A Near-Infrared Mechanically Switchable Elastomeric Film as a Dynamic Cell Culture Substrate
Abstract
:1. Introduction
2. Materials and Methods
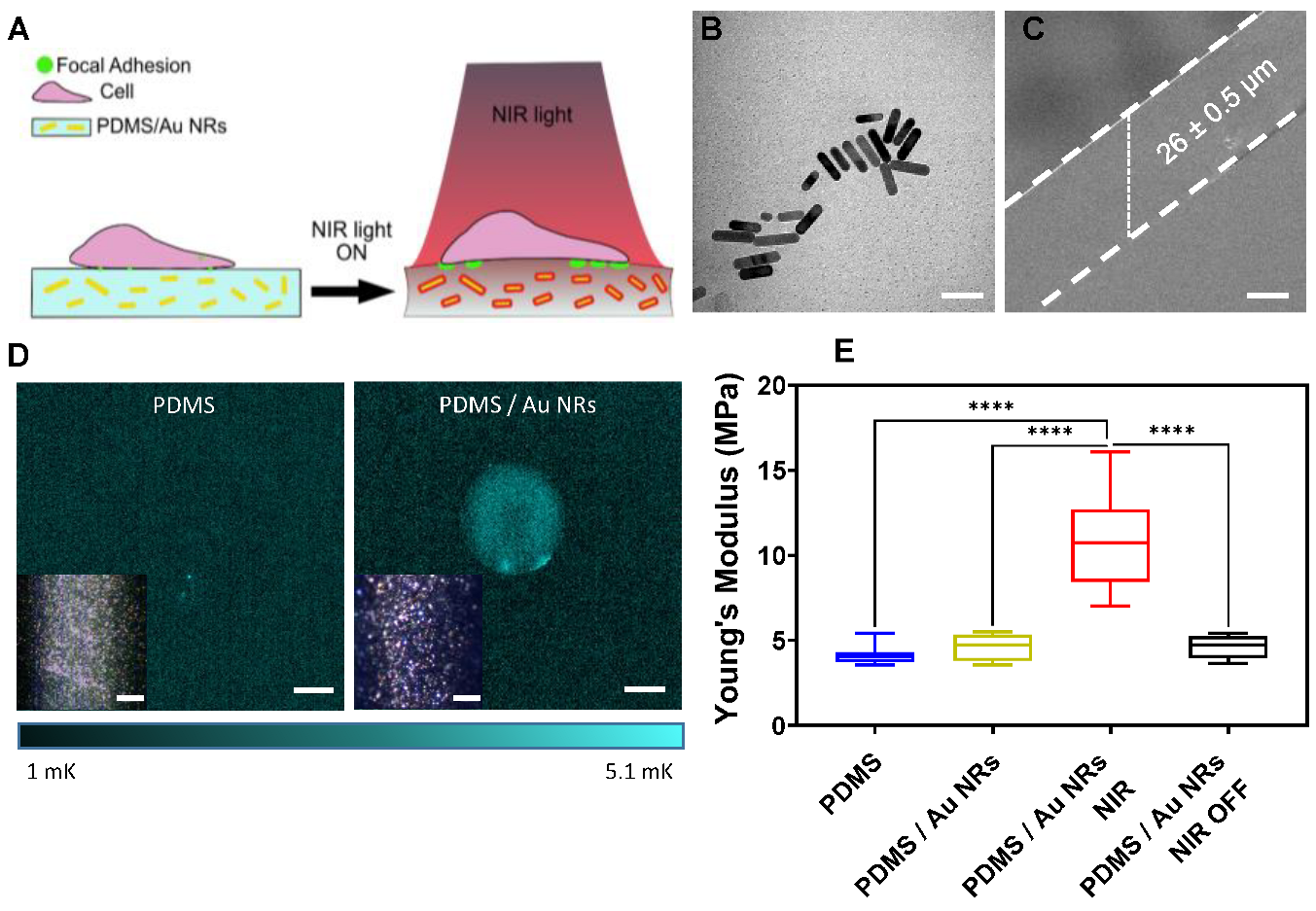
2.1. Preparation and Characterisation of Gold Nanorods (Au NRs)
2.2. Preparation of PDMS and PDMS/Au NRs Films
2.3. Fluorescence-Enhanced Dark Field Microscopy
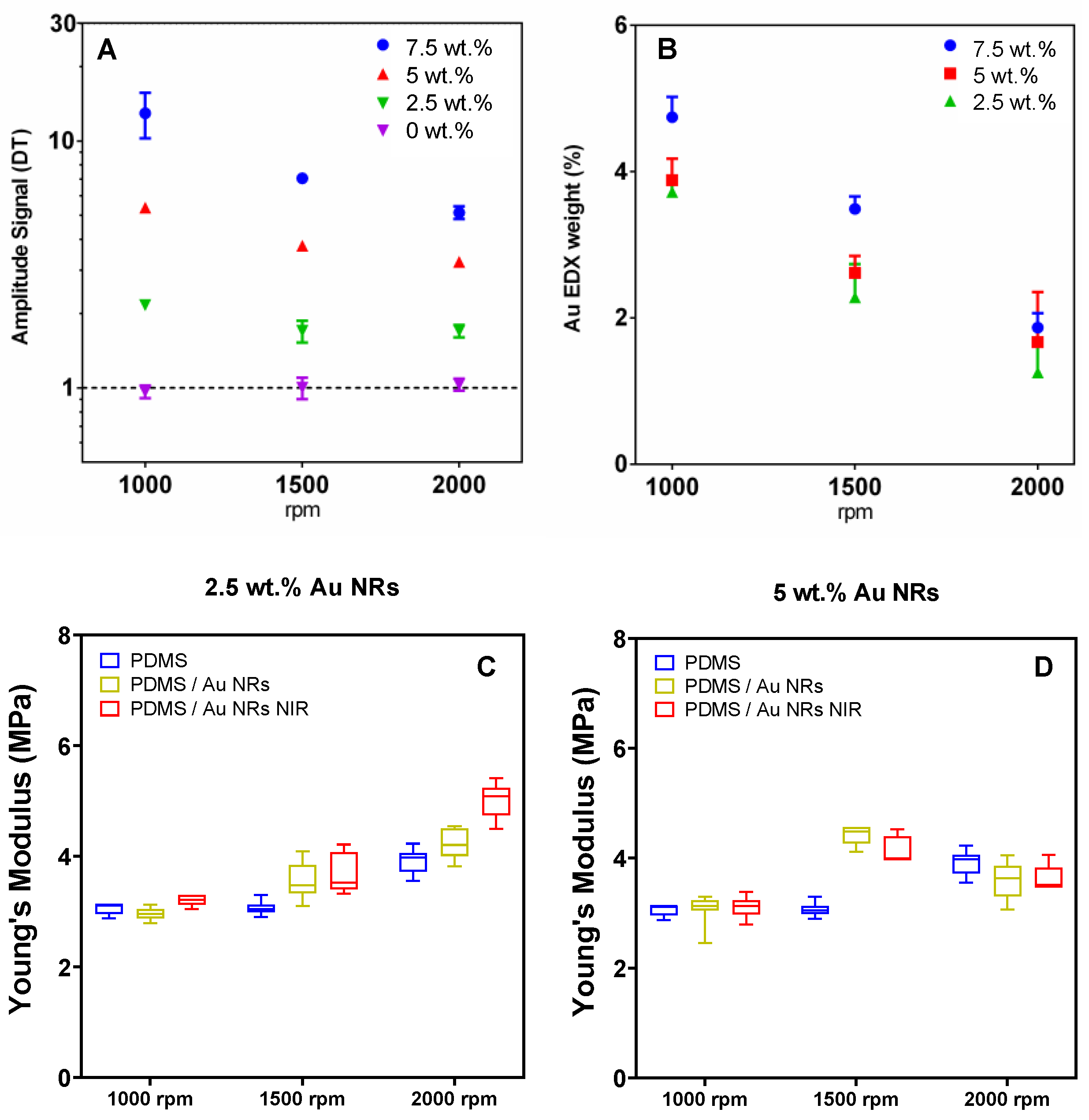
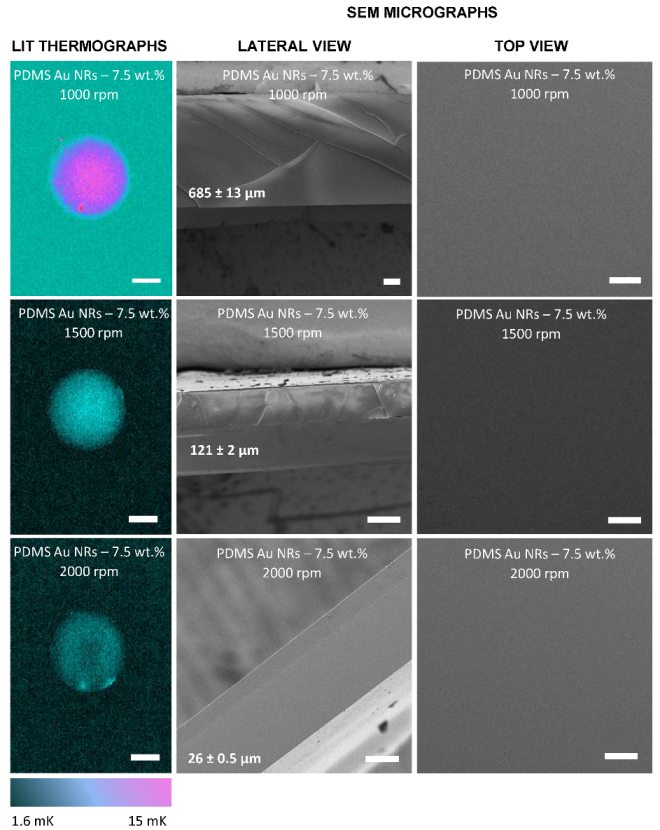
2.4. Lock-In Thermography Analysis (LIT)
2.5. Scanning Electron Microscopy (SEM/EDX) Characterisation
2.6. Film Surface Functionalisation by Collagen Type I and Characterisation
2.7. Mechanical Measurements
2.8. Fibroblasts (NIH/3T3) Culture on the Functionalised Elastomeric Films
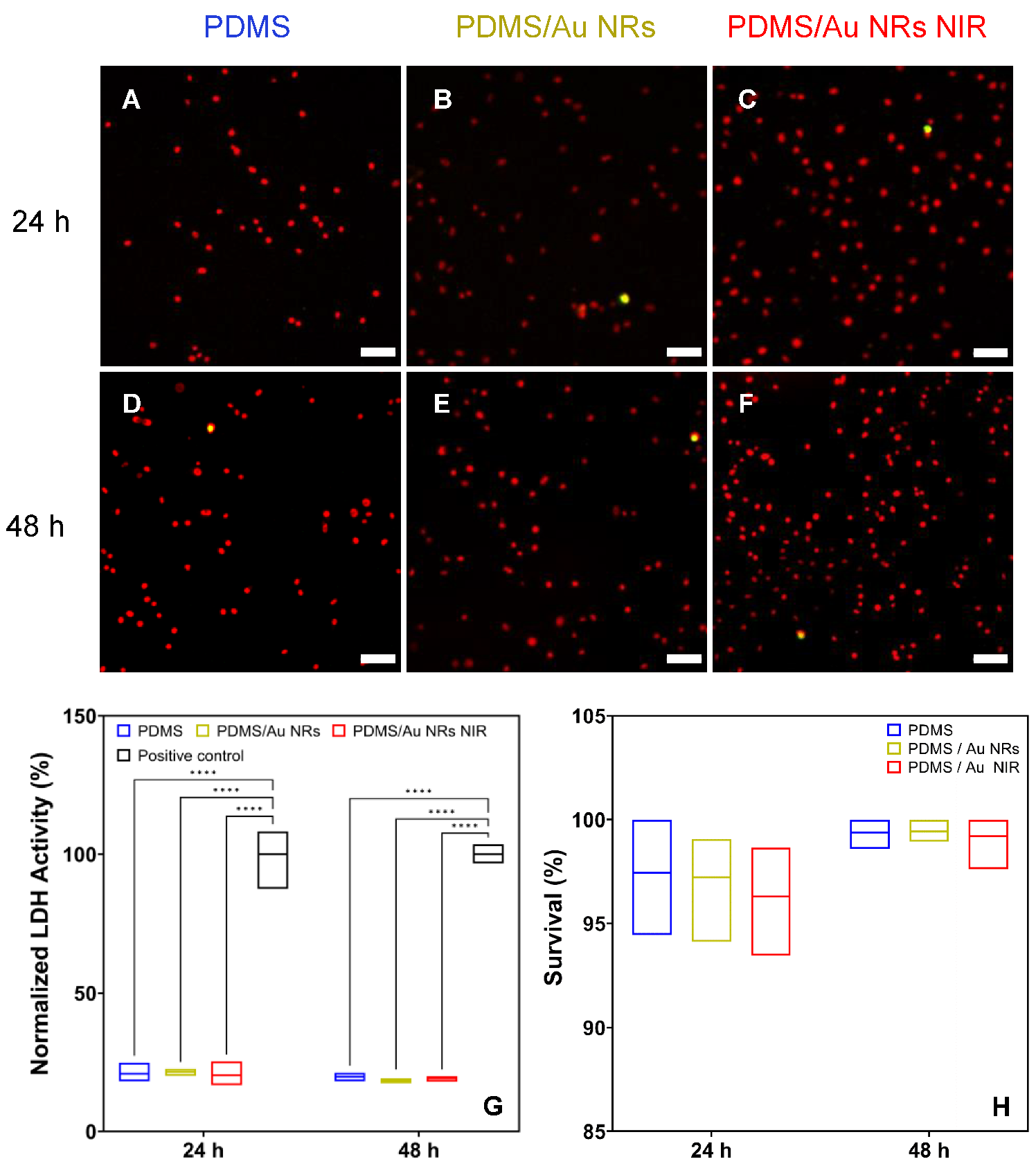
2.9. Analysis of Cell Viability by the Lactate Dehydrogenase (LDH) Assay
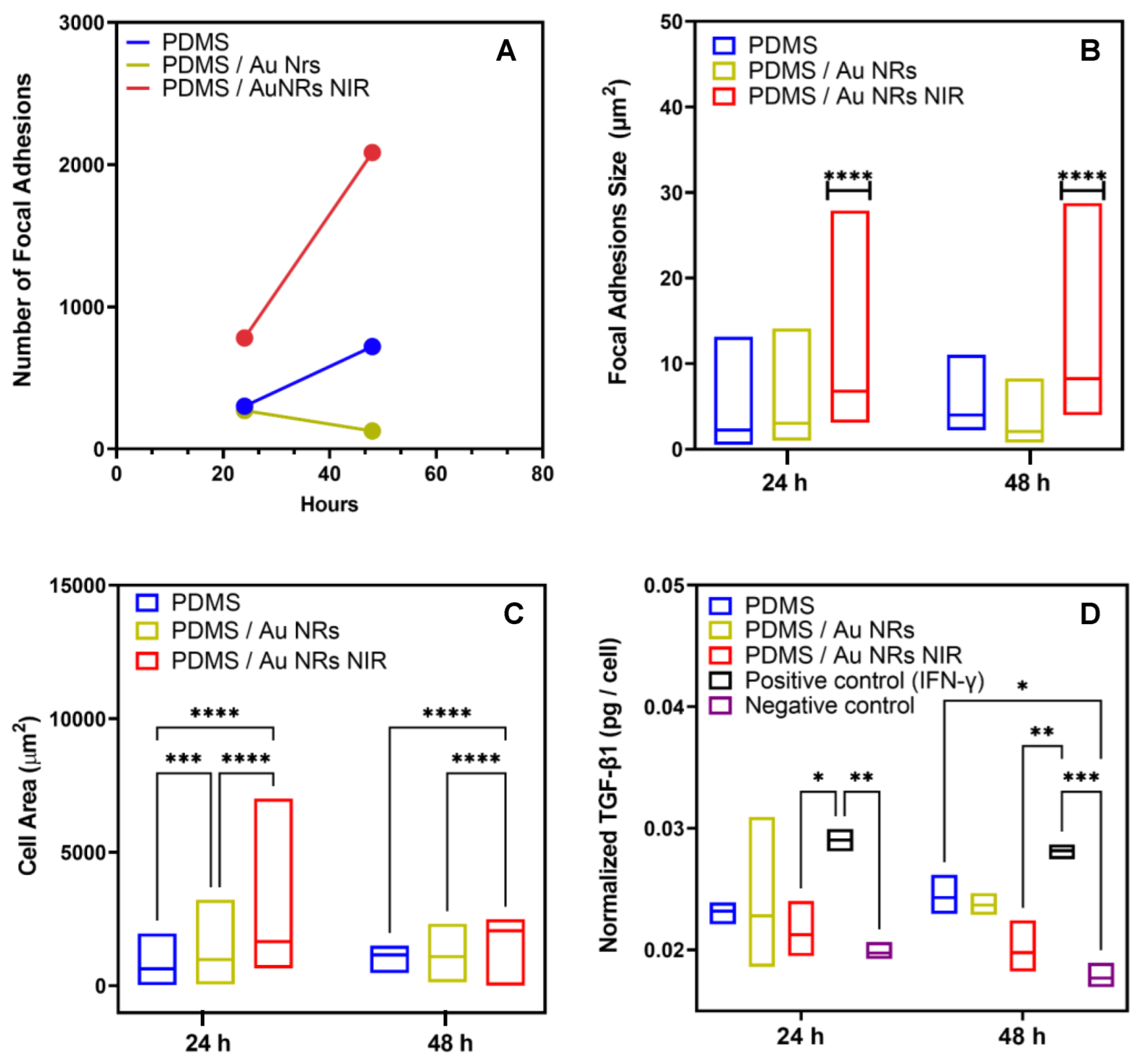
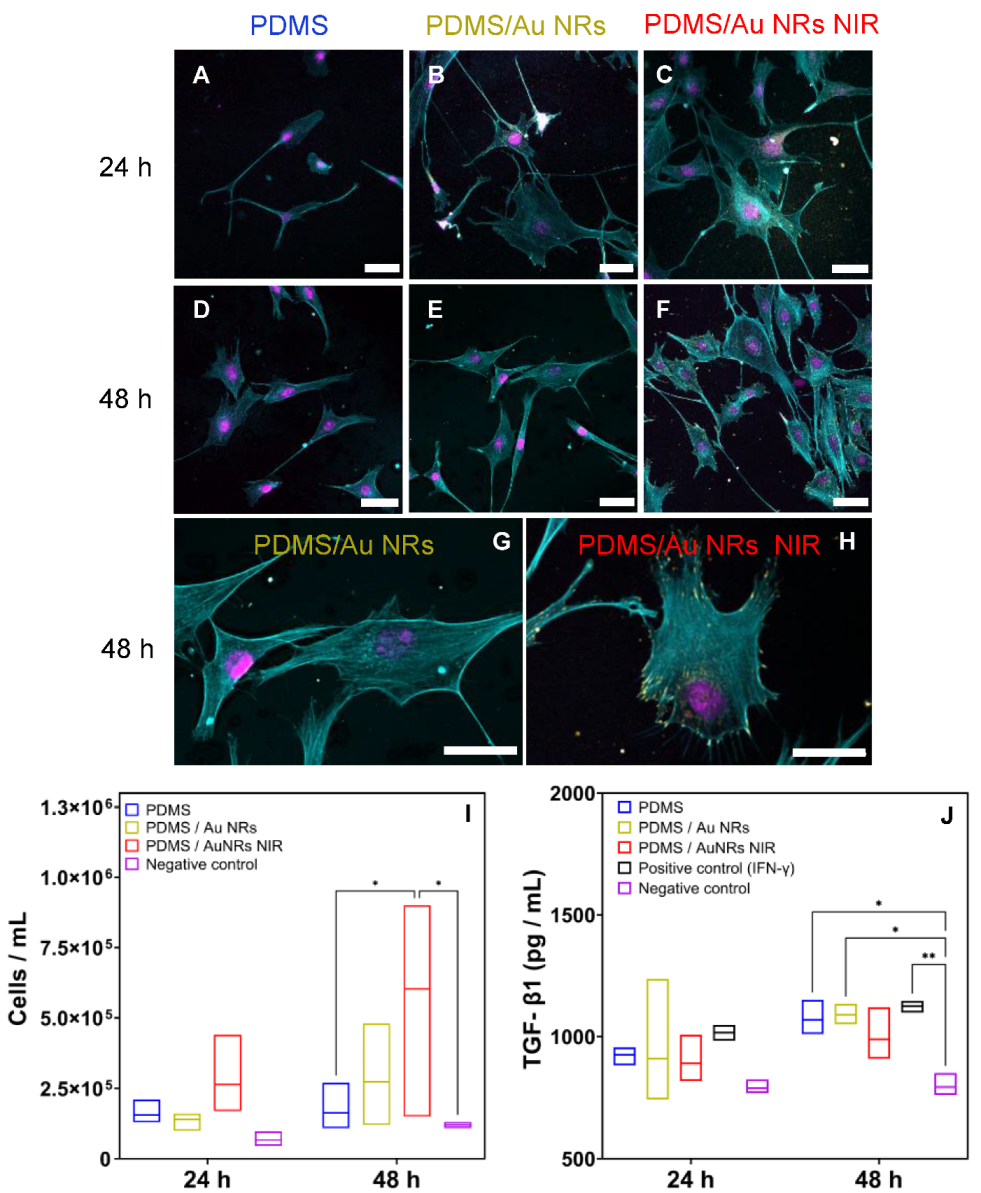
2.10. Growth Factor Assay
2.11. Confocal Laser Scanning Microscopy (cLSM), Immunohistochemistry, and Cell Viability
2.12. Image Analysis
2.13. Statistical Analysis
3. Results and Discussion
3.1. NIR Light Responsiveness of Nanocomposite Films
3.2. Stiffening Effect on Cellular Behaviour
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Lu, P.; Takai, K.; Weaver, V.M.; Zena, W. Extracellular Matrix Degradation and Remodeling in Development and Disease. Old Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S. Fibroblasts in Three Dimensional Matrices: Cell Migration and Matrix Remodeling. Exp. Mol. Med. 2009, 41, 858–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.