Potter, M.; Rosenkrantz, T.; Fitch, R.H. Behavioral and neuroanatomical outcomes in a rat model of preterm hypoxic-ischemic brain Injury: Effects of caffeine and hypothermia. Int. J. Dev. Neurosci. 2018, 70, 46–55. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Chen, J.F.; Cunha, R.A.; Svenningsson, P.; Vaugeois, J.M. Adenosine and brain function. Int. Rev. Neurobiol. 2005, 63, 191–270. [Google Scholar]
- Liu, S.; Zhang, X.; Liu, Y.; Yuan, X.; Yang, L.; Zhang, R.; Zhang, X.; Wang, X.; Xu, F.; Zhu, C. Early application of caffeine improves white matter development in very preterm infants. Respir. Physiol. Neurobiol. 2020, 281, 103495. [Google Scholar] [CrossRef]
- Maitre, N.L.; Chan, J.; Stark, A.R.; Lambert, W.E.; Aschner, J.L.; Key, A.P. Effects of caffeine treatment for apnea of prematurity on cortical speech-sound differentiation in preterm infants. J. Child Neurol. 2015, 30, 307–313. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Tin, W. Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2007, 357, 1893–1902. [Google Scholar] [CrossRef]
- Siahanidou, T.; Spiliopoulou, C. Pharmacological Neuroprotection of the Preterm Brain: Current Evidence and Perspectives. Am. J. Perinatol. 2022, 39, 479–491. [Google Scholar] [CrossRef]
- Yang, L.; Yu, X.; Zhang, Y.; Liu, N.; Xue, X.; Fu, J. Caffeine treatment started before injury reduces hypoxic–ischemic white-matter damage in neonatal rats by regulating phenotypic microglia polarization. Pediatr Res. 2022, 92, 1543–1554. [Google Scholar] [CrossRef]
- Barcelos, R.P.; Souza, M.A.; Amaral, G.P.; Stefanello, S.T.; Bresciani, G.; Fighera, M.R.; Soares, F.A.; de Vargas Barbosa, N. Caffeine intake may modulate inflammation markers in trained rats. Nutrients 2014, 6, 1678–1690. [Google Scholar] [CrossRef]
- Winerdal, M.; Urmaliya, V.; Winerdal, M.E.; Fredholm, B.B.; Winqvist, O.; Aden, U. Single Dose Caffeine Protects the Neonatal Mouse Brain against Hypoxia Ischemia. PLoS ONE 2017, 12, e0170545. [Google Scholar] [CrossRef]
- Di Martino, E.; Bocchetta, E.; Tsuji, S.; Mukai, T.; Harris, R.A.; Blomgren, K.; Aden, U. Defining a Time Window for Neuroprotection and Glia Modulation by Caffeine After Neonatal Hypoxia-Ischaemia. Mol. Neurobiol. 2020, 57, 2194–2205. [Google Scholar] [CrossRef]
- Alexander, M.; Smith, A.L.; Rosenkrantz, T.S.; Fitch, R.H. Therapeutic effect of caffeine treatment immediately following neonatal hypoxic-ischemic injury on spatial memory in male rats. Brain Sci. 2013, 3, 177–190. [Google Scholar] [CrossRef]
- Endesfelder, S.; Weichelt, U.; Schiller, C.; Winter, K.; von Haefen, C.; Bührer, C. Caffeine protects against anticonvulsant-induced impaired neurogenesis in the developing rat brain. Neurotox. Res. 2018, 34, 173–187. [Google Scholar] [CrossRef]
- Endesfelder, S.; Zaak, I.; Weichelt, U.; Bührer, C.; Schmitz, T. Caffeine protects neuronal cells against injury caused by hyperoxia in the immature brain. Free Radic. Biol. Med. 2014, 67, 221–234. [Google Scholar] [CrossRef]
- Endesfelder, S.; Weichelt, U.; Schiller, C.; Sifringer, M.; Bendix, I.; Bührer, C. Caffeine Protects Against Anticonvulsant-Induced Neurotoxicity in the Developing Rat Brain. Neurotox. Res. 2017, 32, 460–472. [Google Scholar] [CrossRef]
- Endesfelder, S.; Weichelt, U.; Strauß, E.; Schlör, A.; Sifringer, M.; Scheuer, T.; Bührer, C.; Schmitz, T. Neuroprotection by Caffeine in Hyperoxia-Induced Neonatal Brain Injury. Int. J. Mol. Sci. 2017, 18, 187. [Google Scholar] [CrossRef]
- Bruschettini, M.; Moreira, A.; Pizarro, A.B.; Mustafa, S.; Romantsik, O. The effects of caffeine following hypoxic-ischemic encephalopathy: A systematic review of animal studies. Brain Res. 2022, 1790, 147990. [Google Scholar] [CrossRef] [PubMed]
- Sperlagh, B.; Vizi, E.S. The role of extracellular adenosine in chemical neurotransmission in the hippocampus and basal ganglia: Pharmacological and cinical aspects. Curr. Top. Med. Chem. 2011, 11, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Pedata, F.; Corsi, C.; Melani, A.; Bordoni, F.; Latini, S. Adenosine extracellular brain concentrations and role of A2A receptors in ischemia. Ann. N. Y. Acad. Sci. 2006, 939, 74–84. [Google Scholar] [CrossRef]