-J. Tissue Stiffness Dictates Development, Homeostasis, and Disease Progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orr, A.W.; Helmke, B.P.; Blackman, B.R.; Schwartz, M.A. Mechanisms of Mechanotransduction. Dev. Cell 2006, 10, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, U.S.; Erdmann, T.; Bischofs, I.B. Focal Adhesions as Mechanosensors: The Two-Spring Model. Biosystems 2006, 83, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of Substrate Stiffness on Cell Morphology, Cytoskeletal Structure, and Adhesion. Cell Motil. 2005, 60, 24–34. [Google Scholar] [CrossRef]
- d’Angelo, M.; Benedetti, E.; Tupone, M.G.; Catanesi, M.; Castelli, V.; Antonosante, A.; Cimini, A. The Role of Stiffness in Cell Reprogramming: A Potential Role for Biomaterials in Inducing Tissue Regeneration. Cells 2019, 8, 1036. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Hayward, R.C. Mimicking Dynamic in Vivo Environments with Stimuli-Responsive Materials for Cell Culture. Trends Biotechnol. 2012, 30, 426–439. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakahata, M.; Linke, P.; Kaufmann, S. Stimuli-Responsive Hydrogels as a Model of the Dynamic Cellular Microenvironment. Polym. J. 2020, 52, 861–870. [Google Scholar] [CrossRef]
- Wang, Y.; Shim, M.S.; Levinson, N.S.; Sung, H.-W.; Xia, Y. Stimuli-Responsive Materials for Controlled Release of Theranostic Agents. Adv. Funct. Mater. 2014, 24, 4206–4220. [Google Scholar] [CrossRef]
- Nandivada, H.; Ross, A.M.; Lahann, J. Stimuli-Responsive Monolayers for Biotechnology. Prog. Polym. Sci. 2010, 35, 141–154. [Google Scholar] [CrossRef]
- Turner, J.G.; Og, J.H.; Murphy, C.J. Gold Nanorod Impact on Mechanical Properties of Stretchable Hydrogels. Soft Matter 2020, 16, 6582–6590. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yao, S.; Qiu, F.; Mao, Z.; Wang, B. A Multifunctional Hydrogel Containing Gold Nanorods and Methylene Blue for Synergistic Cancer Phototherapy. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126154. [Google Scholar] [CrossRef]