- Brothers, H.M.; Marchalant, Y.; Wenk, G.L. Caffeine attenuates lipopolysaccharide-induced neuroinflammation. Neurosci. Lett. 2010, 480, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Gessi, S.; Merighi, S.; Sacchetto, V.; Simioni, C.; Borea, P.A. Adenosine receptors and cancer. Biochim. Biophys. Acta 2011, 1808, 1400–1412. [Google Scholar] [CrossRef]
- Eudy, B.J.; da Silva, R.P. Systematic deletion of adenosine receptors reveals novel roles in inflammation and pyroptosis in THP-1 macrophages. Mol. Immunol. 2021, 132, 1–7. [Google Scholar] [CrossRef]
- Pereira-Figueiredo, D.; Nascimento, A.A.; Cunha-Rodrigues, M.C.; Brito, R.; Calaza, K.C. Caffeine and its neuroprotective role in ischemic events: A mechanism dependent on adenosine receptors. Cell. Mol. Neurobiol. 2021, 42, 1693–1725. [Google Scholar] [CrossRef]
- Pintor, A.; Galluzzo, M.; Grieco, R.; Pezzola, A.; Reggio, R.; Popoli, P. Adenosine A2A receptor antagonists prevent the increase in striatal glutamate levels induced by glutamate uptake inhibitors. J. Neurochem. 2004, 89, 152–156. [Google Scholar] [CrossRef]
- Colella, M.; Zinni, M.; Pansiot, J.; Cassanello, M.; Mairesse, J.; Ramenghi, L.; Baud, O. Modulation of Microglial Activation by Adenosine A2a Receptor in Animal Models of Perinatal Brain Injury. Front. Neurol. 2018, 9, 605. [Google Scholar] [CrossRef]
- Madeira, M.H.; Boia, R.; Ambrosio, A.F.; Santiago, A.R. Having a Coffee Break: The Impact of Caffeine Consumption on Microglia-Mediated Inflammation in Neurodegenerative Diseases. Mediat. Inflamm. 2017, 2017, 4761081. [Google Scholar] [CrossRef]
- Lenz, K.M.; McCarthy, M.M. A starring role for microglia in brain sex differences. Neuroscientist 2014, 21, 306–321. [Google Scholar] [CrossRef]
- Clancy, R.M.; Zheng, P.; O’Mahony, M.; Izmirly, P.; Zavadil, J.; Gardner, L.; Buyon, J.P. Role of hypoxia and cAMP in the transdifferentiation of human fetal cardiac fibroblasts: Implications for progression to scarring in autoimmune-associated congenital heart block. Arthritis Rheum. 2007, 56, 4120–4131. [Google Scholar] [CrossRef]
- Fan, R.; Portuguez, M.; Nunes, M. Cognition, behavior and social competence of preterm low birth weight children at school age. Clinics 2013, 68, 915–921. [Google Scholar] [CrossRef]
- Garfinkle, J.; Yoon, E.W.; Alvaro, R.; Nwaesei, C.; Claveau, M.; Lee, S.K.; Shah, P.S. Canadian Neonatal Network Investigators Trends in sex-specific differences in outcomes in extreme preterms: Progress or natural barriers? Arch. Dis. Child.-Fetal Neonatal Ed. 2020, 105, 158–163. [Google Scholar] [CrossRef]
- Kozhemiako, N.; Nunes, A.S.; Vakorin, V.A.; Chau, C.; Moiseev, A.; Ribary, U.; Grunau, R.E.; Doesburg, S.M. Sex differences in brain connectivity and male vulnerability in very preterm children. Hum. Brain Mapp. 2020, 41, 388–400. [Google Scholar] [CrossRef]
- Sanchez, K.; Spittle, A.J.; Cheong, J.L.; Thompson, D.K.; Doyle, L.W.; Anderson, P.J.; Morgan, A.T. Language in 2-year-old children born preterm and term: A cohort study. Arch. Dis. Child. 2019, 104, 647–652. [Google Scholar] [CrossRef]
- Smith, A.L.; Alexander, M.; Rosenkrantz, T.S.; Sadek, M.L.; Fitch, R.H. Sex differences in behavioral outcome following neonatal hypoxia ischemia: Insights from a clinical meta-analysis and a rodent model of induced hypoxic ischemic brain injury. Exp. Neurol. 2014, 254, 54–67. [Google Scholar] [CrossRef]
- Rosenkrantz, T.S.; Hussain, Z.; Fitch, R.H. Sex Differences in Brain Injury and Repair in Newborn Infants: Clinical Evidence and Biological Mechanisms. Front. Pediatr. 2019, 7, 211. [Google Scholar] [CrossRef]
- Smith, A.L.; Garbus, H.; Rosenkrantz, T.S.; Fitch, R.H. Sex differences in behavioral outcomes following temperature modulation during induced neonatal hypoxic ischemic injury in rats. Brain Sci. 2015, 5, 220–240. [Google Scholar] [CrossRef]