- Stowers, R.S.; Allen, S.C.; Suggs, L.J. Dynamic Phototuning of 3D Hydrogel Stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 1953–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, J.; Chen, A.; Zhang, H.; Burt, H.; Chiao, M. Design and Near-Infrared Actuation of a Gold Nanorod–Polymer Microelectromechanical Device for On-Demand Drug Delivery. Micromachines 2018, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold Nanorods: Synthesis, Characterization and Applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Chang, H.-H.; Murphy, C.J. Mini Gold Nanorods with Tunable Plasmonic Peaks beyond 1000 Nm. Chem. Mater. 2018, 30, 1427–1435. [Google Scholar] [CrossRef]
- Murphy, C.J.; Chang, H.-H.; Falagan-Lotsch, P.; Gole, M.T.; Hofmann, D.M.; Hoang, K.N.L.; McClain, S.M.; Meyer, S.M.; Turner, J.G.; Unnikrishnan, M.; et al. Virus-Sized Gold Nanorods: Plasmonic Particles for Biology. Acc. Chem. Res. 2019, 52, 2124–2135. [Google Scholar] [CrossRef]
- Meyer, S.M.; Pettine, J.; Nesbitt, D.J.; Murphy, C.J. Size Effects in Gold Nanorod Light-to-Heat Conversion under Femtosecond Illumination. J. Phys. Chem. C 2021, 125, 16268–16278. [Google Scholar] [CrossRef]
- Chandorkar, Y.; Castro Nava, A.; Schweizerhof, S.; Van Dongen, M.; Haraszti, T.; Köhler, J.; Zhang, H.; Windoffer, R.; Mourran, A.; Möller, M.; et al. Cellular Responses to Beating Hydrogels to Investigate Mechanotransduction. Nat. Commun. 2019, 10, 4027. [Google Scholar] [CrossRef]
- Sutton, A.; Shirman, T.; Timonen, J.V.I.; England, G.T.; Kim, P.; Kolle, M.; Ferrante, T.; Zarzar, L.D.; Strong, E.; Aizenberg, J. Photothermally Triggered Actuation of Hybrid Materials as a New Platform for in Vitro Cell Manipulation. Nat. Commun. 2017, 8, 14700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.N.; Jiang, X.; Ryan, D.; Whitesides, G.M. Compatibility of Mammalian Cells on Surfaces of Poly(Dimethylsiloxane). Langmuir 2004, 20, 11684–11691. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhou, Q.; Liu, C.; Zhang, Y.; Xie, M.; Qiao, W.; Dong, N. Substrate Stiffness Regulates Differentiation of Induced Pluripotent Stem Cells into Heart Valve Endothelial Cells. Acta Biomater. 2022, 143, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Schubert, B.E.; Floreano, D. Variable Stiffness Material Based on Rigid Low-Melting-Point-Alloy Microstructures Embedded in Soft Poly(Dimethylsiloxane) (PDMS). RSC Adv. 2013, 3, 24671–24679. [Google Scholar] [CrossRef] [Green Version]
- Grzybowski, B.A.; Brittain, S.T.; Whitesides, G.M. Thermally Actuated Interferometric Sensors Based on the Thermal Expansion of Transparent Elastomeric Media. Rev. Sci. Instrum. 1999, 70, 2031–2037. [Google Scholar] [CrossRef]
- Wang, W.; Xiang, C.; Zhu, Q.; Zhong, W.; Li, M.; Yan, K.; Wang, D. Multistimulus Responsive Actuator with GO and Carbon Nanotube/PDMS Bilayer Structure for Flexible and Smart Devices. ACS Appl. Mater. Interfaces 2018, 10, 27215–27223. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The Stiffness of Living Tissues and Its Implications for Tissue Engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Dreier, R. Hypertrophic Differentiation of Chondrocytes in Osteoarthritis: The Developmental Aspect of Degenerative Joint Disorders. Arthritis Res. Ther. 2010, 12, 216. [Google Scholar] [CrossRef] [Green Version]
- Chen, J. Recent Development of Biomaterials Combined with Mesenchymal Stem Cells as a Strategy in Cartilage Regeneration. Int. J. Transl. Med. 2022, 2, 456–481. [Google Scholar]
- Scarabelli, L.; Sánchez-Iglesias, A.; Pérez-Juste, J.; Liz-Marzán, L.M. A “Tips and Tricks” Practical Guide to the Synthesis of Gold Nanorods. J. Phys. Chem. Lett. 2015, 6, 4270–4279. [Google Scholar] [CrossRef] [Green Version]
- Monnier, C.A.; Crippa, F.; Geers, C.; Knapp, E.; Rothen-Rutishauser, B.; Bonmarin, M.; Lattuada, M.; Petri-Fink, A. Lock-In Thermography as an Analytical Tool for Magnetic Nanoparticles: Measuring Heating Power and Magnetic Fields. J. Phys. Chem. C 2017, 121, 27164–27175. [Google Scholar] [CrossRef]
- Hengsberger, S.; Kulik, A.; Zysset, P. Nanoindentation discriminates the elastic properties of individual human bone lamellae under dry and physiological conditions. Bone. 2002, 30, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Dogru, S.; Aksoy, B.; Bayraktar, H.; Alaca, B.E. Poisson’s Ratio of PDMS Thin Films. Polym. Test. 2018, 69, 375–384. [Google Scholar] [CrossRef]
- Steinmetz, L.; Geers, C.; Bonmarin, M.; Rothen-Rutishauser, B.; Petri-Fink, A.; Lattuada, M. Experimental and Theoretical Validation of Plasmonic Nanoparticle Heat Generation by Using Lock-In Thermography. J. Phys. Chem. C 2021, 125, 5890–5896. [Google Scholar] [CrossRef]
- Steinmetz, L.; Taladriz-Blanco, P.; Geers, C.; Spuch-Calvar, M.; Bonmarin, M.; Balog, S.; Rothen-Rutishauser, B.; Petri-Fink, A. Lock-In Thermography to Analyze Plasmonic Nanoparticle Dispersions. Part. Part. Syst. Charact. 2019, 36, 1900224. [Google Scholar] [CrossRef] [Green Version]
- Huber, N.; Heerens, J. On the Effect of a General Residual Stress State on Indentation and Hardness Testing. Acta Mater. 2008, 56, 6205–6213. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Yan, J.; Karlsson, A.M. On the Determination of Residual Stress and Mechanical Properties by Indentation. Mater. Sci. Eng. A 2006, 416, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Suresh, S.; Giannakopoulos, A.E. A New Method for Estimating Residual Stresses by Instrumented Sharp Indentation. Acta Mater. 1998, 46, 5755–5767. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R. Thermo-Plasmonics: Using Metallic Nanostructures as Nano-Sources of Heat. Laser Photon. Rev. 2013, 7, 171–187. [Google Scholar] [CrossRef]