- Wood, T.R.; Gundersen, J.K.; Falck, M.; Maes, E.; Osredkar, D.; Løberg, E.M.; Thoresen, M. Variability and sex-dependence of hypothermic neuroprotection in a rat model of neonatal hypoxic–ischemic brain injury: A single laboratory meta-analysis. Sci. Rep. 2020, 10, 10833. [Google Scholar] [CrossRef]
- Vannucci, R.C.; Lyons, D.T.; Vasta, F. Regional cerebral blood flow during hypoxia-ischemia in immature rats. Stroke 1988, 19, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Hovens, I.; Nyakas, C.; Schoemaker, R. A novel method for evaluating microglial activation using ionized calcium-binding adaptor protein-1 staining: Cell body to cell size ratio. Neuroimmunol. Neuroinflam. 2014, 1, 82. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Renuka, R.; Thyagarajan, S. Atlas of the Neonatal Rat Brain; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Hoogland, I.C.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflam. 2015, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.A. Exploring Sex-Related Differences in Microglia May Be a Game-Changer in Precision Medicine. Front. Aging Neurosci. 2022, 14, 868448. [Google Scholar] [CrossRef]
- Yanguas-Casás, N. Physiological sex differences in microglia and their relevance in neurological disorders. Neuroimmunol. Neuroinflam. 2020, 7, 13–22. [Google Scholar] [CrossRef]
- Bordeleau, M.; Carrier, M.; Luheshi, G.N.; Tremblay, M.È. Microglia along sex lines: From brain colonization, maturation and function, to implication in neurodevelopmental disorders. Semin. Cell Dev. Biol. 2019, 94, 152–163. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Hutchinson, M.R.; Bilbo, S.D. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 17835–17847. [Google Scholar] [CrossRef]
- Simoes-Henriques, C.; Mateus-Pinheiro, M.; Gaspar, R.; Pinheiro, H.; Mendes Duarte, J.; Baptista, F.I.; Canas, P.M.; Fontes-Ribeiro, C.A.; Cunha, R.A.; Ambrosio, A.F.; et al. Microglia cytoarchitecture in the brain of adenosine A2A receptor knockout mice: Brain region and sex specificities. Eur. J. Neurosci. 2020, 51, 1377–1387. [Google Scholar] [CrossRef]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783. [Google Scholar] [CrossRef]
- Hill, C.A.; Fitch, R.H. Sex differences in mechanisms and outcome of neonatal hypoxia-ischemia in rodent models: Implications for sex-specific neuroprotection in clinical neonatal practice. Neurol. Res. Int. 2012, 2012, 867531. [Google Scholar] [CrossRef]
- Lang, J.T.; McCullough, L.D. Pathways to ischemic neuronal cell death: Are sex differences relevant? J. Transl. Med. 2008, 6, 33. [Google Scholar] [CrossRef]
- Liu, F.; Li, Z.; Li, J.; Siegel, C.; Yuan, R.; Mccullough, L.D. Sex differences in caspase activation after stroke. Stroke 2009, 40, 1842–1848. [Google Scholar] [CrossRef]
- Hasko, G.; Csoka, B.; Nemeth, Z.H.; Vizi, E.S.; Pacher, P. A(2B) adenosine receptors in immunity and inflammation. Trends Immunol. 2009, 30, 263–270. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McLeod, R.M.; Rosenkrantz, T.S.; Fitch, R.H.; Koski, R.R. Sex Differences in Microglia Activation in a Rodent Model of Preterm Hypoxic Ischemic Injury with Caffeine Treatment. Biomedicines 2023, 11, 185. https://doi.org/10.3390/biomedicines11010185
McLeod RM, Rosenkrantz TS, Fitch RH, Koski RR. Sex Differences in Microglia Activation in a Rodent Model of Preterm Hypoxic Ischemic Injury with Caffeine Treatment. Biomedicines. 2023; 11(1):185. https://doi.org/10.3390/biomedicines11010185
Chicago/Turabian StyleMcLeod, Ruth Mae, Ted S. Rosenkrantz, Roslyn Holly Fitch, and Rachel R. Koski. 2023. "Sex Differences in Microglia Activation in a Rodent Model of Preterm Hypoxic Ischemic Injury with Caffeine Treatment" Biomedicines 11, no. 1: 185. https://doi.org/10.3390/biomedicines11010185
APA StyleMcLeod, R. M., Rosenkrantz, T. S., Fitch, R. H., & Koski, R. R. (2023). Sex Differences in Microglia Activation in a Rodent Model of Preterm Hypoxic Ischemic Injury with Caffeine Treatment. Biomedicines, 11(1), 185. https://doi.org/10.3390/biomedicines11010185