- Loomis, J.; King, B.; Burkhead, T.; Xu, P.; Bessler, N.; Terentjev, E.; Panchapakesan, B. Graphene-Nanoplatelet-Based Photomechanical Actuators. Nanotechnology 2012, 23, 45501. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Arévalo, F.M.; Garnica-Palafox, I.M.; Jagdale, P.; Hernández-Cordero, J.; Rodil, S.E.; Okonkwo, A.O.; Robles Hernandez, F.C.; Tagliaferro, A. Photomechanical Response of Composites Based on PDMS and Carbon Soot Nanoparticles under IR Laser Irradiation. Opt. Mater. Express 2015, 5, 1792–1805. [Google Scholar] [CrossRef]
- Qian, Z.; Ross, D.; Jia, W.; Xing, Q.; Zhao, F. Bioactive Polydimethylsiloxane Surface for Optimal Human Mesenchymal Stem Cell Sheet Culture. Bioact. Mater. 2018, 3, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.; Meckel, T.; Stark, R.W.; Narayan, S. Improved Cell Adhesion under Shear Stress in PDMS Microfluidic Devices. Colloids Surf. B Biointerfaces 2017, 150, 456–464. [Google Scholar] [CrossRef]
- Mofrad, M.R.K.; Golji, J.; Abdul Rahim, N.A.; Kamm, R.D. Force-Induced Unfolding of the Focal Adhesion Targeting Domain and the Influence of Paxillin Binding. Mech. Chem. Biosyst. 2004, 1, 253–265. [Google Scholar] [PubMed]
- Silver, F.H.; Freeman, J.W.; Seehra, G.P. Collagen Self-Assembly and the Development of Tendon Mechanical Properties. J. Biomech. 2003, 36, 1529–1553. [Google Scholar] [CrossRef]
- Bosman, F.T.; Stamenkovic, I. Functional Structure and Composition of the Extracellular Matrix. J. Pathol. 2003, 200, 423–428. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Modzelewska, K.; Kwong, L.; Keely, P.J. Focal Adhesion Regulation of Cell Behavior. Biochim. Biophys. Acta-Mol. Cell Res. 2004, 1692, 103–119. [Google Scholar] [CrossRef]
- Bai, M.; Xie, J.; Liu, X.; Chen, X.; Liu, W.; Wu, F.; Chen, D.; Sun, Y.; Li, X.; Wang, C.; et al. Microenvironmental Stiffness Regulates Dental Papilla Cell Differentiation: Implications for the Importance of Fibronectin–Paxillin−β-Catenin Axis. ACS Appl. Mater. Interfaces 2018, 10, 26917–26927. [Google Scholar] [CrossRef]
- Na, S.; Trache, A.; Trzeciakowski, J.; Sun, Z.; Meininger, G.A.; Humphrey, J.D. Time-Dependent Changes in Smooth Muscle Cell Stiffness and Focal Adhesion Area in Response to Cyclic Equibiaxial Stretch. Ann. Biomed. Eng. 2008, 36, 369–380. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Schwarz, U.S.; Riveline, D.; Goichberg, P.; Tzur, G.; Sabanay, I.; Mahalu, D.; Safran, S.; Bershadsky, A.; Addadi, L.; et al. Force and Focal Adhesion Assembly: A Close Relationship Studied Using Elastic Micropatterned Substrates. Nat. Cell Biol. 2001, 3, 466–472. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Keely, P.J. Mechanical Signaling through the Cytoskeleton Regulates Cell Proliferation by Coordinated Focal Adhesion and Rho GTPase Signaling. J. Cell Sci. 2011, 124, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Abrahamian, A.; Xi, M.-S.; Donnelly, J.J.; Rockey, J.H. Effect of Interferon-γ on the Expression of Transforming Growth Factor-β by Human Corneal Fibroblasts: Role in Corneal Immunoseclusion. J. Interf. Cytokine Res. 1995, 15, 323–330. [Google Scholar] [CrossRef]
- Bobade, C.D.; Nandi, S.; Kale, N.R.; Banerjee, S.S.; Patil, Y.N.; Khandare, J.J. Cellular Regeneration and Proliferation on Polymeric 3D Inverse-Space Substrates and the Effect of Doxorubicin. Nanoscale Adv. 2020, 2, 2315–2325. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Kayyali, U.S.; Sousa, A.M.; Rajan, T.; Lechleider, R.J.; Day, R.M. Transforming Growth Factor-Β1 Effects on Endothelial Monolayer Permeability Involve Focal Adhesion Kinase/Src. Am. J. Respir. Cell Mol. Biol. 2007, 37, 485–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, R.; Wang, M.; Zhao, C.; Shen, M.; Yu, Y.; He, L.; Zhao, Y.; Chen, H.; Shi, X.; Zhou, M.; et al. TFEB-Driven Autophagy Potentiates TGF-β Induced Migration in Pancreatic Cancer Cells. J. Exp. Clin. Cancer Res. 2019, 38, 340. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiaggia, G.; Taladriz-Blanco, P.; Hengsberger, S.; Septiadi, D.; Geers, C.; Lee, A.; Rothen-Rutishauser, B.; Petri-Fink, A. A Near-Infrared Mechanically Switchable Elastomeric Film as a Dynamic Cell Culture Substrate. Biomedicines 2023, 11, 30. https://doi.org/10.3390/biomedicines11010030
Spiaggia G, Taladriz-Blanco P, Hengsberger S, Septiadi D, Geers C, Lee A, Rothen-Rutishauser B, Petri-Fink A. A Near-Infrared Mechanically Switchable Elastomeric Film as a Dynamic Cell Culture Substrate. Biomedicines. 2023; 11(1):30. https://doi.org/10.3390/biomedicines11010030
Chicago/Turabian StyleSpiaggia, Giovanni, Patricia Taladriz-Blanco, Stefan Hengsberger, Dedy Septiadi, Christoph Geers, Aaron Lee, Barbara Rothen-Rutishauser, and Alke Petri-Fink. 2023. "A Near-Infrared Mechanically Switchable Elastomeric Film as a Dynamic Cell Culture Substrate" Biomedicines 11, no. 1: 30. https://doi.org/10.3390/biomedicines11010030
APA StyleSpiaggia, G., Taladriz-Blanco, P., Hengsberger, S., Septiadi, D., Geers, C., Lee, A., Rothen-Rutishauser, B., & Petri-Fink, A. (2023). A Near-Infrared Mechanically Switchable Elastomeric Film as a Dynamic Cell Culture Substrate. Biomedicines, 11(1), 30. https://doi.org/10.3390/biomedicines11010030





